Abstract
The sedative and hypnotic agent THIP is a GABAA receptor agonist that preferentially activates δ-subunit containing GABAA receptors (δ-GABAARs). To clarify the role of δ-GABAARs in mediating the sedative actions of THIP, we have utilised mice lacking the α1 or δ subunit in a combined electrophysiological and behavioural analysis.
Whole-cell patch-clamp recordings were obtained from thalamic ventrobasal (VB) neurones at a holding potential of −60mV. Application of bicuculline to wild type (WT) VB neurones revealed a GABAAR-mediated tonic current of 92 ± 19 pA, which was greatly reduced (13 ± 5 pA) for VB neurones of δ0/0 mice. Deletion of the δ, but not the α1 subunit, dramatically reduced the THIP (1µM)-induced inward current in these neurones (WT = −309 ± 23 pA; δ0/0 = −18 ± 3 pA; α10/0 = −377 ± 45 pA). Furthermore, THIP selectively decreased the excitability of WT and α10/0, but not δ0/0 VB neurones. THIP did not affect the properties of mIPSCs in any of the genotypes. No differences in rotarod performance and locomotor activity were observed across the three genotypes. In WT mice, performance of these behaviours was impaired by THIP in a dose-dependent manner. The effect of THIP on rotarod performance was blunted for δ0/0, but not α10/0 mice. We previously reported deletion of the α1 subunit to abolish synaptic GABAA responses of VB neurones. Therefore, collectively, these findings suggest that extrasynaptic δ-GABAARs vs synaptic α1-GABAARs of thalamocortical neurones represent an important molecular target underpinning the sedative actions of THIP.
Keywords: thalamus, tonic inhibition, sedation, extrasynaptic receptors, gaboxadol
Introduction
Extrasynaptic GABAA receptors incorporating the δ subunit have emerged as an important molecular target for the action of therapeutically useful drugs including intravenous general anaesthetics (e.g.. etomidate) and sedatives e.g. THIP, or gaboxadol (Belelli et al., 2005; Wafford & Ebert, 2006; Orser, 2006; Wafford & Ebert, 2008). The sedative actions of THIP have attracted considerable attention due to the selectivity of this ligand at clinically relevant concentrations (i.e. ~1 µM) for GABAA receptors composed of either αβ, or αβδ subunits vs αβγ2 receptors, where THIP acts as a potent full and a weak partial agonist respectively (Brown et al., 2002; Storustovu & Ebert, 2006). As receptors incorporating only α and β subunits do not readily occur in vivo (although see Mortensen and Smart, 2006), δ-GABAA receptors are probably the behavioural target for this agonist.
Although sedation and hypnosis are not equivalent (Rudolph & Antkowiak, 2004), sedative and hypnotic agents that act primarily via GABAA receptors, including THIP, engage some of the neuronal pathways implicated in sleep (Nelson et al., 2002; Lu & Greco 2006). Uniquely amongst these agents, THIP selectively promotes slow wave sleep, in a manner similar to that observed physiologically following a period of sleep deprivation, without reducing REM sleep (Lancel, 1997; Lancel et al,. 2000). Of the anatomical substrates that mediate the sedative and hypnotic actions of THIP, the thalamus may play an important role, given the thalamic contribution to the generation of oscillations typical of slow wave sleep and that a significant population of extrasynaptic δ-GABAARs occur in thalamocortical neurones (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005; Bright et al., 2007). We demonstrated that in VB neurones THIP induces a large GABAA receptor-mediated extrasynaptic conductance at sub-micromolar concentrations, but has no effect upon the synaptic GABAA receptors in either VB, or nucleus reticularis (nRT) neurones (Belelli et al., 2005). Furthermore, our findings have identified distinct GABAA receptor isoforms, α1β2γ2 and α4β2δ, to mediate the majority of synaptic and extrasynaptic inhibitory transmission respectively, in mouse VB neurones (Belelli et al., 2005; Chandra et al., 2006; Peden et al., 2008).
In the present study we have utilized a combined electrophysiological and behavioural analysis of mice lacking the α1 or δ subunit to explore the contribution of synaptic (i.e. α1-containing) vs extrasynaptic (i.e. δ-containing) GABAA receptors of VB neurones to the sedative properties of THIP. Here we report that for thalamic VB neurones, deletion of the δ subunit has a modest effect on synaptic “phasic” transmission, but greatly decreases the extrasynaptic “tonic” conductance and consequently prevents the THIP-induced cessation of neuronal firing. By contrast, deletion of the α1 subunit abolishes phasic transmission, with no effect on the tonic conductance, or on the actions of THIP to suppress neuronal firing. Behaviourally, the sedative properties of THIP are severely disrupted in δ0/0, but not α10/0 mice. Collectively, these findings demonstrate that THIP reduces thalamic excitability by selectively activating extrasynaptic δ-GABAARs vs synaptic α1-GABAARs and suggest that this action may contribute to the sedative actions of THIP.
Materials and Methods
Breeding of mice
The α10/0 and δ0/0 mice, together with wild type control mice utilized for electrophysiological experiments and the rotarod (α10/0) and locomotor activity (δ0/0) tests were generated on a mixed C57BL6/J-129SvEv (α10/0), or single C57BL6 (δ0/0) background at the Merck Sharp and Dohme Research Laboratories at the Neuroscience Research Centre in Harlow and at the University of Pittsburgh respectively as described previously (Mihalek et al., 1999; Sur et al., 2001). Experiments were conducted on the first two generations of WT, α10/0 and δ0/0 breeding pairs derived from the corresponding heterozygous +/0 mice bred at the University of Dundee. Rotarod experiments conducted on δ0/0 and WT littermate controls, utilized heterozygous breeding pairs of mice as previously described (Mihalek et al., 1999). Mice were of a mixed C57BL6/J and Strain 129S1/X1 genetic background from > F20 generations and were bred at the University of Pittsburgh.
At weaning, mice bred at both institutions were genotyped using either a PCR strategy, or Southern Blot analysis of tail, or ear DNA as previously described (Mihalek et al., 1999; Sur et al., 2001; Herd et al., 2008). Mice were group housed, given free access to standard rodent chow and water, and maintained on a 12 hr. alternating light/dark schedule, with lights on at 7:30 AM.
Electrophysiology
Slice preparation
Thalamic slices were prepared from mice of either sex (P18–27) according to standard protocols (Belelli et al., 2005). Animals were killed by cervical dislocation in accordance with Schedule 1 of the UK Government Animals (Scientific Procedures) Act 1986. The brain was rapidly removed and placed in oxygenated “ice cold” maintenance solution containing (in mM): 225 sucrose, 2.95 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 10 MgSO4, 10 D-glucose, (pH 7.4; 330–340mOsm). The tissue was maintained in this ice-cold solution whilst horizontal 300–350 µm slices were cut using a Vibratome (Intracel, Royston, Herts., UK). The slices were incubated at 32°C for 1 hr. in an oxygenated, extracellular solution containing (in mM): 126 NaCl, 2.95 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 10 D-glucose and 2 MgCl2 (pH 7.4; 300–310 mOsm). Subsequently, slices were maintained at room temperature (20–23°C) before being used for recordings.
