Abstract
Intravesical bacillus Calmette-Guerin (BCG) has been used successfully to treat superficial bladder cancer for 3 decades. However, 20–30% of patients will fail initial BCG therapy and 30–50% of patients will develop recurrent tumors within 5 years. Alternative or complementary strategies for the management of superficial bladder cancer are needed. IL-12 is a potent TH1 cytokine with robust antitumor activity and the ability to potentiate immunological memory. Unfortunately, intravesical IL-12 did not demonstrate anti-tumor efficacy in a recent clinical study of patients with recurrent superficial bladder cancer. We hypothesized that coformulation of IL-12 with chitosan – a biocompatible, mucoadhesive polysaccharide – could improve intravesical IL-12 delivery and provide an effective and durable alternative for the treatment of superficial bladder cancer. In antitumor studies, 88 to 100% of mice bearing orthotopic bladder tumors were cured after 4 intravesical treatments with chitosan/IL-12. In contrast, only 38 to 60% of mice treated with IL-12 alone, and 0% treated with BCG, were cured. Antitumor responses following chitosan/IL-12 treatments were durable and provided complete protection from intravesical tumor rechallenge. Urinary cytokine analysis showed that chitosan/IL-12 induced multiple TH1 cytokines at levels significantly higher than either IL-12 alone or BCG. Immunohistochemistry revealed moderate to intense tumor infiltration by T cells and macrophages following chitosan/IL-12 treatments. Bladder submucosae from cured mice contained residual populations of immune cells that returned to baseline levels after several months. Intravesical chitosan/IL-12 is a well tolerated, effective immunotherapy that deserves further consideration for testing in humans for the management of superficial bladder cancer.
Keywords: immunotherapy, bladder cancer, IL-12, chitosan, intravesical
Introduction
Bladder cancer is the fifth most common cancer in the United States, with an estimated 68,810 new cases and 14,100 deaths in 2008 (1). Global prevalence of bladder cancer is estimated at over 1 million and is steadily increasing (2). In addition, bladder cancer has the highest domestic cost per patient of all cancers, with U.S. medical care expenditures surpassing $4 billion per year (3). Although 70 to 80% of patients are diagnosed with superficial disease, bladder cancer has a high overall rate of recurrence at approximately 65% (4).
Since the pioneering work of Morales et al. in 1976 (5), intravesical BCG has been the standard-of-care immunotherapy for superficial bladder cancer. BCG immunotherapy results in 70–75% and 50–60% complete response rates for carcinoma-in-situ and small residual tumors, respectively (4). Unfortunately, a significant percentage of patients will fail initial BCG therapy and approximately 30–50% of BCG responders will develop recurrent tumors within 5 years (6, 7). Therefore, despite the effectiveness of BCG, additional therapies are needed to limit recurrence and improve survival for both BCG responders and non-responders.
IL-12 is a powerful, proinflammatory cytokine that enhances: 1) TH1 cell differentiation, 2) proliferation of activated T cells and natural killer (NK) cells and 3) cell-mediated immunity. IL-12 was recently ranked third in a comprehensive list of immunotherapeutic agents with high potential for use in treating cancer (8). Preclinically, IL-12 has demonstrated remarkable antitumor effects against a range of malignancies (9, 10). In addition, several investigators have shown that IL-12-based therapies can potentiate immunological memory and mediate protection from tumor rechallenge in preclinical models (11, 12).
Unfortunately, the success of IL-12 in the clinic has been limited by adverse events, including 2 on-study deaths following administration of intravenous IL-12 (13). Many researchers have since supported the notion that a safer, more effective approach for cytokine-based immunotherapies, including IL-12, is through local administration (14, 15). In theory, local administration reduces systemic cytokine-based toxicities and takes advantage of the preferred paracrine mechanism of action of most cytokines.
For the treatment of bladder cancer, local, i.e. intravesical, administration of IL-12-based therapies has demonstrated a significant survival advantage in murine orthotopic bladder cancer models (16, 17). To date, only one clinical study has used IL-12 intravesically in patients with superficial bladder cancer (18). Doses of IL-12 up to 200 μg were administered weekly for 6 weeks without significant systemic toxicity. The authors reported no clinically relevant antitumor or immunologic activity; however, the maximum tolerated dose of intravesical IL-12 was not reached.
In order to improve antitumor efficacy of intravesically administered IL-12, we formulated IL-12 with chitosan, a mucoadhesive biopolymer. Chitosan is a nontoxic, biodegradable, natural polysaccharide derived primarily from the exoskeletons of crustaceans. It is a widely used biomaterial (19, 20) with an established safety profile in humans (21, 22). Three properties of chitosan may potentially enhance the intravesical delivery of IL-12. First, the high polycationic charge and mucoadhesive properties of chitosan may facilitate adherence to the negatively charged bladder mucosa, thereby increasing local retention of IL-12. Second, the high viscosity of chitosan-based solutions may make them resistant to excretion from the bladder. And third, chitosan has been shown to loosen gap junctions and enhance epithelial permeability to drugs and proteins at mucosal surfaces (23).
In this study, we investigated the antitumor activity of intravesically administered chitosan/IL-12 during multiple treatments in an aggressive, bioluminescent orthotopic bladder cancer model. We then compared the antitumor efficacy of chitosan/IL-12 and BCG. We used urinary cytokine analysis and immunohistochemical staining to elucidate mechanisms and uncover potential effector cells responsible for tumor regression, and also tested the durability of chitosan/IL-12-mediated responses to tumor rechallenge.
