Abstract
The CD200 receptor (CD200R) acts as a negative regulator of myeloid cells by interacting with its widely expressed ligand CD200. Using mutants expressed in U937 cells, we show that inhibition is mediated by the PTB domain binding motif (NPLY) in the receptor’s cytoplasmic region. The adaptor protein downstream of tyrosine kinase 2 (Dok2) bound directly to the phosphorylated NPLY motif with a tenfold higher affinity (KD ~ 1 μM at 37°C) than the closely related Dok1. Both of these proteins have been suggested to play a role in CD200R signaling in murine cells. Dok2 was phosphorylated in response to CD200R engagement and recruited RAS p21 protein activator 1 (RasGAP). Knockdown of Dok2 and RasGAP by RNA interference revealed that these proteins are required for CD200R signaling, while knockdown of Dok1 and the inositol 5-phosphatase SHIP did not affect CD200R mediated inhibition. We conclude that CD200R inhibits the activation of human myeloid cells through direct recruitment of Dok2 and subsequent activation of RasGAP, which distinguishes this receptor from the majority of inhibitory receptors that utilize immunoreceptor tyrosine-based inhibitory motifs (ITIM) and recruit phosphatases.
Keywords: Human, Monocyte/Macrophage, Signal Transduction
Introduction
The CD200 receptor (CD200R) is an immunoglobulin (Ig) superfamily transmembrane glycoprotein present on most leukocytes, but with relatively higher expression levels on cells of the myeloid lineage (1, 2). CD200R interacts with its structurally related but more widely expressed ligand CD200 through its N-terminal Ig V-type domain (3). The cytoplasmic tail of CD200R contains three conserved tyrosine residues, while CD200 has only a very short intracellular domain without any known signaling motifs (1, 2). The functional consequences of ligand engagement of CD200R were revealed in vivo in genetically manipulated mice lacking CD200. These mice exhibited a hyperactivated and hyperproliferative myeloid compartment and were more susceptible to induction of auto-immune disorders (4). The phenotype of mice lacking CD200R subsequently confirmed that the effects of CD200 deficiency were, indeed, due to loss of ligand induced inhibitory signalling through the receptor (5). In vitro studies showed that engagement of CD200R caused inhibition of cellular activation in human and mouse mast cells (5), macrophages (6, 7), mixed lymphocyte reactions (8, 9) and basophils (10).
The significance of the CD200/CD200R pathway for control of leukocyte activation is illustrated through its subversion by viruses which inhibit anti-viral host responses by expressing CD200-like proteins that mimic host-derived CD200 (7, 10-13). CD200 is also a marker for various human cancer or cancer stem cells, where it enhances evasion of immune recognition by inhibiting the activation of CD200R bearing leukocytes (9, 14-17).
CD200R is unusual amongst inhibitory receptors, as it does not contain any immunoreceptor tyrosine-based inhibitory motifs (ITIMs). ITIMS are present in a large number of inhibitory receptors and mediate inhibition through the recruitment of protein tyrosine phosphatases such as Src homology 2 domain-containing phosphatase (SHP) 1, SHP2, or the inositol phosphatase SHIP upon phosphorylation (18). The cytoplasmic region of CD200R contains three tyrosine residues of which the membrane distal one is located within a phosphotyrosine-binding (PTB) domain recognition motif (NPxY) (19). Interestingly, the chicken CD200R does contain an ITIM (NVIYNSV) instead of the PTB domain motif (NPLYDTV) found in human, mouse, rat and cow (1, 2, 20) suggesting that the mammalian receptor may possibly have evolved from an ITIM bearing precursor, which has been retained in chicken. The NPxY motif of murine CD200R has been suggested to bind the PTB domain-containing adaptor proteins downstream of tyrosine kinase 1 (Dok1) and Dok2 upon tyrosine phosphorylation, resulting in the recruitment of SHIP and RAS p21 protein activator 1 (RasGAP) (21, 22).
In this study, we investigated the molecular mechanisms of CD200R signaling in human myeloid cells. We show that Dok2 can directly interact with the NPxY motif of human CD200R and that Dok2 and RasGAP, but not Dok1 and SHIP are required for CD200R mediated cellular inhibition.
Materials and Methods
Antibodies
Polyclonal goat (sc-8130) and rabbit anti-human Dok2 (sc-13952), monoclonal mouse anti-human RasGAP (sc-63) and monoclonal mouse anti-human SHIP (sc-8425) antibodies were from Santa Cruz Biotechnology. A polyclonal rabbit anti-human Dok1 antibody (23) was a kind gift from Dominique Davidson and André Veillette. The monoclonal mouse anti-human CD200R antibody OX108 has been described previously (2). Biotinylated mouse monoclonal anti-phosphotyrosine antibody (B1531) and peroxidase conjugated polyclonal anti-mouse, anti-rabbit and anti-goat antibodies and ExtrAvidin® were from Sigma-Aldrich Ltd. Phycoerythrin-conjugated donkey anti-mouse IgG F(ab’)2 fragment (715–116–151) was from Jackson ImmunoReasearch Laboratories Inc.
CD200-COMP
Pentameric human CD200 (CD200-COMP) consisting of the extracellular region of human CD200 (2) linked to domains 3 and 4 of rat CD4 followed by an 11-amino-acid linker sequence (NSGGGSGGGTG) and the rat COMP (cartilage oligomeric matrix protein) oligomerization domain was generated as previously described (24). 293T cells were transiently transfected with pEF-BOS vector containing the CD200-COMP construct, and tissue culture supernatant was collected, concentrated and dialyzed into PBS. Protein activity was tested by surface plasmon resonance (SPR) on a BIAcore™ 3000, which showed strong binding to recombinant human CD200R with binding characteristics similar to those of OX108 mAb (24). Titrations of the concentrate were used in IL-8 assays with CD200R transduced U937 cells as described below to determine optimal working dilutions.