Recordings
Whole-cell voltage-clamp recordings were performed at 35°C from thalamic VB neurones visually identified with an Olympus BX51 microscope (Olympus, Southall, UK) equipped with differential interference contrast/infrared optics as previously described (Belelli et al., 2005). Patch pipettes were prepared from thick walled borosilicate glass (Garner Glass Company, Claremont, California, USA) and had open tip resistances of 3–5 MΩ when filled with an intracellular solution that contained (in mM): 140 CsCl, 10 HEPES, 10 EGTA, 2 Mg-ATP, 1 CaCl2, 5 QX-314 (pH 7.3 with CsOH, 300–305 mOsm). Miniature inhibitory post-synaptic currents (mIPSCs) were recorded using an Axopatch 1D, or 200B amplifier (Axon Instruments, Foster City, CA USA) at a holding potential of −60mV in extracellular solution, that additionally contained 2 mM kynurenic acid (Sigma-Aldrich-RBI, UK), 0.5 µM strychnine and 0.5 µM tetrodotoxin (TTX - TCS Biologicals Ltd, UK) to block ionotropic glutamate receptors, glycine receptors and sodium-dependent action potentials, respectively. For whole-cell recordings, all drugs were applied via the perfusion system (2 – 4ml/min) and allowed to infiltrate the slice for a minimum of 10 min while recordings were acquired.
Perforated patch, current-clamp experiments were performed at 30°C using an Axopatch 200B amplifier. Patch electrodes were prepared as for voltage-clamp experiments and had open tip resistances of 4.5 – 5.5 MΩ when filled with a solution containing (in mM): 145 KCl, 1 MgCl2, 0.1 CaCl2, 1 EGTA, 10 HEPES (pH 7.2–7.3 with KOH, 290 mOsm). The gramicidin-based perforated patch configuration (Kyrozis & Reichling, 1995), which maintains the physiological transmembrane Cl− gradient, was achieved by supplementing the pre-filtered pipette solution with 5–10 µg/ml gramicidin (Sigma-Aldrich-RBI, UK, diluted from a fresh 5–10 mg/ml stock solution in DMSO). The gramicidin-containing pipette solution was sonicated for 2 min. prior to experimentation, and further vortexed between each recording attempt. Electrode tips were filled with gramicidin-free solution, and subsequently back-filled with antibiotic-containing solution. Upon establishment of a high resistance seal (≥ 1GΩ), the progress of perforation was monitored in voltage-clamp mode (holding potential −60mV) by observing the slow development of capacitance transients in response to −5mV hyperpolarising current steps. Due to the high Cl− content of the electrode solution, undesired patch rupture could be monitored as a sudden increase in the amplitude of capacitance transients, followed by the appearance of high frequency spontaneous inward GABA-ergic synaptic currents. Such recordings were immediately discarded. Full perforation (series resistance <80MΩ) was generally achieved within 30 min. and the recording configuration switched to the fast current clamp mode of the amplifier. The voltage drop arising from the series resistance was corrected using the simulated bridge balance circuitry of the amplifier and the estimated liquid junction potential of 3.4 mV (calculated using pClamp v8.2) was left uncorrected. Input resistance was calculated following delivery of small (−20pA) hyperpolarising current steps. Sustained tonic action potential firing was elicited by depolarising the cell to a supra-threshold membrane potential using direct current injection. To investigate the influence of acute GABAAR activation on action potential firing, THIP was applied locally to the recorded cell by placing a second, drug-containing patch pipette approximately 30–40µm from the cell soma. THIP (30 µM in the pipette) was focally applied by pressure ejection using a Picospritzer II system (General Valve, 10ms duration, 7–10 psi). Pressure ejection of drug-free extracellular solution confirmed the lack of any stimulus artefact when using these parameters. Recordings were filtered at 5 kHz and recorded to digital audio tape (DTR1200, Intracell) for offline analysis.
Drugs
THIP-HCl (4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol-HCl, gaboxadol- 10−2M, hereafter referred to as THIP), bicuculline methobromide (10−2 M), and strychnine (10−3 M) were dissolved in water. These stock solutions were diluted in extracellular solution to the desired concentration. With the exception of THIP, which was a generous gift of Bjarke Ebert (Lundbeck), all drugs tested were obtained from either Sigma-Aldrich-RBI UK, or Tocris UK.
Data analysis
Data were recorded onto a digital audio tape using a Biologic DTR 1200 recorder and analyzed offline using the Strathclyde Electrophysiology Software, WinEDR/WinWCP (J. Dempster, University of Strathclyde, UK). Individual mIPSCs were detected using a −4pA amplitude threshold detection algorithm and visually inspected for validity. Accepted events were analysed for peak amplitude, 10–90% rise time, charge transfer and time for events to decay from peak by 90% (T90). To minimize the contribution of dendritically generated currents, which are subject to cable filtering, the analysis was restricted to events with a rise time ≤ 1ms. A minimum of 100 accepted events/cell were digitally averaged by alignment at the mid-point of the rising phase, and the mIPSC decay fitted by either monoexponential (y(t) = Ae(−t/τ)), or biexponential (y(t) = A1e(−t/τ1) + A2e(−t/τ2)) functions using the least squares method, where A is amplitude, t is time and τ is the decay time constant. Analysis of the standard deviation of residuals and the use of the F-test to compare the goodness of the fit revealed that the average mIPSC decay was always best fit with the sum of 2 exponential components. Thus, a weighted decay time constant (τw) was also calculated according to the equation: τw = τ1P1 + τ2P2, where τ1 and τ2 are the decay time constants of the first and second exponential functions and P1 and P2 are the proportions of the synaptic current decay described by each component.
The mIPSC frequency was determined over 10sec. bins for 2 min. with the WinEDR program using a detection method based on the rate of rise of events (≥35–40 pA/ms) and subsequent visual scrutiny. The tonic current was calculated as the difference between the holding current before and after application of bicuculline methobromide (30 µM-Belelli et al., 2005). We previously demonstrated the effects of bicuculline in this respect to be reproduced by 100 µM picrotoxin, a structurally distinct GABAA receptor antagonist (Belelli et al., 2005).
Spike analysis (action potential half width, frequency and inter-event interval) was also performed using the Strathclyde University WinEDR/WCP software.
All results are reported as the arithmetic mean ± standard error of mean (SEM). Statistical significance of the mean data was assessed with the unpaired, or paired Student’s t test as appropriate, using the SigmaStat (SPSS Inc., Chicago, IL USA) software package.
Behavioural tests
Fixed Speed Rotarod
For all experiments, both male and female mice 8– 16 weeks of age and 19–35 gm. in weight at the time of testing, were used. THIP (Sigma-Aldrich-RBI) was diluted in saline and administered into the intraperitoneal cavity. WT, α10/0 and δ0/0 mice were trained on the rotarod at a fixed speed of 6RPM (Ugo Basile, Model 7650 with rod diameter of 6 cm, Stoelting Co., Wood Dale, IL). Training was considered complete when mice were able to remain on the fixed speed rotarod for 180 sec. Training took 4 ± 1 trials on the rotarod. For experiments comparing WT and δ0/0 mice, two groups of animals were used. Two separate groups of mice were tested with either 10 mg/kg, or 30 mg/kg THIP. For experiments comparing WT and α10/0 genotypes, mice were injected with two doses of THIP (10 and 30mg/kg), or its saline vehicle on three separate occasions with at least 48 hr. in between doses. Drugs were administered in a counter balanced repeated measure design over genotype. After injection of THIP, time on the rotarod was measured up to 150 min. post-injection in 15 min. intervals.