Materials and Methods
Animals, cell lines, and reagents
Female C57BL/6 mice 10 to 12 weeks old were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). Mice were housed and maintained under pathogen-free conditions in microisolator cages. Animal care was in compliance with the recommendations of The Guide for Care and Use of Laboratory Animals (National Research Council).
The parental MB49 cell line (murine transitional cell carcinoma) was maintained in Dulbecco’s Modified Eagle Medium containing high glucose and 10% heat-inactivated fetal bovine serum supplemented with nonessential amino acids, 1 mM sodium pyruvate, 2 mM glutamine, and 100 U/mL penicillin/streptomycin. Stable transfection of the MB49 cell line with a luciferase-expressing plasmid (pGL4.20, Promega, Madison, WI) was accomplished using a commercially available kit (Nucleofector Kit R, Amaxa Inc., Walkersville, MD). Cells were selected in complete media containing 2 μg/mL puromycin. The resulting cell line was designated MB49luc.
Recombinant murine IL-12 was purchased from Peprotech (Rocky Hill, NJ). BCG (BCG Live - Intravesical) was purchased from Sanofi Pasteur Limited (Toronto, ONT) and chitosan glutamate (Protosan G 213) was purchased from NovaMatrix (Sandvika, Norway).
Tumor model
Orthotopic bladder tumors were generated via intravesical instillation of MB49luc cells as previously described (24). Briefly, mice were anesthetized with ketamine (15 mg/kg)/xylazine (75 mg/kg), and a 24 G × ¾″ Teflon catheter (Terumo, Somerset, NJ) was inserted into the bladder through the urethra. One hundred microliters of 0.1 μg/mL poly-L-lysine solution (PLL, MW = 70,000 to 150,000) was administered intravesically for 10 min while catheters/syringes were kept in place. The PLL was then suctioned from the bladder and 100 μL containing 75,000 MB49luc cells were instilled for 30 to 45 min. Intravesical treatments containing 100 μL of PBS, BCG (1.35 mg), 1% (w/v) chitosan solution in DPBS, IL-12 (5 μg), or 1% chitosan admixed with IL-12 (5 μg) (chitosan/IL-12) were administered via the same catheterization technique. Mice were anesthetized as before and treatments were instilled for 45 to 60 min.
Bioluminescence imaging
The growth of orthotopically implanted MB49luc tumors was monitored with an IVIS Lumina (Caliper Life Sciences, Alameda, CA). Fifteen min prior to imaging, mice were given 15 mg/kg luciferin salt i.p. Five min prior to imaging, anesthesia was induced with ketamine/xylazine as before, and abdominal areas were shaved.
Serum cytokines
Mice bearing 7-day-old orthotopic MB49luc tumors were treated intravesically or subcutaneously (s.c.) with IL-12 (5 μg) or chitosan/IL-12 (5 μg). After 24 h, serum was collected and analyzed for IL-12 and IFN-γ concentrations via ELISA kits (Pierce/Thermo Scientific, Rockford, IL).
Urinary cytokines
Mice bearing 7-day-old orthotopic MB49luc tumors were treated intravesically with PBS, BCG (1.35 mg), IL-12 (5 μg), or chitosan/IL-12 (5 μg). Mice were allowed to recover in heated cages for 6 h, then transferred to metabolic cages for urine collection. Following the methods of O’Donnell et al. (25), urine was collected in a tube on ice containing a concentrated urine stabilizer solution (2 M Tris-HCl [pH 7.6], 5% BSA, 0.1% sodium azide, and a COMPLETE™ mini protease inhibitor tablet) (Roche Diagnostics, Indianapolis, IN). Urine was collected from 6 to 24 h, from 24 to 48 h, from 48 to 72 h, and from 72 to 96 h following treatment, then centrifuged and stored at −80°C until batch analysis via bead-based ELISA (CBA Inflammation Kit, BD Biosciences, San Diego, CA).
Histology and immunohistochemistry
Bladders were removed and a portion of each was fixed in 10% neutral buffered formalin; the remainders were embedded in OCT for immunohistochemical staining for CD3, CD4, CD8a, and F4/80. All immunohistochemistry procedures were performed at the Pathology/Histotechnology Laboratory at NCI-Frederick, MD. All tissues were scored for relative levels of immune infiltrates by a board certified pathologist.
Results
Tracking tumor growth with a luciferase-expressing orthotopic bladder tumor model
MB49, a cell line derived from transitional cell carcinoma of the murine urinary bladder (26), was stably transfected with the gene encoding firefly luciferase. Luciferase expression allowed for the detection and routine monitoring of orthotopic bladder tumors via serial bioluminescence imaging (supplemental Fig. 1A). Tumors were detectable as early as day 4 after implantation—several days before tumors became palpable or mice became hematuric.
To determine if bioluminescence could be used as a surrogate for tumor burden, cohorts of mice were imaged just prior to sacrifice, after which bladders were removed and weighed. Photon counts increased by 2 orders of magnitude over the first week of tumor growth. After one week, photon counts reached a limit as tumors continued to grow for up to 5 weeks (supplemental Fig. 1B). Our finding that bioluminescence does not correlate well with continued disease progression in an orthotopic bladder tumor model agrees with another recently published study (27). However, bioluminescence imaging is an effective tool for excluding from subsequent studies mice in which tumors did not “take.”
