Abstract
This study investigated the antioxidant activity of methanol (MeOH) and water extracts from roots of Cirsium japonicum in vitro. MeOH extract showed a stronger free radical scavenging activity than water extract. However, both of extracts showed a concentration dependent hydroxyl radical scavenging activity, reducing power and metal chelating ability. MeOH extract had greater phenolic and flavonoid contents than water extract. The antidiabetic activity of these two extracts was evaluated by the α-glucosidase inhibition assay. The water extract showed a considerable α-glucosidase inhibitory activity. To our knowledge, this may be the first time to report the antioxidant and antidiabetic activities in Cirsium japonicum roots.
Keywords: Cirsium japonicum, antioxidant, antidiabetic, methanol and water extracts
Introduction
Polyphenols are a variety of antioxidant compounds that have been used as dietary supplements for the prevention of pathological diseases and for the improvement of human health conditions (Weisburger et al., 1996; Zhang et al., 2006). The bioactivity of phenolics may be related to their ability to chelate metals, inhibit lipoxygenase, and scavenge free radicals (Decker, 1997), and flavonoids can act as free radical scavengers and terminate the radical chain reactions that occur during the oxidation of triglycerides (Das & Pereira, 1990).
Free radicals can lead to a variety of biochemical and physiological lesions (Ames, 1998) and induce degenerative diseases such as coronary artery disease, diabetes, stroke, and cancer (Halliwell et al., 1992). Antioxidants can prevent oxidation of substrates when present at low concentrations compared with that of an oxidizable substrate (Halliwell, 1995). Interest in finding naturally occurring antioxidants in foods or medicines to replace synthetic antioxidants has increased considerably, given that synthetic antioxidants are being restricted due to their side effects (Zheng & Wang, 2001). The antioxidants in some plants play important roles in preventing diseases induced by free radicals (Huxley & Neil, 2003). Therefore, attention has been directed toward the development and isolation of natural antioxidants from plant sources.
Cirsium japonicum is a wild perennial herb native to China, Korea and Japan, and has been used as an antihemorrhagic, antihypertensive and antihepatitis agent. It has been prescribed in treatment of tumors, such as liver cancer, uterine cancer, and leukaemia in folk (Liu et al., 2006) and used as a hemostatic agent in herbal preparations to prevent epistaxis and metrorrhagia and to improve blood circulation (Donguibogam, 1999). Cirsium japonicum extracts contain various flavonoid compounds (Ganzera et al., 2005) and the apigenin and kaempferol derivatives were kinds of the Cirsium-derived flavonoids (Nazaruk & Jakoniuk, 2005). Also, a previous study showed that MeOH extract from Cirsium japonicum leaves had good antioxidant activity (Yin et al., 2008), but little research was found on the antioxidant and antidiabetic activities of Cirsium japonicum roots. Therefore, the objective of this study was to investigate the antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots.
Materials and Methods
Materials
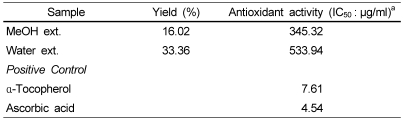
Cirsium japonicum was collected at Chuncheon in September, 2007. Roots of Cirsium japonicum were dried at 60℃ and then 100 g dried powder was refluxed with methanol and water (m/v=1:20) for 3 days at room temperature. After filtering, the residue was re-extracted, filtered, and evaporated using a rotary evaporator at 45℃. The yields of dried extracts were shown in Table 1.
Table 1.
The yield and DPPH free radical scavenging activity of methanol and water extracts from Cirsium japonicum roots
aIC50: the effective concentration at which DPPH radical was scavenged by 50%
Free radical scavenging activity assay
The free radical scavenging activity was measured by the DPPH method (Mensor et al., 2001) with some modifications. Samples at different concentrations (100-500 µg/ml) were added to 0.3 mM DPPH methanol solution. After 30 min at room temperature, the absorbance was measured at 517 nm. The IC50 value was defined as the amount of antioxidant necessary to decrease the initial DPPH concentration by 50%. The capability to scavenge the DPPH radical was calculated using the following equation:
DPPH Scavenging Effect (%)=[(A1-A2/A1)]×100
where A1 is the absorbance of the control reaction and A2 is the absorbance in the presence of the sample. α-Tocopherol and ascorbic acid were used as controls.