Generation of CD200R mutant cell lines
Mutants of human CD200R were generated by overlap extension PCR mutagenesis. Fragments were amplified from the wild-type gene using terminal and internal primers, with the N-terminal primer introducing a Bgl II restriction site and the internal primers overlapping and containing a single base change (A to T) to change a tyrosine to a phenylalanine codon of each of Y291, Y294 and Y302, referred to hereafter as Y1, Y2 and Y3. To generate a truncated mutant of the receptor, a stop codon followed by a Sal I restriction site was inserted, resulting in the removal of the last 40 residues (a.a. 286–325) of the cytoplasmic tail of human CD200R. The resulting PCR products were digested with Bgl II and Sal I and cloned into a BamH I and Xho I cut bicistronic, emerald (Em) enhanced green fluorescent protein (eGFP) expressing pHR-SIN-BX-IRES-Em lentiviral expression vector. The constructs were transfected into 293T cells at 60% confluence in 175 cm2 culture flasks. 15 μg expression vector, 10 μg pCMV-ΔR8.91 packaging vector and 10 μg pMD2G envelope plasmid that had been pre-incubated with polyethylenimine (PEI) at a ratio of 1:7 (w/w) for 15 min at room temperature was added to cell cultures in 20ml X-Vivo media. After overnight incubation, the media was replaced with 20 ml RPMI 1640 containing 5% FCS, penicillin and streptomycin, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. Supernatant containing viral particles was then collected and replaced with fresh media after 24, 48 and 72 h. Cells of the human monocyte-like histiocytic lymphoma line U937 were transduced by incubating for 24–48 h in neat supernatant and then purified based on CD200R expression by magnetic bead isolation using OX108 mAb on an autoMACS™ separator (Miltenyi Biotech). For some experiments, cells were sorted into high and low CD200R expressing populations by fluorescent-activated cell sorting (FACS) based on eGFP expression. Transduced cells were maintained at densities below 5×105 per ml prior to experimental use.
Preparation of IL-4 activated human macrophages
Monocytes (>90% pure) were isolated from healthy donor buffy coats (Bristol Blood Donor Services) by two-step centrifugation over Ficoll and 46% Percoll gradients (25) and differentiated into macrophages for 7 days in X-Vivo 10 supplemented with 1% autologous serum. Macrophages were alternatively activated by culturing for an additional 2 days in the presence of 20 ng/ml recombinant human IL-4 (Peprotech).
Cytokine assays
For IL-8 assays using CD200R transduced U937 cells, OX108 mAb diluted at the indicated concentrations in phosphate buffered saline (PBS) was immobilized overnight at 4°C to wells of a 96-well tissue culture plate. The plate was then blocked with PBS, 5% FCS at room temperature for at least 2 h, followed by addition of 5×104 U937 cells per well. For experiments using soluble OX108 or pentameric human CD200 (CD200-COMP), these reagents were added at the indicated concentrations to wells containing 5×104 U937 cells. In experiments using Maja cells, a CD200 positive human B cell line, to engage CD200R on the surface of U937 cells, 4×105 Maja cells that had been irradiated at 30 Gy were added to 105 U937 cells in the wells of a 96-well tissue culture plate. After 30 min incubation on ice, lipopolysaccharide (LPS), ethanol-killed Neisseria meningitides cells (26) or IFN-γ were added at the indicated concentrations to stimulate IL-8 production. The total volume of media was 200 μl per well. After overnight incubation at 37°C, supernatants were collected and assayed for the presence of IL-8 by ELISA (BD Pharmingen).
RNA interference
SiRNA duplexes containing 2 thymidine 3′ overhangs were purchased from Sigma-Aldrich Ltd. (siDok1, siDok2, siSHIP and siScr) and NBS Biologicals (siRasGAP and siNeg). The sequences were: 5′-GAAUGCUGCACCCGCUACA -3′ for siDok2, 5′-GGUCAUGUUCUCUUUCGAG -3′ for siDok1, 5′-GCUAAGUGCUUUACGAACA -3′ for siSHIP, 5′-ACGCAUGUACACACUCGCG -3′ for a randomly Dok-2 scrambled sequence (siScr) (27) and 5′-AUAAUGGAAAGCAAGCAGUCUUGUGAG-3′ for siRasGAP (28). 106 U937 cells sorted for high expression of wild-type or truncated human CD200R were transfected with 1.5–4 μg siRNA using kit C of the Amaxa nucleoporation system according to the manufacturer’s instructions. In order to maximize transfection efficiency, cells were re-transfected after 36 h. Cells were used in IL-8 cytokine assays with 2.5 μg/ml soluble OX108 and 50 ng/ml LPS as described above, 24–48 h after the second transfection. The remaining cells where washed in PBS and lysed at 2.5 × 107 cells/ml in NP-40 lysis buffer (10 mM Tris-HCl [pH 7.4], 140 mM NaCl, 1 mM EDTA, 10% (v/v) glycerol, 0.02% (w/v) NaN3, 1% (v/v) NP-40, 1 mM sodium pervanadate, 1mM NaF and 10% protease inhibitor cocktail for mammalian cells (Sigma)) for 20 min at 4°C. Lysates were cleared by centrifugation at 16000 g for 10 min at 4°C and equal amounts of protein (as determined by Bradford assay) were resolved under non-reducing conditions on NuPAGE® Bis-Tris gradient (4 to 12%) gels (Invitrogen). Proteins were transferred to nitrocellulose membranes in a Novex XCell II ™ Blot Module and western blotted using the SNAP i.d. ™ Protein Detection System (Millipore) to determine the effect of RNA interference on protein expression.
Immunoprecipitations
U937 cells expressing wild-type or truncated CD200R were pre-incubated on ice for 30 min at ~1.5 × 107 cells/ml in the presence of CD200-COMP concentrate diluted 1:100 in RPMI. Cells were then warmed to 37°C for 5 min, washed in ice-cold PBS containing 1mM sodium pervanadate and lysed for 10 min at 4°C. Lysates were cleared by centrifugation at 16,000 g for 10 min at 4°C and incubated for 30 min at 4°C in the presence of 1 μg of antibody per 107 cells. Protein G Sepharose beads (Amersham) were washed 3 times in PBS and re-suspended as a 50% slurry in lysis buffer of which ~10 μl was added per 107 cells. After incubating at 4°C for 30 min, beads were washed 3 times in PBS 1mM pervanadate. Proteins were eluted by boiling the beads at 95°C for 10 min in non-reducing SDS sample buffer, resolved on gradient gels and transferred and blotted with the indicated antibodies as described. For immunoprecipitations from primary human cells, 2 × 107 IL-4 activated human macrophages were cultured in T175 tissue culture flasks at 37 °C for 30 min in RPMI without supplements followed by addition of OX108 or OX21 mAb at a final concentration of 10 μg/ml. After 7 min at 37 °C, flasks were placed on ice and cultures washed once with ice-cold PBS, 1mM pervanadate. Cells were lysed at 107 cells/ml and immunoprecipitations were performed as described above.
BIAcore analysis
Recombinant human Dok1 PTB domain (amino acids 140–268), Dok2 PTB domain (amino acids 133–259) were provided by Louise Bird (Oxford Module Consortium [www.omc.ox.ac.uk]). Surface plasmon resonance analyses using a BIAcore™ 3000 were carried out essentially as described previously (29). In brief, ~4000 response units (RU) of streptavidin were immobilized at 25°C to CM5 chips by amine coupling followed by immobilization of 50–150 RU of biotinylated peptides (Peptide Protein Research Ltd. and Sigma). Flow cells with non-phosphorylated peptides or with streptavidin only were used as controls. Increasing concentrations of monomeric, recombinant, soluble protein were then passed over the chip at 37°C to determine equilibrium binding. Response units from empty control flow cells were subtracted from those of experimental flow cells and the resulting data points plotted and fitted to hyperbolas. Sequences of peptides from human proteins used in BIAcore experiments are shown in Table I.
