Abstract
This study was purposed to investigate the effect of onion or beet on plasma and liver lipids, erythrocyte Na efflux channels and platelet aggregation in simvastatin (SIM) treated hypercholesterolemic rats. Forty Sprague Dawley rats were divided into four groups and fed 0.5% cholesterol based diets containing 2 mg/kg BW simvastatin or simvastatin with 5% onion or beet powder. Plasma total cholesterol was significantly increased in SIM group compared with the control (p<0.01), and the elevated plasma total cholesterol of SIM group was significantly decreased in SIM-onion and SIM-beet groups (p<0.05). HDL-cholesterol in SIM-beet group was significantly increased compared with other groups (p<0.05). Platelet aggregation in both the maximum and initial slope was significantly decreased in SIM group compared with SIM-onion group (p<0.05). Na-K ATPase was significantly decreased in SIM group compared with the control, SIM-onion and SIM-beet groups (p<0.05). Na passive leak was significantly increased in all groups treated with SIM compared with the control (p<0.05). The total Na efflux was decreased in SIM group and increased in SIM-onion group and the difference between these two groups was significant (p<0.05). There was no difference in intracellular Na among groups. In present study, simvastatin, a HMG CoA reductase inhibitor at dose of 2mg/kg BW/day rather increased plasma total cholesterol in rats, inferring that the action mechanism of simvastatin on cholesterol metabolism differ between rat and human. Onion and beet play favorable roles in cardiovascular system by restoring the reduced Na efflux through Na-K ATPase and Na-K cotransport in SIM treated rats.
Keywords: Simvastatin, onion, beet, antioxidant, hypercholesterolemic rats
Introduction
According to American Heart Association Report, cardiovascular disease (CVD) is a major cause of death in US and the death rate of 35.2% is accounted from CVD in 2005 (Rosamond et al., 2008). Elevated plasma cholesterol is one of the major risk factors in developing cardiovascular diseases, and an intervention study showed that reduction of total cholesterol by 11.5% reduced CVD events by 33% (Nakamura et al., 2007). Statin, a HMG CoA reductase inhibitor is recognized as one of the most popular drug lowering cholesterol in human. Among US adults aged ≥ 40years with hypertension, 14.5% are taking statin medication in NHANES (1999-2002) study (King et al., 2007). Statin is known to deplete coenzyme Q10 (CoQ10) that shares cholesterol biosynthetic pathway, resulting in decreased antioxidant capacity. The reduced form of CoQ10 (CoQH2), ubiquinol in plasma membrane functions as radical scavenger, protecting cell membrane from oxidative damage (Mabuchi et al., 2005). Nitric oxide (NO) that plays a dual role as deleterious radical species and beneficial species can be induced by statin (Fontaine et al., 2002). Statin dependant endothelial nitric oxide synthetase (NOS) affected Na-K ATPase and Na-K cotransport in rat renal tubule (Varela et al., 2004). Statin may influence membrane ion channel by changing membrane lipids, thereby affecting membrane fluidity. Membrane fluidity of erythrocyte was increased with decreasing plasma cholesterol by statin therapy (Broncel et al., 2007). NO inhibited platelet aggregation through cyclic GTP pathway in simvastatin treated rabbit (Chou et al., 2008). A NO donor, nitro-L-arginine methyl ester (L-NAME) or antioxidant quercetin and catechin exerted antiplatelet effects by inhibiting NADPH oxidase and increasing NO (Pignatelli et al., 2006). Betalain pigments which are present in red beet (Beta vulgaris L.) root and prickly pear cactus (Opuntia ficus indica) fruit are recently recognized as bioavailable natural radical scavengers. Tesoriere et al. (2005) reported that betalain metabolite, betanin and indicaxanthin were observed in LDL fraction at 3 hours after ingestion of prickly pear cactus fruit and the resistance of LDL particles to ex vivo induced oxidation was increased. Cationized antioxidants in red beet inhibit lipid peroxidation in membranes or linoleate emulsion and prevent H(2)O(2)-activated LDL oxidation at relatively low concentration (Kanner et al., 2001). Quercetin which is abundant in most fruits and vegetables is the major type of flavonoid in onion (Allium cepa). Yamamoto et al. (2005) have shown that the green-leafy Welsh onion reduced superoxide generation by suppressing the angiotensin II production and then the NADH/NADPH oxidase activity, increasing the NO availability in the aorta and consequently lowering blood pressure in the rats fed with a high fat diet. Continuous ingestion of onion soup inhibits collagen induced platelet aggregation in human by inhibiting collagen stimulated tyrosine phosphorylation (Hubbard et al., 2006)
We hypothesized that statin therapy may affect some cardiovascular related parameters such as erythrocyte efflux channels, platelet aggregation besides cholesterol lowering effect by way of its own function on erythrocyte Na channel and platelet aggregation or by depletion of CoQ10 and induction of NO, and antioxidant rich foods such as onion and beet may reverse or interact statin action on these parameters.
