Abstract
Obesity has become a worldwide health problem. Orlistat, an inhibitor of pancreatic lipase, is currently approved as an anti-obesity drug. However, gastrointestinal side effects caused by Orlistat may limit its use. In this study the inhibitory activities of dandelion (Taraxacum officinale) against pancreatic lipase in vitro and in vivo were measured to determine its possible use as a natural anti-obesity agent. The inhibitory activities of the 95% ethanol extract of T. officinale and Orlistat were measured using 4-methylumbelliferyl oleate (4-MU oleate) as a substrate at concentrations of 250, 125, 100, 25, 12.5 and 4 µg/ml. To determine pancreatic lipase inhibitory activity in vivo, mice (n=16) were orally administered with corn oil emulsion (5 ml/kg) alone or with the 95% ethanol extract of T. officinale (400 mg/kg) following an overnight fast. Plasma triglyceride levels were measured at 0, 90, 180, and 240 min after treatment and incremental areas under the response curves (AUC) were calculated. The 95% ethanol extract of T. officinale and Orlistat, inhibited, porcine pancreatic lipase activity by 86.3% and 95.7% at a concentration of 250 µg/ml, respectively. T. officinale extract showed dose-dependent inhibition with the IC50 of 78.2 µg/ml. A single oral dose of the extract significantly inhibited increases in plasma triglyceride levels at 90 and 180 min and reduced AUC of plasma triglyceride response curve (p<0.05). The results indicate that T. officinale exhibits inhibitory activities against pancreatic lipase in vitro and in vivo. Further studies to elucidate anti-obesity effects of chronic consumption of T. officinale and to identify the active components responsible for inhibitory activity against pancreatic lipase are necessary.
Keywords: mouse, pancreatic lipase, Taraxacum officinale, triglyceride
Introduction
Obesity, defined as the excessive accumulation of body fat that may impair health, has become a worldwide epidemic (Mokdad et al., 2003). Obesity is a major risk factor for cardiovascular disease including heart disease and stroke, diabetes mellitus, musculoskeletal disorders such as osteoarthritis, and some cancers such as breast, endometrial, prostate, and colon cancer (Aronne, 1998). The fundamental cause of obesity is an energy imbalance between calorie intake and expenditure. Therefore, inhibition of digestion and absorption of dietary fat, the most concentrated source of energy, can be useful in treatment of obesity. In fact, Orlistat, a potent competitive inhibitor of gastric and pancreatic lipase, is available as an anti-obesity drug (Ballinger & Peikin, 2002; Hochuli et al., 1987; Jandacek & Woods, 2004; Weibel et al., 1987). However, this medication is associated with a greater incidence of gastrointestinal side effects (Birari & Bhutani, 2007; Weigle, 2003). Therefore, numerous studies have evaluated natural products with pancreatic lipase inhibitory activity, including plant materials, as an alternative anti-obesity agent (Arai et al., 1999; Han et al., 2001; Han et al., 2002; Kim, 2007; Kurihara et al., 2003; Kurihara et al., 2006). Dietary teasaponin (Han et al., 2001), saponins from Platycodi Radix (Han et al., 2002), and onion skin extract (Kim, 2007) were reported to decrease postprandial plasma triglyceride response after lipid load and showed anti-obesity effects in animals fed high fat diets.
Dandelion (Taraxacum officinale) is a herbaceous perennial plant of the family Asteraceae (Compositae). T. officinale has been used as a phytomedicine due to its choleretic, antirhemetic, diuretic, and anti-inflammatory properties (Bisset, 1994). It has been reported that T. officinale exhibits anti-inflammatory activity (Mascolo et al., 1987) and anti-tumor activity (Williams et al., 1996). Antioxidant activity of T. officinale has been demonstrated in vitro (Hu & Kitts, 2005) and in vivo (Cho et al., 2003). T. officinale has been reported to have hypolipidemic effects in rats fed a high cholesterol diet (Cho et al., 2000) and streptozotocin-induced diabetic rats (Cho et al., 2002).
However, the effect of this plant on the activity of pancreatic lipase has not yet been examined. Thus, in this study we measured the pancreatic lipase inhibitory activity of T. officinale in vitro and in vivo to evaluate its possible use as an anti-obesity agent.
Materials and Methods
Reagents
Orlistat was obtained from Roche Korea (Seoul, Korea). Corn oil was purchased from Cheiljedang (Seoul, Korea). Porcine pancreatic lipase, 4-methylumbelliferyl oleate (4-MU oleate), as well as the assay kit for triglycerides and all other reagent grade chemicals were purchased from Sigma (St. Louis, MO, USA).