Measurement of total phenolic and flavonoid contents
Determination of total phenolic content was made using Folin-Ciocalteau's phenol reagent (Athukorala et al., 2006). Sample (1 ml), 0.5 ml of Folin-Ciocalteau's phenol reagent (2 N), and 2 ml of Na2CO3 (5%) were mixed and the reaction mixture was allowed to proceed for 5 min at room temperature, before dilution with 5 ml of deionized water. Each sample was mixed thoroughly and placed in dark for 1 h and the absorbance was measured at 725 nm with a UV-vis spectrophotometer. Tannic acid equivalent (mg/g) was determined from a standard concentration curve. All tests were performed in independent triplicates (n=3) and data were expressed as mean ± SD.
Flavonoid content was determined according to the aluminum chloride colorimetric method (Chang et al., 2002) with some modifications. Quercetin was used as a standard to make the calibration curve. The sample solution (0.5 ml) was mixed with 1.5 ml of 95% ethanol, 0.1 ml of 10% aluminum chloride hexahydrate, 0.1 ml of 1 M potassium acetate, and 2.8 ml of distilled water. After incubation at room temperature for 40 min, the absorbance of reaction mixture was measured at 415 nm. The same amount of distilled water substituted for the amount of 10% aluminum chloride as the blank. Using a seven point standard curve (0-500 µg/ml), the flavonoid content of extracts was determined in independent triplicate (n=3).
Hydroxyl radical scavenging activity assay
The scavenging activity of methanol and water extracts from roots of Cirsium japonicum on the hydroxyl radical was measured by the deoxyribose method (Hagerman et al., 1998). Hydroxyl radicals were generated by direct addition of iron (II) salts to the reaction mixture. The reaction mixture contained 200 µl of 10 mM FeSO4·7 H2O, 200 µl of 10 mM EDTA, 200 µl of 10 mM 2-deoxyribose, 200 µl of 10 mM H2O2, mixed with 1.0 ml of 100 mM phosphate buffer (pH 7.4) containing 200 µl of the samples and incubated for 4 h at 37℃. The color was developed by addition of 1% TBA followed by ice-cold 2.8% TCA and heating in boiling water for 10 min. After cooling, it was centrifuged at 395 ×g for 5 min and the absorbance at 532 nm was measured. α-Tocopherol and BHT were used as the positive control.
The scavenging activity on hydroxyl radicals (HRSA) was expressed as:
HRSA (%)=[1-(As-Ac) / Ab]×100%
where As was the absorbance with the presence of deoxyribose and samples; Ab was the absorbance with the presence of deoxyribose but without samples; and Ac was the absorbance with the presence of samples but without deoxyribose. All tests were performed in independent triplicate (n=3) and data were expressed as mean ± SD.
Reducing power assay
Various concentrations of methanol and water extracts (1 ml) were mixed with 2.5 ml of 1% sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide and the mixture was incubated at 50℃ for 30 min. After 2.5 ml of 10% TCA were added, the mixture was centrifuged at 3,000 rpm for 10 min. The upper layer (2.5 ml) was mixed with 2.5 ml deionized water and 0.5 ml of 0.1% of ferric chloride, and the absorbance was measured at 700 nm. The assays were carried out in triplicate and the results were expressed as mean values ± SD. α-Tocopherol was used as a standard.
Ferrous ions chelating activity assay
Different concentrations of methanol and water extracts from roots of Cirsium japonicum (10-2,000 µg/ml) were added to a solution of 2 mM FeCl2 (0.05 ml). The reaction was initiated by adding 5 mM ferrozine (0.2 ml) and the mixture was shaken vigorously and left standing at room temperature for 10 min. After the mixture had reached equilibrium, the absorbance of the solution was measured spectrophotometrically at 562 nm. EDTA was used as a positive control.