Table I.
Peptides used in BIAcore experiments1
| Peptide | Sequence |
|---|---|
| CD200R pY0 | Biotin-DEMQPYASYTEKNNPLYDTTN |
| CD200R pY1 | Biotin-DEMQP(pY)ASYTEKNNPLYDTTN |
| CD200R pY3 | Biotin-DEMQPYASYTEKNNPL(pY)DTTN |
| CD200R pY1,2 | Biotin-DEMQP(pY)AS(pY)TEKNNPLYDTTN |
| CD200R pY1, 3 | Biotin-DEMQP(pY)ASYTEKNNPL(pY)DTTN |
| Dok1 pY146 | Biotin-EMLENSL(pY)SPTW |
| Dok2 pY139 | Biotin-CMEENEL(pY)SSAV |
| SHIP pY917 | Biotin-TEIINPN(pY)MGVGP |
| SHIP pY1022 | Biotin-EMFENPL(pY)GSLSS |
pY = phosphotyrosine.
Results
Generation of cell lines expressing mutant and wild-type human CD200R
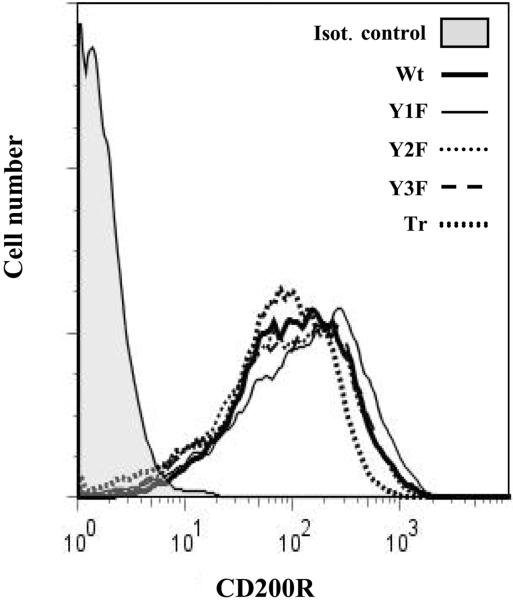
To generate a cellular system in which to dissect the signaling pathway of human CD200R, we used lentiviral transduction to create stable lines of U937 cells expressing wild-type or mutant (cytoplasmic domain truncated or with each of its three tyrosines mutated to phenylalanines) human CD200R. No endogenous CD200R was detected on untransduced U937 cells (data not shown). After transduction, cells expressing high levels of CD200R were purified by magnetic (MACS) bead isolation, which resulted in stable cell lines expressing similar levels of wild-type or mutant receptor (Fig. 1).
Fig. 1.
CD200R mutants are expressed on transduced U937 cells. U937 cells were lentivirally transduced to express wild-type (Wt) or signaling deficient (cytoplasmic domain truncated [Tr] or with each of its three tyrosines mutated to phenylalanines [Y1F, Y2F, Y3F]) human CD200R. Expression of wild-type and mutant CD200R is shown after magnetic (MACS) beads separation using OX108 mAb. Cells were stained with OX108 followed by phycoerythrin-conjugated anti-mouse IgG F(ab’)2.
CD200R engagement causes inhibition of IL-8 secretion from activated U937 cells
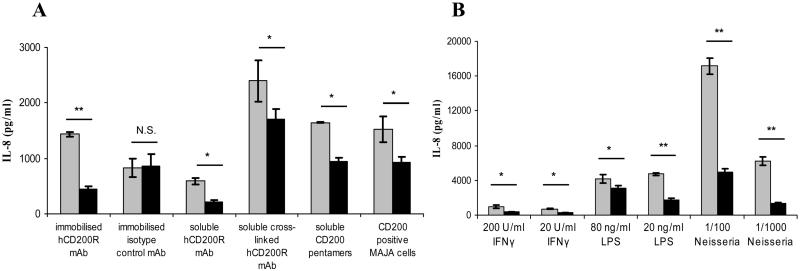
One previous study showed that ligation of CD200R on transfected U937 cells inhibits the secretion of various pro-inflammatory mediators including the neutrophil-chemoattractant IL-8 (CXCL8) (6). We confirmed this finding using U937 cells expressing wild-type and truncated human CD200R (Fig. 2). Generation of cells expressing a signaling deficient form of CD200R allows for the use of the same extracellular conditions in both samples and controls. This eliminates potential artefacts caused by different degrees of cell-cell contact or differences in Fc-receptor stimulation by antibodies. The inhibitory effect we observed did not depend on the method of receptor ligation; plate-bound or soluble mAb with or without secondary crosslinking antibody, soluble multimeric CD200 or CD200 expressing cells (Fig. 2A) or the type of activation stimulus; LPS, IFN-γ or ethanol-killed N. meningitides (Fig. 2B). U937 cells express no or minimal levels of CD200 and cells were maintained at densities which minimized cell contact. The inhibition caused by soluble reagents (CD200-COMP and OX108), which can block the CD200-CD200R interaction (data not shown and M. Foster-Cuevas and A. Neil Barclay, unpublished) is, therefore, not due to blocking the engagement of CD200R by endogenously expressed CD200, but rather represents an agonistic effect. Crosslinking with OX108 mAb thus mimics the effect of natural ligand engagement in our assays.
Fig. 2.
CD200R engagement inhibits activation of U937 cells. (A) Wild-type (black) and truncated (grey) CD200R were engaged on U937 cells using different reagents as indicated. Plate-bound OX108 or isotype control were immobilized at 40 μg/ml, soluble OX108 was used at 10 μg/ml and crosslinking anti-mouse IgG at 20 μg/ml. Cells were then stimulated overnight with 20 ng/ml LPS and culture supernatants assayed for IL-8 by ELISA. (B) Wild-type or truncated CD200R was engaged on U937 using 100 μg/ml plate-bound OX108 mAb and cells were stimulated overnight using different reagents as indicated. Neisseria = ethanol-killed Neisseria meningitides diluted in culture medium. * = p < 0.05 according to two-tailed student’s t-test, ** = p < 0.005, N.S. = non-significant. Results are expressed as means of duplicate or triplicate wells ± standard deviation and are representative of two or more independent experiments.
The effect of CD200R ligation on other cytokines was also investigated. LPS stimulation of CD200R transduced U937 cells did not elicit TNF and IL-1β secretion. IL-10 secretion was detectable and, similar to IL-8, was inhibited by soluble or plate-bound OX108 or CD200-COMP (data not shown). However, the effect on IL-10 was more variable and less pronounced than that on IL-8 secretion.
