Abstract
Modification of NMDA receptor function and trafficking contributes to the regulation of synaptic transmission and is important for several forms of synaptic plasticity. Here, we report that NMDA receptor subunits NR2A and NR2B have two distinct clusters of palmitoylation sites in their C-terminal region. Palmitoylation within the first cluster on a membrane proximal region increases tyrosine phosphorylation of tyrosine-based internalization motifs by Src family protein tyrosine kinases, leading to enhanced stable surface expression of NMDA receptors. In addition, palmitoylation of these sites regulates constitutive internalization of the NMDA receptor in developing neurons. In marked contrast, palmitoylation of the second cluster in the middle of C-terminus by distinct palmitoyl transferases causes receptors to accumulate in the Golgi apparatus and reduces receptor surface expression. These data suggest that regulated palmitoylation of NR2 subunits differentially modulate receptor trafficking and may be important for NMDA receptor dependent synaptic plasticity.
Introduction
Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system. The ionotropic glutamate receptors (GluRs) are classified into several groups, namely, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionate), KA (kainate), δ (delta) and NMDA (N-methyl-D-aspartate) receptors (Hollmann and Heinemann, 1994; Seeburg, 1993). The NMDA receptor plays a crucial role in synaptic plasticity, synaptogenesis and excitotoxicity (Constantine-Paton et al., 1990). NMDA receptors consist of three families of homologous subunits, NR1, NR2 and NR3 (Cull-Candy and Leszkiewicz, 2004; Dingledine et al., 1999; Mori and Mishina, 1995). The NR1 subunit is encoded by a single gene and is obligatory for functional NMDA receptors. NR2 subunits are composed of four members (NR2A-2D) and determine the functional and spatiotemporal diversity of NMDA receptors. Most central NMDA receptors are NR1/NR2 assemblies (Abe et al., 2004; Carroll and Zukin, 2002; Wenthold et al., 2003a; Wenthold et al., 2003b). The NR1 subunit is ubiquitously expressed in neurons throughout the brain whereas the different NR2 subunits have more restricted patterns of distribution (Monyer et al., 1994). In contrast to most other GluRs, NMDA receptor channels exhibit high permeability to Ca2+ and voltage-dependent block by Mg2+. The NR3 subunits (NR3A and NR3B) decrease Mg2+ sensitivity and Ca2+ permeability and reduce agonist-induced current responses (Cavara and Hollmann, 2008; Cull-Candy and Leszkiewicz, 2004). These properties underlie NMDA receptor-dependent plasticity in the central nervous system (Collingridge et al., 2004; Lau and Zukin, 2007; Stephenson, 2001).
Many studies have shown that post-translational modifications of GluRs, such as phosphorylation, play critical roles in the regulation of synaptic plasticity (Derkach et al., 2007; Malinow and Malenka, 2002; Shepherd and Huganir, 2007; Song and Huganir, 2002; Swope et al., 1999). Phosphorylation of AMPA receptors modulates AMPA receptor ion channel properties as well as the trafficking of AMPA receptors to the postsynaptic membrane. Similarly, phosphorylation of serine and tyrosine residues on NMDA receptor subunits is a well-established biochemical mechanism for the modulation of receptor function (Collingridge et al., 2004; Lan et al., 2001; Leonard and Hell, 1997; Li et al., 2002; Nakazawa et al., 2001; Nakazawa et al., 2006; Omkumar et al., 1996; Skeberdis et al., 2001; Vissel et al., 2001). Another type of protein modification that regulates localization and function of proteins is post-translational palmitoylation (Bijlmakers and Marsh, 2003; El-Husseini Ael and Bredt, 2002; Resh, 1999), the covalent attachment of palmitate to proteins via thioester bonds at cysteine residues. Like phosphorylation, this modification is labile and reversible. Post-translational palmitoylation of proteins plays important roles in the regulation of protein targeting to membranes and synapses. Some neuronal ionotropic receptors (Drisdel et al., 2004; Hayashi et al., 2005; Keller et al., 2004; Pickering et al., 1995; Rathenberg et al., 2004), neuronal metabotropic G-protein coupled receptors (Alaluf et al., 1995; Ng et al., 1993; Ng et al., 1994a; Ng et al., 1994b; Ponimaskin et al., 2002; Ponimaskin et al., 2001) and ion channels (Chien et al., 1996; Qin et al., 1998; Schmidt and Catterall, 1987) are reversibly palmitoylated. We recently reported that all four types of AMPA receptor subunits GluR1-4 are palmitoylated on two distinct sites, in their transmembrane domain (TMD) 2 and in their C-terminal domain (Hayashi et al., 2005). TMD 2 palmitoylation is increased by the palmitoyl acyl transferase (PAT) GODZ and causes accumulation of the receptor in the Golgi apparatus. Palmitoylation on the C-terminal domain inhibits AMPA receptor interaction with the 4.1N protein, which stabilizes AMPA receptor expression on the cell surface, and regulates endocytosis and the insertion (Lin et al., 2009) of AMPA receptors. Moreover, depalmitoylation of the AMPA receptor in neurons is regulated by glutamate treatment. Among other ionotropic GluRs, palmitoylation of GluR6 KA receptor has been reported at its C-terminal cysteine residues, Cys827 and Cys840 (Pickering et al., 1995). In this instance, palmitoylation regulated protein kinase C (PKC)-mediated phosphorylation of GluR6 but did not affect KA channel properties.
In this paper, we demonstrate that the NMDA receptor subunits NR2A and NR2B are palmitoylated in cortical neurons on two distinct cysteine clusters. Palmitoylation of the first cysteine cluster (Cys cluster I) controls stable expression and constitutive internalization of surface NMDA receptor. The second cysteine cluster (Cys cluster II) is palmitoylated by GODZ and depalmitoylation of this cluster regulates surface delivery of NMDA receptors. Moreover, the palmitoylation of the NR2 subunits is dynamically regulated by neuronal activity. These data indicate that palmitoylation of the NR2 subunits plays an important role in the regulation of NMDA receptor trafficking. NMDA receptors are critical for the regulation of synaptic plasticity and activity dependent development of neuronal circuits and thus palmitoylation of the NR2 subunits may have important effects on brain function and development.
Results
Palmitoylation of the NMDA receptor subunits NR2A and NR2B in neurons
The NR1 subunit is essential for functional NMDA receptors (Dingledine et al., 1999; Mori and Mishina, 1995). As shown in our previous work (Hayashi et al., 2005), the NR1 subunit is not palmitoylated in cultured cortical neurons. However, the palmitoylation status of NR2 subunits had not been investigated. The NR2A and NR2B subunits have long intracellular C-terminal tails, which contain many cysteine residues. To test whether NR2 subunits were palmitoylated, cultured cortical neurons were metabolically labeled with [3H]-palmitic acid. Metabolic labeling showed that both endogenous NR2A and NR2B were palmitoylated (Fig. 1A). The AMPA receptor GluR2/3 subunits, which we previously reported to be palmitoylated, were used as a positive control. Palmitoylation occurs on sulfhydryl groups of intracellular cysteine residues in target proteins. To examine if the [3H]-palmitate was linked to the receptor subunits via hydroxylamine-sensitive thioester bonds (Bizzozero, 1995), duplicate immunoprecipitated samples of [3H]-palmitate-labeled endogenous NR2A and NR2B from cultured cortical neurons were treated with hydroxylamine (NH2OH) or Tris-HCl buffer (Fig. 1B). The [3H]-palmitate labeling of these NR2 subunits was eliminated by treatment with hydroxylamine and not by control Tris-HCl buffer, indicating that the labeling was due to palmitoylation at cysteine residues via thioester bonds. We also assessed NR2 palmitoylation in neuronal culture using an Acyl-biotinyl exchange (ABE) assay. ABE uses an exchange of biotin for thioester-linked acyl modifications, in which the resultant biotinylated proteins are affinity-purified using neutravidin-conjugated beads (Wan et al., 2007: see Experimental Procedures). This assay confirmed that both NR2A and NR2B are palmitoylated in cultured cortical neurons (Fig. 1C).