Locomotor Activity
The locomotor activity of 18 male mice (9 δ+/+ and 9 δ0/0) aged between 12 and 16 weeks (20–35g) was measured using an activity monitor (Benwick Electronics, Norfolk, UK) employing infrared light beams to detect movement. To habituate the animals to the testing box each animal was placed in the box for 20 min. for 6 days using a counter balanced design. On each day the locomotor activity was logged in 5 min. bins.
Following 6 days of habituation each mouse was injected (i.p.) with one of three doses of THIP (2mg/kg, 5mg/kg, or 10mg/kg), or saline vehicle (0.9% NaCl). Locomotor activity was measured for 20 min. starting 30 minutes after the injection. Each mouse received all four treatments using a counter balanced design with 48hr. intervals between each injection.
Data analysis
Statistical analysis was performed using SPSS. One-way ANOVAs with repeated measures with genotype as the between subjects factor and time (for rotarod tests) and time and day/drug treatment (for locomotor activity test) respectively as the within subjects factors were used to investigate interactions and significant differences between groups. In the rotarod experiments comparing α10/0 and WT mice in which mice received more than one drug treatment, the drug treatment was used as an additional within subjects variable. The Mauchly’s test for sphericity was used to test the assumption of equal variances (i.e. the variances in each experimental condition are equal) and when this assumption was violated (Mauchly’s test result is < 0.05) the Huynh-Feldt Correction for heterogeneity of variance was applied (Mauchly, 1940; Field, 2005). Post-hoc analyses were performed using paired and unpaired t-tests where appropriate.
Results
The influence of δ- and α1-subunit deletion on synaptic and extrasynaptic inhibition in thalamic VB neurones
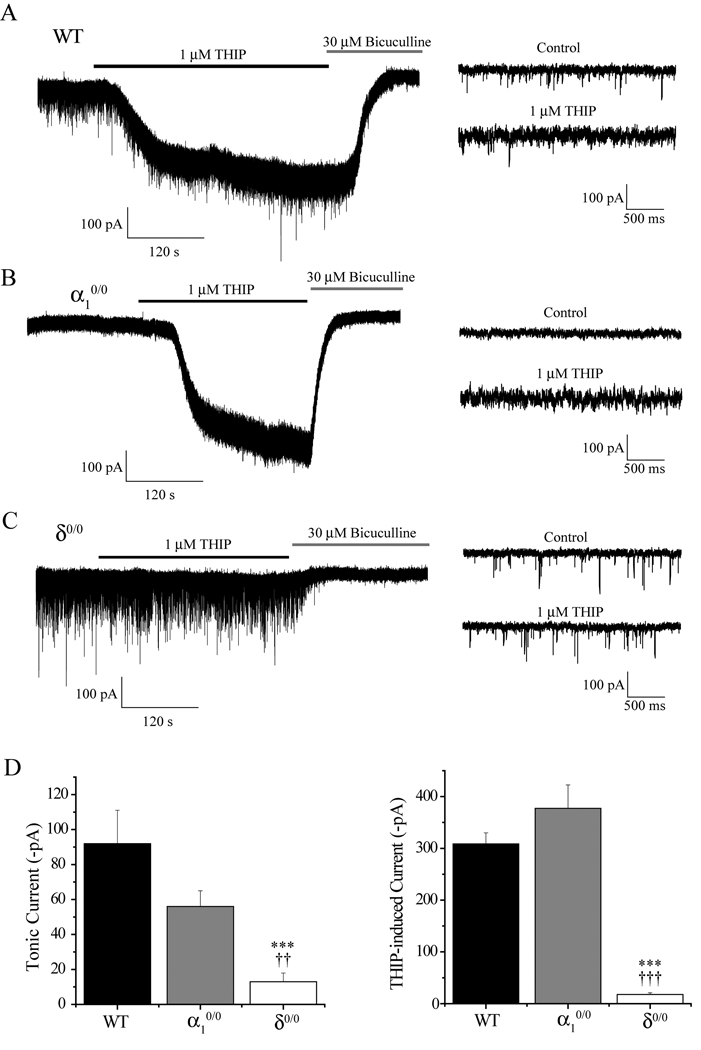
To investigate the relative subcellular distribution of the α1 and δ subunits in thalamic VB neurones, we used the whole-cell voltage clamp technique to compare tonic and phasic inhibition of WT neurones with that recorded from neurones obtained from mice lacking either the α1 (α10/0), or the δ subunit (δ0/0). We have previously reported that deletion of the α1 subunit completely abolished GABAAR synaptic transmission in VB neurones of ≥ P18 mice (Peden et al., 2008). By contrast, the properties of mIPSCs derived from δ0/0 mice were only modestly influenced by deletion of the δ subunit, with a small reduction in the peak amplitude (P = 0.0005) and an increase in the τw (P = 0.0003) observed relative to WT (Table 1, unpaired t-test). All other mIPSC properties recorded from δ0/0 neurones were indistinguishable from WT (Table 1; P > 0.05). Conversely, deletion of the δ, but not the α1 subunit, severely disrupted extrasynaptic thalamic transmission. Thus, in agreement with our previous findings (Belelli et al., 2005; Peden et al., 2008), the bath application of bicuculline to define the resting tonic current (Itonic) revealed an Itonic of 92 ± 19 pA, n = 5 in VB neurones derived from WT mice (P = 0.008, paired Student’s t test, Figure 1). By comparison, for δ0/0 VB neurones, the outward current induced by 30 µM bicuculline was significantly reduced vs WT (δ0/0 Itonic = 13 ± 5 pA, n = 9, P = 0.0002 vs WT; unpaired t test, Figure 1). Similarly, the membrane noise of the δ0/0 neurones was significantly lower than those of WT (RMS noise, WT = 7.7 ± 1 pA; δ0/0 = 4.7 ± 0.2 pA, P = 0.0005 unpaired t test). Moreover, for δ0/0 neurones, the RMS noise was only modestly, albeit significantly, reduced by bicuculline, (4.4 ± 0.1 pA, P = 0.009 paired t test). Thus, α1 and δ subunits are incorporated into separate synaptic and extrasynaptic receptor pools. Consistent with a synaptic localization of α1-GABAARs, in α10/0 VB neurones, the δ-preferring agonist THIP (1µM) evoked a large bicuculline-sensitive inward current, which was not significantly different from that recorded from WT mice (α10/0 = −377 ± 45pA, n = 4; WT = −309 ± 23 pA, n = 11, P = 0.17 unpaired t test, Figure 1A, B & D). However, in VB neurones derived from δ0/0 mice, the inward current induced by 1µM THIP was significantly reduced relative to WT (δ0/0 = −18 ± 3 pA, n = 7 vs WT, P < 0.000001, unpaired t test; Figure 1A, C & D). Collectively, these results are consistent with the proposal that extrasynaptic receptors in VB neurones incorporate the δ, but not the α1 subunit.
Table 1.