Materials and Methods
Animals and diets
Forty of twelve weeks old Sprague Dawley rats (Orient Bio Co., Ltd, Korea) were divided into four groups and fed the following diets: Control; 0.5% cholesterol based diet: Simvastatin (Choongwae Pharm. Co., Korea); the control diet plus 2 mg/kg BW simvastatin: Sim-onion; the simvastatin diet plus 5% onion powder: Sim-beet; the simvastatin diet plus 5% beet powder. Rats had free access to water and were housed individual cages in a room maintained at 20-25℃ with a 12 hour dark-light cycle. After 4 weeks ad libitum feeding, blood samples were obtained by cardiac puncture into heparinized vacuum tubes, and platelet aggregation and erythrocyte Na efflux were performed with fresh blood. Liver samples were prepared for microscopic examination and plasma and liver samples were stored at -70℃ for later assays.
Platelet aggregation
Platelet aggregation was measured using a Chronolog Whole Blood Aggregometor (model 500-Ca, Havertown, Pennsylvania, USA), of which the instrumental principle is based on the increase in impedance (Ω) across two platinum electrodes as platelet aggregation proceeds. The whole blood was diluted with isotonic saline (1:4) to give platelet concentration of approximately 200,000 platelets/µl. Adenosine diphosphate (ADP, 2 µM) was added to initiate aggregation, and three readings of impedance changes were averaged for each rat.
Plasma and liver lipid assays
Plasma total cholesterol, HDL-cholesterol and triglyceride were assayed using enzymatic kits (Asan Pharmaceuticals, Korea). Ten µl of plasma was used for the assays of total cholesterol and triglyceride. A 200 µl sample of plasma was incubated with dextran sulfate to precipitate apo B containing lipoprotein and 50 µl of the supernatant was used for HDL cholesterol.
Liver lipids were extracted by a modified Folch method (Folch et al., 1957). One gram of liver tissue was homogenized for 5 min in 6ml of Folch solution [chloroform (2): methanol (1)] and 2ml H2O. After centrifugation for 10 min, the lower phase that contains liver lipids was separated. Lower phase of lipid fractions was assayed after treating with triton X-100:chloroform (25 µl:475 µl) for total cholesterol or with methanol for triglyceride, using enzymatic kits (Asan Pharmaceuticals, Korea).
Na Efflux Channels
Red cell preparation:
Blood was centrifuged at 1,000 ×g for 10 minutes, and the plasma and buffy coat were removed. Red blood cells were washed 5 times with a cold isotonic washing solution [150 mM choline chloride, 10 mM Tris-4 morpholinopropane sulfonic acid (MOPS), pH 7.4 at 4℃], and centrifuged at 1,000 ×g for 5 minutes after each wash. The RBC pellet was resuspended in the choline chloride washing to give 40-50% hematocrit, which was also measured. A 50ul aliquot of the RBC suspension was added to 5ml of 0.025% acationox (a metal free detergent, Scientific products, McGraw Park, Illinois, USA) to be used for determination of intracellular Na concentrations.