Figure 1. Palmitoylation of endogenous NMDA receptor subunits NR2A and NR2B.
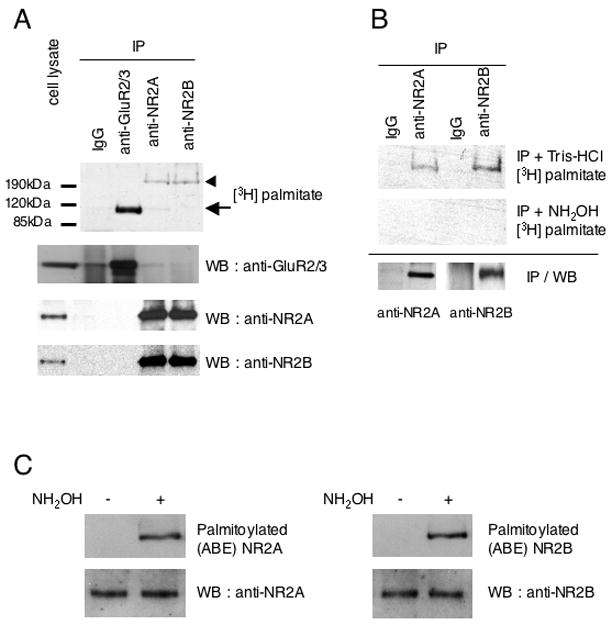
(A) Palmitoylation of endogenous GluR2/3, NR2A and NR2B was examined in metabolically labeled cultured cortical neurons. Immunoprecipitation (IP) and detection of incorporated [3H]-palmitate into GluR2/3 and NR2A and NR2B (top). IP and expression of each protein was confirmed by western blotting (WB) (bottom). (B) Hydroxylamine treatment of palmitoylated NR2A and NR2B to confirm thioester linkage to intracellular cysteines. [3H]-palmitate content of Tris-HCl-treated (top) and hydroxylamine (NH2OH)-treated (middle) IP samples are shown. IP of each NR2 protein from cell lysates of metabolically labeled cultured cortical neurons was confirmed by WB (bottom). (C) Palmitoylation of endogenous NR2A and NR2B was examined in cultured cortical neurons using a biotin switch assay for palmitoylation (Wan et al., 2007: see Experimental Procedures). Palmitoylated (Acyl-Biotinyl Exchanged) NR2 (top) and total protein expression (cell lysate) of NR2 protein (bottom) were confirmed by WB.
Activity-dependent change of NR2 palmitoylation in neurons
Previously we showed that the AMPA receptor subunits GluR1 and GluR2 were depalmitoylated when cortical neurons were stimulated by 10 μM glutamate for 1 hr (Hayashi et al., 2005). Using anti-NR2A and anti-NR2B antibodies, stimulation-dependent changes of NR2 palmitoylation were also studied. Treatment of neurons with glutamate (10 μM, 1 hr at 37°C) decreased the palmitoylation of NR2 without affecting total NR2 protein amounts (Fig. 2A, B). These results indicate that similar to the palmitoylation of AMPA receptor, activation of glutamate receptors can regulate NR2 palmitoylation. Moreover, activity-dependent changes of NR2 palmitoylation were examined using the biotin switch assay for palmitoylation (Fig. 2C, D). Treatment of neurons with tetrodotoxin (TTX, 1 μM, 30 min at 37°C), to inhibit the intrinsic neuronal network, increased NR2 palmitoylation (Fig. 2C), whereas treatment with bicuculline (20 μM, 30 min at 37°C), to increase the intrinsic neuronal network activity by suppressing the inhibitory action of GABAA receptors, conversely decreased the palmitoylation of NR2 without affecting total NR2 protein amounts (Fig. 2D). These results demonstrate that neuronal activity can dynamically regulate NR2 palmitoylation.
Figure 2. Activity-dependent change of endogenous NR2 palmitoylation.
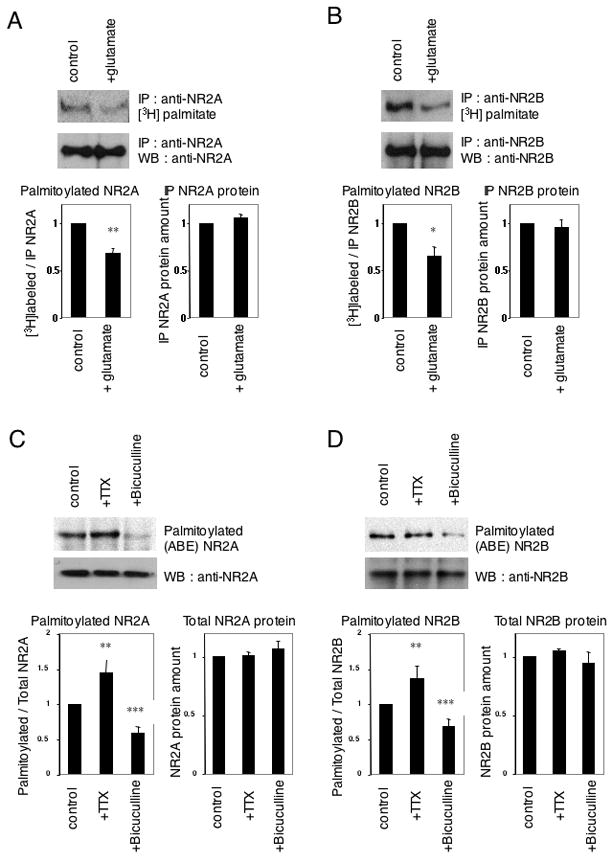
(A, B) Glutamate-induced change of NR2A (A) and NR2B (B) palmitoylation. Cultured cortical neurons were treated with 10 μM glutamate for 1 hr at 37°C. A representative example of an IP with each anti-NR2 antibody and [3H]palmitate incorporation (top) or IP and WB with each anti-NR2 antibody (middle) were shown. Quantified data of ratio of [3H]palmitate-labeled NR2 to total NR2 protein amount (bottom, left, n = 4, respectively, Student t-test, *p < 0.05, **p < 0.01 compared with control) and immunoprecipitated (IP) NR2 protein amount (bottom, right, n = 4, respectively). (C, D) Effects of treatment with tetrodotoxin (TTX) and bicuculline on NR2A (C) and NR2B (D) palmitoylation. Cultured cortical neurons were treated with 1 μM TTX or 20 μM bicuculline for 30 min at 37°C. A representative example of an in vitro acyl-biotinyl exchanged (ABE) sample, followed by purification with streptavidin-conjugated beads and WB with each anti-NR2 antibody is shown (top). Total protein expression (cell lysate) of each protein was confirmed by WB (middle). Quantified data of ratio of palmitoylated NR2 to total NR2 protein amount (bottom, left, n = 4, respectively, Student t-test, **p < 0.01, ***p < 0.001 compared with control) and total NR2 protein amount (bottom, right, n = 4, respectively).