All tests and analyses were run in triplicate. The percentage of inhibition of ferrozine-Fe2+ complex formation is given by the formula:
Inhibition (%)=[(A0-A1)/A0]×100%
where A0 was the absorbance of the control and A1 was the absorbance in the presence of samples and standards. The control did not contain FeCl2 or ferrozine, complex formation molecules.
α-Glucosidase inhibitory assay
One hundred microliters of 3 mM pNPG in 0.2 M sodium phosphate buffer (pH 6.8) was added as a substrate to the mixture of 50 µl of α-glucosidase (0.15 unit/ml) and 50 µl of sample to start the reaction. The reaction was conducted at 37℃ for 15 min and stopped by the addition of 750 µl of 0.1 M Na2CO3. α-Glucosidase activity was assessed by measuring the release of p-nitrophenol from pNPG at 405 nm. Acarbose was used as a positive control. All tests were performed in independent triplicate (n=3) and data were expressed as mean ± SD.
Statistical analysis
All tests were performed in independent triplicate (n=3) and data were expressed as mean ± SD. Statistical significance was evaluated by one-way analysis of variance using SPSS 7.5 (SPSS Institute, Cary, NC, USA) and individual comparisons were obtained by Duncan's multiple-range test which was used to determine the difference of means (p<0.05).
Results
Free radical scavenging activity
The free radical scavenging activity of MeOH and water extracts from roots of Cirsium japonicum, measured by the DPPH method, was shown in Table 1. MeOH extract had stronger antioxidant activity (IC50: 345.32 µg/ml) than water extract (IC50: 533.94 µg/ml). However, α-Tocopherol and ascorbic acid which were used as positive control showed better radical scavenging effect (IC50: 7.61 and 4.54 µg/ml, respectively).
Total phenolic and flavonoid contents
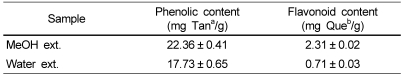
We measured the phenolic and flavonoid contents to investigate if they may contribute to the antioxidant activity of the extracts. As shown in Table 2, the phenolic content in MeOH and water extracts was 22.36 and 17.73 mg Tan/g, respectively. The flavonoid content in MeOH and water extracts was 2.31 and 0.71 mg Que/g, respectively. Obviously, both phenolic and flavonoid contents of MeOH extract were higher than those of water extract.
Table 2.
Total phenolic and flavonoid contents of methanol and water extracts from Cirsium japonicum roots
aTannic acid (Tan) was used as a standard for measuring of the total phenolic content.
bQuercetin (Que) was used as a standard for measuring of the flavonoid contet.
Hydroxyl radical scavenging activity
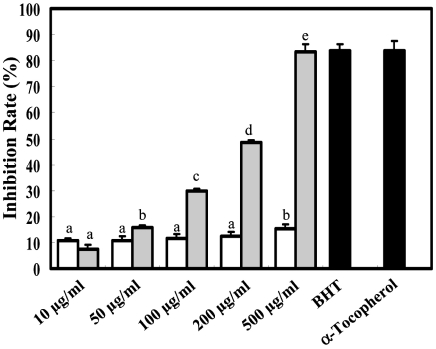
The hydroxyl radical scavenging activity of the extracts from roots of Cirsium japonicum was increased with increasing sample concentrations (Fig. 1). MeOH extract showed considerable hydroxyl radical scavenging activity; although the activity was less than that of the positive controls at the same concentration (α-Tocopherol and BHT scavenged 83.7 and 84.1% of the available free radicals, respectively, at the concentration of 100 µg/ml). MeOH extract had better hydroxyl radical scavenging activity than water extract at the same concentration. MeOH extract showed a significant dose-dependent scavenging activity, especially, and it reached up to 83.5% at the concentration of 500 µg/ml.