Antitumor activity and survival with chitosan/IL-12 vs. IL-12 alone
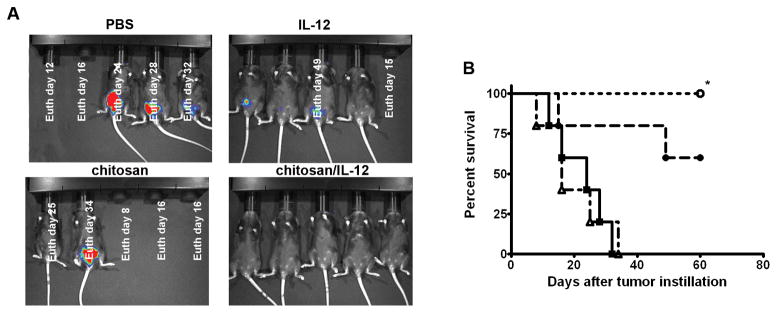
Mice bearing orthotopic bladder tumors were treated intravesically on days 7, 14, 21, and 28 post-tumor implantation with PBS, IL-12 (5 μg), chitosan, or chitosan/IL-12 (5 μg). After 3 treatments, bioluminescence imaging on day 22 revealed that 100% of mice treated intravesically with chitosan/IL-12 appeared tumor-free. In the IL-12 alone group, 1 mouse died prior to imaging and 3 of 4 mice had measurable disease (Fig. 1A). Chitosan alone had no antitumor activity. At 60 days post-tumor implantation, 100% of mice treated with chitosan/IL-12 had survived vs. 60% of mice treated with IL-12 alone (Fig. 1B). No overt toxicity attributable to any of the intravesical treatments was observed.
Figure 1. Antitumor activity of intravesical immunotherapies.
C57BL/6 mice (n = 5) bearing orthotopic MB49luc tumors were treated with PBS, chitosan, IL-12 (5 μg), or chitosan/IL-12 (5 μg) on days 7, 14, 21, and 28 post-tumor implantation. A, bioluminescence and photographic overlay images of mice at day 22. White text on the images indicates day of euthanasia based on humane criteria. B, overall survival curves of mice treated as above with PBS (■), chitosan (△), IL-12 (5 μg) (●), or chitosan/IL-12 (5μg) (○). *, P < 0.005 (log-rank test) vs. PBS and chitosan groups.
Comparison of chitosan/IL-12, IL-12 alone, and BCG
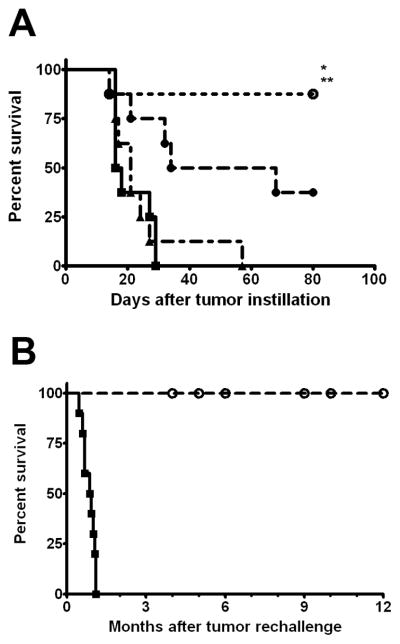
To compare chitosan/IL-12 with standard BCG immunotherapy, mice bearing orthotopic bladder tumors were treated intravesically on days 5, 12, 19, and 26 post-tumor implantation with PBS, BCG (1.35 mg) (28, 29), IL-12 (5 μg) alone, or chitosan/IL-12 (5 μg). As in our previous experiments, intravesical chitosan/IL-12 led to long-term survival and cures in the overwhelming majority (88%) of treated mice (Fig. 2A). IL-12 alone was less effective, with 3 of 8 mice surviving up to 80 days post-tumor implantation. Mice receiving intravesical BCG showed no significant difference in survival over mice receiving intravesical PBS, and none survived > 60 days. No overt toxicity was observed in any mouse receiving PBS, IL-12, or chitosan/IL-12. Mice receiving BCG exhibited grades 1 to 2 toxicities, with hunched habitus and ruffled fur for 24 h post-treatment before returning to normal.
Figure 2. Comparison of survival with intravesical chitosan/IL-12 vs. BCG.
A, C57BL/6 mice (n = 8) bearing orthotopic MB49luc tumors were treated with PBS (■), BCG (1.35 mg) (▲), IL-12 (5 μg) (●), or chitosan/IL-12 (5 μg) (○) on days 5, 12, 19, and 26 post-tumor implantation. *, P < 0.06 (log-rank test) vs. IL-12 group; **, P < 0.005 (log-rank test) vs. PBS and BCG groups. B, 18 mice (from this and other experiments) that were cured with intravesical chitosan/IL-12 immunotherapy were rechallenged orthotopically with the original dose of MB49luc cells. Age-matched naïve mice (■) were challenged at the same conditions.
Intravesical chitosan/IL-12 generated durable immunological memory
Pathology reports on the bladders of 4 mice surviving long-term (> 60 days) following chitosan/IL-12 treatments revealed no residual tumor. Thus, mice that survived > 60 days with no tumor visible via bioluminescence imaging were deemed cured. In separate experiments, 18 mice that were cured following intravesical chitosan/IL-12 treatments were rechallenged intravesically with the original dose of tumor cells. All of the cured mice immediately rejected tumor rechallenge (i.e., tumors failed to establish), while 100% of naïve mice developed tumors. Mice that rejected rechallenge survived for at least one year with no further evidence of disease (Fig. 2B).