Identification of C-terminal palmitoylation sites of NR2A and NR2B
Next, we determined the sites of palmitoylation using site-specific mutagenesis. There are 12 intracellular cysteine residues in NR2A and 17 in NR2B. Seven of these sites in the intracellular C-terminal region of NR2 subunits are conserved between NR2A and NR2B. Of these seven conserved cysteines, the first three cysteines (NR2A-Cys848, Cys853, Cys870; NR2B-Cys849, Cys854, Cys871) are located in the juxta-membrane region just past TMD 4; we named this group of cysteine residues “Cys cluster I”. Another cysteine cluster, “Cys cluster II” exists in the middle of NR2A/2B C-terminal tails (NR2A-Cys1214, Cys1217, Cys1236, Cys1239; NR2B-Cys1215, Cys1218, Cys1239, Cys1242, Cys1245) (Fig. 3A). To test if these consensus cysteines were palmitoylated, we generated a NR2A hepta CS mutant (7CS) where all seven cysteines in Cys cluster I and II were mutated to non-palmitoylatable serines (NR2A C848S/C853S/C870S/C1214S/C1217S/C1236S/C1239S). In these experiments, the NR1-1a subunit was co-transfected with NR2A 7CS into HEK 293T cells in order to form functional receptors (Scott et al., 2003; Scott et al., 2001). Labeling experiments with [3H]-palmitate showed that the NR2A 7CS mutations completely eliminated NR2A palmitoylation in transfected HEK 293T cells (Fig. 3B). These data indicate that NR2A is palmitoylated on one or more of the seven consensus cysteine residues. To narrow down the regions of NR2A that are palmitoylated, we next made a series of NR2A multiple CS mutants in Cys cluster I (C848S/C853S/C870S) (3CS) and Cys cluster II (C1214S/C1217S/C1236S/C1239S) (4CS). Labeling experiments using these mutants showed that NR2A palmitoylation was dramatically decreased in the NR2A 3CS mutant (Fig. 3C). This result indicates that palmitoylation sites lie within a homologous C-terminal region just after the TMD 4 (Cys cluster I). Finally, we determined the exact cysteines that were palmitoylated in NR2A subunit. In an experiment using a series of CS mutants within the Cys cluster I (C848S, C853S, C870S, C848S/C853S, 3CS), we detected palmitoylation signals in each single CS mutant and even in double CS mutant (C848S/C853S). Palmitoylation was eliminated only when all three cysteines were mutated in the 3CS mutant, suggesting that each of the three cysteines within the Cys cluster I can be palmitoylated in HEK 293T cells (Fig. 3D). The NR2B triple CS mutant (3CS), whose corresponding cysteine residues in Cys cluster I were substituted with serine (C849S/C854S/C871S), was not palmitoylated, similar to NR2A (Fig. 3E). These data indicate that both NR2A and NR2B are palmitoylated in their Cys cluster I. With longer exposures, a weak [3H]-palmitate signal was detected in the NR2A 3CS and NR2B 3CS mutant, suggesting that NR2A and NR2B contains additional minor palmitoylation site(s) (data not shown). Co-immunoprecipitation experiments in transfected HEK 293T cells showed that CS mutations in both Cys cluster I and II did not affect the proper assembly of the NR1 with either NR2A subunits (Supplemental Fig. S1A) or NR2B subunits (Supplemental Fig. S1B).
Figure 3. Palmitoylation sites of NR2A and NR2B.
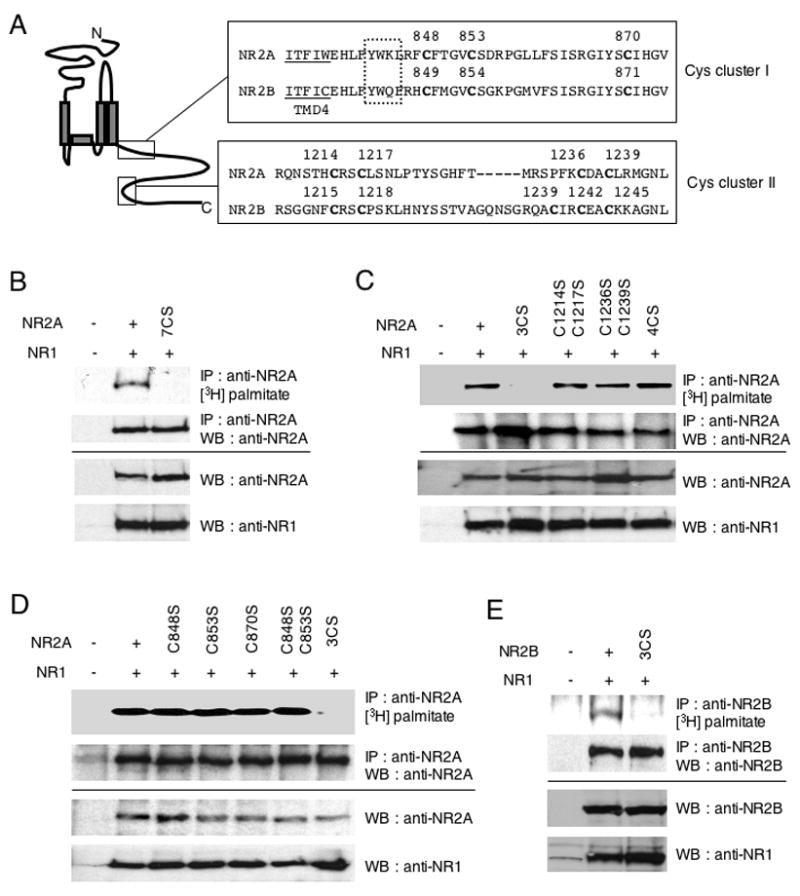
(A) C-terminal amino acid sequences of NMDA receptor subunits NR2A and NR2B, containing consensus cysteine clusters. Boxes indicate conserved cysteine residues (Cys cluster I and II). Transmembrane domain (TMD) 4 is underlined. Dotted box indicates AP-2 binding motif (YXXΦ). (B–E) Identification of NR2A and NR2B palmitoylation sites using a series of CS mutants. NR1/NR2A wild type and NR1/NR2A 7CS (B), or NR1/NR2A 3CS, NR1/NR2A C1214SC1217S, NR1/NR2A C1236SC1239S, NR1/NR2A 4CS (C), or NR1/NR2A C848S, NR1/NR2A C853S, NR1/NR2A C870S, NR1/NR2A C848SC853S, NR1/NR2A 3CS (D) and NR1/NR2B wild type and NR1/NR2B 3CS (E) were cotransfected into HEK 293T cells. Transfected cells were metabolically labeled with [3H]-palmitate and then solubilized and lysates were immunoprecipitated with anti-NR2A or anti-NR2B antibodies. The incorporated [3H]-palmitate signals were shown (upper top). Immunoprecipitation (IP, second panels) and total protein expression (cell lysate) of each protein (third and fourth panels) was confirmed by western blotting (WB).
