Abstract
The effect of diet supplemented with red beet (Beta vulgaris L.) leaf on antioxidant status of plasma and tissue was investigated in C57BL/6J mice. The mice were randomly divided into two groups after one-week acclimation, and fed a high fat (20%) and high cholesterol (1%) diet without (control group) or with 8% freeze-dried red beet leaf (RBL group) for 4 weeks. In RBL mice, lipid peroxidation determined as 2-thiobarbituric acid-reactive substances (TBARS value) was significantly reduced in the plasma and selected organs (liver, heart, and kidney). Levels of antioxidants (glutathione and β-carotene) and the activities of antioxidant enzyme (glutathione peroxidase) in plasma and liver were considerably increased, suggesting that antioxidant defenses were improved by RBL diet. Comet parameters such as tail DNA (%), tail extent moment, olive tail moment and tail length were significantly reduced by 25.1%, 49.4%, 35.4%, and 23.7%, respectively, in plasma lymphocyte DNA of RBL mice compared with control mice, and indicated the increased resistance of lymphocyte DNA to oxidative damage. In addition, the RBL diet controlled body weight together with a significant reduction of fat pad (retroperitoneal, epididymal, inguinal fat, and total fat). Therefore, the present study suggested that the supplementation of 8% red beet leaf in high fat high cholesterol diet could prevent lipid peroxidation and improve antioxidant defense system in the plasma and tissue of C57BL/6J mice.
Keywords: Antioxidant, DNA, mice, red beet leaf, TBARS value
Introduction
Reactive oxygen species (ROS) are highly reactive molecules due to having unpaired valence shell electrons, and react with all classes of biological molecules resulting in oxidative stress (Aviram, 2000; Parthasarathy et al., 1999; Stehbens, 1999). In human and animal body, ROS can be neutralized by antioxidant defense systems including antioxidant enzymes (Fang et al., 2002) and antioxidant compounds (Catapano et al., 2000; Duthie & Bellizzi, 1999). However, excessive ROS production and the depleted antioxidant defenses lead to oxidative stress and induce oxidative damage, causing pathological dysfunction in the organism (Urso & Clarkson, 2003). Further, the oxidative stress is involved in the pathogenesis of chronic diseases such as neurodegenerative disease and coronary heart disease (CHD) (Nishimura et al., 2000; Urso & Clarkson, 2003). Evidence from the cohort studies supported the view that a sufficient intake of fruits and vegetables is inversely associated with the risk of chronic diseases (Dauchet et al., 2006; Dyun & Pivonka, 2000) and a number of possible mechanisms have been proposed with antioxidant nutrients through lowering oxidative stress (Ames et al., 1995). Therefore, much attention has been focused on natural antioxidants in fruits and vegetables (Ames et al., 1995).
Red beet (Beta vulgaris L.) leaf is a good source of natural antioxidants such as betalains, flavonoids, polyphenols, vitamins, and folic acid. Total phenol content in red beet is the highest among 23 vegetables that have been studied (Vinson et al., 1998). Betalains comprise betacyanins (red-violet pigments) and betazanthins (yellow pigments). In red beet leaf, ROS induced a synthesis of betacyanin which could act as ROS scavengers, limiting damage caused by bacterial infection and wounding (Sepúlveda-Jiménez et al., 2004). Betanin, the main betacyanin, showed strong antioxidant effects in lipid peroxidation of membranes and inhibition of LDL oxidation, in which the oxidation rate was better than that by catechin (Kanner et al., 2001).
Most studies that have assessed the beneficial effect of red beet have been limited to the root. Red beet leaf is consumed in salad with other vegetables world-wide. In Korea, leafy vegetables including red beet leaf are normally provided for wrapping cooked rice or meat, and such a diet is popular for a meat-containing menu. The supplementation of 8% leafy vegetable powder in atherogenic diet showed a prevention of lipid peroxidation and an increase of the antioxidant defense system in plasma and liver of mice (Lee et al., 2009). However, little is known about the biochemical effect of red beet leaf. Therefore, in the present study, C57BL/6J mice were fed high fat high cholesterol diet supplemented with red beet leaf (freeze-dried, 8% of diet) for 4 weeks, and its protective effect against oxidative damage was evaluated by antioxidant biomarkers in blood and liver tissue.
Materials and Methods
Materials
Red beet leaf (Beta vulgaris L.) was obtained from an organic farm (Gongju, Korea) and freeze-dried. The contents of total phenolic compounds and β-carotene contents in freeze-dried red beet leaf were determined with the methods described by Teow et al. (2007). The betanin and α-tocopherol contents were analyzed in accordance with the methods described by Sepúlveda-Jiménez et al. (2004) and Lee et al. (2006), respectively. Glutathione reductase (Type III from bakers yeast), reduced glutathione, oxidized glutathione, tetramethoxypropane, 5,5'-dithio-2-nitrobenzoic acid (DTNB), NADP, NADPH, thiobarbituric acid (TBA) and bovine serum albumin were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). All reagents used were analytical grade.