Chitosan facilitated higher serum IL-12 and IFN-γ levels following intravesical administration
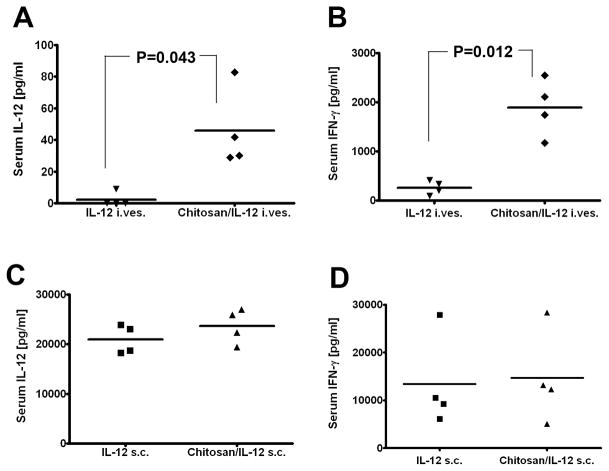
Mice bearing 7-day-old orthotopic bladder tumors were treated intravesically or s.c. with IL-12 alone or chitosan/IL-12. Serum IL-12 and IFN-γ levels were measured 24 h after administration. With intravesical administration, chitosan/IL-12 enhanced serum IL-12 levels 20-fold (Fig. 3A). IFN-γ, produced by several immune cell populations in response to IL-12, was also significantly enhanced when chitosan was used to deliver IL-12 intravesically (Fig. 3B). Importantly, serum cytokine levels resulting from intravesical administration were far below levels produced by s.c. administration (Fig. 3A, B vs. Fig. 3C, D). The average serum IL-12 and IFN-γ levels following intravesical chitosan/IL-12 were 516-fold and 7.8-fold lower, respectively, than the average levels following s.c. chitosan/IL-12.
Figure 3. Serum IL-12 and IFN-γ levels following s.c. and intravesical administrations of IL-12 and chitosan/IL-12.
C57BL/6 mice bearing orthotopic MB49luc tumors were treated intravesically with IL-12 (5 μg) alone or chitosan/IL-12 (5 μg) on day 7. A, serum IL-12 and B, serum IFN-γ levels were measured via ELISA 24 h after treatment. Similarly, C57BL/6 mice were injected s.c. with IL-12 (5 μg) alone or chitosan/IL-12 (5 μg). C, serum IL-12 and D, serum IFN-γ levels were measured via ELISA 24 h after s.c. injections. Each data point represents one mouse. Reported P values were obtained through unpaired t-tests with Welch’s correction assuming unequal variances.
Chitosan/IL-12 induced production of multiple urinary cytokines
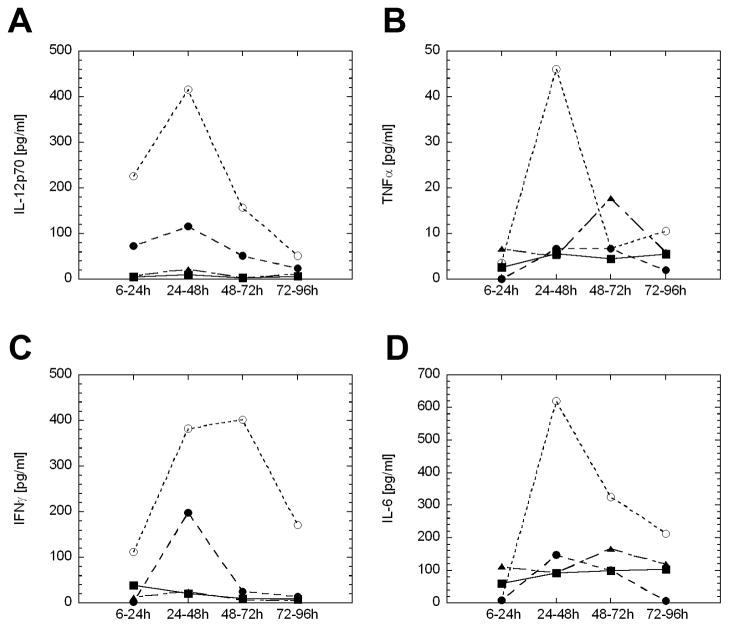
Mice bearing orthotopic bladder tumors were treated intravesically on day 7 post-tumor implantation with PBS, BCG (1.35 mg), IL-12 (5 μg), or chitosan/IL-12 (5 μg). Urine was collected over 4 days in 4 discrete increments following treatment. Mice receiving chitosan/IL-12 had the highest urinary concentrations of IL-12p70, IFN-γ, TNFα, and IL-6 (Fig. 4). IL-12 alone induced these same cytokines, but at much lower levels. BCG treatment resulted in significant urinary levels of TNFα and IL-6. There were no differences in urinary levels of IL-10 or MCP-1 among the treatment groups (data not shown). With one exception, cytokine production peaked within 24 to 48 h in all treatment groups (IFN-γ levels were slightly higher at 48 to 72 h in the chitosan/IL-12 group).
Figure 4. Urinary cytokine levels following intravesical immunotherapy.
C57BL/6 mice (n = 5) bearing orthotopic MB49 luc tumors were treated with PBS (■), BCG (1.35 mg) (▲), IL-12 (5 μg) (●), or chitosan/IL-12 (5 μg) (○) on day 7 post-tumor implantation. Following treatment, mice were placed in metabolic cages and urine was collected over 96 h (see Materials and Methods). Each data point represents an average of 2 pools of mice (n = 2 and n = 3) for each treatment group.