Palmitoylation of the NR2 in its C-terminal region by GODZ
PATs were originally cloned by genetic studies in yeast (Erf2, Akr1 and Ykt6) (Dietrich et al., 2004; Dietrich and Ungermann, 2004; Lobo et al., 2002; Roth et al., 2002). Erf2 and Akr1 have an aspartate-histidine-histidine-cysteine (DHHC)-cysteine rich domain, which is conserved in proteins from yeast to mammals. A large family of mammalian DHHC-PATs, 23 in mouse and 22 in human, have been identified (Fukata et al., 2004; Huang et al., 2004; Ohno et al., 2006). A Golgi apparatus-specific protein with a DHHC zinc finger domain (GODZ) has been reported to have PAT activity towards both AMPA and GABAA receptors (Hayashi et al., 2005; Keller et al., 2004; Uemura et al., 2002). We therefore examined if GODZ may also function as a neuronal PAT for the NMDA receptor. Palmitoylation of NR2A was enhanced by co-expression of GODZ in transfected HEK 293T cells (Fig. 4A). In contrast, the catalytically inactive mutant of GODZ (DHHS) (Hayashi et al., 2005) did not cause this increase (Fig. 4A). However, although mutation of NR2A Cys cluster I (3CS) decreased NR2A palmitoylation, it did not eliminate palmitoylation of NR2A in the presence of GODZ. These results indicate that GODZ palmitoylates NR2A at additional sites besides the Cys cluster I (Fig. 4A). Mutation of cluster II (NR2A 4CS) decreased palmitoylation, indicating that GODZ palmitoylates Cys cluster II (Fig. 4B). A low level of palmitoylation of NR2A was still seen in the NR2A 7CS mutant indicating that GODZ also palmitoylates sites outside of Cys cluster I and II (Fig. 4C). Similar results were obtained with NR2B 3CS, a penta-CS mutant of Cys cluster II (5CS) and octa-CS mutant on Cys cluster I and II (8CS) (Fig. 4D). These data indicate that overexpression of GODZ catalyzes palmitoylation of cluster II in both the NR2A and NR2B subunits. GODZ had no effects on GluR6 (Supplemental Fig. S2A). Endogenous GODZ expression in both 11 DIV and 22 DIV cultured cortical neurons was confirmed by western blotting with anti-GODZ antibodies (Supplemental Fig. S2B).
Figure 4. Palmitoylation of NR2A and NR2B by the palmitoyl acyl transferase GODZ.
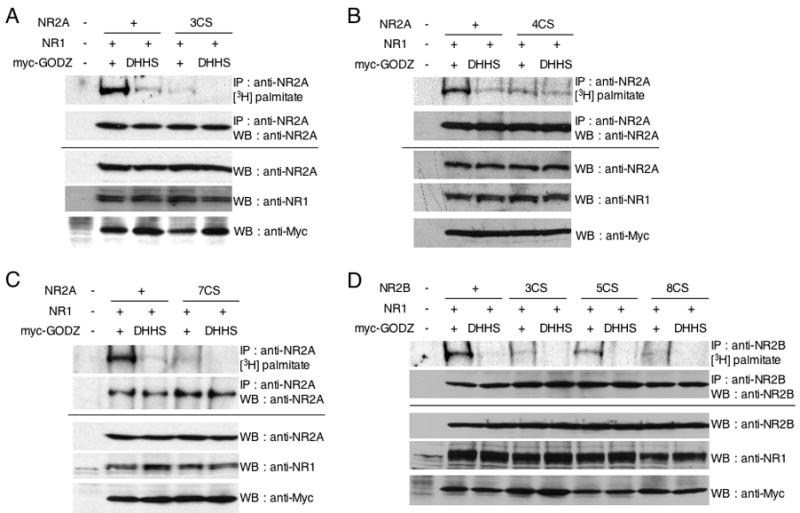
(A) Palmitoylation of NR2A C-terminus in NR1/NR2A and NR1/NR2A 3CS by cotransfected palmitoyl acyl transferase GODZ or its inactive mutant GODZ(DHHS). (B) Palmitoylation of NR2A in NR1/NR2A and NR1/NR2A 4CS by cotransfected GODZ or GODZ(DHHS). (C) Palmitoylation of NR2A in NR1/NR2A and NR1/NR2A 7CS by cotransfected GODZ or GODZ(DHHS). (D) Palmitoylation of NR2B in NR1/NR2B, NR1/NR2B 3CS, NR1/NR2B 5CS and NR1/NR2B 8CS by cotransfected GODZ or GODZ(DHHS). Transfected HEK 293T cells were labeled with [3H]-palmitate and were then immunoprecipitated with anti-NR2A or anti-NR2B antibodies. The incorporated [3H]-palmitate signals were shown (upper top). Immunoprecipitation (IP, upper bottom) and total protein expression (cell lysate) of each protein (lower panels) were confirmed by western blotting (WB).
Palmitoylation-dependent tyrosine phosphorylation of NR2
The functional effect of palmitoylation of NR2A and NR2B was initially examined in heterologous cells. Conserved membrane-proximal motifs in the C-terminus of NR2A (Y842WKL) and NR2B (Y843WQF), directly adjacent to Cys cluster I are necessary and sufficient to drive the internalization of NMDA receptors (Fig. 3A) (Lau and Zukin, 2007; Scott et al., 2004). These regions contain a consensus binding motif (YXXΦ, Φ represents a large hydrophobic residue) for the μ2 subunit of the clathrin adaptor protein AP-2. Previous studies have shown NR2A surface expression is regulated by Src protein tyrosine kinase (PTK) mediated phosphorylation at NR2A Tyr842 (Vissel et al., 2001). Tyrosine dephosphorylation triggers stimulation dependent NMDA receptor internalization. We therefore investigated whether palmitoylation of Cys cluster I might affect tyrosine phosphorylation of NR2A and thus regulate receptor surface expression. NR1/NR2A or NR1/NR2A 3CS were cotransfected into HEK 293T cells with or without the Src family PTK Fyn and tyrosine phosphorylation of NR2A subunit was examined by immunoblotting with anti-phosphotyrosine antibody (PY20). The result showed that Fyn-dependent tyrosine phosphorylation of wild type NR2A was markedly higher than that of the NR2A Cys cluster I (3CS) mutant (Fig. 5A). There was no significant change of tyrosine phosphorylation between NR2A wild type and NR2A 4CS mutant, indicating that Fyn-mediated tyrosine phosphorylation of NR2A was not affected by palmitoylation in Cys cluster II (Supplemental Fig. S1C).
Figure 5. Palmitoylation regulates tyrosine phosphorylation of NR2A and NR2B by the Src family tyrosine kinase Fyn.