Na efflux:
Four ml each of the RBC suspension were added to 40ml MgCl2 medium with and without ouabain (70 mM MgCl2, 10 mM KCl, 85 mM sucrose, 10 mM glucose, 10 mM Tris MOPS pH 7.4 at 37℃, 1 mM ouabain) for determination of Na efflux via Na-K ATPase. Two ml of the RBC suspension was added to 40 ml of choline chloride medium with and without furosemide (150 mM choline chloride, 10 mM glucose, 1 mM ouabain, 10m Tris-MOPS pH 7.4 at 37℃, 1 mM furosemide) for determination of Na efflux via Na-K cotransport. The RBCs in each medium were mixed and aliquoted into 12 tubes. Tubes in duplicate were transferred to an ice bath after incubation at 37℃ in a shaking water bath for 0, 2, 4, 6, 8, and 10 minutes for Na-K ATPase and 0, 10, 20, 30, 40 and 50 minutes for the Na-K cotransport. Tubes were centrifuged at 1,000 ×g for 5 minutes and then the supernatant was removed and measured for Na concentration using Atomic Absorption Spectrophotometer (Shimadzu model AA6701F).
Calculation:
Ouabain sensitive Na-K ATPase can be blocked by ouabain and furosemide sensitive Na-K cotransport can be blocked by furosemide. Sodium efflux via Na-K ATPase was calculated as the difference between the efflux into MgCl2 medium with ouabain and the efflux into MgCl2 medium without ouabain; Na-K cotransport was calculated as the difference between the efflux into choline chloride medium with furosemide and the efflux into choline chloride medium without furosemide; Na passive leak was calculated as the efflux into choline chloride medium that contained ouabain and furosemide. For the determination of the efflux via Na-K ATPase with 4 ml erythrocyte suspension added to 40 ml MgCl2 medium, the following calculation was used (Kang et al., 1990).
Na µg/(ml×min)×60 min×µmole/23 µg×[44 ml-(4 ml×hct)/(4 ml×hct)]×=µmole/ml rbc/hour (mmole/ℓ rbc/hour)
For the determination of intracellular Na concentration with 50 µl red cell suspension added to 5ml 0.02% acationox, the following calculation was used;
Na µg/(ml×min)×60 min×µmol/23 µg×101/hct = µmole/ml rbc (mmole/ℓ rbc)
Histological examination
Two parts of liver and aorta samples each were obtained from four rats per group. After being washed with saline, samples were fixed in 10% formalin for 48 hours and dehydrated in xylene for 18 hours. Samples were then embedded in paraffin and cut into 4-5 um cross section. Tissues were stained with hematoxylineosin for microscopic observation.
Statistical analysis
Values were statistically analyzed using the SAS package (SAS, 1994). Analysis of variance was conducted in a completely randomized block design. Duncan's multiple range test was applied to compare individual means when F-value was significant (p<0.05).
Results
Weight gain and food efficiency
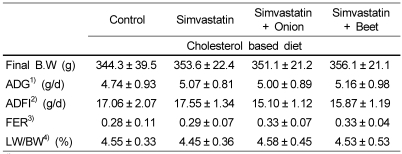
The final body weight along with average daily gain (ADG) was not different among groups (Table 2). The feed efficiency ratio (FER) tended to be a little higher in fiber containing SIM-onion and SIM-beet groups. There was no difference in liver/body weight ratio (LW/BW).
Table 2.
Effects of onion and beet on growth rate and feed intake in simvastatin treated hypercholesterolemic rats
1)ADG: Average daily gain
2)ADFI: Average daily feed intake
3)FER : Feed Efficiency Ratio
4)LW/BW: Liver/Body weight ratio
Values are means ± SD of 10 rats.
Plasma and liver cholesterol and triglyceride
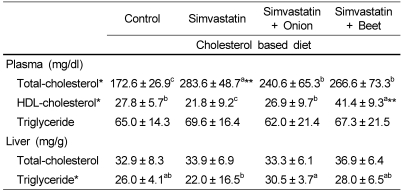
All groups fed diets containing simvastatin were significantly increased in plasma total cholesterol and the difference between the control and SIM group was significant (p<0.01) (Table 3). Plasma total cholesterol in SIM-onion and SIM-beet groups was significantly decreased compared with SIM group (p<0.05). HDL-cholesterol was significantly increased in SIM-beet group compared with other groups (p<0.05). There was virtually no difference in plasma triglyceride among groups.
Table 3.
Effects of onion and beet on plasma and liver lipids in simvastatin treated hypercholesterolemic rats
**Values compared with the lowest value in the same row differ (p<0.01).
*Values in the same row not sharing the same superscript differ (p<0.05).
Values are means ± SD of 10 rats.