Intravesical chitosan/IL-12 induced intense and lasting lymphocytic infiltration
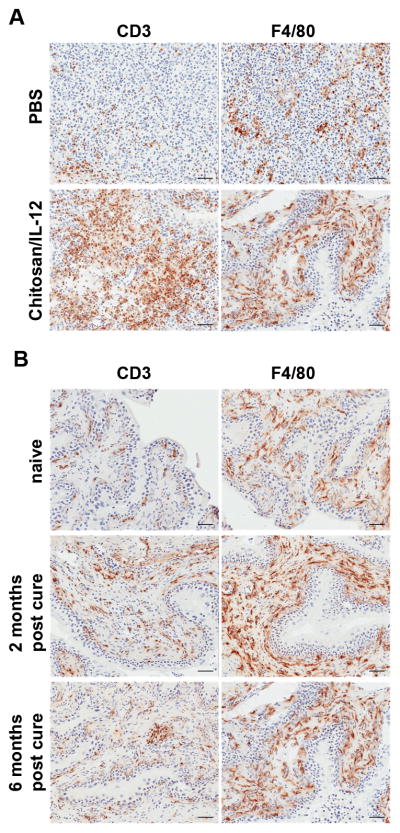
Mice bearing orthotopic bladder tumors were treated intravesically with either PBS or chitosan/IL-12 (5 μg) on days 7 and 14 post-tumor implantation. Mice were sacrificed 48 h after the second treatment and tumors were stained for immune infiltrates. PBS-treated tumors contained minimal lymphocytic infiltrates, while chitosan/IL-12-treated tumors showed moderate multifocal lymphocytic infiltration (Fig. 5A). Immunohistochemical staining revealed that CD3+ and F4/80+ (macrophage) cells increased significantly with chitosan/IL-12 immunotherapy, suggesting that T cells and macrophages are likely effector cells mediating tumor regression during chitosan/IL-12 immunotherapy. An analysis of distribution showed that immune cells in PBS-treated tumors were constrained to the periphery, while chitosan/IL-12-treated tumors show marked infiltration by immune cells.
Figure 5. Immunohistochemistry of bladder tissues during and after intravesical immunotherapy.
A, mice bearing orthotopic bladder tumors were treated intravesically on days 7 and 14 post-tumor implantation with either PBS or chitosan/IL-12 (5 μg). Mice were sacrificed 48 h after the second treatment and tumors were stained for CD3 and F4/80 infiltrates. B, bladders from naïve mice or mice that were tumor-free or cured for 2 months or 6 months after chitosan/IL-12 treatments were stained for CD3 and F4/80. Bar = 50 μm.
In a related study, bladders from naïve mice and cured mice were stained to monitor the maintenance of effector cell populations in the bladder. Mice cured for 2 months showed significant residual CD3+ and F4/80+ populations in the bladder submucosa (Fig. 5B). Effector cell levels in mice cured for 6 months decreased, but were still greater than in naïve mice. A summary of tumor infiltrates during treatment and resident effector cells after treatment, including CD4+ and CD8a+ subsets, is shown in Table 1.
Table 1.
Infiltrate scores in bladder tumors and submucosa duringa and afterb intravesical chitosan/IL-12 immunotherapy.
| Tumor infiltrates after 2 intravesical treatmentsa |
Resident populations in submucosab |
||||
|---|---|---|---|---|---|
| PBS | chitosan/IL-12 | naïve | 2 months post-cure | 6 months post-cure | |
| CD3 | +/++c,d | +++/++++ | ++ | ++++ | +++ |
| CD4 | + | +/+++ | + | +++/++++ | ++/+++ |
| CD8a | − | ++/+++ | −/+ | ++/+++ | ++ |
| F4/80 | +++ | +++/++++ | +++ | ++++ | ++++ |
Mice bearing orthotopic bladder tumors were treated intravesically on days 7 and 14 post-tumor implantation with either PBS or chitosan/IL-12 (5 μg). Mice (n = 3 per group) were sacrificed 48 h after the second treatment and tumor infiltrates were scored by a board certified pathologist.
Bladders from naïve mice (n = 2) or mice that were tumor-free for 2 months (n = 2) or 6 months (n = 2) after chitosan/IL-12 treatments were scored by a board certified pathologist.
Infiltrates scored as negative (−), minimal (+), mild (++), moderate (+++) and intense (++++).
Only unique scores are reported.
Discussion
Our antitumor studies demonstrate that intravesical chitosan/IL-12 is superior to both intravesical IL-12 alone and BCG, the standard-of-care immunotherapy for the treatment of superficial bladder cancer in a murine model. Intravesical treatments with chitosan/IL-12 consistently resulted in 88 to 100% of mice being cured of established, aggressive orthotopic bladder tumors. IL-12 alone was less effective (38 to 60% survival), a finding similar to those in published studies using either recombinant IL-12 protein (16) or IL-12 gene therapy (17) in orthotopic bladder cancer models. Transfection of MB49 cells with the luciferase construct allowed for visualization of tumor growth as early as 4 days post-tumor implantation. Tumors did not become palpable or show any evidence of hematuria for at least one week, and often up to 10 days post-tumor implantation. The enhanced sensitivity of bioluminescence imaging was useful for excluding mice in which tumors do not “take.”
In our study, BCG had minimal antitumor activity and no long-term survival advantage against orthotopic MB49 tumors. In previous studies, BCG treatment initiated the day after tumor implantation conferred a nominal 10 to 15% survival advantage (30, 31). Therefore, the survival advantage mediated by intravesical BCG in this model is abrogated when treatment is withheld until tumors are better established (day 5 post-tumor implantation).
Immunological memory is particularly important for bladder cancer, which has the highest recurrence rate of any cancer. Thus, perhaps more important than the high rate of tumor regression mediated by intravesical chitosan/IL-12 is the complete protection from tumor rechallenge it confers, suggesting that chitosan/IL-12 immunotherapy can generate durable immunological memory. In contrast, others have demonstrated that intravesical BCG does not mediate protective immunity, and no CTL activity could be measured from spleens of BCG-cured mice (32, 33). In yet another study, intravesical IL-12 gene therapy, but not BCG, conferred protection from tumor rechallenge (17).