Animal experiments
Male C57BL/6J mice (6-8 weeks of age, Damul Science Co. Deajeon) weighing 25-30 g were kept in polycarbonate cages (identified with ear punch method) with controlled temperature (23 ± 3℃) and humidity level (55 ± 10%) under a 12 h light:dark cycle. The mice were fed pelleted commercial diet (Samyang Co., Seoul, Korea) for the first week. After acclimation, the mice were randomly divided into two treatment groups of 8 mice each, and allowed free access to water and assigned diets for 4 weeks. The control diet contained high fat (20% fat and 1% cholesterol) and AIN 93 mineral and vitamin mixtures (Table 1). The experimental diet (RBL) was supplemented with 8% freeze-dried red beet leaf, and its diet composition of starch, sucrose, cellulose, mineral mixture and vitamin mixture was adjusted to be isoenergetic with control diet (Table 1). The chemical composition of red beet leaf (in dry matter) used in the present study was presented in Table 1.
Table 1.
Composition of experimental diets
aAIN 93 mineral mixture (g/kg): Calcium Carbonate 357.00, Potassium Phosphate (monobasic) 196.00, Potassium Citrate H2O 70.78, Sodium Chloride 74.00, Potassium Sulfate 46.60, Magnesium Oxide 24.00, Ferric Citrate, U.S.P. 6.06, Zinc Carbonate 1.65, Manganous Carbonate 0.63, Cupric Carbonate 0.30, Potassium Iodate 0.01, Sodium Selenate 0.01025, Ammonium Paramolybdate 4H2O 0.00795, Sodium Metasilicate 9H2O 1.45, Chromium Potassium Sulfate 12H2O 0.275, Lithium Chloride 0.0174, Boric Acid 0.0815, Sodium Flouride 0.0635, Nickel Carbonate 0.0318, Ammonium Vanadate 0.0066, Sucrose finely powdered 221.026
bAIN 93 Vitamin mixture (g/kg): Niacin 3.00, Calcium Pantothenate 1.60, Pyridoxine HCl 0.70, Thiamine HCl 0.60, Riboflavin 0.60, Folic Acid 0.20, Biotin 0.02, Vitamin E Acetate (500 IU/g) 15.00, Vitamin B12 (0.1%) 2.50, Vitamin A Palmitate (500,000 IU/g) 0.80, Vitamin D3 (400,000 IU/g) 0.25, Vitamin K1/Dextrose Mix (10 mg/g) 7.50, Sucrose 967.23
cRed beet leaf composition (in dry weight): energy 321 kcal/100 g, fiber 34.3%, β-carotene 69.27 mg/100 g, α-tocopherol 5.17 mg/100 g, total phenols 1,292 mg/100 g, betanin 106.4 mg/100 g
The body weight of mice and food intake were measured daily, and the feed efficiency ratio (FER) was calculated throughout the experiment. The feces were collected for 3 days before sacrifice. Animal experiment was conducted in compliance with 'Guide for Care and Use of Laboratory Animals' of the National Institutes of Health Guidelines (NIH, 1996). After 4 weeks, mice were fasted for 12 h and then anesthetized during the post-absorptive period between 08.00 a.m. and 10.00 a.m. The selected organs (i.e., heart, kidneys, brain and liver) were rapidly removed and washed in saline buffer, weighed, and stored immediately in liquid nitrogen for lipid peroxidation and antioxidant marker assays. Fat pads (R, retroperitoneal; M, mesenteric; E, epididymal; I, inguinal; S, spleenic) were also collected and weighed. Blood was drawn from the vena cava into heparin tubes and centrifuged at 2,500 rpm and 4℃, and the plasma was stored in liquid nitrogen for further assays.
Plasma and fecal lipid profiles
The concentration of plasma total cholesterol, HDL-cholesterol, and triglyceride were determined using a Hitachi 7020 automatic blood analyzer. Fecal lipids were extracted using the methods described by Folch et al. (1957), and their cholesterol concentrations were determined using a kit purchased from Yeongdong Pharmaceutical Co. (Seoul, Korea). Optical density was measured with a spectrophotometer (Pharmacia Biotech, Cambridge, England).