Liver total cholesterol was not different between any two groups, but liver triglyceride was significantly different between SIM group and SIM-onion group (p<0.05).
Whole blood platelet aggregation
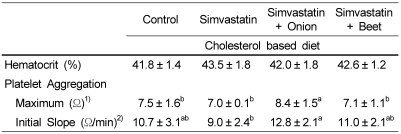
Hematocrit was not different between any two groups (Table 4). Platelet aggregation in both the maximum aggregation and initial slope was significantly increased in SIM-onion group compared with simvastatin group (p<0.05).
Table 4.
Effects of onion and beet on platelet aggregation in simvastatin treated hypercholesterolemic rats
1)Maximum aggregation is ohm at the point where aggregate dissociated.
2)Initial slope is ohm change for the first one minute of aggregation.
Values in the same row not sharing the same superscript differ (p<0.05)
Values are means ± SD of 10 rats.
Na efflux channels
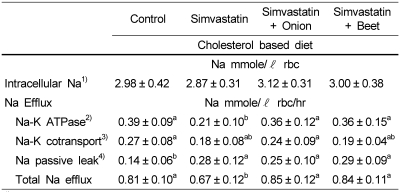
Erythrocyte Na efflux through Na-K ATPase was significantly decreased in SIM group compared with the control, SIM-onion and SIM-beet group (p<0.05). Na efflux through Na-K cotransport was also decreased in SIM group compared with the control and SIM-onion group. Na passive leak was significantly increased in all groups treated with simvastatin compared to the control (p<0.05). The total Na efflux was decreased significantly in SIM group compared with all other groups (p<0.05). There was no statistical difference in intracellular Na among groups.
Histological examination of liver and aorta
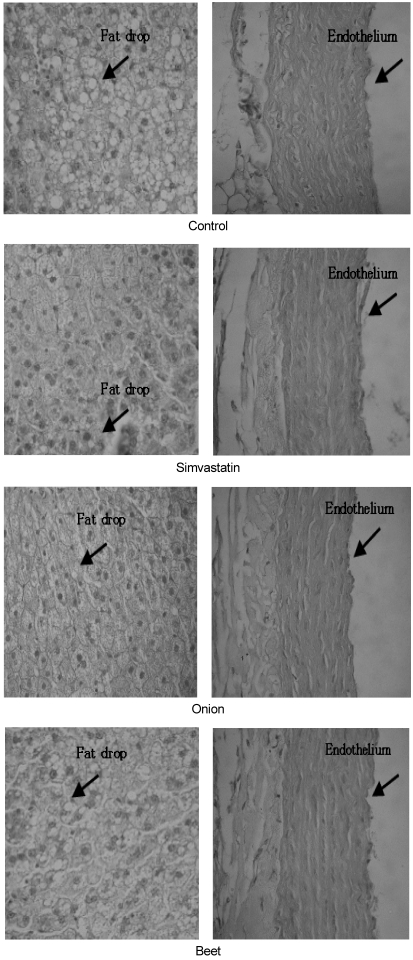
Despite of higher plasma cholesterol, fat accumulation of liver tissue in simvastatin and SIM-onion groups appeared less than that of the control (Fig. 1). Endothelium of aorta tissue of the control looked more rumpled than that of groups treated with simvastatin.
Fig. 1.