Our finding that intravesical chitosan/IL-12 produced greater serum levels of IL-12 and IFN-γ than intravesical IL-12 alone supports the hypothesis that chitosan’s unique properties can enhance local IL-12 delivery. It is likely that chitosan enhances both retention through mucoadhesive interactions and penetration through increased epithelial permeability. In previous reports, chitosan has been shown to enhance local delivery of drugs to and across multiple mucosal surfaces, including nasal (34), buccal (35), intestinal (36), and vaginal (37). Chitosan has also been shown to enhance the permeability of the bladder to small hydrophilic drugs (38, 39). In the present study, this enhanced permeability may have allowed IL-12 better access to resident immune cells in the submucosa. The relative importance of increasing epithelial permeability vs. IL-12 retention is currently under investigation.
The difference in IFN-γ levels between intravesical and s.c. administrations is not as great as the difference in IL-12 levels. In our experience, serum IFN-γ reaches a plateau at moderate IL-12 doses. For instance, at 24 h, serum IFN-γ levels with 1 μg of IL-12 s.c. are similar to levels with 5 μg of IL-12 s.c. (10,897 ± 4,004 pg/mL vs. 13,431 ± 4,894 pg/mL). It should be noted that although IL-12 was detected in serum following intravesical chitosan/IL-12 administration, the concentrations were 516 times lower than levels measured following s.c. injections.
Urinary cytokine analysis revealed that chitosan/IL-12 induced significant production of important TH1 cytokines (IL-12p70, IFN-γ, and TNFα) (Fig. 4). The induction of multiple urinary cytokines has long been thought to play a role in intravesical BCG immunotherapy (25, 40). A previous study looked extensively into a range of urinary cytokines produced by tumor-free mice in response to intravesical BCG (29). Our measurements of urinary TNFα, IFN-γ, and IL-10 in response to BCG were similar. However, we measured significantly lower values for IL-12p70 and IL-6. Two procedural differences between the studies may account for this discrepancy. First, our mice had MB49 tumors, which Lattime et al. showed to be highly immunosuppressive (41, 42). Second, our instillation volume was 100 μL rather than the 200 μL used by Saban et al. (29). The larger volume, which is close to the maximum capacity of the murine bladder, would have likely disrupted the integrity of the bladder wall and possibly resulted in a more effective BCG infection. In yet another study, no increases of urinary IFN-γ or IL-12 were detected between 4 h and 24 h after repeated BCG instillation (43). In the present study, chitosan/IL-12 induced much higher levels of TH1 cytokines than BCG. The ability of chitosan/IL-12 to induce far greater levels of cytokines than BCG correlates with its superior antitumor activity. Based on these results, urinary cytokines may be an important prognostic indicator of clinical efficacy in potential trials involving intravesical chitosan/IL-12.
Immunohistochemical staining of treated bladder tumors provided further insight into the mechanism of action of chitosan/IL-12. In our experience, 2 intravesical treatments with chitosan/IL-12 were necessary to observe initial signs of tumor regression; i.e., stable or decreasing bioluminescence and reversal of hematuria. Thus, the optimal time to look for differences in tumor infiltrates was after the second treatment. Indeed, tumors treated twice with chitosan/IL-12 showed moderate to intense staining for CD3 and F4/80 infiltrates (Fig. 5A). Within the CD3 compartment, CD8 staining was greater than CD4 staining (Table 1). This is in contrast to preclinical and clinical studies with BCG in which CD4+ cells outnumber CD8+ cells (30, 43). Our results point to CD8+ T cells and macrophages as potential effector cells mediating the regression of MB49 tumor. Subset depletion studies are planned to confirm this hypothesis.
Combining immunohistochemistry results with urinary cytokine measurements, it appears likely that IL-12 induces a TH1 cytokine cascade that is produced initially by resident immune cells (most likely T cells and macrophages). These cytokines can (a) have direct cytotoxic and antitumor effects; (b) recruit additional immune cells to the tumor; (c) enhance the proliferation of pre-activated T cells; and (d) reverse immune suppression. In particular, IFN-γ has anti-angiogenic properties (44), is directly cytotoxic when combined with other cytokines such as TNFα (10), can upregulate MHC I and II molecules on tumor cells, including the MB49 cell line (45), and can activate macrophage effectors (46) and rescue immunosuppressive tumor-associated macrophages (47, 48). Chitosan enhances all of these effects through better local delivery of IL-12, which leads to higher cytokine production.
In additional immunohistochemistry studies, we found that a residual population of T cells and macrophages remains in the bladder submucosa after a tumor is completely eradicated (Fig. 5B). These populations begin to approach normal (naïve) levels 6 months after tumor cure. These residual effectors may be responsible for the complete protection from tumor rechallenge among cured mice. The relative contributions of innate (macrophages) and adaptive (antigen-specific CD8+ cells) immune systems during this rejection are currently being studied. Nevertheless, because of the high rate of recurrence of bladder cancer, the ability of an intravesical immunotherapy to maintain a resident effector population in the bladder is a distinct benefit.
Additional sections of naïve and cured bladder tissues were stained with hematoxylin and eosin to document any pathological changes associated with chitosan/IL-12 treatment. There were no pathological differences between the cured and naïve bladders as determined by a board certified pathologist. This suggests that chitosan/IL-12 is likely a well tolerated immunotherapy with no long-term pathology. Confirmatory toxicology and pathology studies are planned.