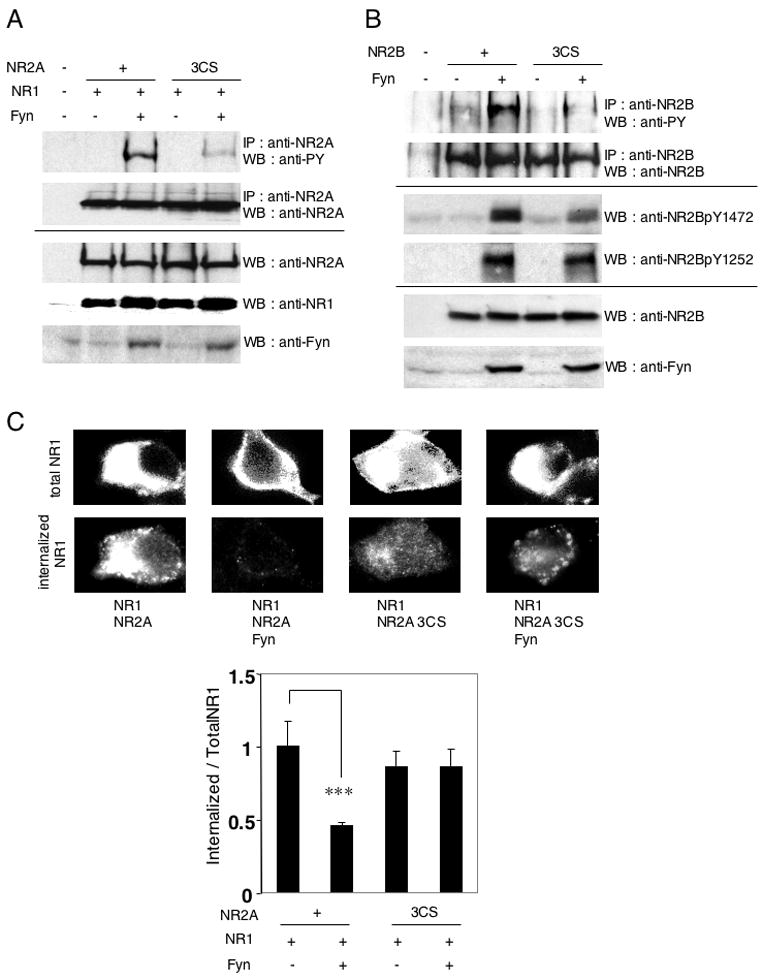
(A) Fyn-mediated tyrosine phosphorylation of NR2A in NR1/NR2A and NR1/NR2A 3CS receptors. HEK 293T cells were cotransfected with NR2A or NR2A 3CS and NR1 in the presence or absence of Fyn. Transfected cells were solubilized and then immunoprecipitated with anti-NR2A antibodies, followed by probing with phosphotyrosine antibody (PY20) (upper top panel). Immunoprecipitation (IP, upper bottom) and total protein expression (cell lysate) of each protein (lower panels) were confirmed by western blotting (WB). (B) Fyn-mediated tyrosine phosphorylation of NR2B and NR2B 3CS. The phosphotyrosine signals (PY20) were shown (upper top). IP of NR2B (top), site-specific tyrosine phosphorylation (NR2BpY1472 and NR2BpY1252, middle) and total protein expression (cell lysate) of each protein (bottom) were confirmed by WB. (C) Effect of Fyn on NMDA receptor endocytosis in transfected HEK 293T cells. Representative patterns of total and constitutive internalized NR1 were shown (top). The ratio of fluorescence intensities of internalized anti-NR1 antibodies to total NR1 expression in transfected HEK 293T cells (bottom, four bars (NR1/NR2A, NR1/NR2A/Fyn, NR1/NR2A 3CS, and NR1/NR2A 3CS/Fyn), n = 22, n = 28, n = 29, n = 34 cells, respectively, F = 5.276, p < 0.002, ANOVA). ***p < 0.001 compared with combination without Fyn. Error bars indicate sem.
We also examined tyrosine phosphorylation of NR2B and NR2B 3CS mutant by Fyn in transfected HEK 293T cells (Fig. 5B). The Fyn-dependent tyrosine phosphorylation of the wild type NR2B subunit, similar to NR2A, was markedly higher than that of the NR2B 3CS mutant. NR2B contains an additional YXX motif in its C-terminus (Y1472EKL) and this Tyr1472 is the major tyrosine phosphorylation site in NR2B for Src family PTKs. Activity-dependent phosphorylation of NR2B at Tyr1472 by Fyn PTK suppresses clathrin-mediated endocytosis of NMDA receptors (Nakazawa et al., 2001; Prybylowski et al., 2005; Yaka et al., 2002). Fyn-dependent phosphorylation of Tyr1472 is also required for proper localization of NR2B-containing receptors at synapses in the hippocampus (Prybylowski et al., 2005) and amygdala (Nakazawa et al., 2006). We therefore also examined tyrosine phosphorylation of Tyr1472 of the wild type and NR2B 3CS mutant. Immunoblotting with site-specific phosphoantibodies revealed that Fyn-dependent phosphorylation of NR2B Tyr1472 was decreased in the NR2B 3CS mutant. Interestingly, phosphorylation of NR2B on Tyr1252 was not altered in the NR2B 3CS mutant. These results suggest that palmitoylation of Cys cluster I may influence the NR2B C-terminal structure and the accessibility of Src family PTKs to their substrates, or may regulate Src family PTKs association with the C-terminal region. There was no significant change of tyrosine phosphorylation between NR2B wild type and NR2B 5CS mutant, indicating that Fyn-mediated tyrosine phosphorylation of NR2B was not affected by palmitoylation in Cys cluster II (Supplemental Fig. S1D).
To investigate the influence of NR2 palmitoylation on tyrosine phosphorylation dependent internalization of the NMDA receptor, NR1/NR2A and NR1/NR2A 3CS were cotransfected into HEK 293T cells with or without Fyn (Fig. 5C). Surface expressing NR1 was immunostained with polyclonal anti-NR1 N-terminus antibodies at 4°C for one hour and returned to 37°C for 15 min. Cells were then fixed and permeabilized, and total NR1 was stained with monoclonal anti-NR1 C-terminal antibody. Total NR1 expression and the internalized anti-NR1 N-terminus antibodies were visualized by staining with fluorescent-conjugated secondary antibodies. The ratio of the average intensity of internalized anti-NR1 antibodies (Alexa594-red intensity) to that of total NR1 level (Alexa488-green intensity) was calculated. The results showed that Fyn inhibited NR1/NR2A endocytosis, but Fyn had less effect on NR1/NR2A 3CS (Fig. 5C). These data indicated that NR2A palmitoylation upregulates Src family PTKs-dependent tyrosine phosphorylation of NR2A and inhibits consequent AP-2 mediated internalization of NMDA receptors.
Regulation of NMDA receptor surface expression by C-terminal palmitoylation in neurons
We next investigated whether palmitoylation affected the steady state membrane trafficking of the NMDA receptor in neurons. We initially examined the steady state surface expression of N-terminally GFP-tagged NR2B (GFP-NR2B WT) and its palmitoylation-deficient mutant on Cys cluster I (GFP-NR2B 3CS) in neurons. These constructs were transfected into cultured cortical neurons at 9 days in vitro (DIV) and examined 2 days after transfection at 11 DIV. Phosphorylation at Tyr1472 of GFP-NR2B WT and 3CS mutant were examined in transfected neurons (Fig. 6A). Tyrosine phosphorylation of GFP-NR2B WT was higher than that of GFP-NR2B 3CS, similar to the result of Fyn-dependent NR2B phosphorylation in transfected HEK 293T cells (Fig. 5B). Surface expression of GFP-NR2B WT and GFP-NR2B 3CS mutant was visualized by live staining with polyclonal anti-GFP antibodies, followed by addition of Alexa594-conjugated anti-rabbit IgG secondary antibodies. Representative patterns of GFP-NR2B WT and its GFP-NR2B 3CS mutant are shown (Fig. 6B). The ratio of Alexa594 to GFP fluorescence intensities indicated the proportion of surface to total GFP-NR2B. Compared with GFP-NR2B WT, the GFP-NR2B 3CS mutant showed decreased levels of surface expression (Fig. 6C). Similar results were observed in older cultured cortical neurons (22 DIV), which were transfected at 16 DIV and examined for surface expression 6 days after transfection (Fig. 6D). Moreover, N-terminally GFP-tagged NR2A (GFP-NR2A WT), had a higher level of surface expression than its Cys cluster I mutant (GFP-NR2A 3CS) in cultured cortical neurons at 22 DIV (Supplemental Fig. S3A). These results suggest that steady state surface expression of NMDA receptors is influenced by palmitoylation on Cys cluster I of NR2A and NR2B in neurons.
Figure 6. Effect of Cys cluster I palmitoylation of NR2B on the steady state surface expression and constitutive internalization of NMDA receptors.