Microscopic appearance of liver tissue and aorta tissue (×400)
Discussion
Higher food efficiency ratio in SIM-onion and SIM-beet can be attributed to short chain fatty acids produced from fermentation of fibers in onion and beet. Some of undigested natural fiber can be fermented to propionate and acetate and absorbed in the large intestine (Lambo-Fodje et al., 2006). Plasma total cholesterol was increased in all groups treated with simvastatin opposing to our expectation. Condo et al. (1996) reported atorvastatin at 1, 3, 10 or 20 mg/kg BW in guinea pig decreased plasma and liver cholesterol with reduced HMG CoA reductase in a dose-dependent manner. Pravastatin or fluvastatin at dose of 2 mg/kg or 4 mg/kg BW respectively did not change plasma total cholesterol in rats (Chen et al., 2003). Pravastatin at high dose as 40 mg/kg or fluvastatin 20 mg/kg BW (Bełtowski et al., 2002) in Wistar rats and rosuvastatin at 20 mg/kg Bw in SHR rats (Susic et al., 2003) had no effect on plasma HDL, total cholesterol and triglyceride. However, simvastatin 2 mg/kg BW we used in the present study did act on cholesterol metabolism causing an increase in plasma total cholesterol and a decrease in HDL cholesterol. Simvastatin, 80 mg/day or 1.8 mg/kg BW per day in human is the maximal approved dose and effective in lowering cholesterol (Lutjohann et al., 2004). Rat model may differ from human and guinea pigs in the cholesterol synthesis compartment, the plasma distribution of cholesterol and the processing of lipoprotein in plasma compartment. Unlike human in whom approximately 65% of total endogenous cholesterol is synthesized in extrahepatic tissues, the major site of cholesterol synthesis in the rat is liver (West et al., 2004). According to Fernandez (2001), plasma LCAT (lecithin-cholesterol acyltransferase) and CETP (cholesteryl ester transfer protein) in human and guinea pig are very active, while those enzymes in rat or mice are negligible therefore the plasma cholesteryl ester in rat is produced by liver ACAT (Acyl CoA-cholesterol acyltransferase) and released. The majority of circulating cholesterol in human and guinea pig is transported in LDL, but cholesterol in rats is carried by HDL fraction (Fernandez, 2001). West et al., (2004) reported that simvastatin is a lipophilic drug, which crosses membrane layers and influences cholesterol synthesis in extrahepatic tissues as well as in the liver, but there was a differential effect among species on HMG CoA reductase activity and mRNA synthesis. The different action mode among species can explain the discrepancy in hypocholesterolemic effect of statins. Onion and beet were effective in reducing plasma total cholesterol, which may be attributed to the fiber in onion and beet. Short chain fatty acids (SCFA) produced by microflora in the large intestine suppress cholesterol synthesis in rat liver and intestine (Hara et al., 1999), and SCFA produced from sugar beet fiber decreased plasma cholesterol by interrupting enterohepatic bile circulation (Hara et al., 1998). S-methyl cysteine sulfoxide in onion family suppresses HMG CoA reductase (Kumari & Augusti, 2007). Onion and beet in the present study showed a favorable effect of increasing HDL-cholesterol which is beyond any reasonable explanation. There is a report that daily consumption of 300 gram tomato for one month increased HDL cholesterol in human (Blum et al., 2006).
Induction of nitric oxide (NO) by statin is one factor for suppressing platelet aggregation (Chou et al., 2008), while depletion of plasma antioxidant ubiquinol by statin therapy is another factor for activating platelet aggregation (Pignatelli et al., 2006). Theoretically, statin itself could hardly affect platelet aggregation as shown in the present study. Platelet aggregation may be controlled by much more complicated mechanism than we thought. Most of previous studies have reported that onion has antiplatelet effect (Briggs et al., 2001; Hubbard et al., 2006), but onion in the present study increased platelet aggregation in the initial slope and the maximum. Thiosulfinates have been implicated as a principle source of the antiplatelet property of raw onion and garlic juice (Briggs et al., 2001). Hubbard et al. (2006) showed that antioxidant quercetin in onion is involved in tyrosine phosphorylation in the signaling pathway causing reduced platelet aggregation. Antiplatelet compounds such as herb extracts can suppress platelet activity in vitro at high concentration but at low concentration, those can stimulate platelet aggregation. Healthy platelets can be dissociated from their aggregate under certain physiological condition. Platelet dissociation and survival after aggregation depends on the concentration of platelet suppressing compounds in medium (Chew et al., 2001). Previous studies (Briggs et al., 2001; Chew et al., 2001) that had antiplatelet effects of onion were conducted in the in vitro system, while the effect of onion on platelet aggregation in the present study was performed in vivo. Differences in experimental protocols and species must be taken into consideration for interpretation on platelet activity.