Fig. 1.
Scavening effect on hydroxyl radical of methanol and water extracts from Cirsium japonicum roots. a-Tocopherol and BHT at the concentration of 100 µg/ml were used as the positive control. Data were presented as the mean ± SD (n=3). (□: water ext., ▒: MeOH ext.)
Reducing power
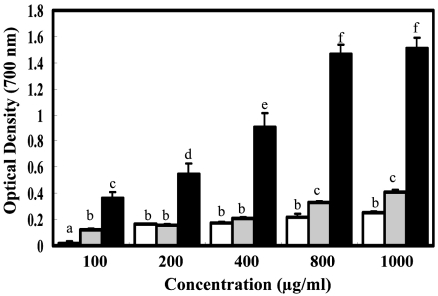
The reducing power indicates compounds that are electron donors, which can act as primary and secondary antioxidants (Yen & Chen, 1995). The reducing power of MeOH extract was increased with increasing amount of sample (OD value: 0.105-0.409), but values remained lower than that for α-tocopherol (OD value: 0.366-1.513) (Fig. 2). Moreover, MeOH extract had higher reducing activity than water extract, and this fact might be associated with the relationship between the antioxidant activity and the reducing power of extracts.
Fig. 2.
Reducing power of methanol and water extracts from Cirsium japonicum roots. a-Tocopherol was used as the positive control. Data were presented as the mean ± SD (n=3). (□: water ext., ▒: MeOH ext., ■: a-tocopherol)
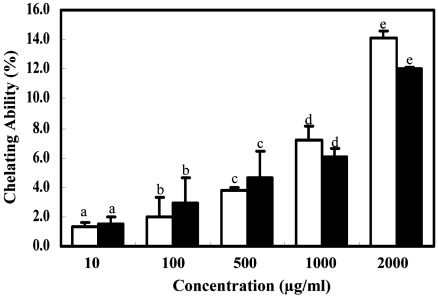
Ferrous ions chelating activity
Ferrozine can quantitatively form complexes with Fe2+. In the presence of other chelating agents, the complex formation is disrupted with the result that the red color of the complexes decreases. The absorbance of Fe2+-ferrozine complex was decreased dose-dependently from 10 to 2,000 µg/ml for both extracts and EDTA, although the iron chelating ability of both extracts was moderate (Fig. 3). Obviously, both extracts had a steady increase on metal chelating activity with increasing concentrations. However, EDTA showed high chelating ability of 95.82% at 100 µg/ml.
Fig. 3.
Ferrous ions chelating ability of methanol and water extracts from Cirsium japonicum roots. EDTA at the concentration of 100 µg/ml was used as the positive control. Data were presented as the mean ± SD (n=3). (□: water ext., ■: MeOH ext.)
α-Glucosidase inhibitory effect
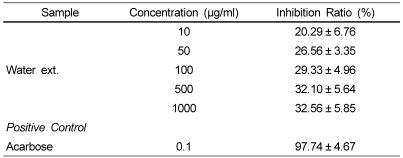
In order to determine if Cirsium japonicum extracts possess antidiabetic properties, we studied the activity of MeOH and water extracts. Water extract demonstrated a moderate but not high inhibitory activity on α-glucosidase (Table 3), and MeOH extract had no inhibitory activity. Water extract showed the inhibitory activity ranging from 20.09% to 32.56% on α-glucosidase from 10 to 1,000 µg/ml, which was increased steadily with increasing sample concentrations. However, the α-glucosidase inhibitor, acarbose showed 97.74% inhibitory activity at the concentration of 0.1 µg/ml.
Table 3.
The a-glucosidase inhibitory activity of water extract from Cirsium japonicum roots
Each value was expressed as mean ± SD (n=3).