Summary of the properties of mIPSC recorded from VB neurones of WT and δ0/0 mice. The majority of neurones derived from α10/0 mice (aged P18–P27) are devoid of mIPSCs and are therefore not included.
| WT (n = 19) | δ0/0 (n = 25) | |
| Peak Amplitude (pA) | −66 ± 2 | −82 ± 3* |
| Rise Time (ms) | 0.5 ± 0.01 | 0.5 ± 0.01 |
| Charge Transfer (fC) | 263 ± 11 | 296 ± 15 |
| T90 (ms) | 5.5 ± 0.1 | 5.3 ± 0.1 |
| τw (ms) | 3.8 ± 0.1 | 3.2 ± 0.1* |
| Frequency (Hz) | 16.8 ± 1.9 | 21.7 ± 2.0 |
P < 0.05 WT vs δ0/0.
Figure 1. Deletion of the δ, but not the α1 subunit, reduces the tonic current and the THIP-induced current in VB neurones.
(A, B & C) Left panels: Whole-cell voltage-clamp recordings from WT (A), α10/0 (B) and δ0/0 (C) VB neurones illustrating the effect of THIP (1 µM, black bars) and bicuculline (30 µM, grey bars) on the holding current. THIP induced a large, bicuculline-sensitive increase of the tonic current of WT and α10/0, but of δ0/0 neurones. Right panels: Expanded traces from the same recordings, illustrating an increased membrane noise in the presence of THIP in WT and α10/0, but not δ0/0 neurones. Note also the absence of mIPSCs in recordings from α10/0 neurones (D) Bar graphs summarising the tonic current (left) and the THIP-induced current (right) recorded from WT (black bars), α10/0 (grey bars) and δ0/0 neurones (open bars). Data were obtained from 4 to 17 recordings. Asterisks and daggers denote significant differences (*** P < 0.001 WT vs δ0/0; †† P < 0.01 α10/0vs δ0/0, ††† P <0.001 α10/0 vs δ0/0 unpaired t test).
Deletion of the δ, but not the α1 subunit, abolishes the THIP-induced suppression of tonic action potential firing in VB neurones
Thalamocortical relay neurones exhibit three characteristic action potential (AP) firing patterns arising within distinct membrane potential windows: at hyperpolarised membrane potentials, low threshold burst firing predominates, whereas tonic, or high threshold burst firing modes are observed at relatively depolarised membrane potentials (Jahnsen & Llinas, 1984, Steriade et al., 1993, Hughes et al., 2002; 2004). Here, we have investigated the effect of THIP on VB neurones during tonic AP firing and assessed the influence of α1 or δ subunit deletion, on this response using the respective mice. To preserve a physiological Cl− driving force, current-clamp recordings were performed using the gramicidin-based perforated patch technique (Kyrozis & Reichling, 1999). Under control conditions, VB neurones derived from WT mice displayed a resting membrane potential (RMP) of −75 ± 1 mV and an input resistance (IR) of 382 ± 27 MΩ (n = 27). The RMP of VB neurones was not significantly influenced by deletion of either the α1 or the δ subunit (α10/0 = −72 ± 1 mV, n = 10; δ0/0 = −73 ± 1 mV, n = 21, P > 0.1 vs WT for either strain). Similarly, the IR of VB neurones derived from α10/0 mice (425 ± 60 MΩ, n = 10) was not significantly different from WT (P = 0.44 vs WT Figure 2E). By contrast, in mice lacking the δ subunit, the IR was significantly increased (493 ± 41 MΩ,n = 21, P = 0.02 vs WT, Figure 2E). At the relatively hyperpolarised membrane potentials observed in VB neurones under control conditions, spontaneous low threshold AP bursts were observed only rarely (not shown). To investigate the effect of THIP on the tonic AP mode, which corresponds to the in vivo firing pattern observed during awake behavioural states, VB neurones were slowly depolarised by injection of constant direct current (DC) via the recording electrode until single, repetitive APs were elicited at an average frequency of 4–8Hz. The shape of the AP spike, as measured by the duration of the half width did not significantly change across the three genotypes (half-width, WT = 0.7 ± 0.04 ms; α10/0 = 0.7 ± 0.04 ms; δ0/0 = 0.7 ± 0.03 ms; P = 0.88, one way ANOVA). In thalamic neurones derived from WT mice, focal application of THIP (30 µM) to the cell soma from a local pressure ejection pipette evoked a rapid hyperpolarisation of the membrane potential and an abrupt, but reproducible cessation of AP discharge (inter-event interval [IEI] pre-THIP = 0.2 ± 0.01s, post-THIP = 3.9 ± 0.4 s, n = 10, P = 0.00001, paired t-test, Figure 2A & 2D), followed by a progressive recovery of the AP frequency to pre-application levels upon agonist dispersal (Figure 2A). Similarly, the local application of THIP to VB neurones derived from α10/0 mice induced a temporary blockade of tonic AP firing (pre-THIP IEI = 0.2 ± 0.02 s, post THIP IEI = 3.5 ± 0.3 s, n = 7, P = 1 × 10−5, paired t-test, Figure 2B & D). Importantly, for equivalent tonic AP firing frequencies (WT = 5.1 ± 0.3 Hz, n =10; α10/0 = 5.0 ± 0.5 Hz, n =7), the duration of THIP-induced AP cessation was not significantly different between the strains (P > 0.05, unpaired t-test). By contrast, in mice lacking the δ subunit, the effect of THIP on tonic AP firing was completely abolished (pre-THIP IEI = 0.2 ± 0.01 s, post THIP IEI = 0.3 ± 0.1 s, n = 9, P = 0.40, paired t-test, Figure 2C & D). Indeed, the post-THIP IEI recorded from δ0/0 neurones was significantly different from both WT (P < 0.000001) and α10/0 (P < 0.000001, unpaired t test) cells. Furthermore, THIP did not change the half-width of APs recorded from VB neurones derived from δ0/0 mice.
Figure 2. The effect of THIP on tonic action potential firing in VB neurones is abolished in δ0/0, but not α10/0 mice.
(A, B & C) Gramicidin-perforated patch current clamp recordings (left panels) and associated action potential (AP) frequency plots (right panels) from WT (A), α10/0 (B) and δ0/0 (C) VB neurones illustrating the effect of focal application of THIP (arrows) on tonically firing APs. The values to the left of each trace indicate the approximate membrane potential (mV) from which firing was elicited by injection of a depolarising direct current. Focal application of THIP induces a transient cessation of tonic AP firing in WT and α10/0 neurones, but not of δ0/0 cells. (D) Bar graph summarising the AP inter-event interval (IEI) before (black bars) and after (open bars) the focal application of THIP to WT, α10/0 and δ0/0 VB neurones. Data were obtained from 7 to 10 cells. Asterisks and daggers denote significant differences (*** P < 0.001 pre-THIP vs post THIP, paired t-test; ††† P < 0.001 WT post THIP IEI vs δ0/0 post THIP IEI, unpaired t test). (E) Bar graph summarising the input resistance of VB neurones recorded from WT (black bar), α10/0 (grey bar) and δ0/0 (open bar) mice. Data were obtained from 10 to 27 cells. Asterisk denotes a significant difference for WT vs δ0/0 (* P < 0.05, unpaired t test).
Collectively, these results illustrate that extrasynaptic δ-GABAARs, but not synaptic α1-GABAARs, mediate the effects of THIP on tonic AP firing in VB neurones.