To date, the only clinical trial to date using intravesical IL-12 Failed to show clinically relevant antitumor or immunological effects in patients with recurrent superficial bladder cancer (18) which contrasts the preclinical data reported here (Figs. 1B, 2A) and elsewhere (16, 17). There are at least two possible explanations for this discordance. First, the dose of IL-12 delivered intravesically in humans may have been insufficient to induce an effective immune/antitumor response. The findings of this (supplemental Fig. 2) and other studies (16, 49) have reported dose-dependent effects of intravesical IL-12-based therapies in murine models. In the clinical study, the highest dose used was 200μg which is equivalent, based on bladder volume, to approximately 0.07μg in mice. At this dose, even when formulated with chitosan, IL-12 would not be expected to improve survival in the MB49 model. Although this is not a perfect comparison because of the aggressiveness of the murine tumor, it does indicate that there may be a threshold dose for IL-12 to induce a clinically relevant immune response. Based on our study, it is reasonable to predict that chitosan would substantially reduce this threshold dose in humans.
A second possible explanation is that IL-12 may be less potent in humans than in mice. Systemic (i.v. or s.c.) IL-12 therapy has shown remarkable antitumor effects in numerous murine models that have not been duplicated in clinical trials although those trials have reported sporadic complete responses, significant percentages of stable disease (10) as well as enhanced NK cytolytic activity and T cell proliferation (50). However, two on-study deaths in a Phase II study have overshadowed the modest antitumor and immunological effects generated by systemically administered IL-12. Taken together, the modest antitumor efficacy and well documented toxicity in humans indicate that the therapeutic window for systemically administered IL-12 is more narrow for humans than mice.
The disappointment of early trials brings into question the translatability of any new IL-12-based monotherapy, such as the one introduced here. However, it is important to note that local administrations of IL-12 can limit systemic toxicity and offer an opportunity to expand the therapeutic window of IL-12. Although clinical success of local IL-12 therapies is far from guaranteed, promising approaches deserve consideration for human testing without prejudices based on earlier disappointments from systemic IL-12 trials.
In sum, intravesical chitosan/IL-12 is a promising immunotherapy for the treatment of superficial bladder cancer. We found chitosan/IL-12 to be superior to intravesical IL-12 alone and BCG in terms of survival and induction of TH1 urinary cytokines. Furthermore, chitosan/IL-12 immunotherapy, unlike BCG, mediates complete protection from tumor rechallenge. Taken together, we believe these studies form the rationale for clinical studies employing intravesical chitosan/IL-12 for the treatment of superficial bladder cancer.
Supplementary Material
Acknowledgments
The authors thank Garland Davis, Bertina Gibbs, LaJuan Chase and Curtis Randolph for their superior technical assistance. We also thank Bonnie L. Casey for editorial assistance, Dr. R. Ingrid Fernando for assistance with cell line transfections, Dr. Alfredo Molinolo for the use of slide imaging equipment, and Dr. Miriam Anver for scoring and interpretation of IHC results.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Simons MP, O’Donnell MA, Griffith TS. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol. 2008;26:341–5. doi: 10.1016/j.urolonc.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 5.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 6.Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 7.Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol. 1999;161:1124–7. [PubMed] [Google Scholar]
- 8.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–68. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 9.Brunda MJ, Luistro L, Rumennik L, et al. Antitumor activity of interleukin 12 in preclinical models. Cancer Chemother Pharmacol. 1996;38 (Suppl):S16–21. doi: 10.1007/s002800051031. [DOI] [PubMed] [Google Scholar]
- 10.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 11.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–706. [PubMed] [Google Scholar]
- 12.Zitvogel L, Tahara H, Robbins PD, et al. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol. 1995;155:1393–403. [PubMed] [Google Scholar]
- 13.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–8. [PubMed] [Google Scholar]
- 14.Salem ML, Gillanders WE, Kadima AN, et al. Review: novel nonviral delivery approaches for interleukin-12 protein and gene systems: curbing toxicity and enhancing adjuvant activity. J Interferon Cytokine Res. 2006;26:593–608. doi: 10.1089/jir.2006.26.593. [DOI] [PubMed] [Google Scholar]
- 15.Egilmez NK, Kilinc MO, Gu T, Conway TF. Controlled-release particulate cytokine adjuvants for cancer therapy. Endocr Metab Immune Disord Drug Targets. 2007;7:266–70. doi: 10.2174/187153007782794335. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell MA, Luo Y, Hunter SE, Chen X, Hayes LL, Clinton SK. Interleukin-12 immunotherapy of murine transitional cell carcinoma of the bladder: dose dependent tumor eradication and generation of protective immunity. J Urol. 2004;171:1330–5. doi: 10.1097/01.ju.0000109742.88380.a2. [DOI] [PubMed] [Google Scholar]