Discussion
DPPH is a stable free radical that accepts an electron or hydrogen radical and becomes a stable diamagnetic molecule (Loo AY et al., 2007; Soares et al., 1997). A deep purple color with an absorption maximum at 517 nm is formed from DPPH solution, but it generally fades when some antioxidants are present in the solution. In addition, antioxidant activity had a linear relationship with the total phenolic or anthocyanin content in some plants (Kalt et al., 1999), which can be validated by the following research. MeOH extract had better reduction capacity of DPPH radical than water extract (Table. 1). A strong positive correlation has been reported between total polyphenol content and DPPH free radical scavenging activity (Oki et al., 2002; Siriwardhana & Shahidi, 2002). The antioxidant activity was derived from some flavonoid-type compounds, which are one of the most diverse and widespread groups of natural phenolics (Cakir et al., 2003). Therefore, the higher phenolic content in MeOH extract might account for the better results found in their reducing power and scavenging effect on DPPH and hydroxyl radicals. Hydroxyl radical is the most reactive free radical among the reactive oxygen species, which can be formed from superoxide anion and hydrogen peroxide in the presence of metal ions. Hydroxyl radical scavenging activity is related to the levels of phenolic compounds present in the scavenger. Also, phenolic compounds which originate from plant resources have multiple biological effects such as antioxidant activity and mainly attribute antioxidant activity to their redox properties (Osawa, 1994; Siriwardhana et al., 2003). MeOH showed effective scavenging activity on hydroxyl radical as the result of high phenolic content (Fig. 1). The reducing properties are associated with the antioxidant activity. Extracts from roots of Cirsium japonicum had moderate reducing activity (Fig. 2) in a dose-dependent manner, although this activity was lower than that of α-tocopherol. Chelating agents were reported to be effective as secondary antioxidants because they can reduce the redox potential thereby stabilizing the oxidized form of the metal ion (Gordon, 1990). Measurement of the rate of color reduction allows estimation of the chelating activity of the coexisting chelator (Yamaguchi et al., 2000). Both extracts and standard compound (EDTA) interfered with the formation of ferrous and ferrozine complex, suggesting that they have chelating activity and capture ferrous ion before ferrozine. MeOH and water extracts demonstrated iron binding capacity, suggesting that the antioxidant activity may be related to the capacity for iron binding.
One therapeutic approach for treating diabetes is to decrease the postprandial hyperglycemia. This is done by retarding the absorption of glucose through the inhibition of the carbohydrate-hydrolyzing enzymes α-glucosidase in the digestive tract. Inhibitors of these enzyme delay carbohydrate digestion, causing a reduction in the rate of glucose absorption and consequently blunting the postprandial plasma glucose rise (Rhabasa & Chiasson, 2004). Inhibitors of intestinal α-glucosidase have been used in the treatment of noninsulin-dependent diabetes mellitus (NIDDM) and represented at the huge proportion of antidiabetic drug market (Inzucchi, 2002). The present findings suggest that water extract from roots of Cirsium japonicum may be potential but not necessary candidates for treating diabetes by inhibiting glucosidase activity (Table 3).
In conclusion, in this investigation, we tested the antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots. Based on these results, Cirsium japonicum can be considered as a potential source of functional food and pharmaceutical agent.
