Abstract
Retinoic acids (RAs) modulate growth, differentiation, and apoptosis in normal, pre-malignant & malignant cells. In the present study, the effects of RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) on the cell signal transduction of human breast cancer cells have been studied. The relationship between RAs and an enzymatic antioxidant system was also determined. Estrogen-receptor (ER) positive MCF-7 and ER-negative MDA-MB-231 human breast cancer cells were treated with different doses of each RA isomers, all-trans RA, 13-cis RA, or 9-cis RA. Treatment of RA isomers inhibited cell viability and induced apoptosis of MCF-7 cells as a result of increased caspase activity in cytoplasm and cytochrome C released from mitochondria. All-trans RA was the most effective RA isomer in both cell growth inhibition and induction of apoptosis in MCF-7 cells. However, no significant effect of RA isomers was observed on the cell growth or apoptosis in ER-negative MDA-MB-231 cells. In addition, activities of antioxidant enzymes such as catalase and glutathione peroxidase were decreased effectively after treatment of RA in MCF-7 cells, whereas SOD activity was rarely affected. Thus, the present data suggest that all-trans RA is the most potential inducer of apoptosis and modulator of antioxidant enzymes among RA isomers in MCF-7 human breast cancer cells.
Keywords: Breast cancer cells, RA Isomers, apoptosis, antioxidant enzymes
Introduction
Among the many investigations of breast cancer, there has been much interest recently in retinoic acid (RA) metabolites used in i) cellular growth process, ii) activating gene transcription by specific nuclear receptors, iii) inhibiting cancer cell growth by several different pathways (Freemantle et al., 2003; Marill et al., 2003; Napoli, 1996; Ralhan & Kaur, 2003; Smith et al., 1992). Previous studies also suggest an intimate relationship between cancer and serum RA level; possibly in prevention of cancer cell proliferation or development into second malignancy in breast and liver cancer (Gronemeyer & Miturski, 2001; Manor et al., 2003; Prakash et al., 2001). Treatment with RA in other types of cancer, ischemia or pathophysiology of acne has been shown to be effective as well (Brodowicz et al., 1999; Freemantle et al., 2003; Nelson et al., 2006; Sato et al., 2008). Many studies have been conducted on molecular and cellular mechanisms of RA in the past ten years. In particular, the roles of RA in control and gene expression of cancer cell's initiation and apoptosis, in multiple signal pathways inside cancer cells, and in the correlation between cancer cells and RA receptors have been investigated (Chambon, 1996; Hayashi et al., 2001; Marill et al., 2003). However, recent clinical experiments that used RA show no effect in reducing first stage or second stage lung cancer for cigarette smokers. In addition, due to the potential toxicity of RA, detailed research is needed on the effect of physiological dose of RA through a close examination of its mechanisms at the cellular level (Freemantle et al., 2003; Ralhan & Kaur, 2003; Windhorst & Nigra, 1982).
When free radicals and reactive oxygen species (ROS) accumulate in the process of aerobic cell metabolism, it consequently results in diseases and aging by inducing damage to tissues and cells. ROS are also known to be involved in cancer growth, cancer cell regeneration and differentiation, and cell signaling systems (Drisko et al., 2003). The enzymatic defense mechanisms with superoxide dismutase (SOD), catalase, and glutathione peroxidase participate in converting superoxide anion to H2O2, and then to H2O. Although turnover volume for catalase is very high, its affinity to H2O2 is relatively low. Therefore, when H2O2 accumulates in cells in even low concentration, it can cause oxidative damage to DNA, which then induces cancer or cell death (Nordberg & Arner, 2001). According to studies of relationship between RA and antioxidation, RA stabilizes protein levels in brain cells and the activity of SOD at the mRNA level, thereby reduces oxidative stress and apoptosis (Ahlemeyer et al., 2001). On the other hand, according to other researches, among RA isomers, all-trans RA suppresses cell growth of malignant myeloblastic leukemia and induces its apoptosis, and is known to control the activity levels of antioxidant enzymes in rat sertoli cells. Many different aspects of cellular level mechanisms of RA are currently being discovered. However, inconsistency of the results requires more in-depth research (Conte da Frota et al., 2006; Xu et al., 2002).
In this report, we have investigated to measure the influence of RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) on apoptosis and on enzymatic antioxidant system in breast cancer cell lines using the activity levels of caspases and antioxidant enzymes.