Preparation of the ethanol extract
Young leaves of T. officinale were obtained from a local market in Changnyung-gun, Korea. The leaves were freeze-dried, powdered, and extracted with ten volumes of 95% ethanol for 12 hr three times at room temperature. The solvent was removed by rotary evaporation at 40℃.
Measurement of pancreatic lipase inhibitory activity in vitro
The inhibitory activity against pancreatic lipase was measured using 4-MU oleate as a substrate (Thompson, 1978). The reaction mixture consisted of 50 µL 0.1 mM 4-MU oleate, 20 µL McIlvane buffer (0.1 M citrate-Na2 HPO4, pH 7.4), and 5 µL of sample solution. Porcine pancreatic lipase (25 µL) was added to the reaction mixture and the final volume was adjusted to 0.1 ml. After the mixture was incubated at 37℃ for 10 min, the amount of 4-MU released by the lipase was measured using a fluorescence multi-detection reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at an excitation wavelength of 320 nm and an emission wavelength of 450 nm. The inhibitory activities of the T. officinale. extract and Orlistat, a positive control against pancreatic lipase were measured at concentrations of 250, 125, 100, 25, 12.5 and 4 µg/ml. The measurements were performed in triplicate, and the IC50 value, the concentration of the extract that results in 50% inhibition of maximal activity was determined.
Animals
Five week-old male ICR mice weighing between 20 and 25 g were purchased from Bio Genomics, Inc. (Seoul, South Korea). All animals were fed a commercial chow ad libitum for 2 wk after arrival. Mice were housed individually in stainless steel wire-bottomed cages and located in a room where temperature (23-27℃), humidity (50-60%), and lighting cycle (12 hr light/dark cycle) were controlled. Mice (28-35 g, n=16) were then randomly divided into two groups.
Measurement of plasma triglyceride levels after oral administration of a lipid emulsion to mice
Following an overnight fast, the animals were fed corn oil emulsion (5 ml/kg) alone or oil emulsion with 95% ethanol extract of T.officinale (400 mg/kg). The oil emulsion was composed of 80 mg cholic acid, 2 mg cholesteryl oleate, 6 ml saline, and 6 ml corn oil. Food was withheld during the test. Blood samples were collected from the ophthalmic venous plexus 0, 90, 180, and 240 min after the oral administration and centrifuged at 2,000 × g for 15 min. Plasma triglyceride levels were measured using a commercial triglyceride assay kit and were expressed as increments from the baseline. Incremental areas under the response curves (AUC) were calculated using the trapezoidal rule with fasting levels as the baseline. The experiments were performed according to the guidelines of animal experimentation approved by the Animal Resource Center at Inje University, Korea.
Statistics
All data are expressed as mean ± standard deviation (SD). All statistical analyses were performed using SAS (version 8.02). Differences in incremental plasma triglyceride levels and AUC between control group and T. officinale group were assessed using Student's t-test. Significance was defined as p<0.05.
Results
Dose-dependent inhibition of pancreatic lipase activity
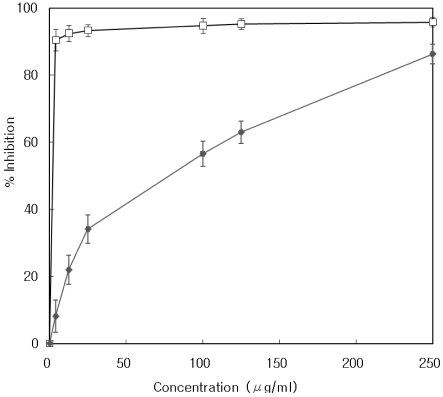
The inhibitory activities of 95% ethanol extract of T. officinale against porcine pancreatic lipase are shown in Fig. 1. The T. officinale extract inhibited pancreatic lipase activity by 86.3, 63.0, 56.5, 34.1, 21.9, and 8.1% at concentrations of 250, 125, 100, 25, 12.5 and 4 µg/ml in vitro, respectively. Orlistat, a pancreatic lipase inhibitor used as an anti-obesity agent, inhibited the enzyme activity by 95.7 and 90.4% at concentrations of 250 and 4 µg/ml, respectively. The T. officinale extract and Orlistat had IC50 values of 78.2 µg/ml and 0.22 µg/ml, respectively.
Fig. 1.
Dose-dependent inhibition of pancreatic lipase activity of Taraxacum officinale. extract and Orlistat. The inhibitory activities of the 95% ethanol extract of Taraxacum officinale or Orlistat were measured at concentrations of 250, 125, 100, 25, 12.5 and 4 µg/ml. □, Orlistat; ♦, T. officinale. Values represent mean ± SD of triplicate measurements.