References
- 1.Ames B. Micronutrients prevent cancer and delay aging. Toxicol Lett. 1998;102:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 2.Athukorala Y, Kim KN, Jeon YJ. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol. 2006;44:1065–1074. doi: 10.1016/j.fct.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Cakir A, Mavi A, Yildirim A, Duru ME, Harmandar M, Kazaz C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J Ethnopharmacol. 2003;87:73–83. doi: 10.1016/s0378-8741(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food Drug Analysis. 2002;10:178–182. [Google Scholar]
- 5.Das NP, Pereira TA. Effects of flavonoids on thermal autoxidation of palm oil: structure- activity relationships. J Am Oil Chem Soc. 1990;67:255–258. [Google Scholar]
- 6.Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997;55:396–407. doi: 10.1111/j.1753-4887.1997.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 7.Donguibogam. Seoul. Republic of Korea: Bubin Publishers Co.; 1999. p. 1942. [Google Scholar]
- 8.Ganzera M, Pocher A, Stuppner H. Differentiation of Cirsium japonicum and Cirsium setosum by TLC and HPLC-MS. Phytochem Anal. 2005;16:205–209. doi: 10.1002/pca.846. [DOI] [PubMed] [Google Scholar]
- 9.Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London. UK: Elsevier Applied Science; 1990. pp. 1–18. [Google Scholar]
- 10.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Antioxidant characterization: methodology and mechanism. Biochem Pharmacol. 1995;49:1341–1348. doi: 10.1016/0006-2952(95)00088-h. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants, and human disease: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 13.Huxley RR, Neil H. The relationship between dietary flavonol intake and coronary heart disease mortality: a metaanalysis of prospective cohort studies. Eur J Clin Nutr. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 14.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002;287:360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 15.Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999;47:4638–4644. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- 16.Liu SJ, Luo X, Li DX, Zhang J, Qiu DL, Liu W, She L, Yang ZR. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. International Immunopharmacology. 2006;6:1387–1393. doi: 10.1016/j.intimp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Loo AY, Jain K, Darah I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem. 2007;104:300–307. [Google Scholar]
- 18.Mensor LL, Menezes FS, Leitao GG, Reis AS, Dos Santos TC, Coube CS, Leitao SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 19.Nazaruk J, Jakoniuk P. Flavonoid composition and antimicrobial activity of Cirsium rivulare (Jacq.) all flowers. J Ethnopharmacol. 2005;102:208–212. doi: 10.1016/j.jep.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Oki T, Masuda M, Furuta S, Nishibia Y, Terahara N, Suda I. Involvement of anthrocyanins and other phenolic compounds in radical scavenging activity of purple-fleshed sweet potato cultivas. Food Chem. 2002;67:1752–1756. [Google Scholar]
- 21.Osawa T. Novel Natural Antioxidants for Utilization in Food and Biological Systems. Tokyo. Japan: Japan Scientific Scoieties Press; 1994. pp. 241–251. [Google Scholar]
- 22.Rhabasa LR, Chiasson JL. Alpha-glucosidase inhibitors. In: Defronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International textbook of diabetes mellitus. 3rd ed. Vol. 1. UK: John Wiley; 2004. [Google Scholar]
- 23.Siriwardhana N, Lee KW, Kim SH, Ha WJ, Jeon YJ. Antioxidant activity of Hizikia fusiformis on reactive oxygen scavenging and lipid peroxidation inhibition. Food Science and Technology International. 2003;9:339–346. [Google Scholar]
- 24.Siriwardhana SSKW, Shahidi F. Antiradical activity of extracts of almond and its by-products. J Am Oil Chem Soc. 2002;79:903–908. [Google Scholar]
- 25.Soares JR, Dins TCP, Cunha AP, Ameida LM. Antioxidant activity of some extracts of Thymus zygis. Free Radic Res. 1997;26:469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 26.Weisburger JH, Hara Y, Sulan L, Luo FQ, Pittman B, Zang E. Tea polyphenols as inhibitors of mutigenicity of major classes carcionogens. Mutat Res. 1996;371:57–63. doi: 10.1016/s0165-1218(96)90094-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi F, Ariga T, Yoshimira Y, Nakazawa H. Antioxidant and anti-glycation of carcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:180–185. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- 28.Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agicr Food Chem. 1995;43:27–32. [Google Scholar]
- 29.Yin Y, Heo SI, Wang MH. Antioxidant and anticancer activities of methanol and water extracts from leaves of Cirsium japonicum. J Appl Biol Chem. 2008;51:160–164. [Google Scholar]
- 30.Zhang X, Koo J, Eun JB. Antioxidant activities of methanol extracts and phenolic compounds in Asian pear at different stages of maturity. Food Sci Biotechnol. 2006;15:44–50. [Google Scholar]
- 31.Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]