Materials and Methods
Chemicals and reagents
All-trans RA, 13-cis RA, and 9-cis RA were purchased from Sigma Chemical Co. (St. Louis, MO). Growth media RPMI-1640 and other growth supplements, heat-inactivated fetal bovine serum (FBS), penicillin streptomycin, Hank's Balanced Salt Solutions (HBSS) were obtained from Gibco-BRL (Eggenstein, Germany). A spectrophotometric assay kit for SOD activity and a colormetric kit for apoptosis were purchased from R&D Systems (Wiesbaden, Germany). Caspase-8&-9 fluorescent assay kits were purchased from Peptron Co. (Daejeon, Korea).
Cell culture and treatment of cells
Human breast ER-positive cells MCF-7 and ER-negative cells MDA-MB-231 obtained from the American Type Culture Collection (Rockville, MD) were grown in Roswell Park Memorial Institute (RPMI) medium-1640 containing HEPES, sodium bicarbonate, penicillin streptomycin, and 10% fetal bovine serum at 37℃ in a humidified atmosphere of 5% CO2 in air. Around 80% confluent cells were passaged by standard trypsinization procedures. Cells were seeded at a density of 3×104 cells /P-100 dish. The RA isomer solutions dissolved in ethanol (final ethanol concentration was 0.1%) were diluted in culture medium to final concentrations of 10, 100, and 1,000 nM. Cells were treated for up to 4 days. All solutions containing RA isomers were handled under yellow light. The range of RA concentration was 10 nM~1 µM. Considering the potential toxicity and resistance of retinoic acid, the concentration over 1 µM was not used. Media in the presence and absence of RA isomers were changed every 48 hrs and cells were harvested and counted with a haemocytometer. Each sample was counted in duplicate, and each experiment was done in triplicate (Prakash et al., 2001).
MTT assay
Following incubation with various reagents, the rate of cell viability was assessed by colormetric measurement of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) reduction (Rishi et al., 2003). Values from each treatment were calculated as a percentage relative to the untreated control (defined as 100% survival).
Apoptosis detection assay
MCF-7 and MDA-MB-231 cells placed in dishes filled with 3.7% buffered formaldehyde solution for 7 min. at room temperature (RT) were washed twice with 1X PBS. The cells were post-fixed in 100% methanol for 20 min and washed twice with 1X PBS. A portion of 50 µl of cytonin solution was added to each dish and the cells were incubated for 15 min at RT. Fixed cells were washed with 200 µl of DNasa-free water, to which 50 µl of 2.5% H2O2 solution was added and the cells were incubated for 5 min at RT. After washing the cells again, 150 µl of 1X TdT labeling buffer was added, and the cells were incubated for 5 min. The cells were washed, and 50 µl of labeling reaction mixture was added followed by incubation at 37℃ for 1 hr. The cells were placed in 150 µl of 1X TdT stop buffer for 5 min and washed twice with 1X PBS for 2 min. The cells were placed in 50 µl of streptavidin-HRP solution and incubated at RT for 10 min. Cells were washed four times with 200 µl of PBS and 0.1% Tween 20. The cells were then placed in 100 µl of TACS-Sapphire and incubated at RT for 30 min in the dark. The reaction was stopped by adding 100 µl of 2N HCl. The absorbance of apoptotic cells, which could be detected by labeling of terminal deoxynucleotidyl transferase (TdT) and TACS-Sapphire, was measured at 450 nm (Kuypers et al., 1996; Pigault et al., 1994)
Caspase activity assay
To determine caspase-3 activity, western blot analysis (Nelson et al., 2006) was used. Fluorometiric assay was used to measure activities of caspase-8 &-9 (Kuida, 2000; Stennicke & Salvesen, 2000; Villa et al., 1997).
Determination of cytochrome C releasere
The release of cytochrome C into the cytosol of MCF-7 cells treated with 1 µM of RA isomers for 4 days was measured by a western blot (Nagase et al., 2001) For the detection of cytochrome C, the lysate from the last centrifugation and mitochondrial fractions were subjected to 12% SDS-PAGE. Proteins in the gel were transferred onto prewetted polyvinylidene difluoride (PVDF)-nitrocellulose filters (Bio-Rad, Cambridge, MA). Membranes were blocked with 5% non-fat, dry milk in TBST at RT for 1hr to reduce nonspecific antibody binding. Membranes were washed with PBS three times and incubated with cytochrome C specific antibody. Specific bands of interest were detected by Enhanced Chemiluminescence (ECL; Amersham, Buckinghamshire, U.K.).