Effect of T. officinale extract on postprandial triglyceride response
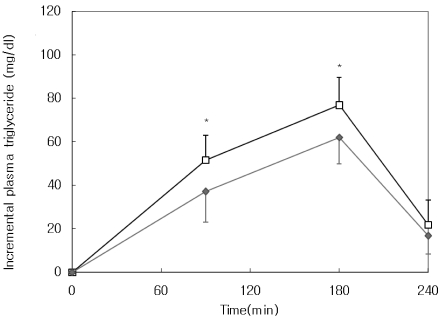
Plasma triglyceride responses to a single oral dose of corn oil emulsion (5 ml/kg) alone or the oil emulsion with T. officinale extract (400 mg/kg) in ICR mice are shown in Fig. 2. Incremental plasma triglyceride levels of mice that consumed the oil emulsion alone reached a peak (76.9 ± 12.6 mg/dl) at 180 min. Consumption of T. officinale extract significantly decreased incremental plasma triglyceride levels at 90 and 180 min (p<0.05). The AUC for triglyceride response was significantly lower in the T. officinale group (8,504 ± 1,950 mg·min/dl) than in the control group (11,068 ± 2,054 mg·min/dl, p<0.05, Table 1).
Fig. 2.
Increase in plasma triglyceride after administration of Taraxacum officinale extract to mice. In the control group (□), corn oil emulsion (5 ml/kg) was administered orally to mice after an overnight fast. In the Taraxacum officinale. group (♦), corn oil emulsion (5 ml/kg) plus 95% ethanol extract of T. officinale. (400 mg/kg) was administered orally to mice after an overnight fast. Values represent mean ± SD. *Significantly different at P<0.05
Table 1.
Area under the curve (AUC) of postprandial triglyceride responses of mice
Control group: Corn oil emulsion (5 ml/kg) was administered orally to mice after an overnight fast.
Taraxacum officinale group: Corn oil emulsion (5 ml/kg) plus 95% ethanol extract of T. officinale. (400 mg/kg) was administered orally to mice after an overnight fast. AUC: Area under the response curves.
Values represent mean ± SD (n=8).
*Significantly different at p<0.05
Discussion
Pancreatic lipase is the key enzyme for dietary fat digestion (Olshansky et al., 2005), and inhibition of the enzyme could be an effective way to alter fat absorption. In fact, Orlistat, pancreatic lipase inhibitor and Sibutramine, an appetite suppressor are the only two anti-obesity medications currently approved (Jandacek & Woods, 2004). However, because Orlistat can result in undesirable side effects, such as fecal incontinence, flatulence, and steatorrhea, its use may be limited (Birari & Bhutani, 2007; Weigle, 2003). Therefore, it may be worthwhile to search the natural substances that show potent inhibitory activity against pancreatic lipase and have fewer side effects. Alpinia officinarum (Shin et al., 2003), grape seed extract (Moreno et al., 2003), green tea extract (Juhel et al., 2000), mango leaf and stem bark extracts (Moreno et al., 2006), and onion skin (Kim, 2007) showed potent inhibitory activity against pancreatic lipase.
In this study, we investigated the inhibitory activity of T. officinale against porcine pancreatic lipase in vitro. At a concentration of 250 µg/ml, the T. officinale extract exhibited 90.2% of the inhibition activity of Orlistat against pancreatic lipase, and T. officinale inhibited pancreatic lipase in a dose-dependent manner with an IC50 of 78.2 µg/ml (Fig. 1). Orlistat showed IC50 of 0.22 µg/ml Orlistat was reported to show IC50 of 0.14 µg/ml (Weibel et al., 1987). We determined the effect of T. officinale on postprandial blood triglyceride in mice after consumption of corn oil emulsion load. The T. officinale extract (400 mg/kg) significantly suppressed incremental plasma triglyceride at 90 and 180 min (Fig. 2). Arai et al. (1999) reported that n-hexane fraction isolated from Eugenia uniflora extract showed pancreatic lipase inhibitory activity in vitro and improving effect on postprandial hypertriglyceridemia in mice administered with corn oil emulsion. Kurihara et al. (2003) reported that Cyclocarya paliurus (Batal) Iljinskaja extract with pancreatic lipase inhibitory activity inhibited postprandial plasma triglyceride increase at 180 min after administration of lard oil or olive oil in mice without influencing postprandial plasma free fatty acid levels suggesting that utilization of triglyceride was not changed. In this study T. officinale extract significantly decreased AUC for the postprandial triglyceride response curve. These data demonstrate that T. officinale exerts pancreatic lipase inhibitory activity in vivo to decrease postprandial triglyceride levels. AUC after a test meal is extensively used as index of the postprandial triglyceride response (Karamanos et al., 2001). The limitation of this study is that we could not distinguish contribution of inhibition of dietary fat absorption and increase of triglyceride clearance to the reduction of postprandial plasma triglyceride response. Further study to measure the effect of T. officinale on postprandial plasma free fatty acid and lipoprotein lipase activity would be necessary to determine the effect on triglyceride clearance.