CD200R signaling is dependent on its third intracellular tyrosine residue
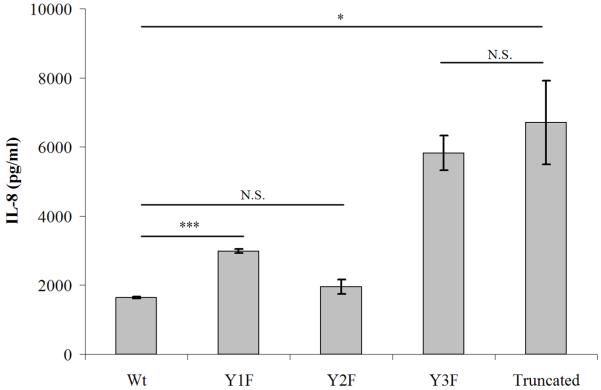
To determine the relative contribution of each of the three cytoplasmic tyrosine residues of human CD200R to signaling, we incubated U937 cells expressing wild-type or mutant (truncated, Y1F, Y2F and Y3F) CD200R in the presence of plate-bound OX108 mAb and 20 ng/ml LPS. IL-8 secretion was strongly inhibited in the presence of wild-type CD200R, while the Y3F mutation completely abrogated this effect. The Y1F mutation relieved inhibition only slightly, while the Y2F mutation had no functional effect (Fig. 3). Together, these results show that CD200R mediates active inhibitory signaling which is dependent on its third intracellular tyrosine residue. The first tyrosine plays a minor role and the second one is dispensable in this assay.
Fig. 3.
The innhibitory effect of CD200R is mainly mediated by its third intracellular tyrosine. CD200R (wild-type and mutants) was engaged on U937 cells using 100 μg/ml plate-bound OX108 mAb and stimulated and assayed as in Fig. 2. * = p < 0.05, *** = p < 0.0005, N.S. = non-significant. Results are expressed as means of triplicate wells ± standard deviation and are representative of six experiments. No significant effects of tyrosine mutations were observed in the absence of OX108 mAb or in the presence of an isotype control mAb (data not shown).
The third intracellular tyrosine residue of CD200R binds Dok2 with higher affinity than Dok1
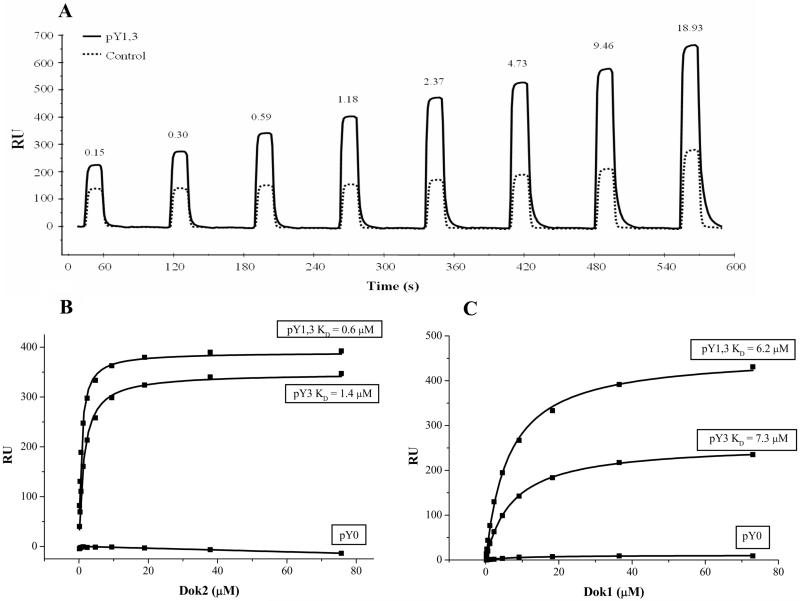
Previous studies suggest that the cytoplasmic domain of murine CD200R, when phosphorylated, can recruit the adaptor molecules Dok1 and Dok2 (21, 22). We used SPR to determine whether these interactions occur in human cells and whether they are direct or indirect. We measured direct binding of both Dok proteins to phosphorylated peptides corresponding to parts of the human CD200R cytoplasmic domain (Fig. 4). Dok2 and Dok1 both bound to two phosphopeptides (pY3 and pY1,3), but Dok2 bound with a tenfold higher affinity (KD ~ 1 μM at 37°C; Fig. 4B) than Dok1 (Fig. 4C). The difference in binding affinity between Dok1 and Dok2 was not due to differences in protein activity, as Dok1 bound more strongly than Dok2 to several other peptides (Table II and data not shown). The interaction between CD200R and Dok2 was dependent on phosphorylation of the functionally most important third tyrosine residue. Neither Dok1 nor Dok2 bound strongly to the first or second tyrosine when these were phosphorylated alone or in combination (Table II), although co-phosphorylation of the first tyrosine caused a slight increase in affinity compared to phosphorylation of the third tyrosine alone (Fig. 2).
Fig. 4.
Dok2 binds the cytoplasmic tail of CD200R with higher affinity than Dok1. Singly (pY3) or doubly (pY1,3) phosphorylated peptides corresponding to parts of the cytoplasmic tail of human CD200R were immobilized on a BIAcore chip. (A) Binding of various concentrations (μM) of recombinant PTB domain of human Dok2 passed over flow cells containing immobilized unphosphorylated (pY0) control or pY1,3 phosphopeptide. (B) and (C), Equilibrium binding values at each concentration for Dok2 (B) and Dok1 (C) binding to pY3 and pY1,3 phosphopeptides. The hyperbolas represent best fits used for affinity calculations. Results are representative of two independent experiments.
Table II.
Dissociation constants of interactions between Dok PTB domains and biotinylated peptides2
| Dok1 PTB | Dok2 PTB | |
|---|---|---|
| CD200R pY0 | N.B. | N.B. |
| CD200R pY1 | 14 μM | N.B. |
| CD200R pY1,2 | 8.4 μM | N.B. |
| CD200R pY3 | 7.3 μM | 1.4 μM |
| CD200R pY1,3 | 6.2 μM | 0.6 μM |
| Dok1 pY146 | 6.9 μM | N.B. |
| Dok2 pY139 | 3.3 μM | N.B. |
| SHIP pY917 | 13 μM | > 30 μM* |
| SHIP pY1022 | 13 μM | N.B. |
N.B. = no binding (KD > 50 μM or no specific interaction detectable).
Interaction was dependent on high peptide density and was not observed under standard conditions. Results are representative of at least two experiments.