- 17.Horinaga M, Harsch KM, Fukuyama R, Heston W, Larchian W. Intravesical interleukin-12 gene therapy in an orthotopic bladder cancer model. Urology. 2005;66:461–6. doi: 10.1016/j.urology.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Weiss GR, O’Donnell MA, Loughlin K, Zonno K, Laliberte RJ, Sherman ML. Phase 1 study of the intravesical administration of recombinant human interleukin-12 in patients with recurrent superficial transitional cell carcinoma of the bladder. J Immunother. 2003;26:343–8. doi: 10.1097/00002371-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Di Martino A, Sittinger M, Risbud MV. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–84. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 21.Read RC, Naylor SC, Potter CW, et al. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23:4367–74. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.McNeela EA, Jabbal-Gill I, Illum L, et al. Intranasal immunization with genetically detoxified diphtheria toxin induces T cell responses in humans: enhancement of Th2 responses and toxin-neutralizing antibodies by formulation with chitosan. Vaccine. 2004;22:909–14. doi: 10.1016/j.vaccine.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Dodane V, Amin Khan M, Merwin JR. Effect of chitosan on epithelial permeability and structure. Int J Pharm. 1999;182:21–32. doi: 10.1016/s0378-5173(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 24.Loskog A, Ninalga C, Hedlund T, Alimohammadi M, Malmstrom PU, Totterman TH. Optimization of the MB49 mouse bladder cancer model for adenoviral gene therapy. Lab Anim. 2005;39:384–93. doi: 10.1258/002367705774286475. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-gamma in response to bacillus Calmette-Guerin. J Immunol. 1999;163:4246–52. [PubMed] [Google Scholar]
- 26.Summerhayes IC, Franks LM. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979;62:1017–23. [PubMed] [Google Scholar]
- 27.Jurczok A, Fornara P, Soling A. Bioluminescence imaging to monitor bladder cancer cell adhesion in vivo: a new approach to optimize a syngeneic, orthotopic, murine bladder cancer model. BJU Int. 2008;101:120–4. doi: 10.1111/j.1464-410X.2007.07193.x. [DOI] [PubMed] [Google Scholar]
- 28.Gunther JH, Jurczok A, Wulf T, et al. Optimizing syngeneic orthotopic murine bladder cancer (MB49) Cancer Res. 1999;59:2834–7. [PubMed] [Google Scholar]
- 29.Saban MR, Simpson C, Davis C, et al. Discriminators of mouse bladder response to intravesical Bacillus Calmette-Guerin (BCG) BMC Immunol. 2007;8:6. doi: 10.1186/1471-2172-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemensberger J, Bohle A, Brandau S. IFN-gamma and IL-12 but not IL-10 are required for local tumour surveillance in a syngeneic model of orthotopic bladder cancer. Clin Exp Immunol. 2002;127:20–6. doi: 10.1046/j.1365-2249.2002.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold J, de Boer EC, O’Donnell MA, Bohle A, Brandau S. Immunotherapy of experimental bladder cancer with recombinant BCG expressing interferon-gamma. J Immunother. 2004;27:116–23. doi: 10.1097/00002371-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–23. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 33.Gan YH, Zhang Y, Khoo HE, Esuvaranathan K. Antitumour immunity of Bacillus Calmette-Guerin and interferon alpha in murine bladder cancer. Eur J Cancer. 1999;35:1123–9. doi: 10.1016/s0959-8049(99)00057-x. [DOI] [PubMed] [Google Scholar]
- 34.Yu S, Zhao Y, Wu F, et al. Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int J Pharm. 2004;281:11–23. doi: 10.1016/j.ijpharm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Sandri G, Rossi S, Ferrari F, Bonferoni MC, Muzzarelli C, Caramella C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur J Pharm Sci. 2004;21:351–9. doi: 10.1016/j.ejps.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J Control Release. 2003;89:151–65. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 37.Valenta C. The use of mucoadhesive polymers in vaginal delivery. Adv Drug Deliv Rev. 2005;57:1692–712. doi: 10.1016/j.addr.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Kerec M, Bogataj M, Veranic P, Mrhar A. Permeability of pig urinary bladder wall: the effect of chitosan and the role of calcium. Eur J Pharm Sci. 2005;25:113–21. doi: 10.1016/j.ejps.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Grabnar I, Bogataj M, Mrhar A. Influence of chitosan and polycarbophil on permeation of a model hydrophilic drug into the urinary bladder wall. Int J Pharm. 2003;256:167–73. doi: 10.1016/s0378-5173(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 40.Bohle A, Brandau S. Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer. J Urol. 2003;170:964–9. doi: 10.1097/01.ju.0000073852.24341.4a. [DOI] [PubMed] [Google Scholar]
- 41.Yang AS, Lattime EC. Tumor-induced interleukin 10 suppresses the ability of splenic dendritic cells to stimulate CD4 and CD8 T-cell responses. Cancer Res. 2003;63:2150–7. [PubMed] [Google Scholar]
- 42.Halak BK, Maguire HC, Jr, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911–7. [PubMed] [Google Scholar]
- 43.Shintani Y, Sawada Y, Inagaki T, Kohjimoto Y, Uekado Y, Shinka T. Intravesical instillation therapy with bacillus Calmette-Guerin for superficial bladder cancer: study of the mechanism of bacillus Calmette-Guerin immunotherapy. Int J Urol. 2007;14:140–6. doi: 10.1111/j.1442-2042.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 44.Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–6. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell MA, Luo Y, Hunter SE, Chen X, Hayes LL, Clinton SK. The essential role of interferon-gamma during interleukin-12 therapy for murine transitional cell carcinoma of the bladder. J Urol. 2004;171:1336–42. doi: 10.1097/01.ju.0000109751.60921.da. [DOI] [PubMed] [Google Scholar]
- 46.Tsung K, Dolan JP, Tsung YL, Norton JA. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 2002;62:5069–75. [PubMed] [Google Scholar]
- 47.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–62. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 48.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–9. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 49.Teicher BA, Ara G, Buxton D, Leonard J, Schaub RG. Optimal scheduling of interleukin 12 and chemotherapy in the murine MB-49 bladder carcinoma and B16 melanoma. Clin Cancer Res. 1997;3:1661–7. [PubMed] [Google Scholar]
- 50.Robertson MJ, Cameron C, Atkins MB, et al. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin Cancer Res. 1999;5:9–16. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.