Cholesterol lowering agent statin may decrease the membrane ratio of cholesterol/phospholipid, which may cause increase in membrane fluidity and increase in Na-K ATPase. Higher membrane cholesterol and lower Na-K ATPase were observed in the erythrocyte of hyperlipidemic patients (Broncel et al., 2007). Broncel et al. (2007) reported that fluvastatin at 80 mg/day in human decreased plasma total cholesterol and triglyceride and increased erythrocyte Na-K ATPase and Na-K cotransport. Statin in the present study rather increased plasma cholesterol, consequently increased membrane rigidity, and decreased Na-K ATPase. Statin induced nitric oxide synthetase (NOS) increasing cellular NO which in turn activates Na-K ATPase (Varela et al., 2004). On the other hand, Bełtowski et al. (2007) reported that NO decreased renal Na-K ATPase by stimulating cGMP and superoxide radicals and increased Na-K ATPase by depleting NO in leptin induced obese rats. Exposure of enterocytes to a NO donor, S-nitroso-N-acetlypenicillamine (SNAP), induced enterocyte injury in cultured intestinal epithelial cells, causing decrease in Na-K ATPase (Suzuki et al., 2005). This discrepancy in the effect of NO and oxygen radical on Na-K ATPase may be attributed to the dual effects of NO. Quercetin rich in onion and betalain rich in beet are recognized to be bioavailable antioxidants and prevent membranes from damage by free radicals. Oxidative damage of membrane causes alterations on membrane channels. Decrease in platelet membrane fluidity and Na-K ATPase were observed in patients with ischemic stroke (Nanetti et al., 2008).
In conclusion, despite reports that statins at even high dose as 20-40 mg/kg did not affect plasma total cholesterol in rats or mice, simvastatin at 2 mg/kg BW in the present experiment was enough to act on cholesterol metabolism, increasing plasma total cholesterol in rats. This dose is comparable to the maximum approved dose effectively lowering plasma cholesterol in human. The discrepancy on these results can be explained by the species difference in the metabolic pathway of cholesterol metabolism. Unlike antiplatelet action of onion in previous studies (Briggs et al., 2001; Chew et al., 2001; Hubbard et al., 2006), onion in the present study acted not as platelet suppressant but as stimulant where the platelets with high dissociation would have high survival rate after aggregation. In this study, simvastatin, which is a structural analog of HMG CoA thereby inhibiting HMG CoA reductase, increased plasma cholesterol and reduced Na-K ATPase and Na-K cotransport, and consequently decreased the total Na efflux. The results indicate that simvastatin apparently plays different roles in the cholesterol metabolism in rat differently from those in human.
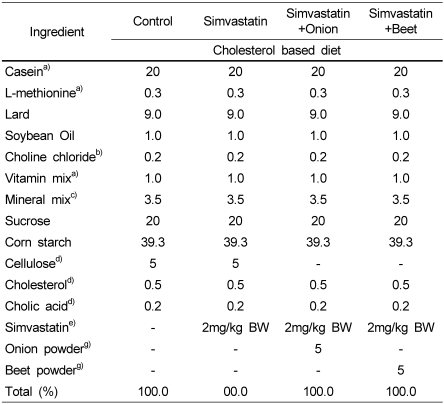
Table 1.
Composition of experimental diets (%)
a)Teklad, Harlan Madison WI, USA
b)Junsei Chemical Co., Ltd.
c)Mineral mixture (g/100 g) : CaHPO4 50.0, NaCl 7.4, K3C6H5O7 H2O 22.0, K2SO4 5.2, MgO 2.4, Manganous carbonate (43-48% Mn) 0.35, Ferric citrate (16.7% Fe) 0.6, Zinc carbonate (70% Zn) 0.16, Cupric carbonate (53-55% Cu) 0.03, KIO3 0.001, Na2SeO3 · 5H2O 0.001, CrK(SO4)2 · 12H2O 0.055, Sucrose 11.804
d)Sigma Chemical Co., USA
e)Simvastatin, Choongwae Pharm. Co., Korea. 2 mg/kg BW, calculated from the daily food consumption
g)Onion and beet after cut and freeze dried were powdered (Jeju Agri Devel Tech Exten Center).
Table 5.
Effects of onion and beet on Na efflux in simvastatin treated hypercholesterolemic rats
1)Intracellular Na ; upper values are for intact red blood cells. (Na mmole/ℓ rbc).
2)Na-K ATPase is ouabain sensitive Na efflux through Na-pump (Na mmole/ℓ rbc/hr).
3)Na-K cotransport is furosemide sensitive Na efflux through Na-pump (Na mmole/ℓ rbc/hr).
4)Na-passive is Na efflux through passive sodium channel in intact red blood cells.