Hierarchy of interactions suggests indirect recruitment of Dok1 and SHIP
Dok1 and Dok2 have been reported to form phosphorylation dependent homo- and heterodimers resulting from an interaction between their PTB domains and Tyr146 of Dok1 or Tyr139 of Dok2 (30, 31). To investigate the possibility of indirect recruitment of Dok1 to the CD200R signaling complex, we measured interactions between both Dok proteins and peptides corresponding to sequences surrounding these tyrosines. Dok2 did not bind to either of the two peptides. The PTB domain of Dok1, on the other hand, was found to interact with both peptides, but with a slight preference for the Dok2 derived sequence (Table II). This suggests that Dok1 may be recruited to CD200R indirectly via Dok2.
Dok1 has been shown to interact directly with the inositol phosphatase SHIP, and this interaction was dependent on both, the PTB domain of Dok1 and the SH2 domain of SHIP (32). The C-terminal part of SHIP contains two NPxY motifs that are likely to be involved in the formation of this complex. We measured interactions between Dok proteins and phosphopeptides corresponding to sequences surrounding these NPxY motifs. Dok2 did not bind to the sequence surrounding Tyr1022 and only very weakly (KD > 30μM) to the sequence surrounding Tyr917 of SHIP. The PTB domain of Dok1 bound both of the SHIP NPxY motifs, although this interaction was not particularly strong (Table II). Sequence analysis of Dok1 and Dok2 in the context of a published SHIP SH2 domain consensus sequence (33) suggests that SHIP is more likely to bind to Dok1 (especially Tyr377 and Tyr398) than Dok2.
Engagement of CD200R on U937 cells causes phosphorylation of Dok2 and recruitment of RasGAP
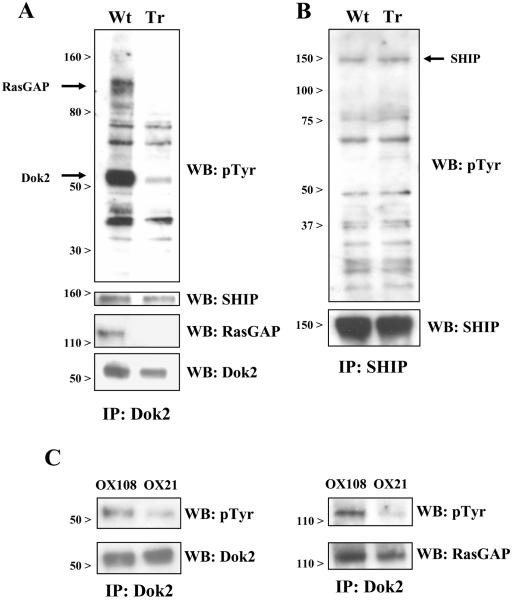
As recruitment of downstream effector molecules via Dok2 depends on phosphorylation of its C-terminal tail (34), we tested whether CD200R engagement results in phosphorylation of Dok2 in human myeloid cells. Wild-type or truncated CD200R was engaged on the surface of U937 cells using soluble CD200-COMP, followed by lysis and immunoprecipitation of Dok2. Analysis of precipitates by western blot showed that engagement of wild-type but not truncated CD200R caused phosphorylation of Dok2 (Fig. 5A).
Fig. 5.
Phosphorylation of Dok2 and recruitment of RasGAP following engagement of CD200R. U937 cells expressing wild-type or truncated CD200R were incubated for 5 min at 37°C in the presence of CD200-COMP. Cells were then lysed and Dok2 (A) or SHIP (B) was immunoprecipitated from lysates. Immunoprecipitates were blotted with biotinylated anti-phosphotyrosine mAb. Membranes were then stripped and re-blotted with specific antibodies against the indicated proteins. Results are representative of at least three independent experiments. (C) IL-4 activated human macrophages were treated with CD200R mAb (OX108) or isotype control (OX21) for 7 min, followed by Dok2 immunoprecipitation and Western blotting with the indicated antibodies. Results are representative of two independent experiments conducted with macrophages from three different donors.
Concomitant with phosphorylation of Dok2, engagement of CD200R resulted in recruitment of RasGAP (Fig. 5A). Phosphorylation of Dok2 and recruitment of RasGAP were dependent on signalling by the intact cytoplasmic region of CD200R. The levels of SHIP co-precipitated with Dok2 from lysates of cells expressing wild type or truncated CD200R were the same (Fig. 5A), suggesting that SHIP co-precipitation was non-specific. Lack of a specific effect of CD200R on SHIP activity was confirmed by immunoprecipitation of SHIP (Fig. 5B), which showed no difference in SHIP phosphorylation or its interaction with other phosphoproteins in response to CD200R engagement (Fig. 5B).
To determine whether Dok2 and RasGAP are also involved in CD200R signaling in primary human cells, Dok2 was immunoprecipitated from IL-4 activated macrophages that had been treated with CD200R mAb (OX108) or isotype control (OX21). Treatment of macrophages with OX108 resulted in phosphorylation of Dok2 and recruitment and phosphorylation of RasGAP. RasGAP also co-precipitated with Dok2 in lysates of OX21 treated cells, but phosphorylation of Dok2 and RasGAP was substantially stronger with OX108 treatment (Fig. 5 C). No phosphoproteins corresponding to the molecular weight of Dok1 or SHIP were detectable (data not shown).
Dok2 and RasGAP are essential for CD200R signaling
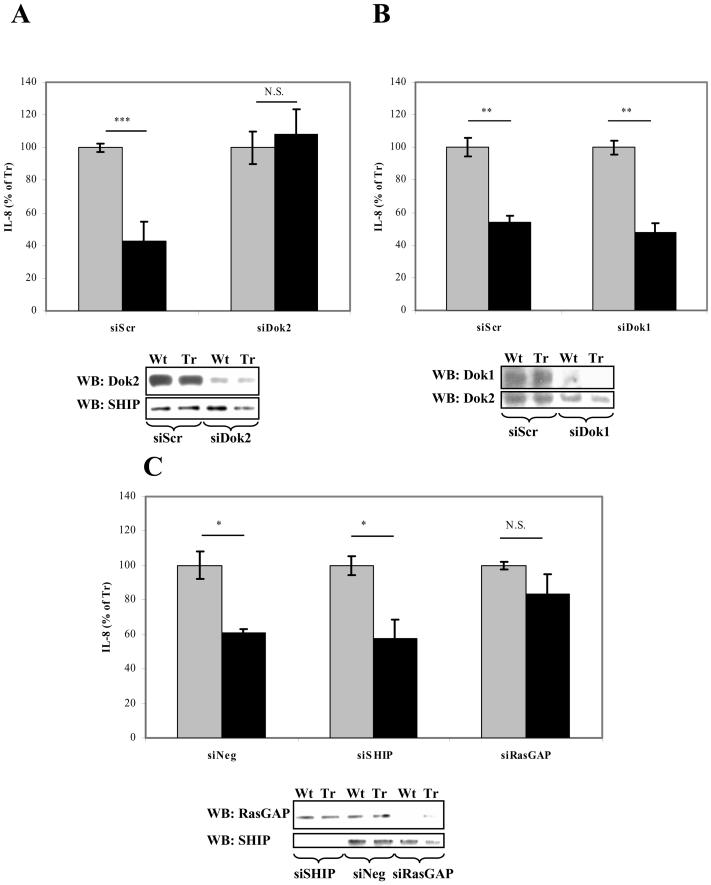
Our functional analysis of mutant receptors and SPR binding data indicate that Dok2 binding to phosphorylated Y3 and recruitment of RasGAP is essential for signalling by CD200R, while Dok1 and SHIP are less important. To test this, we used RNA interference to knock down expression of Dok1, Dok2, SHIP and RasGAP in CD200R transduced U937 cells and assess the effects on CD200R signalling. We compared the effects of the RNAi on CD200R mAb (OX108) induced CD200R signalling in cells expressing wild type and truncated receptor (Fig. 6). Knockdown of Dok2 completely abrogated CD200R mediated inhibition of IL-8 secretion from LPS stimulated U937 cells (Fig. 6A). In contrast, knockdown of Dok1 did not affect CD200R signalling in this assay (Fig. 6B).