Antioxidant enzyme activity assay
Activities of SOD, catalase and glutathione peroxidase were measured by spectrophotometry (Arruda et al., 1996; Bai et al., 1999; Sutherland & Learmonth, 1997).
Statistical analysis
For statistical analysis, each experiment was carried out in triplicate sets and the SAS program was used. Data were expressed as mean ± SE. The differences among mean values were assessed by one-way analysis of variance (ANOVA) coupled with Duncan's multiple range test or student's t test. A p-value <0.05 was considered to be statistically significant.
Results
Effect of RA isomer concentrations on breast cancer cell growth
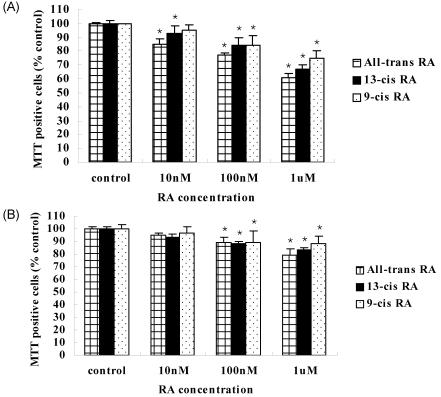
In the ER-positive MCF-7 cell line, the cancer cell growth was blocked by the high concentration of RA. In particular, all-trans RA showed greater inhibition of cancer cell growth than other isomers did, showing the lowest cell survival rate of 61% at 1 µM concentration. This result was followed by 13-cis RA, which showed a 67% cell survival and 9-cis RA resulted in 75% survival, which showed the least influence in breast cancer cell growth. On the other hand, in the ER-negative MDA-MB-231 cell line, all-trans RA did show a better suppression of cancer cell growth (79% cell survival) than the other isomers similar to shown in ER-positive MCF-7 cell line. The effect on cell growth inhibition in the ER-negative MDA-MB-231 cells was not as significant as in ER-positive MCF-7 cell line (Fig. 1).
Fig. 1.
Effect of retinoic acid (RA) isomer concentrations on the growth of human breast cancer MCF-7 cells (A) and MDA-MB-231 cells (B). MCF-7 cells and MDA-MB-231 cells were seeded at 3×104 cells and incubated with various concentrations of RA isomers for 4 days. The viability of Cells were measured by MTT assay. Values are means ± S.D. of three independent experiments. *P<0.05: compared to the control
Changes in apoptosis by RA isomers
In both ER-positive MCF-7 and ER-negative MDA-MB-231 cell lines, as the concentration of RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) became higher, the number of cell deaths increased (Fig. 2). All-trans RA in the MCF-7 cell line at 1 µM concentration showed the greatest suppression of cancer cell growth up to 41%, followed by 13-cis RA up to 37%, and 9-cis RA up to 28%. In the MDA-MB-231 cell line, the induction of apoptosis was considerably lower, with all-trans RA showing 18% cell death, 13-cis RA showing 16%, and 9-cis RA showing 11% (Fig. 2). These results indicate that RA isomers are closely related to sensitivity and resistance of breast cancer cells. According to several research results, the ER positive breast cancer cells are known to be related to sensitivity of breast cancer cells due to RA, and exert influence on cell growth inhibition, while ER-negative breast cancer cells are reported to have more effect on cells' resistance than on sensitivity (Freemantle et al., 2003; Gallagher, 2002).
Fig. 2.