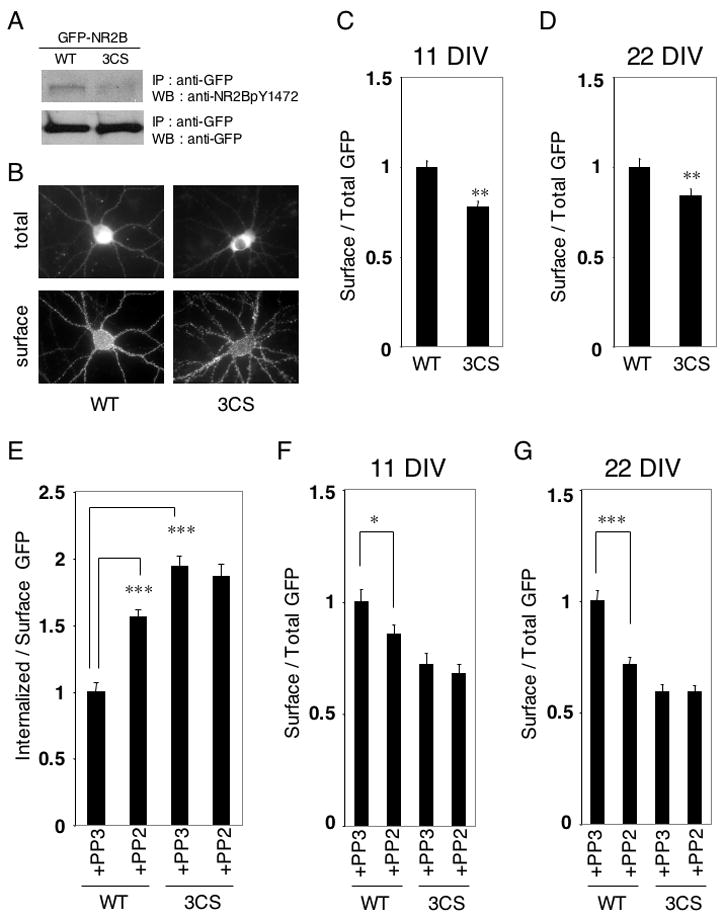
(A) Phosphorylation at Tyr1472 of GFP-NR2B WT and GFP-NR2B 3CS transfected cultured cortical neurons. (B) Representative patterns of surface expression of GFP-NR2B WT and GFP-NR2B 3CS mutant in transfected cultured cortical neurons. Total GFP-NR2B distribution (top) and surface expression of GFP-NR2B (bottom) were shown. (C) Ratio of surface to total expression of GFP-NR2B at 11 DIV (WT and 3CS, n = 16 neurons respectively). Student t-test. **p < 0.01 compared with WT. (D) Ratio of surface to total expression of GFP-NR2B at 22 DIV (WT and 3CS, n = 16 neurons respectively). Student t-test. **p < 0.01 compared with WT. (E) Regulation of NR2B constitutive internalization by its palmitoylation on Cys cluster I. The effect of Src family PTK-specific inhibitor PP2 (20 μM) or the inactive structural analog PP3 (20 μM) on internalization of GFP-NR2B WT (WT) and GFP-NR2B 3CS (3CS) in transfected cultured cortical neurons at 11 DIV (n = 12 neurons respectively). Cultured cortical neurons were treated with PP2 or PP3 for 30 min and the ratio of fluorescence intensities of internalized anti-GFP antibodies to surface GFP expression of GFP-NR2B were examined. ***p < 0.001. (F, G) Effects of treatment with Src family PTK specific inhibitor PP2 (20 μM) and its inactive analog PP3 (20 μM) for 30 min on steady state surface expression of GFP-NR2B WT and GFP-NR2B 3CS in transfected cultured cortical neurons were examined. (F) Ratio of surface to total GFP expression of GFP-NR2B at 11 DIV (four bars (WT+PP3, WT+PP2, 3CS+PP3, 3CS+PP2), n = 12 neurons respectively, F = 9.837, p < 0.0001, ANOVA). *p < 0.05 compared with PP3 treatment. (G) Ratio of surface to total GFP expression of GFP-NR2B at 22 DIV (four bars (WT+PP3, WT+PP2, 3CS+PP3, 3CS+PP2), n = 12 neurons respectively, F = 27.90, p < 0.0001, ANOVA). ***p < 0.001 compared with PP3 treatment. Error bars indicate sem.
Palmitoylation-dependent NMDA receptor constitutive internalization in developing neurons
Unlike AMPA receptors, synaptic NMDA receptors are stable components of the postsynaptic density. However, surface-expressed NMDA receptors have been shown to internalize in primary cultured neurons, especially early in development (Lavezzari et al., 2004; Roth et al., 2002). At early stages of development, NMDA receptors undergo robust endocytosis, which progressively decreases in more mature cultures (Roche et al., 2001). We therefore examined the effects of the Cys cluster I palmitoylation on constitutive internalization of surface NR2B. Surface expression of the GFP-NR2B WT and the GFP-NR2B 3CS mutant in transfected cortical neurons were labeled by live-staining with anti-GFP antibodies and the neurons were then incubated in media for 15 min at 37°C. After incubation, the neurons were fixed, and the internalized anti-GFP antibodies were visualized by staining with Alexa594-conjugated secondary antibodies. The ratio of the average intensity of internalized anti-GFP antibodies to that of surface GFP-NR2B level was calculated. Constitutive internalization of GFP-NR2B WT was lower than that of the palmitoylation-deficient GFP-NR2B 3CS mutant in young neurons (11 DIV) (Fig. 6E). To examine whether tyrosine phosphorylation interacted with this palmitoylation-regulated internalization in neurons as predicted from our HEK 293T cells, we used the Src family PTK-specific inhibitor PP2. Constitutive internalization of GFP-NR2B WT at 11 DIV was enhanced by the Src family PTK-specific inhibitor PP2, compared with its inactive structural analog PP3. In contrast, PP2 had little effect on constitutive internalization of GFP-NR2B 3CS (Fig. 6E). These results show that the NR2 palmitoylation on its Cys cluster I regulates constitutive endocytosis of NMDA receptors during synaptic development, most likely by regulating tyrosine phosphorylation of tyrosine-based internalization motifs.
Palmitoylation-dependent regulation of Src family PTK-mediated NMDA receptor surface expression
Finally, we tested the influence of palmitoylation of Cys cluster I on Src family PTK-mediated NR2B surface expression. The GFP-NR2B WT and the GFP-NR2B CS mutant were transfected into cultured cortical neurons at 9 DIV. At DIV 11 the neurons were treated with Src family PTK-specific inhibitor PP2 (20 μM) or its inactive structural analog PP3 (20 μM) and receptor surface expression was examined. Surface expression of GFP-NR2B WT was decreased by PP2 treatment (Fig. 6F). In contrast, surface expression of GFP-NR2B 3CS was not affected by PP2. Similar results were observed in older cultured cortical neurons at 22 DIV (Fig. 6G), which were transfected at 16 DIV and examined for surface expression 6 days after transfection. Non-palmitoylated NR2B was less tyrosine phosphorylated (Fig. 5B) and Src family PTK had little effect on surface expression of NR2B 3CS mutant. In addition, PP2 treatment showed similar effects on GFP-NR2A WT, but not on GFP-NR2A 3CS, in cultured cortical neurons at 22 DIV (Supplemental Fig. S3B). This result suggests that NR2 internalization through tyrosine-based internalization motif in the membrane proximal region (Vissel et al., 2001) is also regulated by palmitoylation on Cys cluster I. The Src family PTK-specific inhibitors PP2 (Supplemental Fig. S3D) and SU6656 (Supplemental Fig. S3E) also suppressed the surface expression of endogenous NR2A. These data indicated that palmitoylation on Cys cluster I regulated Src family PTK-mediated tyrosine phosphorylation of NR2A and NR2B and surface expression of NMDA receptors.