Determination of lipid peroxidation by 2-thiobarbituric acid reactive substances (TBARS)
The liver, heart, and kidney were placed on ice, and homogenized with 50 mM sodium phosphate buffer using a tissue homogenizer. Each 1 mL of the tissue homogenates and plasma samples was mixed with 8.1% sodium dodecyl sulfate (SDS, 1 mL), 20% acetic acid (2 mL) and 0.75% TBA (1 mL). The mixture was boiled for 30 min and centrifuged at 14,000 rpm for 10 min. The absorbance of the malondialdehyde (MDA)-TBA adduct formed was measured colorimetrically at 533 nm with a spectrophotometer (Bidlack & Tappel, 1973). A standard curve was prepared with tetramethoxypropane and TBA, and then the MDA values were calculated and expressed as TBARS values.
Determination of total glutathione level
Total glutathione (GSH) content was enzymatically determined by the method of Floreani et al. (1997) with a slight modification. Liver tissue (0.2 g) was pulverized in a cooled ceramic percussion motor with 6% metaphosphoric acid, and the mixture was centrifuged (25,000 rpm and 20 min) at 4℃. The supernatant (50 µL) was mixed with 100 mM phosphate buffer (pH 7.4, 39 µL) containing 5 mM ethylenediaminetetraacetic acid (EDTA), 10 mM 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB, 25 µL) and 5 mM NADPH (80 µL). After equilibration for 3 min at 25℃, the reaction was started by adding glutathione reductase (two units). The formation of DTNB was continuously recorded with an ultraviolet/visible (UV/VIS) spectrophotometer at 412 nm. The total content of GSH in the liver was calculated from a standard curve obtained by plotting the known amount of GSH vs. the rate of change of absorbance at 412 nm.
Determination of antioxidant enzyme activities in liver and plasma
Liver tissues were homogenized in a 20 mM phosphate buffer containing 0.1 M KCl, 1 mM EDTA, and 0.5% Triton X-100 (pH 7.4). The homogenates were centrifuged for 30 min at 25,000 rpm and 4℃, and the supernatant was used for the following enzyme assays. Glutathione S-transferase (GST) activity was determined as previously described (Habig et al., 1974) using 1-chloro-2,4-dinitrobenzene (CDNB, a substrate) in the presence of 0.1 mM GSH. The formation of dinitrophenyl thioether by GST at 37℃ was monitored for 3 min with a UV/VIS spectrophotometer in absorbance at 340 nm. GST activity was expressed as the mean ± standard deviation of quadruplicate analysis. For determination of the activity of glutathione reductase (GR), the supernatant was mixed with 1 M glutathione disulfide (GSSG), and 5 mM NADPH in 0.1 M phosphate/ 0.5 mM EDTA buffer (pH 7.0), and the formation of NADP+ was monitored with a UV/VIS spectrophotometer at 340 nm (Floreani et al., 1997; Pinto et al., 1984).
The activity of glutathione peroxidase (GPx) was determined by mixing the supernatant with 1 mM EDTA, 100 mM GSH, 5 mM NADPH, and one unit of glutathione reductase in 0.1 M phosphate buffer (pH 7.0). After being incubated for 3 min, 10 mM cumene hydroperoxide was added and the oxidation of NADPH into NADP+ was monitored spectrophotometrically at 340 nm, in which one unit of GPx leads the formation of 1 µmole NADP+ per min (Tappel et al., 1978). For the assay of superoxide dismutase (SOD), the supernatant was mixed with 1 mM xanthine, 0.2 mM cytochrome, and 0.05 M potassium cyanide in 0.05 M potassium phosphate/ 0.1 mM EDTA buffer. Xanthine oxidase was added into the reaction mixture, and the SOD activity was spectrophotometrically determined (550 nm) as the inhibition rate of reduction of cytochrome by superoxide radical (McCord & Fridovich, 1969). The activities of GR, GPx, and SOD were expressed as an international unit/mg of liver tissue. For the assay of plasma paraoxonase (PON), the mice plasma (5 µL) was transferred to the buffer containing 20 mmol Tris/HCl and 1 mM CaCl2. After adding 0.2 M phenylacetate, the activity of PON was determined with the rate of formation of phenol by monitoring the increase at 270 nm. One unit of PON activity is equal to 1 µM of phenol per min (Gan et al., 1991).
Determination of β-carotene content in plasma
The mice plasma (100 µL) was mixed with ethanol (500 µL) and butylated hydroxytoluene (BHT) in methanol (91 mmol, 25 µL) in a test tube. Hexane (3 mL) was added into the reaction mixture and vortexed for 30s. The organic phase containing β-carotene was separated by distillated water and centrifuged at 1,500 rpm for 2 min. The upper phase was collected and hexane was evaporated under nitrogen stream. The residue was dissolved in isopropanol/acetonitrile (50:50, v/v), and injected into a high-performance liquid chromatography (HPLC) equipped with UV detector set at 450 nm. A Nova-Pak C18 column (4 µm, 150 × 3.9 mm internal diameter, Waters, Milford, MA) was used for the separation. Elution was performed with an isocratic mobile phase of acetonitrile/isopropanol/methanol (68:20:12, v/v/v) with 0.02% ammonium acetate, and the flow rate was set at 1.0 mL/min. The amount of β-carotene was determined with a calibration curve. β-apo-8'-carotenal was used as an internal standard, and triplicate analysis was performed.