Effect of retinoic acid (RA) isomer concentrations on apoptosis of human breast cancer MCF-7 cells (A) and MDA-MB-231 cells (B). Apoptotic cells were detected the most at 1 µM all-trans RA. Results are expressed as relative % of control value. Values are means ± S.D. of three independent experiments. *p<0.05: compared to the control group
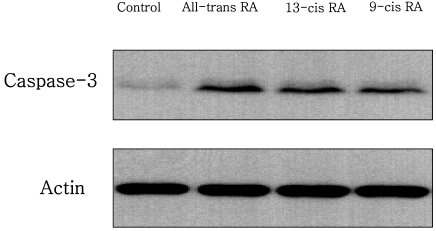
Changes in caspase-3 activity and inhibition by RA isomers
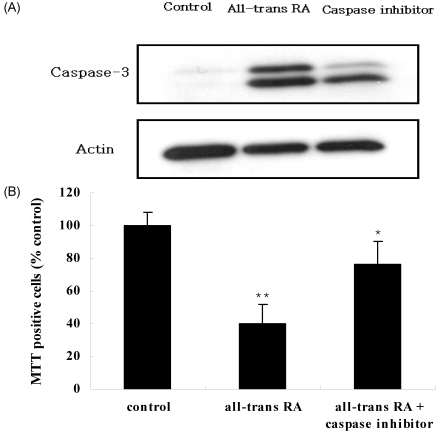
The effect of RA isomers on cell death of the ER-positive MCF-7cell line was the maximum at 1 µM of RA isomers. Examining with a western blot, the changes in the activity of caspase-3, which can be the major effector inducing apoptosis, showed that all-trans RA increased caspase-3 activity most significantly compared to controls, and it was followed by 13-cis RA and 9-cis RA (Fig. 3). To verify the effect of RA on the increase of caspase-3 activity, an experiment was carried out in which caspase-3 inhibitor, Ac-DEVD-CHO, was treated with all-trans RA to determine whether the increase in caspase-3 activity could be blocked (Fig. 4). In addition, to find out whether it sped up the cancer cell growth again, the viability of MCF-7 cells was measured. The results showed that the cancer cell growth was increased again from 40% to 76% when cells were treated with caspase-3 inhibitor, Ac-DEVD-CHO (Fig. 4).
Fig. 3.
Effect of retinoic acid (RA) isomers on caspase-3 activity in MCF-7 cells. MCF-7 cells were seeded at 3×104 cells and incubated with 1 µM RA isomers. Caspase-3 activity was measured by a Western blot using caspase cleavage specific antibody.
Fig. 4.
Effect of all-trans retinoic acid (RA) on caspase inhibiton of MCF-7 cells. MCF-7 cells were seeded at 3×104 cells and incubated with 1 µM all-trans RA. Caspase inhibitor was used by Ac-DEVD-CHO. A: Caspase-3 activity by caspase inhibitor in ER-positive MCF-7 cells. B: Effect of caspase inhibitor on viability of MCF-7 cells. Results are expressed as relative % of control value. Values are means ± S.D. of three independent experiments. *p<0.05, **p<0.01: compared to the control group
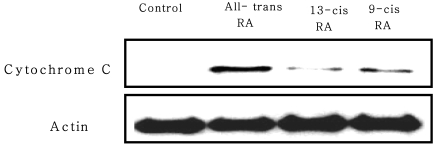
Changes in Cytochrome C release in mitochondria by RA isomers
Cytochrome C release in mitochondria was measured using a western blot. The results showed that all-trans RA exerted the most influence on the cytochrome C release, similar to the results for caspase-3 activity. However, 9-cis RA and 13-cis RA released cytochrome C in mitochondria, more so with 9-cis RA than with 13-cis RA. This indicates that in programmed cell death, RA isomers act differently in mechanisms of apoptosis (Fig. 5).
Fig. 5.
Effect of retinoic acid (RA) isomers on cytochrome C release from mitochondria in MCF-7 cells. MCF-7 cells were seeded at 3×104 cells and incubated with 1 µM RA isomers. Released cytochrome C was measured by a Western blot. Actin was a protein marker that sould not change between samples.
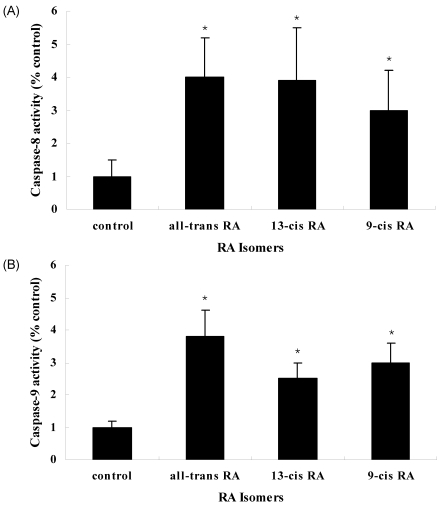
Changes in caspase-8 and caspase-9 activities by RA isomers
For inducing apoptosis, pro-caspase-8 of the death receptors is activated first followed by other caspases activations resulted in caspase cascade. In consistent with other studies, all-trans RA treatment resulted in a four-fold increase in the caspase-8 activity in the MCF-7 cell line. It is plausible that the activation of caspase-3 by all-trans RA arises from the increase in the activity of caspase-8, which is acting as an initiator of apoptosis. The activation of caspase-3 affects the death-receptor pathway resulted in apoptosis induction (Fig. 6). Furthermore, cytochrome C released in mitochondria (which can be called a key regulator of caspase cascade) combines with Apaf-1 and pro-caspase-9 to form apoptosome, which can activate caspase-3. All-trans RA treatment induced, like other caspase activities, the highest increase in the capase-9 activity of mitochondrial pathway, where apoptosis pathway advances. This result may be connected with cytochrome C being released in mitochondria. All-trans RA seems to activate both of the two main pathways that induce apoptosis and thus induce cell death.