Accumulation of Cys cluster II-palmitoylated NR2 in the Golgi apparatus
We next examined the effect of GODZ on NR2 subcellular localization using immunocytochemical techniques. Our previous data showed that both GODZ and its catalytically inactive mutant, GODZ (DHHS), colocalized with the Golgi-apparatus marker GM-130 and were concentrated in the Golgi apparatus in transfected HEK 293T cells (Hayashi et al., 2005) (See Fig. 7). Cotransfection of NR1 and NR2A in HEK cells results in a diffuse localization of NR2A. However, cotransfection of NR1/NR2A with GODZ resulted in the NR2A subunit being localized with GODZ in the Golgi apparatus. In contrast, cotransfection of NR1/NR2A with the catalytically inactive GODZ (DHHS) mutant, or cotranfection of NR1 and the Cys cluster II mutant, NR2A 4CS, with GODZ, resulted in a diffuse distribution of NR2A in cells (Fig. 7A). The majority of NR2A was colocalized with GODZ when these proteins were coexpressed (Fig. 7B, upper panels). Moreover, NR2A accumulated with NR1 in the Golgi apparatus (Supplemental Figs. S4A and S4B), while in contrast, the Cys cluster II mutant, NR2A 4CS, did not colocalize with GODZ (Fig. 7B, lower panels). Similar results were obtained for the NR2B and NR2B 5CS mutant (Figs. 7C and 7D). In addition, the Cys cluster I mutants, NR2A 3CS (Supplemental Figs. S4C and S4D) and NR2B 3CS (Supplemental Figs. S4E and S4F), behaved similarly to wildtype NR2 subunits and colocalized with GODZ in the Golgi apparatus. These data demonstrate that palmitoylation of the NR2A and NR2B subunits on Cys cluster II, but not Cys cluster I, leads to accumulation of the receptor in the Golgi apparatus and suggests that Cys cluster II depalmitoylation may trigger receptor trafficking to the cell surface.
Figure 7. GODZ-mediated accumulation of NR2 in the Golgi apparatus.
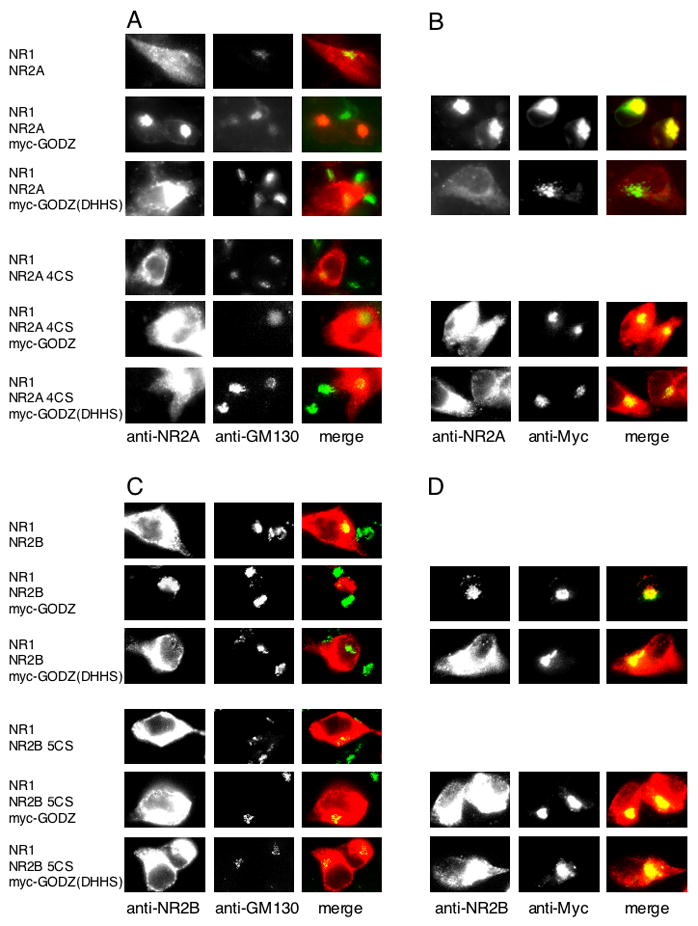
(A) Colocalization of NR2A with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2A with or without myc-GODZ or myc-GODZ(DHHS) (upper panels) and with NR1 and NR2A 4CS with or without myc-GODZ or myc-GODZ(DHHS) (lower panels). (B) Colocalization of NR2A with GODZ in HEK 293T cells cotransfected with NR1 and NR2A with myc-GODZ or myc-GODZ(DHHS) (upper panels) and with NR1 and NR2A 4CS with myc-GODZ or myc-GODZ(DHHS) (lower panels). (C) Colocalization of NR2B with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2B with or without myc-GODZ or myc-GODZ(DHHS) (upper panels) and with NR1 and NR2B 5CS with or without myc-GODZ or myc-GODZ(DHHS) (lower panels). (D) Colocalization of NR2B with GODZ in HEK 293T cells cotransfected with NR1 and NR2B with myc-GODZ or myc-GODZ(DHHS) (upper panels) and with NR1 and NR2B 5CS with myc-GODZ or myc-GODZ(DHHS) (lower panels).
Suppression of NMDA receptor surface expression by GODZ-regulated palmitoylation
As shown above, GODZ-mediated palmitoylation of Cys cluster II causes NR2 to localize to the Golgi apparatus in transfected heterologous cells. To examine whether this regulation also occurred in neurons, we analyzed whether GODZ suppressed the surface expression of endogenous NMDA receptors in cultured cortical neurons (Fig. 8A). Cultured cortical neurons were transfected with GODZ-GFP or GODZ(DHHS)-GFP and surface expression of endogenous NR2A was measured by live staining with polyclonal anti-NR2A N-terminal antibodies, followed by labeling with Alexa594-conjugated anti-rabbit IgG antibodies. While GODZ (DHHS) mutant had no effect on NR2A surface expression, wild type GODZ dramatically reduced NR2A surface expression (Fig. 8A). These data suggest that GODZ-induced palmitoylation on Cys cluster II region of NR2 subunits inhibits NMDA receptor surface expression in neurons.
Figure 8. GODZ-mediated suppression of NR2 surface expression through its palmitoylation on Cys cluster II.