Determination of DNA damage of hepatocyte and lymphocyte
Liver tissue was homogenized at 600 rpm with 50 mM Hank's balanced salt solution with 1 mM EDTA (pH 7.4). Cells were washed twice prior to gel electrophoresis. The endogenous DNA damage in lymphocytes and hepatocytes was analyzed by alkaline single-cell gel electrophoresis (Comet assay) with a slight modification (Singh et al., 1988).
Statistical analysis
Data are presented as the mean ± standard deviation, and statistical analyses were performed using Statistical Analysis System software (SAS, Cary, NC) (SAS Institute, 2000). The differences between means were assessed by the Student's t-test, and statistical significance was defined at p<0.05.
Results
Food intake, weight gain, FER, and weight of selected organs and fat pads
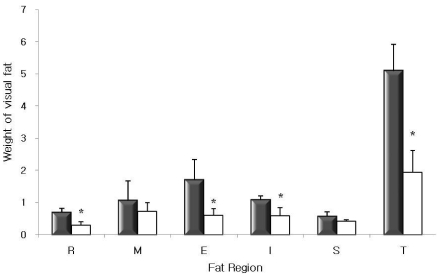
After four weeks of 20% fat and 1% cholesterol diet supplemented with or without 8% freeze-dried red beet leaf, food intake, FER, and fecal weight were not significantly different between control and RBL mice (p>0.05) (Table 2). The weight gain of RBL mice was markedly decreased by 32.5% compared to control mice (p<0.05). The weights of the selected organs (liver, heart, brain, and kidney) were not significantly changed by RBL diet, whereas the visible fat deposition was markedly decreased in RBL mice. The fat pads from retroperitoneal (R), epididymal (E), and inguinal (I) tissue were significantly reduced in RBL mice by 56.5%, 64.1%, and 44.9%, respectively (p<0.05). Hence, a significant reduction of total fat pad (T, 61.9%) was found in RBL mice (p<0.05) (Fig. 1).
Table 2.
Weight gain, food Intake, FER, and fecal and organ weights of C57BL/6J mice after four weeks of experimental feeding
*Values are means ± SD.
n=8
Values within the same column are significantly different by Student's t-test (p<0.05).
Fig. 1.
Weight of fat pad (g/100 g body weight) of C57BL/6J mice after four weeks of experimental diet. ■ Control □ RBL; R: retroperitoneal; M: mesenteric; E: epididymal; I: inguinal; S: spleenic; T: total fat pad. *Values are means ± SD; n=8. Values are significantly different by Student's t-test (p<0.05).
Plasma and fecal lipid profiles
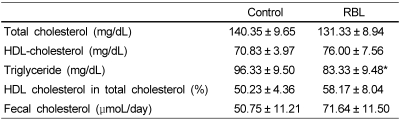
As shown in Table 3, compared with control mice, the concentrations of plasma total cholesterol was reduced by 6.5% and HDL-cholesterol concentration was increased by 7.3% in RBL mice, but no significant differences were found (p>0.05). Plasma triglyceride concentration was significantly reduced by 13.4% in RBL mice (p<0.05). Fecal excretion of cholesterol (µmol/day) was increased by 41.2% in RBL mice, but it was not significant (p=0.09) (Table 3).
Table 3.
Plasma lipid profile and fecal cholesterol of C57BL/6J mice after four weeks of experimental feeding
*Values are means ± SD.
n=8
Values within the same column are significantly different by Student's t-test (p<0.05).
Lipid peroxidation
The status of lipid peroxidation of plasma and organs in mice was assessed as the content of MDA by TBARS value (Table 4). A significant reduction of TBARS value was observed in plasma (42.0%), liver (33.2%), heart (25.9%), and kidney (20.0%) of RBL mice (p<0.05), and showed that the red beet leaf decreased lipid peroxidation in the mice fed high fat and high cholesterol diet.
Table 4.
TBARS value in plasma and selected organs of C57BL/6J mice after four weeks of experimental feeding
*Values are means ± SD.
n=8
Values within the same column are significantly different by Student's t-test (p<0.05).