Fig. 6.
Knockdown of Dok2 and RasGAP abrogates CD200R signaling in U937 cells. U937 cells expressing wild-type (black) or truncated (grey) CD200R were transfected twice with siRNA against Dok2 (A), Dok1 (B), SHIP or RasGAP (C). 24–48 h after the second transfection, cells were incubated overnight in the presence of 2.5 μg/ml soluble OX108 mAb and 50 ng/ml LPS and the supernatants assayed for IL-8. The remaining cells were lysed and lysates were western blotted to assess the degree and specificity of protein knockdown. A scrambled (siScr) Dok2 siRNA sequence or a non-targeting control siRNA sequence (siNeg) was used as negative control. * = p < 0.05, ** = p < 0.005, *** = p < 0.0005, N.S. = non-significant. Results are expressed as means of triplicate wells normalized to the amount of IL-8 produced by cells expressing the truncated CD200R ± standard deviation. Results are representative of at least three independent experiments.
Knockdown of RasGAP also abrogated CD200R signalling consistent with this being an effector enzyme recruited by CD200R via Dok2. Knockdown of SHIP, on the other hand, had no specific effect on CD200R mediated inhibition (Fig. 6C), although it increased overall IL-8 production by about 30–50% (data not shown). Thus our results suggest essential roles for Dok2 and RasGAP, but not Dok1 and SHIP in human CD200R signalling.
Discussion
The CD200R mediates inhibitory signals but is distinguished from the large number of other leukocyte inhibitory receptors that function by recruiting phosphatases through ITIM motifs (18). We show that the membrane distal tyrosine in CD200R is essential for CD200R mediated inhibition of IL-8 secretion in U937 cells and that a phosphotyrosine peptide from this region binds the adaptor Dok2 through its PTB domain. The affinity of Dok2 for this peptide (KD ~ 1 μM at 37°C) is within the range of other functional interactions between phosphotyrosine peptides derived from cell surface receptors and cytoplasmic adaptor or signaling proteins that have been measured at physiological temperature (29, 35). It is about tenfold weaker than that reported for the PTB domain of Src homology 2 domain containing transforming protein (Shc), but these measurements were made at lower temperatures (36), and Shc does not bind strongly to CD200R ((19) and our unpublished observations). The direct interaction between CD200R and Dok2 is thus likely to be crucial in initiating signaling, and its involvement is confirmed by our RNA interference experiments (Fig. 6).
Studies on murine CD200R suggested that Dok1 and Dok2 are involved in CD200R signaling as both were precipitated from mouse mast cells using phosphopeptides corresponding to sequences in the cytoplasmic domain of murine CD200R and were phosphorylated and co-precipitated CD200R in response to receptor engagement (21). We also observed phosphorylation of Dok2 (Fig. 5A) and Dok1 (unpublished) in response to CD200R engagement in human cells. However we found that Dok1 bound much more weakly to CD200R peptides than Dok2, and RNA interference of Dok1 expression had no effect on CD200R signaling in our assays (Fig. 6). Dok1 and Dok2 form phosphorylation dependent homo- and heterodimers (30, 31), and our BIAcore experiments show that Dok1 has a twofold higher affinity for phosphorylated Dok2 than for CD200R. Dok1 is thus more likely to be recruited to CD200R via Dok2 than by direct association with CD200R.
The first two intracellular tyrosine residues of CD200R are also conserved between human and mouse. An inhibitory role for the phosphorylated membrane proximal tyrosine demonstrated in mouse CD200R signalling (22) was substantiated in our human model. The contribution of Y1 to CD200R signalling was greater in mouse cells (22) than we observed in our human system. This may either be due to species differences or variations in assay sensitivity. Neither Dok1 nor Dok2 bound strongly to CD200R peptides phosphorylated at the first and/or second tyrosine residues although co-phosphorylation of Y1 resulted in a slightly higher affinity of both Dok proteins for Y3. Phosphorylation of the first tyrosine may thus stabilize interactions with the third. In neither mouse (22) nor human in vitro models, was an effect of the second tyrosine detected.
Inhibitory signalling of CD200R was functionally dependent on Dok2, as knockdown of Dok2 completely abrogated CD200R mediated inhibition of IL-8 secretion. Knockdown of Dok1 had virtually no effect. Thus the functional data are consistent with the hierarchy of interactions established through quantitative biochemistry. Dok1 and 2 play a role in various inhibitory signaling pathways, downstream of tyrosine kinases and growth factor receptors (summarized in (37)) and are involved in the negative regulation of B-cell receptor (38) and T cell-receptor signaling (27, 39). The primary signalling mechanisms of these receptors are mediated by activating tyrosine kinases, while the Dok proteins only play secondary, inhibitory roles in down-regulating or terminating these pathways. Thus CD200R appears to be unusual in that it uses a Dok protein as the initiators of its primary signaling pathway.
Upon phosphorylation of their C-terminal tyrosine residues, Dok1 and Dok2 can recruit SH2 domain containing proteins, most notably RasGAP (40, 41). RasGAP recruitment is essential for the ability of Dok1/2 to inhibit Ras-ERK signaling (30, 34). This interaction involves five tyrosines in Dok1 (42) and at least two tyrosines in Dok2 (34) indicating that RasGAP recruitment is the primary function of these adaptor proteins. RasGAP is one of several Ras GTPase activating proteins and an important negative regulator of the Ras-ERK and PI3K signaling pathways (summarized in (43)). The functional significance of these pathways can be inferred from the observation that mutations or mis-expression of Ras are found in approximately 30% of all human cancers (44). Our immunoprecipitation and RNA interference experiments suggest that recruitment of RasGAP is the primary mechanism by which Dok2 affects cellular activation in response to CD200R engagement in human myeloid cells.