Deletion of the δ but not the α1 subunit reduces the ataxic effects of THIP on the rotarod
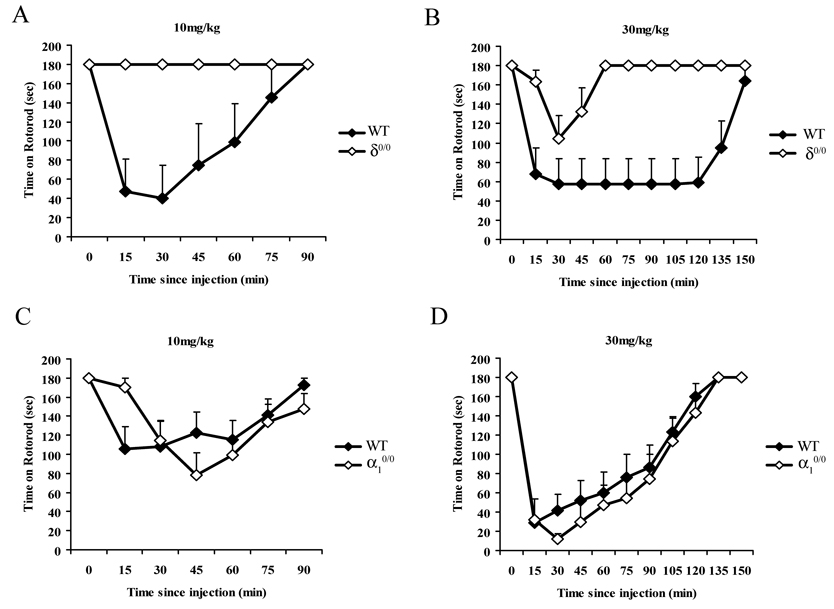
To assess the sedative effects of THIP and the role played by δ-GABAA receptors, we initially investigated the actions of THIP in a test of forced motor activity (i.e. the rotarod test). Previous studies in our laboratory have shown that 5mg/kg THIP does not affect rotarod performance of wild type mice (data not shown, F(6,24) = 0.93; P = 0.46). Therefore, recovery from ataxia induced by two different doses of THIP (10 and 30 mg/kg) known to impair performance in wild type mice was measured using a fixed speed rotarod in 2 separate experiments (Figure 3). There were no significant effects of sex on the responses to THIP. Therefore the data were combined between sexes. δ0/0 mice were completely insensitive to the ataxic effect of THIP at 10 mg/kg [genotype (F(1,45) = 13.3; P = 0.005] (Fig 3A). In a separate experiment, the higher dose of 30 mg/kg THIP impaired performance by the δ0/0 mice on the rotarod, however, the deficiency was significantly less than for WT mice [genotype (F(1,152) = 15.1; P = 0.001] (Fig 3B). Collectively, these data demonstrate that δ0/0 mice have a substantially reduced sensitivity to the ataxic effects of THIP.
Figure 3. Deletion of the δ, but not the α1 subunit reduces the ataxic effects of THIP.
Ataxic effects of THIP were measured by the fixed speed rotarod (6 r.p.m). The data are presented as means ± SEM of 5 to 11 observations and show the mean time spent on the rotarod at different times after the injection. Saline injections had no effects on rotarod performance in any of the strains (data not shown). The ataxic response was either eliminated, or greatly reduced in δ0/0 mice (open diamonds) compared with WT controls (filled diamonds) at 10 mg/kg (A; P < 0.01, n = 6 for δ0/0 and 5 for WT), and at 30 mg/kg (B; P < 0.005, n = 11 for δ0/0 and 10 for WT). By contrast, deletion of the α1 subunit did not affect the ataxic effects of THIP, either at the 10 mg/kg (C; P > 0.1), or the 30 mg/kg dose (D; P > 0.05, n = 8 for both α10/0 and WT at both doses).
To evaluate the contribution of α1-GABAARs to the sedative-ataxic actions of THIP, the rotarod performance of α10/0 mice was investigated following THIP administration. As illustrated by Figure 3C & D, impairment of the rotarod performance by THIP is not affected by deletion of the α1 subunit. Thus, global analysis revealed a significant reduction in the amount of time spent on the rotarod following injection with THIP (drug, F(2,28) = 62.02, P < 0.000001). A significant effect of time since injection (time, F(10,140) = 69.92, P < 0.000001) and a dose of THIP/time since injection interaction (drug × time, F(20,280) = 19.82, P < 0.000001) was also detected, which is attributable to the different recovery profiles for each dose. There was no overall effect of genotype (genotype, F(1,14) = 1.11, P = 0.31), suggesting both WT and α10/0 mice are susceptible to the ataxic effects of 10 and 30mg/kg THIP.
The reduction in locomotor activity induced by THIP is blunted by deletion of the δ subunit
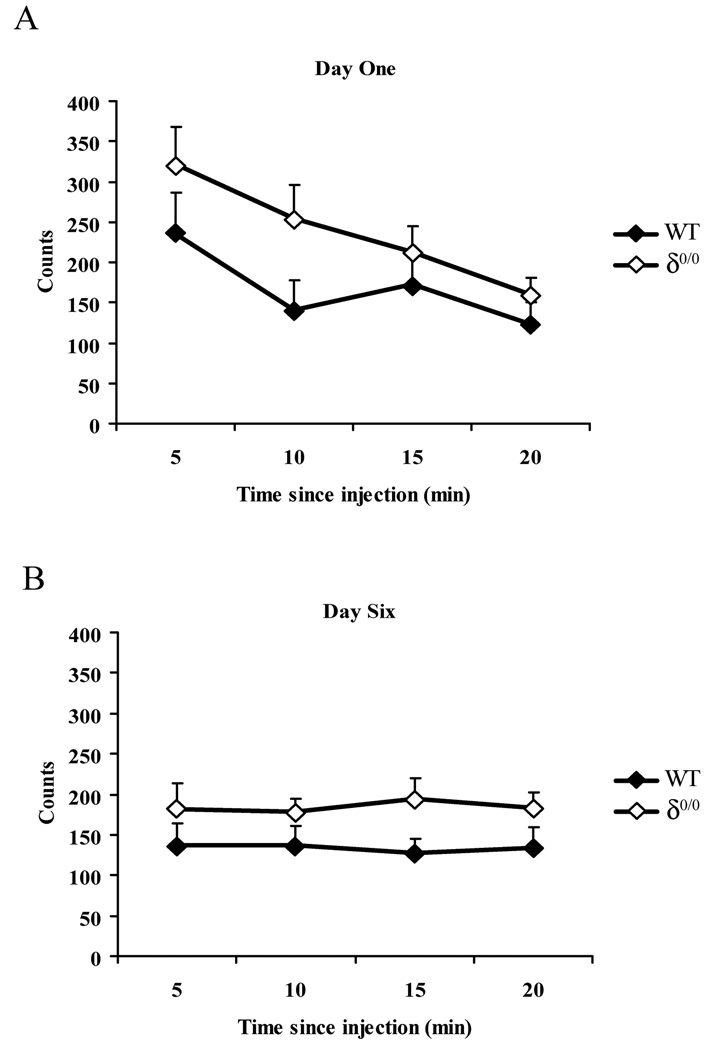
Sedative and hypnotic agents commonly impair performance on the rotarod test. However, interpretation of the results from this test may be compromised if the drug, as THIP does, also impairs motor coordination because it elicits ataxia (Storustovu & Ebert, 2006). Thus, the impact of the δ subunit deletion on the actions of THIP was also evaluated on spontaneous motor activity in a locomotor activity chamber. Preliminary global analysis of basal spontaneous activity revealed there was a significant reduction in activity over days (F(5,80) = 3.3, P = 0.009) and within a session (F(3,48) = 12.04, P = 0.000005). There was a significant interaction between days and the time bin within a session (F(15,240) = 5.1, P < 0.000001). Mice habituated both acutely within the first session and between sessions and such habituation was not influenced by genotype (F(1,16) = 0.6, P = 0.46). On day 1 activity reduced during the session (F(3,48) = 17.8, P < 0.000001- Figure 4A), however by day 6 initial activity during the first 5 min. of the trial was lower than for day 1 (t = 4.8, d.f. = 17, P = 0.0002) and activity no longer declined significantly during the session (F(3,48) = 0.024, P = 1.0 - Figure 4B). These data demonstrate that the mice habituated both acutely within the first session and between sessions as anticipated (Leussis & Bolivar, 2006) and that this habituation was not influenced statistically by genotype. Global analysis of the activity of mice treated with THIP (Figure 5) showed a significant effect of the drug (F(3,48) = 6.8, P = 0.001), but not of time (F(3,48) = 1, P = 0.38). There was a main effect of strain (F(1,16) = 7.9, P = 0.012) and a significant interaction between drug and genotype (F(3,48) = 5.4, P = 0.003). Upon further analysis, WT mice showed a significant reduction in locomotor activity (F(3,24) = 17.4, P = 0.000003) with increasing doses of THIP. THIP diminished activity of WT mice at doses as low as 2mg/kg (t =−3.6, d.f. = 8 P = 0.007, Paired t test). However this drug had no effect on the locomotor activity of δ0/0 mice (F(3,24) = 0.55, P = 0.66).