Antioxidants and antioxidant enzymes
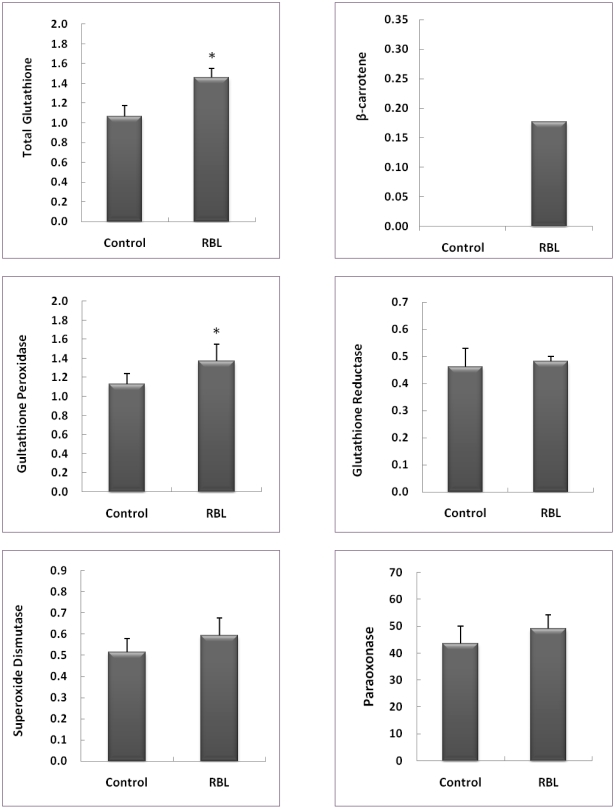
The concentration of plasma β-carotene was 0.177 nmole/mL in RBL mice, whereas β-carotene was not detected in the plasma of control mice (Fig. 2). In liver tissue, the content of GSH was significantly higher in RBL mice compared to control mice, showing a 36.4% increase (p<0.05), and the activity of liver GPx was significantly higher in RBL mice (21.2%, p<0.05). Furthermore, GR and SOD in liver showed no significantly different activities between control and RBL mice (Fig. 2). PON in the plasma of RBL mice was found to be 12.6% higher in activity than in RBL mice, but not significantly (p>0.05).
Fig. 2.
Antioxidants and antioxidant enzymes in liver and plasma of C57BL/6J mice after four weeks of experimental feeding. Total glutathione (GSH, mg/mL), glutathione peroxidase (GPx, unit/prot), glutathione reductase (GR, unit/prot), and superoxide dismuthase (SOD, unit/prot) in liver; paraoxonase (PON, U) and β-carotene (nmole/mL) in plasma. *Values are means ± SD; n=8. Mean values in RBL mice are significantly different from those in control mice by Student's t-test (p<0.05).
Oxidative damage of hepatocyte and lymphocyte DNA
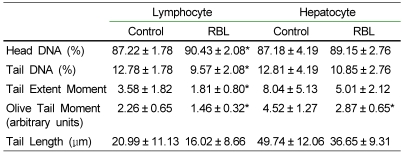
The supplementation of 8% of red beet leaf diet significantly reduced the oxidative damage of lymphocytes in the plasma of RBL mice and showed that the Comet parameters such as tail DNA (%), tail extent moment, and olive tail moment (OTM) were significantly reduced by 25.1%, 49.4%, and 35.4%, respectively, in RBL mice compared with control mice (p<0.05) (Table 5). Hepatocyte DNA was also influenced by dietary red beet leaf, resulting in a markedly decreased OTM (36.5%) in RBL mice (p<0.05). The other parameters such as tail DNA (%), tail extent moment and tail length were not significantly changed, but showed the decreased trend in RBL mice (p>0.05) (Table 5).
Table 5.
Hepatocyte and lymphocyte DNA oxidative damage of C57BL/6J mice after four weeks of experimental feeding
*Values are means ± SD.
n=8
Values within the same row are significantly different by Student's t-test (p<0.05).
Discussion
The intake of red beet leaf diet showed a significant body fat-lowering effect leading to decreased body weight and a significant reduction of fat pads (retroperitoneal, epididymal, and inguinal fat) in C57BL/6J mice, whereas the weights of major organs (brain, liver, heart and kidney) were not affected. A higher amount of fecal cholesterol in RBL mice was observed, and such more secretion of cholesterol into feces may come from a resistance to digestion and absorption by dietary fiber contained in red beet diet. Red beet leaf consists of soluble fiber (12.7 g/100 g in dry matter) and insoluble fiber (21.6 g) (Kim & Kim, 2004). It is generally accepted that higher intake of vegetables is consistently associated with lowering body fat because the dietary fibers contained in vegetables are very effective in controlling weight.