T. officinale leaf was reported to contain flavonoids such as luteolin (Williams et al., 1996) and it was demonstrated flavonoids could have pancreatic lipase inhibitory activity (Moreno et al., 2006; Shin et al., 2003; Yamamoto et al., 2000). Further study to identify the active compounds which inhibit pancreatic lipase from T. officinale leaf could be valued.
Obesity is associated with increased risks for some chronic degenerative diseases and decreased life expectancy (Mokdad et al., 2003; Olshansky et al., 2005). A combination of diet therapy, exercise, and behavior modification is the standard regimen for the treatment of obesity, and medication therapy can be used in conjunction with such lifestyle changes. Since only a few medications have been approved as effective and safe (Jandacek & Woods, 2004), extensive studies have been carried out to develop alternative anti-obesity drugs. Our data suggest that T. officinale has potential to be used as an anti-obesity agent.
In conclusion, T. officinale showed strong pancreatic lipase inhibitory activity in vitro and in vivo. Further studies to elucidate anti-obesity effects of chronic consumption of T. officinale and to identify the active components responsible for inhibitory activity against pancreatic lipase are necessary.
Footnotes
This study was supported (in part) by a grant from the Ministry of Knowledge Economy (MKE) and the Korea Institute of Industrial Technology Evaluation & Planning (ITEP) through the Biohealth Products Research Center (BPRC) of Inje University.
References
- 1.Arai I, Amagaya S, Komatsu Y, Okada M, Hayashi T, Kasai M, Arisawa M, Momose Y. Improving effects of the extracts from Eugenia uniflora on hyperglycemia and hypertriglyceridemia in mice. J Ethnopharmacol. 1999;68:307–314. doi: 10.1016/s0378-8741(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 2.Aronne LJ. Modern medical management of obesity: the role of pharmaceutical intervention. J Am Diet Assoc. 1998;98:S23–S26. doi: 10.1016/s0002-8223(98)00706-8. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger A, Peikin SR. Orlistat: its current status as an anti-obesity drug. Eur J Pharmacol. 2002;440:109–117. doi: 10.1016/s0014-2999(02)01422-x. [DOI] [PubMed] [Google Scholar]
- 4.Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 2007;12:879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Bisset NG. Herbal Drugs and Phytopharmaceuticals, Medpharm. Germany: Stuttgart; 1994. Taraxaci radix cum herba; pp. 486–489. [Google Scholar]
- 6.Cho SY, Park JY, Oh YJ, Jang JY, Park EM, Kim MJ. Effect of dandelion leaf extracts on lipid metabolism in rats fed high cholesterol diet. J Korean Soc Food Sci Nutr. 2000;29:676–682. [Google Scholar]
- 7.Cho SY, Park JY, Park EM, Choi MS, Lee MK, Jeon MJ, Jang MK, Kim MJ, Park YB. Alternation of hepatic antioxidant enzyme activities and lipid profile in streptozotocin-induced diabetic rats by supplementation of dandelion water extract. Clin Chim Acta. 2002;317:109–117. doi: 10.1016/s0009-8981(01)00762-8. [DOI] [PubMed] [Google Scholar]
- 8.Cho SY, Oh YJ, Park JY, Lee MK, Kim MJ. Effect of dandelion (Taraxacum officinale) leaf extracts on hepatic antioxidative system in rats fed high cholesterol diet. J Korean Soc Food Sci Nutr. 2003;32:458–463. [Google Scholar]
- 9.Han LK, Kimura Y, Kawashima M, Takaku T, Taniyama T, Hayashi T, Zheng YN, Okuda H. Anti-obesity effects in rodents of dietary teasaponin, a lipase inhibitor. Int J Obes Relat Metab Disord. 2001;25:1459–1464. doi: 10.1038/sj.ijo.0801747. [DOI] [PubMed] [Google Scholar]
- 10.Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from Platycodi Radix ameliorate high fat diet-induced obesity in mice. J Nutr. 2002;132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- 11.Hochuli E, Kupfer E, Maurer R, Meister W, Mercadal Y, Schmidt K. Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. II. Chemistry and structure elucidation. J Antibiot (Tokyo) 1987;40:1086–1109. doi: 10.7164/antibiotics.40.1086. [DOI] [PubMed] [Google Scholar]