The inositol phosphatase SHIP has been shown to be phosphorylated and bind Dok1 in response to CD200R signaling in mouse mast cells (21). We did not observe any effect of CD200R engagement on SHIP phosphorylation or its interaction with other phosphoproteins in our human system. Moreover, knockdown of SHIP had no effect on CD200R signaling in our RNA interference experiments. In agreement with studies in mouse cells (21), BIAcore experiments (Table II) and the SHIP SH2 domain consensus sequence (33) suggest that SHIP is more likely to interact with Dok1 than Dok2. Neither Dok1 nor SHIP, however, was found to play an important role in CD200R mediated inhibition of IL-8 secretion in our assays. The interaction between Dok1 and SHIP has been shown to be dependent on both, the PTB domain of Dok1 and the SH2 domain of SHIP (32). Quantitative binding analyses show that the affinity of the PTB domain of Dok1 for two phosphorylated NPxY motifs in SHIP is rather weak (KD ~ 13 μM at 37°C; Table II), but binding of the SHIP SH2 domain to phosphorylated tyrosine residues in Dok1 may stabilize this complex. SHIP is thus unlikely to bind to Dok1 if the latter’s PTB domain is already occupied, but may form complexes with phosphorylated free Dok1.
Our results show that CD200R signals through a pathway involving recruitment and activation of RasGAP by the adaptor molecule Dok2. This pathway is novel in so far as it causes cellular inhibition independent of phosphatases and because it uses Dok2 as a primary signal transducer rather than a modulator of other pathways.
Acknowledgements
We thank Oreste Acuto for helpful discussions and for providing the pHR-SIN-BX-IRES-Em lentiviral expression vector, Louise Bird and the Oxford Module Consortium for providing recombinant Dok1 and Dok2 PTB domains, Fernando Martinez for providing human macrophages, Deborah Hatherley and Sam Scheuringer for generating the mutant CD200R constructs, Subhankar Mukhopadhyay for ethanol-killed Neisseria meningitides and Nigel Rust for cell sorting.
Abbreviations used in this paper
- CD200R
CD200 receptor
- Dok
downstream of tyrosine kinase
- PTB
phosphotyrosine binding
- RasGAP
RAS p21 protein activator 1
- RU
response units
- SH2
SRC homology 2
- Shc
Src homology 2 domain-containing transforming protein
- SHP
SRC homology 2 domain-containing phosphatase
- siRNA
small interfering RNA
- SPR
surface plasmon resonance
Footnotes
This study was supported by an Oxford University Clarendon Fund award to RM and by the UK Medical Research Council (ref. G0601169 and G0400808).
References
- 1.Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 2.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 3.Hatherley D, Barclay AN. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur. J. Immunol. 2004;34:1688–1694. doi: 10.1002/eji.200425080. [DOI] [PubMed] [Google Scholar]
- 4.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 5.Cherwinski HM, Murphy CA, Joyce BL, Bigler ME, Song YS, Zurawski SM, Moshrefi MM, Gorman DM, Miller KL, Zhang S, Sedgwick JD, Phillips JH. The CD200 receptor is a novel and potent regulator of murine and human mast cell function. J. Immunol. 2005;174:1348–1356. doi: 10.4049/jimmunol.174.3.1348. [DOI] [PubMed] [Google Scholar]
- 6.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 7.Foster-Cuevas M, Wright GJ, Puklavec MJ, Brown MH, Barclay AN. Human herpesvirus 8 K14 protein mimics CD200 in down-regulating macrophage activation through CD200 receptor. J. Virol. 2004;78:7667–7676. doi: 10.1128/JVI.78.14.7667-7676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorczynski RM. Transplant tolerance modifying antibody to CD200 receptor, but not CD200, alters cytokine production profile from stimulated macrophages. Eur. J. Immunol. 2001;31:2331–2337. doi: 10.1002/1521-4141(200108)31:8<2331::aid-immu2331>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, Ravey EP, Qin F, Bowdish KS. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiratori I, Yamaguchi M, Suzukawa M, Yamamoto K, Lanier LL, Saito T, Arase H. Down-regulation of basophil function by human CD200 and human herpesvirus-8 CD200. J. Immunol. 2005;175:4441–4449. doi: 10.4049/jimmunol.175.7.4441. [DOI] [PubMed] [Google Scholar]
- 11.Cameron CM, Barrett JW, Liu L, Lucas AR, McFadden G. Myxoma virus M141R expresses a viral CD200 (vOX-2) that is responsible for down-regulation of macrophage and T-cell activation in vivo. J. Virol. 2005;79:6052–6067. doi: 10.1128/JVI.79.10.6052-6067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voigt S, Sandford GR, Hayward GS, Burns WH. The English strain of rat cytomegalovirus (CMV) contains a novel captured CD200 (vOX2) gene and a spliced CC chemokine upstream from the major immediate-early region: further evidence for a separate evolutionary lineage from that of rat CMV Maastricht. J. Gen. Virol. 2005;86:263–274. doi: 10.1099/vir.0.80539-0. [DOI] [PubMed] [Google Scholar]
- 13.Langlais CL, Jones JM, Estep RD, Wong SW. Rhesus rhadinovirus R15 encodes a functional homologue of human CD200. J. Virol. 2006;80:3098–3103. doi: 10.1128/JVI.80.6.3098-3103.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, Mohler T, De Vos J, Rossi JF, Goldschmidt H, Klein B. CD200 is a new prognostic factor in Multiple Myeloma. Blood. 2006;108:4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki BT, Mistree T, Hurt EM, Kalathur M, Farrar WL. Co-expression of the toleragenic glycoprotein, CD200, with markers for cancer stem cells. Biochem. Biophys. Res. Commun. 2007;364:778–782. doi: 10.1016/j.bbrc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D, Frederickson S, Maruyama T, Wild MA, Nolan MJ, Wu D, Springhorn J, Bowdish KS. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J. Immunol. 2007;178:5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- 17.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 18.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol. Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith MJ, Hardy WR, Murphy JM, Jones N, Pawson T. Screening for PTB domain binding partners and ligand specificity using proteome-derived NPXY peptide arrays. Mol. Cell. Biol. 2006;26:8461–8474. doi: 10.1128/MCB.01491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viertlboeck BC, Hanczaruk MA, Schmitt FC, Schmitt R, Gobel TW. Characterization of the chicken CD200 receptor family. Mol. Immunol. 2008;45:2097–2105. doi: 10.1016/j.molimm.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Phillips JH. Identification of tyrosine residues crucial for CD200R-mediated inhibition of mast cell activation. J. Leukoc. Biol. 2006;79:363–368. doi: 10.1189/jlb.0705398. [DOI] [PubMed] [Google Scholar]