In normal metabolic processes, cells continuously produce ROS and the cells can also protect themselves against ROS through a defense mechanism of antioxidants and antioxidant enzymes (Fang et al., 2002). However, oxidative stress is caused by an imbalance between the production of ROS and neutralization by antioxidant defense system (Urso & Clarkson, 2003). It is believed that high fat diet induces oxidative stress, which plays an active part in development of many chronic diseases like CHD. In the present study, four weeks of feeding 20% fat and 1% cholesterol diet induced atherogenic profiles in the C57BL/6J mice because their levels of plasma cholesterol and triglyceride were above the normal level of C57BL/6J mice fed 5% fat diet which was done in the study of Nicolle et al. (2004). Red beet leaf was a rich source of bioactive components such as β-carotene, α-tocopherol, betanin, fiber, and polyphenols, and these compounds have highly effective antioxidant properties. In our study, the red beet leaf diet remarkably reduced lipid peroxidation in blood and the major organs including liver, heart, and kidney, and showed a positive antioxidative effect in RBL mice. The β-carotene was found only in plasma of RBL mice because β-carotene was not contained in control diet. In addition, the improved antioxidative defense was shown as the marked increase of GSH, antioxidant nutrient, and the increase of antioxidant activity of liver GPx in RBL mice compared to control mice. Similarly, 15% of lyophilized apple supplementation resulted in a reduction of lipid peroxidation in rats fed cholesterol-enhanced diet (Aprikian et al., 2001), suggesting that vegetable intake can provide a protection against oxidative stress.
GSH plays an important role in antioxidant defense mechanism by scavenging ROS, removing lipid peroxides, and preventing oxidation of biomolecules (Tanaka et al., 2001; Wu et al., 2004). In response, GSH ultimately prevents the buildup of oxidized fats that may contribute to CHD. However, a GSH deficiency could contribute to oxidative stress and result in many pathological diseases including cancer, liver and heart disease. GSH is also the cofactor for the GPx, which is the major antioxidant enzyme. GPx detoxifies endogenously produced hydrogen peroxide or other peroxides. PON located on HDL in serum is highly effective in preventing LDL oxidation through breakdown of lipid peroxides before accumulated on LDL (Durrington et al., 2001). Under oxidative stress, oxidative modifications of LDL take place, which are associated with an incidence of atherosclerosis. Red beet leaf contained antioxidant nutrients including betanin, flavonoids, polyphenols, and vitamins (Lichenthäler & Marx, 2005). In the present study, betanin, β-carotene and α-tocopherol were contained as 8.51, 5.54 and 0.41 mg/100 g RBL diet, respectively. Betanin is considered as the compound with potential antioxidative properties in the mice. Tesoriere et al. (2003) reported betanin to bind LDLs in ex vivo plasma and increase their resistance to copper-induced oxidation. Kanner et al. (2001) found that betanin inhibited LDL oxidation catalyzed by H2O2-activated metmyoglobin, and was better than catechin, a well known antioxidant. β-carotene and α-tocopherol are also effective chain-breaking antioxidants in tissues and LDLs, and appeared to be protective nutrients against the development of CHD (Sulli et al., 1998). β-carotene inhibited the oxidative modification of LDL induced by copper and human monocyte macrophages, resulting in the reduced lipid peroxidation and decreased macrophages degradation (Jialal et al., 1991). Therefore, it appears that the red beet leaf supplementation leads to reduced oxidative stress by improving antioxidant capacity and suppressing lipid peroxidation in C57BL/6J mice.
DNA, containing the genetic instructions, can be damaged by ROS. If the DNA damage is not adequately repaired, errors in DNA replication ensues, which can prelude chronic diseases such as CHD, cancer, and neurologic disorders. Liver serves as the major detoxifying organ in the body, and lymphocytes play a number of roles in the immune system that fight infections and diseases. The oxidative damage of hepatocyte DNA can be an important risk factor leading to development of cancer during chronic active hepatitis and other persistent inflammatory diseases (Hagen et al., 1994). The oxidative DNA damage in lympocytes has been involved in the pathogenesis of neurodegenerative diseases such as Alzheimer and Parkinson's disease (Mecocci et al., 1988; Petrozzi et al., 2001). In the present study, DNA damage was determined with a comet assay and its comet parameters are valuable makers of oxidative stress. The Comet parameters (tail DNA, tail extent moment, OTM, and tail length) in lymphocyte and hepatocyte were improved in RBL mice compared with control mice. Red beet leaf diet was more effective in the reduction of DNA oxidative damage on plasma lymphocytes resulting in significantly decreased Comet parameters (p<0.05). Our study showed that the levels of antioxidants (i.e., GSH and β-carotene) and the activities of antioxidant enzymes (i.e., GPx and PON) were inversely related to oxidative DNA damage, indicating that the red beet leaf can provide antioxidant system to protect DNA against attack from endogenously produced ROS. Like our results, cactus pear fruit containing high levels of betalains and vitamin C decreased DNA damage in human lymphocytes (Siriwadhana et al., 2006). According to the study of Riso et al. (1999), tomato-rich diet significantly reduced DNA tail moment by 33%-42%, and increased the resistance of lymphocyte DNA to oxidative damage in females. Moreover, high intake of fruits and vegetables containing a high concentration of antioxidants induces a significant decrease in oxidative damage of DNA, supporting the view that fruits and vegetables exert a protective effect on oxidative damage to DNA (Duthie et al., 1996).