- 12.Hu C, Kitts DD. Dandelion (Taraxacum officinale) flow extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro. Phytomedicine. 2005;12:588–597. doi: 10.1016/j.phymed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Jandacek RJ, Woods SC. Pharmaceutical approaches to the treatment of obesity. Drug Discov Today. 2004;15:874–880. doi: 10.1016/S1359-6446(04)03244-1. [DOI] [PubMed] [Google Scholar]
- 14.Juhel C, Armand M, Pafumi Y, Rosier C, Vandermander J, Lairon D. Green tea extract (AR25) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro. J Nutr Biochem. 2000;11:45–51. doi: 10.1016/s0955-2863(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 15.Karamanos BG, Thanopoulou AC, Roussi-Penesi DP. Maximal post-prandial triglyceride increases reflects post-prandial hypertriglyceridaemia and is associated with the insulin resistance syndrome. Diabet Med. 2001;18:32–39. doi: 10.1046/j.1464-5491.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim HY. Effect of onion (Allium cepa) skin extract on pancreatic lipase and body weight-related parameters. Food Science and Biotechnology. 2007;16:434–438. [Google Scholar]
- 17.Kurihara H, Asami S, Shibata H, Fukami H, Tanaka T. Hypolipemic effect of Cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice. Biol Pharm Bull. 2003;26:383–385. doi: 10.1248/bpb.26.383. [DOI] [PubMed] [Google Scholar]
- 18.Kurihara H, Shibata H, Fukui Y, Kiso Y, Xu JK, Yao XS, Fukami H. Evaluation of the hypolipidemic property of Camellia sinensis Var. ptilophylla on postprandial hypertriglyceridemia. J Agric Food Chem. 2006;54:4977–4981. doi: 10.1021/jf0603681. [DOI] [PubMed] [Google Scholar]
- 19.Mascolo N, Autore G, Capasso F, Menghini A, Fasulo MP. Biological screening of Italian medical plants for anti-inflammatory activity. Phytother Res. 1987;1:28–31. [Google Scholar]
- 20.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 21.Moreno DA, Ilic N, Poulev A, Brasaemle DL, Fried SK, Raskin I. Inhibitory effects of grape seed extract on lipases. Nutrition. 2003;19:876–879. doi: 10.1016/s0899-9007(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 22.Moreno DA, Ripoll C, Ilic N, Poulev A, Aubin C, Raskin I. Inhibition of lipid metabolic enzymes using Mangifera indica extracts. J Food Agric Environ. 2006;4:21–26. [Google Scholar]
- 23.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1135–1137. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 24.Shin JE, Han MJ, Kim DH. 3-Methylethergalangin isolated from Alpinia officinarum inhibits pancreatic lipase. Biol Pharm Bull. 2003;26:854–857. doi: 10.1248/bpb.26.854. [DOI] [PubMed] [Google Scholar]
- 25.Thompson ABR. Disturbance in lipid metabolism. New York, USA: American Physiological Society; 1978. p. 31. [Google Scholar]
- 26.Weibel EK, Hadvary P, Hochuli E, Kupfer E, Lengsfeld H., Lipstatin An inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity. J Antibiot (Tokyo) 1987;40:1081–1085. doi: 10.7164/antibiotics.40.1081. [DOI] [PubMed] [Google Scholar]
- 27.Weigle DS. Pharmacological therapy of obesity: past, present, and future. J Clin Endocrinol Metab. 2003;88:2462–2469. doi: 10.1210/jc.2003-030151. [DOI] [PubMed] [Google Scholar]
- 28.Williams CA, Goldstone F, Greenham J. Flavonoids, cinnamic acids and coumarins from the different tissues and medical preparations of Taraxacum officinale. Phytochemistry. 1996;42:121–127. doi: 10.1016/0031-9422(95)00865-9. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Shimura S, Itoh Y, Ohsaka T, Egawa M, Inoue S. Anti-obesity effects of lipase inhibitor CT-II, an extract from edible herbs, Nomame Herba, on rats fed a high-fat diet. Int J Obes Relat Metab Disord. 2000;24:758–764. doi: 10.1038/sj.ijo.0801222. [DOI] [PubMed] [Google Scholar]