Fig. 6.
Effect of retinoic acid (RA) isomers on caspase -8 (A) & -9 (B) activities in MCF-7 cells. MCF-7 cells were seeded at 3×104 cells and incubated with 1 µM RA isomers. All-trans RA was the most effector of RA isomers. The activities of caspase-8 &-9 were measured by using fluorogenic substrates Ac-IETD-AMC (caspase-8) and Ac-LEHD-PNA (caspase-9). Results are expressed as relative % of activities of caspase-8 &-9. Values are means ± S.D. of three independent experiments. *p<0.05: compared to the control group
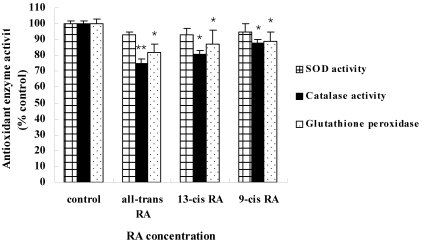
Changes in the activities of SOD, catalase, and glutathione peroxidase by RA isomers
The treatment of RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) at 1 µM concentration in ER-positive MCF-7 cells showed no significant difference in the activity of antioxidant enzyme SOD as compared to those of control (Fig. 7). On the other hand, when all-trans RA was treated, the catalase activity showed 75% of the control value. 9-cis RA caused only a 12% difference with that of the control (Fig. 7). The activity of glutathione peroxidase showed no significant difference among RA isomer treatments. In the case of all-trans RA, the activity of glutathione peroxidase was decreased 18% as compared to that of the control.
Fig. 7.
Effect of retinoic acid (RA) isomers on antioxidant defense enzymes of MCF-7 cells. MCF-7 cells were seeded at 3×104 cells and incubated with 1 µM RA isomers. SOD activites did not show a significant change, but catalase & GPx activities decreased the most by all-trans RA. Results are expressed as relative % of enzymes' activities. Values are means ± S.D. of three independent experiments. *p<0.05, **p<0.01: compared to the control group
Discussion
Many studies have uncovered therapeutic abilities of RA in breast cancer by either blocking cancer regeneration or inducing cancer apoptosis (Freemantle et al., 2003; Marill et al., 2003). Although scientists have focused on molecular and cellular mechanisms of RA for the last decade, research on RA's multiple signal pathways is still scarce. The current study focused on the effect of RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) on the cell growth and cell death in breast cancer cell lines, ER-positive MCF-7 cells and ER-negative MDA-MB-231 cells, as well as on the effect of antioxidant systems in the cell lines. In ER-positive MCF-7 cell line, as the concentration of RA isomer treatment increased, the growth of cancer cells was inhibited as compared to that of the control. All-trans RA specifically displayed the most significant suppression of cancer cell growth over the other isomers. While all-trans RA showed stronger suppression of cancer cell growth than the other isomers in ER-negative MDA-MB-231 cell line similar to the effect on the ER-positive MCF-7 cell line, it produced a higher cell survival rate than that of 9-cis RA in ER-positive MCF-7cell line. It has been reported that breast cancer is closely related to the influence of hormones and cancer cell growth shows significant differences depending on the protein binding affinity of hormone receptors (Hara et al., 2000; Marill et al., 2003; Napoli, 1996; Ott & Lachance, 1979; Paik et al., 2003; Soprano et al., 2004). ER-positive breast cancer cells are known to respond more sensitively to RA isomers in their inhibition of cancer cell growth in contrast to ER-negative breast cancer cells, which are known to influence cancer cell resistance (Gallagher, 2002; Nagpal & Chandraratna, 2000; Xu et al., 1999).