- 23.Lemay S, Davidson D, Latour S, Veillette A. Dok-3, a novel adapter molecule involved in the negative regulation of immunoreceptor signaling. Mol. Cell. Biol. 2000;20:2743–2754. doi: 10.1128/mcb.20.8.2743-2754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voulgaraki D, Mitnacht-Kraus R, Letarte M, Foster-Cuevas M, Brown MH, Barclay AN. Multivalent recombinant proteins for probing functions of leucocyte surface proteins such as the CD200 receptor. Immunology. 2005;115:337–346. doi: 10.1111/j.1365-2567.2005.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locati M, Deuschle U, Massardi ML, Martinez FO, Sironi M, Sozzani S, Bartfai T, Mantovani A. Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J. Immunol. 2002;168:3557–3562. doi: 10.4049/jimmunol.168.7.3557. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay S, Peiser L, Gordon S. Activation of murine macrophages by Neisseria meningitidis and IFN-gamma in vitro: distinct roles of class A scavenger and Toll-like pattern recognition receptors in selective modulation of surface phenotype. J. Leukoc. Biol. 2004;76:577–584. doi: 10.1189/jlb.0104014. [DOI] [PubMed] [Google Scholar]
- 27.Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O, Michel F. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J. Exp. Med. 2006;203:2509–2518. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pamonsinlapatham P, Gril B, Dufour S, Hadj-Slimane R, Gigoux V, Pethe S, L’Hoste S, Camonis J, Garbay C, Raynaud F, Vidal M. Capns1, a new binding partner of RasGAP-SH3 domain in K-Ras(V12) oncogenic cells: modulation of cell survival and migration. Cell. Signal. 2008;20:2119–2126. doi: 10.1016/j.cellsig.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson NG, Simmonds SJ, Puklavec MJ, Brown MH. Direct and indirect interactions of the cytoplasmic region of CD244 (2B4) in mice and humans with FYN kinase. J. Biol. Chem. 2007;282:25385–25394. doi: 10.1074/jbc.M704483200. [DOI] [PubMed] [Google Scholar]
- 30.Songyang Z, Yamanashi Y, Liu D, Baltimore D. Domain-dependent function of the rasGAP-binding protein p62Dok in cell signaling. J. Biol. Chem. 2001;276:2459–2465. doi: 10.1074/jbc.M005504200. [DOI] [PubMed] [Google Scholar]
- 31.Boulay I, Nemorin JG, Duplay P. Phosphotyrosine binding-mediated oligomerization of downstream of tyrosine kinase (Dok)-1 and Dok-2 is involved in CD2-induced Dok phosphorylation. J. Immunol. 2005;175:4483–4489. doi: 10.4049/jimmunol.175.7.4483. [DOI] [PubMed] [Google Scholar]
- 32.Dunant NM, Wisniewski D, Strife A, Clarkson B, Resh MD. The phosphatidylinositol polyphosphate 5-phosphatase SHIP1 associates with the dok1 phosphoprotein in bcr-Abl transformed cells. Cell. Signal. 2000;12:317–326. doi: 10.1016/s0898-6568(00)00073-5. [DOI] [PubMed] [Google Scholar]
- 33.Sweeney MC, Wavreille AS, Park J, Butchar JP, Tridandapani S, Pei D. Decoding protein-protein interactions through combinatorial chemistry: sequence specificity of SHP-1, SHP-2, and SHIP SH2 domains. Biochemistry. 2005;44:14932–14947. doi: 10.1021/bi051408h. [DOI] [PubMed] [Google Scholar]
- 34.Lock P, Casagranda F, Dunn AR. Independent SH2-binding sites mediate interaction of Dok-related protein with RasGTPase-activating protein and Nck. J. Biol. Chem. 1999;274:22775–22784. doi: 10.1074/jbc.274.32.22775. [DOI] [PubMed] [Google Scholar]
- 35.Hassan NJ, Simmonds SJ, Clarkson NG, Hanrahan S, Puklavec MJ, Bomb M, Barclay AN, Brown MH. CD6 regulates T-cell responses through activation-dependent recruitment of the positive regulator SLP-76. Mol. Cell. Biol. 2006;26:6727–6738. doi: 10.1128/MCB.00688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou MM, Harlan JE, Wade WS, Crosby S, Ravichandran KS, Burakoff SJ, Fesik SW. Binding affinities of tyrosine-phosphorylated peptides to the COOH-terminal SH2 and NH2-terminal phosphotyrosine binding domains of Shc. J. Biol. Chem. 1995;270:31119–31123. doi: 10.1074/jbc.270.52.31119. [DOI] [PubMed] [Google Scholar]
- 37.Liang X, Wisniewski D, Strife A, Shivakrupa, Clarkson B, Resh MD. Phosphatidylinositol 3-kinase and Src family kinases are required for phosphorylation and membrane recruitment of Dok-1 in c-Kit signaling. J. Biol. Chem. 2002;277:13732–13738. doi: 10.1074/jbc.M200277200. [DOI] [PubMed] [Google Scholar]
- 38.Yamanashi Y, Tamura T, Kanamori T, Yamane H, Nariuchi H, Yamamoto T, Baltimore D. Role of the rasGAP-associated docking protein p62(dok) in negative regulation of B cell receptor-mediated signaling. Genes Dev. 2000;14:11–16. [PMC free article] [PubMed] [Google Scholar]
- 39.Yasuda T, Bundo K, Hino A, Honda K, Inoue A, Shirakata M, Osawa M, Tamura T, Nariuchi H, Oda H, Yamamoto T, Yamanashi Y. Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int. Immunol. 2007;19:487–495. doi: 10.1093/intimm/dxm015. [DOI] [PubMed] [Google Scholar]
- 40.Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 41.Yamanashi Y, Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell. 1997;88:205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 42.Kashige N, Carpino N, Kobayashi R. Tyrosine phosphorylation of p62dok by p210bcr-abl inhibits RasGAP activity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2093–2098. doi: 10.1073/pnas.040547997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakrabarty K, Heumann R. Prospective of Ras signaling in stem cells. Biol Chem. 2008;389:791–798. doi: 10.1515/BC.2008.104. [DOI] [PubMed] [Google Scholar]
- 44.Pamonsinlapatham P, Hadj-Slimane R, Lepelletier Y, Allain B, Toccafondi M, Garbay C, Raynaud F. P120-Ras GTPase activating protein (RasGAP): a multi-interacting protein in downstream signaling. Biochimie. 2009;91:320–328. doi: 10.1016/j.biochi.2008.10.010. [DOI] [PubMed] [Google Scholar]