Figure 4. Deletion of the δ subunit does not influence locomotor habituation.
Locomotor activity of δ0/0 (open diamonds) and WT controls (closed diamonds) measured on days one and six of habituation. The data are presented as mean ± SEM photobeam counts measured over 20 min. for 9 observations. Both genotypes showed a significant reduction in activity over time within session on day one (A; P < 0.01, n = 9 for δ0/0 and for WT), but not on day six (B; P > 0.05), suggesting both genotypes habituated to the testing environment.
Figure 5. Deletion of the δ subunit abolishes the sedative effect of THIP.
A bar graph illustrating that the reduction of locomotor activity following injection with THIP is abolished in δ0/0 mice (open bars), compared with WT controls (filled bars) at all doses (2, 5 and 10mg/kg) tested. (P < 0.01, n = 9 for δ0/0 and for WT). All mice were given i.p. injections of vehicle or THIP using a counter-balanced design with at least 48hr between each injection. The data are presented as mean ± SEM photobeam counts measured over 20 min. for 9 observations. Each bar indicates the mean and the SEM of the activity of mice. * P < 0.05, ** P < 0.01 when compared with vehicle-treated animals; †† P < 0.05, ††† P < 0.01 when compared with WT.
Collectively these findings are consistent with the hypothesis that the reduction of both spontaneous and forced activity observed following injection with THIP at these doses is mediated by the δ-containing GABAA receptors.
Discussion
The actions of THIP have been extensively scrutinised both at a molecular and behavioural level in an attempt to relate the unique behavioural profile of this sedative and hypnotic agent to specific molecular target(s) (Wafford & Ebert, 2006). A preferential interaction of THIP with GABAARs incorporating the δ subunit has been clearly documented both in recombinant expression systems and in native neurones (Brown et al., 2002; Belelli et al., 2005; Drasbeck & Jensen, 2006). However, the specific anatomical substrate(s) underpinning the sedative actions of THIP remains a matter of debate, although the thalamus has been proposed to play a significant role (Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005). In an attempt to clarify this issue, we employed a combined electrophysiological and behavioural approach to characterise the role of GABAA receptor-mediated synaptic and extrasynaptic transmission in the actions of THIP upon thalamic excitability and to relate these findings to the sedative effects of THIP in mice harbouring a deletion of a subunit putatively expressed either at synaptic (i.e. α1) or extrasynaptic (i.e. δ) locations in thalamic relay neurones (Belelli et al., 2005; Peden et al., 2008).
As we previously reported for DG granule cells (Herd et al., 2008), the properties of synaptic currents recorded from VB neurones were generally unaffected by deletion of the δ subunit with the exception of a modestly increased peak amplitude and a reduced decay, which may reflect an upregulation of the synaptically located γ2 subunit and, in WT, a modest contribution of perisynaptically located δ-GABAAR to the overall synaptic decay, respectively (Hamann et al., 2002; Korpi et al., 2002; Rossi and Hamann, 1998). In contrast to the phasic conductance, the tonic inhibitory conductance was reduced by > 85% vs WT. Similarly, the THIP-induced extrasynaptic conductance was dramatically reduced in δ0/0 mice. Although both α1 and α4 subunits are abundantly expressed in the thalamic relay cells, the α4 and β2 subunits putatively partner the δ isoform at extrasynaptic locations in VB neurones (Belelli et al., 2005; Jia et al., 2005; Chandra et al., 2006). In agreement with this suggestion, the dramatic reduction both in the thalamic tonic inhibitory current and the THIP-induced extrasynaptic conductance produced by deletion of the δ subunit was equivalent to that previously described for α40/0 mice (Chandra et al., 2006). Furthermore, consistent with synaptic vs extrasynaptic expression, deletion of the α1 subunit did not affect either the resting tonic conductance (Peden et al., 2008), or the THIP-induced current (present study).
Extrasynaptic inhibition mediated by GABAARs plays an important role in regulating the excitability of individual neurones and neural networks, where it may regulate the frequency of network oscillations (Walker and Semyanov, 2008). Consistent with this notion, in perforated patch current-clamp recordings, THIP (30µM), applied focally from a pressure ejection pipette, produced a rapid hyperpolarization and a transient cessation of tonic action potential discharge. This effect was blunted in δ0/0, but not in α1 0/0 neurones, thus directly implicating extrasynaptic δ-GABAARs, but not synaptic α1-GABAARs, in the actions of THIP upon thalamic excitability. Furthermore, although the exact concentration of THIP that transiently activates the δ-GABAARs cannot be accurately determined when using brief focal application, the lack of activity exhibited by this drug in VB neurones derived from δ0/0 mice indirectly confirms that the applied concentration selectively targets extrasynaptic receptors, without activating synaptic receptors.
The THIP-induced hyperpolarization of VB neurones is qualitatively similar to that previously determined for 100nM and 300nM THIP under different recording conditions (Cope et al., 2005; Chandra et al., 2006). Furthermore, the loss of the THIP-induced hyperpolarization in VB neurones derived from δ0/0 mice, is similar to that reported for α40/0 VB neurones (Chandra et al., 2006). Collectively, these findings demonstrate for the first time that synaptically located α1-receptor isoforms are not primarily implicated in the action of THIP upon thalamic excitability and identify conclusively the α4βδ isoform as the selective molecular target of the actions of this drug in thalamic VB neurones.