The current study indicated that the inhibition of breast cancer cell growth was associated with apoptosis as shown in the MCF-7 cell line where all-trans RA induced the highest level of apoptosis, followed by 13-cis RA and 9-cis RA. It seemed that there was no significant cell death effect in the MDA-MB-231 cell line. In accordance with the previous study (Prakash et al., 2001), all-trans RA induced greater apoptosis than the other RA isomers in the current study. In normal cells, all-trans RA arrested the G1 phase of the cell cycle without apoptosis induction, whereas in breast cancer cell line, MCF-7, RA not only arrested G0/G1 phases but also induced apoptosis at low concentrations (Dietze et al., 2002; Dimberg & Oberg, 2003; Rishi et al., 2003; Seewaldt et al., 1995). In addition, it has been reported that RA isomers control the Bcl-2 family protein (Pepper et al., 2002; Pettersson et al., 2002). Therefore, it is plausible that the effects mentioned above seem to be associated with the caspase activities and cytochrome C as shown in the current study. RA isomers may affect the regulation of Bcl-2 family protein and the release of cytochrome C in mitochondria (thus called a key regulator of caspase cascade). Cytochorme C combines with Apaf-1 and pro-caspase-9 to form apoptosome, which activates caspase-3 resulted in the activation of the apoptosis pathway. It is interesting to note that the current study indicates that RA isomer treatments incrase the activities of caspase-9 and -3. Furthermore, apoptosis is activated when pro-caspase-8 of the death receptor is firstly activated and successively activates other caspases, thereby activating a caspase cascade. When apoptosis is induced, the typical morphological change is known to occur due to the division of key cellular protein that results from the caspase cascade (Hengartner, 2000). In this study, the morphological change was also occurred by treatment of RA isomers (Data not shown) and the activity of caspase-8 was also increased in breast cancer cells. Caspases involved in apoptosis are known to be largely classified as initiator caspase-2, -8, -9, -10 and effector caspase-3, -6, -7 (Hengartner, 2000; Melino et al., 1997). Overall, the results obtained from the current study by treating RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) in estrogen receptor (ER) positive MCF-7 cell line verify that caspase-8, -9, and -3 are acting as initiator and main effectors thereby inducing apoptosis, that cytochrome C derived and released in mitochondria also has influence, and that all-trans RA showed the most significant influence among the RA isomers.
Regarding the effect of RA isomers on antioxidant systems, low concentrations of RA (0.1-100 nM) did not show any effect on the activity of antioxidant enzymes, whereas high concentrations (1-10 µM) controlled the activity of antioxidant enzymes (Conte et al., 2006). Furthermore, in the neuronal apoptosis case, RA is reported to help the antioxidant system by enhancing the protein levels of SOD-1 (cytosolic Cu-&Zn-SOD) and SOD-2 (Mn-SOD), and reducing the concentration of ROS within cells (Ahlemeyer et al., 2001). In this paper, however, RA isomers did not show any effect on the SOD activity in breast cancer cells, which implied no differences in the protein levels of SOD. In addition, 1 µM of all-trans RA concentration showed differences in the activities of catalase and glutathione peroxidase while showing no difference in the activity of SOD. These observations led to the hypothesis that manipulation of cellular redox state may modulate caspase-mediated death. By using the ratio of SOD and catalase & GPx, H2O2 can be assumed to be accumulated in the breast cancer cells treated with all-trans RA. Even if H2O2 concentration in cells is a small molar concentration such as 10 µM, it nevertheless can induce apoptosis and activates caspase-3, which acts as the main effector of the cell death program (Raloff, 2000; Ueda et al., 2002) contributing to apoptosis induction. The release of cytochrome C into the cytosol by oxidative stress can also stimulate the increase of caspase-3 activity in intracellular level and regulate the apoptosis process (Ott et al., 2007).
In conclusion, all-trans RA among RA isomers may be the most potential inducer of apoptosis and modulator of antioxidant enzymes in ER-positive MCF-7 human breast cancer cells.
Footnotes
This work was supported by Korea Research Foundation Grant KRF-2003-042-C20116.