In conclusion, the supplementation of 8% freeze-dried red beet leaf in a high fat and high cholesterol diet was associated with the reduced lipid peroxidation, improved antioxidant status, and decreased oxidative damage to DNA in the blood and tissues. In addition, red beet leaf could control body weight by reducing fat pads in C57BL/6J mice. These beneficial effects might be contributed by antioxidant components, other various micronutrients and fibers contained in the red beet leaf. Therefore, our results demonstrated that a regular intake of red beet leaf is a better way to improve oxidative damage and possibly contributes to reduced risk of developing chronic diseases.
Footnotes
This study was financially supported by research fund of Chungnam National University in 2006.
References
- 1.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci U S A. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprikian O, Levrat-Verny M, Besson C, Busserolles J, Rémésy C, Demigné C. Apple favourably affects parameters of cholesterol metabolism and of anti-oxidative protection in cholesterol-fed rats. Food Chem. 2001;75:445–452. [Google Scholar]
- 3.Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000;33:S85–S97. [PubMed] [Google Scholar]
- 4.Bidlack WT, Tappel AL. Damage to microsomal membrane by lipid peroxidation. Lipids. 1973;8:177–182. doi: 10.1007/BF02544631. [DOI] [PubMed] [Google Scholar]
- 5.Catapano AL, Maggi FM, Tragni E. Low density lipoprotein oxidation, antioxidants, and atherosclerosis. Curr Opin Cardiol. 2000;15:355–363. doi: 10.1097/00001573-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 7.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–480. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 8.Duthie GG, Bellizzi MC. Effects of antioxidants on vascular health. Br Med Bull. 1999;55:568–577. doi: 10.1258/0007142991902637. [DOI] [PubMed] [Google Scholar]
- 9.Duthie SJ, Ma A, Ross MA, Collins AR. Antioxidant supplementation decreases oxidative DNA damage in human lymphocytes. Cancer Res. 1996;56:1291–1295. [PubMed] [Google Scholar]
- 10.Dyun MAS, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. J Am Diet Assoc. 2000;200:1511–1521. doi: 10.1016/S0002-8223(00)00420-X. [DOI] [PubMed] [Google Scholar]
- 11.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 12.Floreani M, Petrone M, Debetto P, Palatini P. A comparison between different methods for determination of reduced and oxidized glutathione in mammalian tissues. Free Radic Res. 1997;26:449–455. doi: 10.3109/10715769709084481. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Gan KN, Smolen A, Eckerson HW, LaDu BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–106. [PubMed] [Google Scholar]
- 15.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 16.Hagen TN, Huang S, Curnutte J, Flower P, Martinez V, Wehr CM, Ames BN, Chisari FV. Extensive oxidative DNA damage in hepatocytes of transgenic mica with chronic active hepatistis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1994;91:12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jialal I, Norkus EP, Cristol L, Grundy SM. Beta-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1991;1086:134–138. doi: 10.1016/0005-2760(91)90164-d. [DOI] [PubMed] [Google Scholar]
- 18.Kanner J, Harel S, Granit R. Betalains - A new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49:5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Kim DJ. The composition of dietary fiber on new vegetables. Journal of the Korean Society of Food Science and Nutrition. 2004;33:852–856. [Google Scholar]
- 20.Lee JH, Felipe P, Yang YH, Kim MY, Kwon OY, Sok DE, Kim HC, Kim MR. Effects of dietary supplementation with red-pigmented leafy lettuce (Lactuca sativa) on lipid profiles and antioxidant status in C57BL/6J mice fed a high-fat high-cholesterol diet. Br J Nutr. 2009;101:1246–1254. doi: 10.1017/S0007114508073650. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Lee KT, Akoh CC, Chung SK, Kim MR. Antioxidant evaluation and oxidative stability of structured lipids from extravirgin olive oil and conjugated linoleic acid. J Agric Food Chem. 2006;54:5416–5542. doi: 10.1021/jf0603735. [DOI] [PubMed] [Google Scholar]
- 22.Lichetnthäler R, Marx F. Total oxidant scavenging capacities of common European fruit and vegetable juices. J Agric Food Chem. 2005;53:103–110. doi: 10.1021/jf0307550. [DOI] [PubMed] [Google Scholar]