Values in the same row not sharing the same superscript differ (p<0.05)
Values are means ± SD of 10 rats.
Footnotes
This work was supported by the research grant of KRF-2008-521-F00064.
References
- 1.Bełtowski J, Borkowska E, Wójcicka G, Marciniak A. Regulation of renal ouabain-resistant Na+-ATPase by leptin, nitric oxide, reactive oxygen species, and cyclic nucleotides: implications for obesity-associated hypertension. Clin Exp Hypertens. 2007;29:189–207. doi: 10.1080/10641960701361585. [DOI] [PubMed] [Google Scholar]
- 2.Bełtowski J, Wójcicka G, Jamroz A. Differential effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors onplasma paraoxonase 1 activity in the rat. Pol J Pharmacol. 2002;54:661–671. [PubMed] [Google Scholar]
- 3.Blum A, Merei M, Karem A, Blum N, Ben-Arzi S, Wirsansky I, Khazim K. Effects of tomatoes on the lipid profile. Clin Invest Med. 2006;29:298–300. [PubMed] [Google Scholar]
- 4.Briggs WH, Folts JD, Osman HE, Goldman IL. Administration of raw onion inhibits platelet-mediated thrombosis in dogs. J Nutr. 2001;131:2619–2622. doi: 10.1093/jn/131.10.2619. [DOI] [PubMed] [Google Scholar]
- 5.Broncel M, Balcerak M, Cieślak D, Duchnowicz P, Chojnowska-Jezierska J. Effect of fluvastatin extended release on the protein-lipid structure of erythrocyte membrane and C-reactive protein in patients with hyperlipidemia. Pol Merkur Lekarski. 2007;22:107–111. [PubMed] [Google Scholar]
- 6.Chen J, Nagasawa Y, Zhu BM, Ohmori M, Fujimura A, Hashmoto K. Pravastatin prevents arrhythmias induced by coronary artery ischemia/reperfusion in anesthetized normocholesterolemic rats. J Pharmacol Sci. 2003;93:87–94. doi: 10.1254/jphs.93.87. [DOI] [PubMed] [Google Scholar]
- 7.Chew DP, Bhatt DL, Topol EJ. Oral glycoprotein IIb/IIIa inhibitors: why don't they work? Am J Cardiovasc Drugs. 2001;1:421–428. doi: 10.2165/00129784-200101060-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chou TC, Lin YF, Wu WC, Chu KM. Enhanced nitric oxide and cyclic GMP formation plays a role in the anti-platelet activity of simvastatin. Br J Pharmacol. 2008;153:1281–1287. doi: 10.1038/bjp.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condo K, Vergara-Jimenez M, Krause BR, Newton RS, Fernandez ML. Hypocholesterolemic action of atorvastatin are associated with alterations on hepatic cholesterol metabolism and lipoprotein composition in guinea pig. J Lipid Res. 1996;37:2372–2382. [PubMed] [Google Scholar]
- 10.Fernandez ML. Guinea pigs as models for cholesterol and lipoprotein metabolism. J Nutr. 2001;131:10–20. doi: 10.1093/jn/131.1.10. [DOI] [PubMed] [Google Scholar]
- 11.Folch J, Lee M, Sloane Stanley GH. A simple method for the isolation and purification or total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 12.Fontaine D, Fontaine J, Dupont I, Dessy C, Carpentier Y, Berkenboom G. Chronic hydroxymethylglutaryl coenzyme A reductase inhibition and endothelial function of the normocholesterolemic rat: comparison with angiotensin-converting enzyme inhibition. J Cardiovasc Pharmacol. 2002;40:172–180. doi: 10.1097/00005344-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hara H, Haga S, Aoyama Y, Kiriyama S. Short-Chain Fatty Acids Suppress Cholesterol Synthesis in Rat Liver and Intestine. J Nutr. 1999;129:942–948. doi: 10.1093/jn/129.5.942. [DOI] [PubMed] [Google Scholar]
- 14.Hara H, Haga S, Kasai T, Kiriyama S. Fermentation Products of Sugar-Beet Fiber by Cecal Bacteria Lower Plasma Cholesterol Concentration in Rats. J Nutr. 1998;128:688–693. doi: 10.1093/jn/128.4.688. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard GP, Wolffram S, de Vos R, Bovy A, Gibbins JM, Lovegrove JA. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: a pilot study. Br J Nutr. 2006;96:482–488. [PubMed] [Google Scholar]