References
- 1.Ahlemeyer B, Bauerbach E, Plath M, Steuber M, Heers C, Tegtmeier F, Krieglstein J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med. 2001;30:1067–1077. doi: 10.1016/s0891-5849(01)00495-6. [DOI] [PubMed] [Google Scholar]
- 2.Arruda VR, Salles TS, Costa FF, Saad ST. Glutathione peroxidase, reduced glutathione, superoxide dismutase and catalse in red cells of patients with hairy cell leukemia. Neoplasia. 1996;43:99–102. [PubMed] [Google Scholar]
- 3.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 4.Brodowicz T, Wiltschke C, Kandioler-Eckersberger D, Grunt TW, Rudas M, Schneider SM, Hejna M, Budinsky A, Zielinski CC. Inhibition of proliferation and induction of apoptosis in soft tissue sarcoma cells by interferon-alpha and retinoids. Br J Cancer. 1999;80:1350–1358. doi: 10.1038/sj.bjc.6690528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 6.Conte da Frota ML, Jr, Comes da Silva E, Behr GA, Roberto de Oliveira M, Dal Pizzol F, Klamt F, Moreira JC. All trans retinoic acid induces free radical generation and modulate antioxidant enzyme activities in rat sertoli cells. Mol Cell Biochem. 2006;285:173–179. doi: 10.1007/s11010-005-9077-3. [DOI] [PubMed] [Google Scholar]
- 7.Dietze EC, Caldwell LE, Marcom K, Collins SJ, Yee L, Swisshelm K, Hobbs KB, Bean GR, Seewaldt VL. Retinoids and retinoic acid receptors regulate growth arrest and apoptosis in human mammary epithelial cells and modulate expression of CBP/p300. Microsc Res Tech. 2002;59:23–40. doi: 10.1002/jemt.10174. [DOI] [PubMed] [Google Scholar]
- 8.Dimberg A, Oberg F. Retinoic acid-induced cell cycle arrest of human myeloid cell lines. Leuk Lymphoma. 2003;44:1641–1650. doi: 10.1080/1042819031000083316. [DOI] [PubMed] [Google Scholar]
- 9.Drisko JA, Chapman J, Hunter VJ. The use of antioxidant therapies during chemotherapy. Gynecol Oncol. 2003;88:434–439. doi: 10.1016/s0090-8258(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 10.Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher RE. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002;16:1940–1958. doi: 10.1038/sj.leu.2402719. [DOI] [PubMed] [Google Scholar]
- 12.Gronemeyer H, Miturski R. Molecular mechanisms of retinoid action. Cell Mol Biol Lett. 2001;6:3–52. [PubMed] [Google Scholar]
- 13.Hara M, Suzuki S, Mori J, Yamashita K, Kumagai M, Sakuma T, Kakizawa T, Takeda T, Miyamoto T, Ichikawa K, Hashizume K. Thyroid hormone regulation of apoptosis induced by retinoic acid in promyeloleukemic HL-60 cells: studies with retinoic acid receptor-specific and retinoid x receptor-specific ligands. Thyroid. 2000;10:1023–1034. doi: 10.1089/thy.2000.10.1023. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Yokozaki H, Naka K, Yasui W, Lotan R, Tahara E. Overexpression of retinoic acid receptor beta induces growth arrest and apoptosis in oral cancer cell lines. Jpn J Cancer Res. 2001;92:42–50. doi: 10.1111/j.1349-7006.2001.tb01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hengartner MO. The biochemistry of apoptosis (review) Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 16.Kuida K. Caspase-9. Int J Biochem Cell Biol. 2000;32:121–124. doi: 10.1016/s1357-2725(99)00024-2. [DOI] [PubMed] [Google Scholar]
- 17.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipids asymmetry in subpopulations of juman red blood cells using fluorescently labeled annexin V. Blood. 1996;87:1179–1187. [PubMed] [Google Scholar]
- 18.Manor D, Shmidt EN, Budhu A, Flesken-Nikitin A, Zgola M, Page R, Nikitin AY, Noy N. Mammary carcinoma suppressionby cellular retinoic acid binding protein-II. Cancer Res. 2003;63:4426–4433. [PubMed] [Google Scholar]
- 19.Marill J, Idres N, Capron CC, Nguyen E, Chabot GG. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab. 2003;4:1–10. doi: 10.2174/1389200033336900. [DOI] [PubMed] [Google Scholar]
- 20.Melino G, Thiele CJ, Knight RA, Piacentini M. Retinoids and the control of growth/death decisions in human neuroblastoma cell lines. J Neurooncol. 1997;31:65–83. doi: 10.1023/a:1005733430435. [DOI] [PubMed] [Google Scholar]
- 21.Nagase M, Alam MM, Tsushima A, Yoshizawa T, Sakato N. Apoptosis induction by T-2 toxin: activation of caspase-9, caspase-3 and DFF-40/CAD through cytosolic release of cytochrome C in HL-60 cells. Biosci Biotechnol Biochem. 2001;65:1741–1747. doi: 10.1271/bbb.65.1741. [DOI] [PubMed] [Google Scholar]