It is established that at hyperpolarized membrane potentials thalamic relay neurones fire predominantly in the low- threshold bursting mode, which is responsible, physiologically, for the slow oscillations typical of NON-REM sleep and pathologically, for the stereotypical 3Hz spike and wave discharges, characteristic of epileptic absence attacks (Steriade et al., 1993; McCormick and Contreras, 2001). Although not specifically investigated in this study, it is conceivable that the THIP-induced hyperpolarization of VB neurones described here, plays a crucial role in the increase in slow wave sleep associated with the systemic administration of THIP (Lancel, 1997; Lancel et al., 2000). In agreement, a recent study indicates the THIP-induced effects on EEG activity to be prevented by deletion of the δ subunit (Winsky-Sommerer et al., 2007). Furthermore, the hyperpolarization of thalamic neurones is probably implicated in the pro-absence actions of this hypnotic agent evident in animal models of absence epilepsy (Vergnes et al., 1984). Interestingly, neurosteroids may preferentially interact with δ-GABAA receptors and the pro-absence actions of these endogenous GABAAR modulators are significantly reduced in δ0/0 mice (Mihalek et al. 1999). Whether the pro-absence actions of THIP are similarly blunted in δ0/0 mice is presently not known. Analysis of the sedative actions of THIP following deletion of either the δ, or the α1 subunit, in tests of forced and spontaneous motor activity, clearly demonstrates such behaviours to be crucially dependent on δ-containing GABAARs. Overall, these findings are in agreement with a recent report revealing δ0/0 mice to be significantly less sensitive to the hypnotic actions of THIP as measured by the loss of righting reflex (Boehm et al., 2006). Additionally, our findings provide the first demonstration that deletion of a subunit with a widespread synaptic localization, i.e. α1, does not influence the dramatic effects of THIP upon motor activity and furthermore excludes a significant contribution of the α1βδ receptor isoform to the sedative actions of this agonist (Glykys et al., 2007).
Interestingly, the actions of THIP in the tests of spontaneous and forced (i.e. rotarod test) motor activity exhibit different profiles of dose dependence. Thus, in the locomotor activity test, a reduction in activity was apparent at doses of THIP as low as 2mg/kg, whereas for the rotarod, only a dose of 10mg/kg, or greater, produced an impairment in performance. Furthermore, the reduction in locomotor activity elicited by THIP remained constant at all doses tested over the 20 minute period of observation (when analysed in 5 minute bins - data not shown), whereas the ataxic effect was significantly reduced at 45 vs 30 minutes post-injection (Figure 3A). A parsimonious explanation for these findings may simply reside in the intrinsic differences between the two behavioural tasks, as one measures spontaneous activity, whereas the other determines forced motor activity. Thus, a greater effect on the extrasynaptic inhibitory conductance may be required to suppress forced vs spontaneous activity. An alternative, though not mutually exclusive interpretation postulates that the interaction of THIP with δ-GABAARs incorporating distinct α subunit isoforms, i.e. α4 vs α6, may contribute to the differences observed between the tests of spontaneous locomotor activity (i.e. α4-mediated) and rotarod (i.e. α6-mediated) performance. This scenario is credible given that α6βδ GABAA receptors are exclusively expressed in the cerebellum (Pirker et al., 2000; Wisden et al., 1992) and altered GABA-ergic activity in the cerebellum has been associated with deficits in motor co-ordination (impaired rotarod performance) analogous to those reported here (Korpi et al., 1999; Wulff et al., 2007; Cheron et al., 2008). Additionally, based on the potency and efficacy profile exhibited by THIP at recombinant GABAA receptors (Storustovu and Ebert, 2006), Ebert and colleagues have recently predicted that following the administration of a range of THIP doses (similar to those employed in the present study), the peak brain concentration of this drug would result in a level of receptor activation equivalent to 50% of the GABA maximum for the α6βδ isoform, but only ~15–20% for the α4βδ subtype (Cremers et al., 2007; Ebert et al., 2008). Higher doses of THIP and hence, presumably a greater level of receptor occupancy, appear necessary to observe an impairment of rotarod performance. Therefore, these findings are consistent with the suggestion that the cerebellum and the α6βδ receptor isoform in particular may play a prominent, although not necessarily exclusive role in the action of THIP in this test. Indeed, deletion of the α4 subunit also disrupts the deficits induced by THIP in the rotarod test (Chandra et al. 2006). Clearly, the simplest interpretation of these findings is that the α4βδ receptor isoform also plays a role in the THIP-induced impairment on the rotarod performance. In support, the α4βδ receptor isoform is prominently expressed both in the thalamic relay neurones of the VB complex and the dorsal striatum (Wisden et al., 1992), two regions known to play a significant role in motor function (Cicirata et al. 1986; Groenewegen, 2003) and hence, probably complicit in the ataxic effects of THIP.
However, compensatory changes in gene expression of partner subunits as previously described for α60/0 and δ0/0 mice (Jones et al., 1997; Tretter et al., 2001; Korpi et al., 2002), may complicate the interpretation of these findings. Thus, for example, an increased and decreased expression of the γ2 and α4 subunits respectively, coupled with an increased association of the α4 and γ2 subunits across the forebrain of δ0/0 mice have been documented (Korpi et al. 2002). Furthermore, a similar up-regulation of the γ2 subunit has been described in cerebellar granule cells derived from δ0/0 mice (Tretter et al. 2001). The future development of neurone-selective “knock-out” mice may clarify the relative contribution of α6βδ vs α4βδ isoforms to the ataxic effects of THIP (Wulff et al., 2007).
In contrast to the selective localization of α6βδ GABAA receptors to cerebellar granule cells, the α4βδ isoform is expressed in a number of brain areas, but importantly with high levels of expression being detected in the thalamus (Pirker et al., 2000). The presence of δ-GABAARs coupled with the crucial role of the thalamus in the generation of slow-wave oscillations, an activity typically induced by the administration of the δ-GABAAR selective THIP (see above), strongly implicates this structure in the sedative actions of this agonist. In support, available evidence indicates that structurally distinct GABAA receptor agonists and modulators can produce sedation by engaging the endogenous sleep circuitry at different levels (Nelson et al., 2002; Franks, 2008). Moreover, a depression of thalamic activity during sleep is apparent both in EEG recordings and functional brain imaging studies (Braun et al., 1997; Hofle et al., 1997). Thus, of the main pathways known to regulate the sleep-wake cycle, the circuit that utilises the thalamus for the transmission of sensory inputs to the cerebral cortex is conceivably the primary target for THIP. However, within the same circuitry, THIP may additionally interfere with the alternative pathway that bypasses the thalamus to activate both cortical and lateral hypothalamic neurones (Saper et al., 2001). In this regard, it should be noted that GABAA receptors expressed in the hypothalamic histaminergic tuberomammillary nucleus (TMN- Haas et al., 2008) have been causally implicated in the sedative and hypnotic actions of GABA-ergic ligands (Nelson et al., 2002). In accord with these findings, the switch to the sleep state is hypothesised to be dependent upon inhibition of the TMN neurones, a large component of which originates from the "sleep promoting" GABA-ergic neurones of the ventrolateral preoptic nucleus (VLPO, Saper et al., 2001). Interestingly, in common with other GABA-ergic ligands, THIP induces c-FOS activation in the VLPO and in agreement, the sedative effects of THIP are attenuated by lesions of the VLPO (Lu & Greco, 2006). Collectively, these observations indicate that the VLPO contributes to the sedative actions of THIP, yet the specific location(s) of the δ-GABAA receptors activated by the agonist within this sleep circuit pathway remains to be determined. Although the actions of THIP in the VLPO and TMN have not been functionally investigated, immunocytochemical and in situ hybridization studies suggest only low levels of expression of the δ subunit, at least in the VLPO (Volgin, 2008). It is nevertheless conceivable that THIP may promote excitation of VLPO neurones by acting upstream on a pathway normally inhibiting VLPO activity (e.g. noradrenergic and serotonergic neurones of the locus coerelus and dorsal raphe respectively). A better understanding of the actions of THIP at this level of the sleep circuitry, coupled with the generation of mice harboring a region selective deletion of the δ subunit, will clarify the relative contribution played by thalamic δ-GABAA receptors to the sedative actions of THIP.