- 16.Kang JS, Cregor MD, Smith JB. Effect of calcium on blood pressure, platelet aggregation and erythrocyte sodium transport in Dahl salt-sensitive rats. J Hypertens. 1990;8:245–250. [PubMed] [Google Scholar]
- 17.Kanner J, Harel S, Granit R. Betalains-a new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49:5178–5185. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]
- 18.King DE, Mainous AG, Player A, Geesey ME. Use of statins and blood pressure. Am J hypertens. 2007;20:937–941. doi: 10.1016/j.amjhyper.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumari K, Augusti KT. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J Ethnopharmacol. 2007;109:367–371. doi: 10.1016/j.jep.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Lambo-Fodje AM, Oste R, Nyman ME. Short-chain fatty acid formation in the hindgut of rats fed native and fermented oat fibre concentrates. Br J Nutr. 2006;96:47–55. doi: 10.1079/bjn20061797. [DOI] [PubMed] [Google Scholar]
- 21.Lutjohann D, Stroic M, Bertsch T, Lindenthal B, Andersson U, Fassbender K. High doses of simvastatin, pravastatin and cholesterol reduce brain cholesterol synthesis in guinea pig. Steroids. 2004;69:431–438. doi: 10.1016/j.steroids.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Mabuchi H, Higashikata T, Kawashiri M, Inazu A, Koizumi J, Kobayashi J. Reduction of serum ubiquinol-10 and ubiquinone-10 levels by atorvastatin in hypercholesterolemic patients. J Atheroscler Thromb. 2005;12:111–119. doi: 10.5551/jat.12.111. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura H. Primary prevention of cardiovascular diseases among hypercholesterolemic Japanese with a low dose of pravastatin. Atheroscler Suppl. 2007;8:13–17. doi: 10.1016/j.atherosclerosissup.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Nanetti L, Vignini A, Raffaelli F, Silvestrini M, Provinciali L, Mazzanti L. Platelet membrane fluidity and Na+/K+ ATPase activity in acute stroke. Brain Res. 2008;1205:21–26. doi: 10.1016/j.brainres.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Pignatelli P, Di Santo S, Buchetti B, Sanguigni V, Brunelli A, Violi F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. FASEB J. 2006;20:1082–1089. doi: 10.1096/fj.05-5269com. [DOI] [PubMed] [Google Scholar]
- 26.Rosamond W. Heart disease and stroke statistics: A report from the American Heart Association Statistics Committee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 27.Susic D, Varagic J, Ahn J, Slama M, Frohlich ED. Beneficial pleiotropic vascular effects of rosuvastatin in two hypertensive models. J Am Coll Cardiol. 2003;42:1091–1097. doi: 10.1016/s0735-1097(03)00926-4. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Lu Q, Xu DZ, Szabó C, Haskó G, Deitch EA. Na+, K+-ATPase activity is inhibited in cultured intestinal epithelial cells by endotoxin or nitric oxide. Int J Mol Med. 2005;15:871–877. [PubMed] [Google Scholar]
- 29.Tesoriere L, Allegra M, Butera D, Livrea MA. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: potential health effects of betalains in humans. Am J Clin Nutr. 2004;80:941–945. doi: 10.1093/ajcn/80.4.941. [DOI] [PubMed] [Google Scholar]
- 30.Varela M, Garvin JL. Acute and chronic regulation of thick ascending limb endothelial nitric oxide synthase by statins. J Am Soc Nephrol. 2004;15:269–275. doi: 10.1097/01.asn.0000107563.69663.82. [DOI] [PubMed] [Google Scholar]
- 31.West KL, Fernandez ML. Guinea pigs as models to study the hypocholesterolemic effects of drugs. Cardiovasc Drug Rev. 2004;22:55–70. doi: 10.1111/j.1527-3466.2004.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto Y, Aoyama S, Hamaguchi N, Rhi GS. Antioxidative and antihypertensive effects of Welsh onion on rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2005;69:1311–1317. doi: 10.1271/bbb.69.1311. [DOI] [PubMed] [Google Scholar]