- 23.McCord JC, Fridovich I. Superoxide dismutase. An enzymatic function for erythrocuprein. J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 24.Mecocci P, Cherubini A, Polidori MC, Cecchetti R, Chionne F, Senin U. Oxidative stress and lymphocytes in Alzhemimer disease. Arch Gerontol Geriatr. 1998;6:313–316. [Google Scholar]
- 25.National Institutes of Health. National Research Council, "Guide for the Care and Use of Laboratory Animals". Washington DC. USA: National Academy Press; 1996. [Google Scholar]
- 26.Nicolle C, Gueux E, Lab C, Jaffrelo L, Rock E, Mazur A, Amouroux P, Rémésy C. Lyophilized carrot ingestion lowers lipemia and beneficially affects cholesterol metabolism in cholesterol-fed C57BL/6J mice. Eur J Nutr. 2004;43:237–245. doi: 10.1007/s00394-004-0465-3. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura N, Taniguchi Y, Kiriyama S. Plasma cholesterol-lowering effect on rats of dietary fiber extracted from immature plants. Biosci Biotechnol Biochem. 2000;64:2543–2551. doi: 10.1271/bbb.64.2543. [DOI] [PubMed] [Google Scholar]
- 28.Parthasarathy S, Santanam N, Ramachandran S, Meilhac O. Oxidants and antioxidants in atherogenesis: an appraisal. J Lipid Res. 1999;40:2143–2157. [PubMed] [Google Scholar]
- 29.Petrozzi L, Lucetti C, Gambaccini G, Bernardini S, Dotto PD, Migliore L, Scarpato R, Bonuccelli U. Cytogenetic analysis oxidative damage in lymphocytes of Parkinson's disease patients. Neurol Sci. 2001;22:83–84. doi: 10.1007/s100720170058. [DOI] [PubMed] [Google Scholar]
- 30.Pinto MC, Mata AM, Lopes-Barea J. Reversible inactivation of Sacchromyces cerevasiae glutathione reductase under reducing conditions. Arch Biochem Biophys. 1984;228:1–12. doi: 10.1016/0003-9861(84)90040-7. [DOI] [PubMed] [Google Scholar]
- 31.Riso P, Pinder A, Santangelo A, Porrini M. Does tomato consumption effectively increase the resistance of lymphocyte DNA to oxidative damage? Am J Clin Nutr. 1999;69:712–718. doi: 10.1093/ajcn/69.4.712. [DOI] [PubMed] [Google Scholar]
- 32.SAS Institute. SAS Statistics Software, release 8.2. Cary, NC. USA: SAS Institute; 2000. [Google Scholar]
- 33.Sepúlveda-Jiménez G, Rueda-Benítez P, Porta H, Rocha-Soda M. Betacyanin synthesis in red beet (Beta vulgaris) leaves induced by wounding and bacterial infiltration in preceded by an oxidative burst. Physiol Mol Plant Pathol. 2004;64:125–133. [Google Scholar]
- 34.Singh PN, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 35.Siriwardhana N, Shahidi F, Jeon Y. Potential antioxidative effects of cactus pear fruit (Opuntia ficus-indica) extract of radical scavenging and DNA damage reduction in human peripheral lymphocytes. J Food Lipids. 2006;13:445–458. [Google Scholar]
- 36.Stehbens WE. The oxidative stress hypothesis of atherosclerosis: cause or product? Med Hypotheses. 1999;53:507–515. doi: 10.1054/mehy.1999.0801. [DOI] [PubMed] [Google Scholar]
- 37.Sulli KC, Sun J, Giraud DW, Moxley RA, Driskell JA. Effects of β-carotene and α-tocopherol on the levels of tissue cholesterol and triglyceride in hypercholesterolemic rabbits. J Nutr Biochem. 1998;9:344–750. [Google Scholar]
- 38.Tanaka K, Miyazaki I, Fujita N, Haque ME, Asanuma M, Ogawa N. Molecular mechanism in activation of glutathione system by Ropinirole, a Selective Dopamine D2 Agonist. Neurochem Res. 2001;26:31–36. doi: 10.1023/a:1007672414239. [DOI] [PubMed] [Google Scholar]
- 39.Tappel AL, Fleischer S, Packer L. Glutathione peroxidase. Methods Enzymol. 1978;52:506–523. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 40.Teow CC, Truong VD, McFeeters RF, Thompson RL, Pecota KV, Yencho GC. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103:829–838. [Google Scholar]
- 41.Tesoriere L, Butera D, D'arpa D, Gaudio FD, Allegra M, Gentile C, Liverea MA. Increased resistance to oxidation of betalain-enriched human low density lipoproteins. Free Radic Res. 2003;37:689–696. doi: 10.1080/1071576031000097490. [DOI] [PubMed] [Google Scholar]
- 42.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 43.Vinson JA, Hao Y, Su X, Zubik L. Phenol antioxidant quantity and quality in foods: vegetables. J Agric Food Chem. 1998;46:3630–3634. [Google Scholar]
- 44.Wu G, Fang YZ, Yang S, Luption JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]