- 22.Nagpal S, Chandraratna RA. Recent developments in receptor-selective retinoids. Curr Pharm Des. 2000;6:919–931. doi: 10.2174/1381612003400146. [DOI] [PubMed] [Google Scholar]
- 23.Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 24.Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006;126:2178–2189. doi: 10.1038/sj.jid.5700289. [DOI] [PubMed] [Google Scholar]
- 25.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 26.Ott DB, Lachance PA. Retinoic acid-a review. Am J Clin Nutr. 1979;32:2522–2531. doi: 10.1093/ajcn/32.12.2522. [DOI] [PubMed] [Google Scholar]
- 27.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 28.Paik J, Blaner WS, Sommer KM, Moe R, Swisshlem K. Retinoids, retinoic acid receptors, and breast cancer. Cancer Invest. 2003;21:304–312. doi: 10.1081/cnv-120016425. [DOI] [PubMed] [Google Scholar]
- 29.Pepper C, Ali K, Thomas A, Hoy T, Fegan C, Chowdary P, Kell J, Bentley P. Retinoid-induced apoptosis in B-cell chronic lymphocytic leukaemia cells is mediated through caspase-3 activation and is independent of p53, the retinoic acid receptor, and differentiation. Eur J Haematol. 2002;69:227–235. doi: 10.1034/j.1600-0609.2002.02799.x. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555–561. doi: 10.1038/sj.bjc.6600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pigault C, Follenius-Wund A, Schmutz M, Freyssinet JM, Brisson A. Formation of two-dimensional arrays of annexin V on phosphatidylserine-containing liposomes. J Mol Biol. 1994;236:199–208. doi: 10.1006/jmbi.1994.1129. [DOI] [PubMed] [Google Scholar]
- 32.Prakash P, Russell RM, Krinsky NI. In vitro inhibition of proliferation of estrogen-dependent and estrogen independent human breast cancer cells treated with carotenoids or retinoids. J Nutr. 2001;131:1574–1580. doi: 10.1093/jn/131.5.1574. [DOI] [PubMed] [Google Scholar]
- 33.Ralhan R, Kaur J. Retinoids as chemopreventive agents. J Biol Regul Homeost Agents. 2003;17:66–91. [PubMed] [Google Scholar]
- 34.Raloff J. Antioxidants may help cancers thrive. Science News. 2000;157:5. [Google Scholar]
- 35.Rishi AK, Zhang L, Boyanapalli M, Wali A, Mohammad RM, Yu Y, Fontana JA, Hatfield JS, Dawson MI, Majumdar AP, Reichert U. Identification and characterization of a cell cycle and apoptosis regulatory protein-1 as a novel mediator of apoptosis signaling by retinoid CD437. J Biol Chem. 2003;278:33422–33435. doi: 10.1074/jbc.M303173200. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y, Meller R, Yang T, Taki W, Simon RP. Stereo-selective neuroprotection against stroke with vitamin A derivatives. Brain Res. 2008;1241:188–192. doi: 10.1016/j.brainres.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6:1077–1088. [PubMed] [Google Scholar]
- 38.Smith MA, Parkinson DR, Cheson BD, Friedman MA. Retinoids in cancer therapy. J Clin Oncol. 1992;10:839–864. doi: 10.1200/JCO.1992.10.5.839. [DOI] [PubMed] [Google Scholar]
- 39.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 40.Stennicke HR, Salvesen GS. Caspase assays. Methods Enzymol. 2000;322:91–100. doi: 10.1016/s0076-6879(00)22010-7. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland MW, Learmonth BA. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radic Res. 1997;27:283–289. doi: 10.3109/10715769709065766. [DOI] [PubMed] [Google Scholar]
- 42.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxi Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 43.Villa P, Kaufmann SH, Earnshaw WC. Caspase and caspase inhibitors. Trends Biochem Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- 44.Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol. 1982;6:675–682. doi: 10.1016/s0190-9622(82)70056-8. [DOI] [PubMed] [Google Scholar]
- 45.Xu Q, Konta T, Kitamura M. Retinoic acid regulation of mesangial cell apoptosis. Exp Nephrol. 2002;10:171–175. doi: 10.1159/000058343. [DOI] [PubMed] [Google Scholar]
- 46.Xu XC, Liu X, Tahara E, Lippman SM, Lotan R. Expression and up-regulation of retinoic acid receptor-beta is associated with retinoid sensitivity and colony formation in esophageal cancer cell lines. Cancer Res. 1999;59:2477–2483. [PubMed] [Google Scholar]