Acknowledgements
We are grateful to Dr Bjarke Ebert for helpful comments on the manuscript. This work was supported by a Biotechnology and Biological Sciences Research Council project grant [grant number C509923], Biological Sciences Research Council Strategic Studentship [grant number 12019] (NF), Tenovus Scotland, Epilepsy Research UK and NIH grants DE14184 (DC) and AA10422 (GEH).
Abbreviations
- α1-GABAARs
α1 subunit-containing GABAA receptors
- δ-GABAARs
δ subunit containing GABAA receptors
- AP
action potential
- DC
direct current
- DG
dentate gyrus
- GABAARs
γ-aminobutyric acid type A receptors
- IEI
inter-event interval
- IR
input resistance
- Itonic
tonic current
- mIPSCs
miniature inhibitory postsynaptic currents
- RMP
resting membrane potential
- RMS
root mean square
- THIP
4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol
- TMN
tuberomammilary nucleus of the hypothalamus
- VB
ventrobasal thalamic nucleus
- VLPO
ventrolateral preoptic area
- WT
wild type
Reference List
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J.Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, Homanics GE, Blednov YA, Harris RA. δ-Subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur.J.Pharmacol. 2006;541:158–162. doi: 10.1016/j.ejphar.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABAA receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J.Neurosci. 2007;27:2560–2569. doi: 10.1523/JNEUROSCI.5100-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br.J.Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc.Natl.Acad.Sci.U.S.A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Servais L, Dan B. Cerebellar network plasticity: from genes to fast oscillation. Neuroscience. 2008;153:1–19. doi: 10.1016/j.neuroscience.2008.01.074. [DOI] [PubMed] [Google Scholar]
- Cicirata F, Angaut P, Cioni M, Serapide MF, Papale A. Functional organization of thalamic projections to the motor cortex. An anatomical and electrophysiological study in the rat. Neuroscience. 1986;19:81–99. doi: 10.1016/0306-4522(86)90007-2. [DOI] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J.Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers T, Ebert B. Plasma and CNS concentrations of Gaboxadol in rats following subcutaneous administration. Eur.J.Pharmacol. 2007;562:47–52. doi: 10.1016/j.ejphar.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb.Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Ebert B, Anderson NJ, Cremers TI, Rasmussen S, Vogel V, Fahey JM, Sanchez C. Gaboxadol - a different hypnotic profile with no tolerance to sleep EEG and sedative effects after repeated daily dosing. Pharmacol.Biochem.Behav. 2008;90:113–122. doi: 10.1016/j.pbb.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat.Rev.Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat.Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10(1–2):107–120. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAA β subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J.Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Regional cerebral blood flow changes as a function of δ and spindle activity during slow wave sleep in humans. J.Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron. 2002;33:947–958. doi: 10.1016/s0896-6273(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Lorincz M, Cope DW, Blethyn KL, Kekesi KA, Parri HR, Juhasz G, Crunelli V. Synchronized oscillations at α and θ frequencies in the lateral geniculate nucleus. Neuron. 2004;42:253–268. doi: 10.1016/s0896-6273(04)00191-6. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J.Physiol. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J.Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J.Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Koikkalainen P, Vekovischeva OY, Makela R, Kleinz R, Uusi-Oukari M, Wisden W. Cerebellar granule-cell-specific GABAA receptors attenuate benzodiazepine-induced ataxia: evidence from α6-subunit-deficient mice. Eur.J.Neurosci. 1999;11:233–240. doi: 10.1046/j.1460-9568.1999.00421.x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H. Altered receptor subtypes in the forebrain of GABAA receptor δ subunit-deficient mice: recruitment of γ2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J.Neurosci.Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Lancel M. The GABAA agonist THIP increases non-REM sleep and enhances non-REM sleep-specific delta activity in the rat during the dark period. Sleep. 1997;20:1099–1104. doi: 10.1093/sleep/20.12.1099. [DOI] [PubMed] [Google Scholar]
- Lancel M, Langebartels A. γ-aminobutyric acidA (GABAA) agonist 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol persistently increases sleep maintenance and intensity during chronic administration to rats. J.Pharmacol.Exp.Ther. 2000;293:1084–1090. [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci.Biobehav.Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA. Sleep circuitry and the hypnotic mechanism of GABAA drugs. J.Clin.Sleep Med. 2006;2:S19–S26. [PubMed] [Google Scholar]
- Mauchly JW. Significance test for sphericity of a normal n-variate distribution. The Annals of Mathem. Stat. 1940;11:204–209. [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu.Rev.Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc.Natl.Acad.Sci.U.S.A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J.Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat.Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J.Clin.Sleep Med. 2006;2:S12–S18. [PubMed] [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, Homanics GE, Belelli D, Fritschy JM, Lambert JJ. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J.Physiol. 2008;586:965–987. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat.Rev.Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J.Pharmacol.Exp.Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O'Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J.Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Hoger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA receptor δ subunit gene leads to an up-regulation of γ2 subunit-containing receptors in cerebellar granule cells. J.Biol.Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Micheletti G, Depaulis A, Rumbach L, Warter JM. Enhancement of spike and wave discharges by GABAmimetic drugs in rats with spontaneous petit-mal-like epilepsy. Neurosci.Lett. 1984;44:91–94. doi: 10.1016/0304-3940(84)90226-x. [DOI] [PubMed] [Google Scholar]
- Volgin DV. Perinatal alcohol exposure leads to prolonged upregulation of hypothalamic GABAA receptors and increases behavioral sensitivity to gaboxadol. Neurosci.Lett. 2008;439:182–186. doi: 10.1016/j.neulet.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol-a new awakening in sleep. Curr.Opin.Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nat.Rev.Drug Discov. 2008;7:530–540. doi: 10.1038/nrd2464. [DOI] [PubMed] [Google Scholar]
- Walker MC, Semyanov A. Regulation of excitability by extrasynaptic GABAA receptors. Results Probl.Cell Differ. 2008;44:29–48. doi: 10.1007/400_2007_030. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABAA δ-subunit-containing receptors. Eur.J.Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain I. Telencephalon, diencephalon, mesencephalon. J.Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, Farrant M, Wisden W. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABAA receptors. Nat.Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. [DOI] [PMC free article] [PubMed] [Google Scholar]