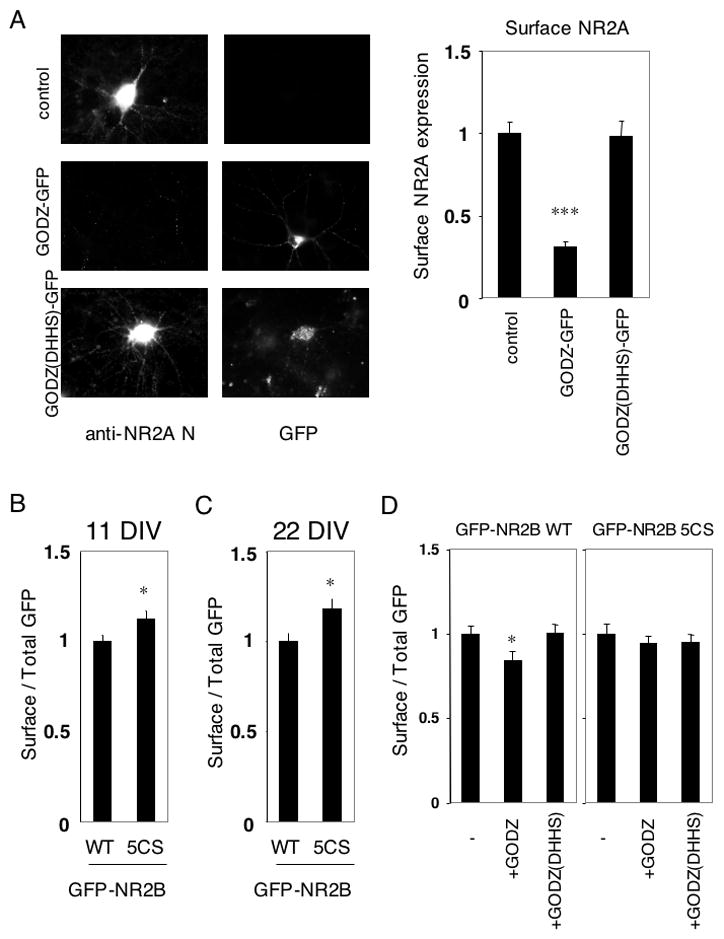
(A) Surface expression of endogenous NR2A in GODZ-GFP transfected cultured cortical neurons. Typical expression patterns of surface NR2A and GODZ-GFP or GODZ(DHHS)-GFP were shown (left). Quantified data of surface expression of endogenous NR2A in transfected cultured cortical neurons at 21 DIV (right, n = 10 neurons respectively, Student t-test. ***p < 0.001 compared with control. (B) Ratio of surface to total GFP expression of GFP-NR2B at 11 DIV (WT and 5CS, n = 16 neurons respectively). (C) Ratio of surface to total GFP expression of GFP-NR2B at 22 DIV (WT and 5CS, n = 16 neurons respectively). Student t-test. *p < 0.05 compared with WT. (D) Effect of myc-GODZ or myc-GODZ(DHHS) inactive mutant on surface expression of GFP-NR2B in transfected cultured cortical neurons at 11 DIV. Ratio of surface to total GFP expression of GFP-NR2B at 11 DIV (WT and 5CS, n = 16 neurons respectively). Student t-test. *p < 0.05 compared with WT. Error bars indicate the sem.
Finally, we examined the steady state surface expression of GFP-NR2B WT and its palmitoylation-deficient Cys cluster II mutant, GFP-NR2B 5CS, in neurons. Compared with GFP-NR2B WT, the GFP-NR2B 5CS mutant showed increased levels of surface expression both at 11 DIV (Fig. 8B) and at 22 DIV (Fig. 8C). Moreover, the GFP-NR2A 4CS mutant also showed increased surface expression in cultured cortical neurons compared to the GFP-NR2A WT (Supplemental Fig. S3C). The increased surface expression of the non-palmitoylated GFP-NR2A 4CS and GFP-NR2B 5CS supports the hypothesis that Cys cluster II depalmitoylation promotes forward trafficking of NMDA receptors to the cell surface. The surface expression of GFP-NR2B WT was reduced by cotransfected GODZ, but not by inactive GODZ(DHHS) mutant, while that of GFP-NR2B 5CS was not influenced by GODZ or GODZ(DHHS) (Fig. 8D). Similar results were obtained with GFP-NR2A WT and GFP-NR2A 4CS (Supplemental Fig. S3F).
Discussion
Here, we report that the NMDA receptor subunits NR2A and NR2B are palmitoylated in vitro and in vivo. The NR2A and NR2B subunits, which are predominantly expressed in forebrain with characteristic but overlapping distribution profiles, have two distinct consensus cysteine clusters in their C-terminus (Cys clusters I and II). Surface expression of the NMDA receptor was regulated by dual palmitoylation of these sites (Fig. 9). In particular, palmitoylation of Cys cluster I controls NMDA receptor expression and stabilization on the cell surface by modulating tyrosine phosphorylation of NR2A and NR2B. Under basal conditions, internalization of NMDA receptors occurs through clathrin-mediated endocytosis, primarily by interactions between AP-2 and tyrosine based internalization motifs (YXXΦ) (Carroll and Zukin, 2002; Lau and Zukin, 2007; Prybylowski et al., 2005). Tyrosine phosphorylation within these motifs by Src family PTK eliminates AP-2 binding. We found that Fyn-mediated tyrosine phosphorylation of NR2A and NR2B was reduced in the NR2A 3CS and NR2B 3CS non-palmitoylation mutants. Our data indicate that Fyn-mediated phosphorylation of tyrosine based internalization motifs in membrane proximal regions is increased by palmitoylation of the adjacent Cys cluster I. In addition, tyrosine phosphorylation at NR2B Tyr1472, a major site for Fyn phosphorylation, is also decreased by mutation of Cys cluster I. Palmitoylation-dependent phosphorylation at NR2B Tyr1472 presumably induces AP-2 dissociation from tyrosine based internalization motif. Moreover, Cys cluster I palmitoylation- and tyrosine phosphorylation-dependent effects on constitutive rapid internalization were observed in neurons. In contrast, palmitoylation of Cys cluster II by GODZ regulates the accumulation of NMDA receptors in the Golgi apparatus and depalmitoylation of these cysteines may be critical for release of the receptor from the Golgi for surface delivery. The mechanism of this Golgi retention is not clear but may require a palmitoylation specific interaction of the NR2 subunits with Golgi specific proteins.
Figure 9. Model of the role of NMDA receptor palmitoylation .
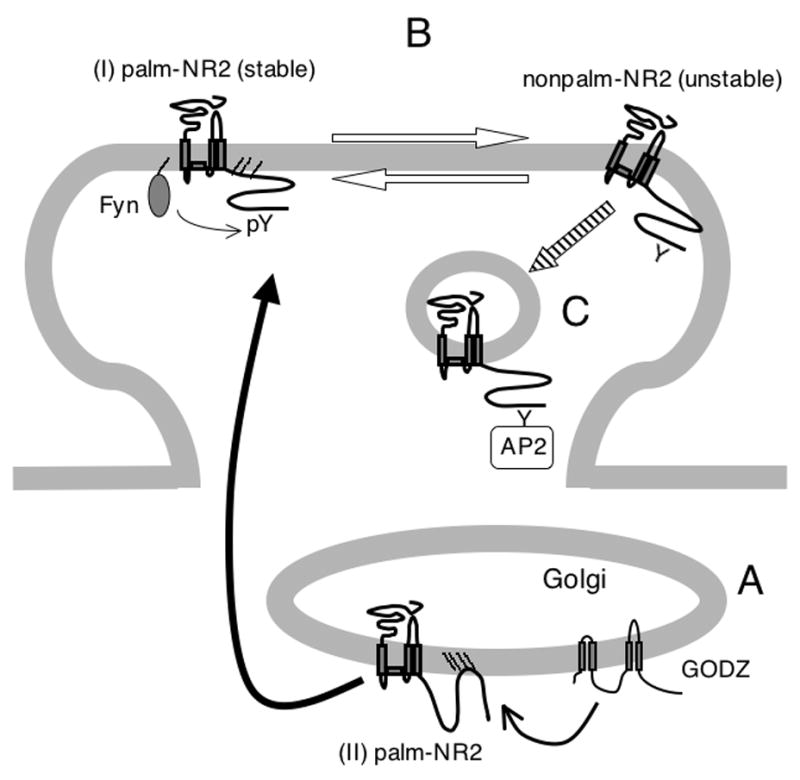
(A) GODZ increases palmitoylation of NMDA receptors on the NR2A/2B subunit Cys cluster II region ((II) palm-NR2) in Golgi apparatus. Depalmitoylation of the NR2A/2B subunit Cys cluster II may regulate release of NMDA receptors from the Golgi for surface delivery as shown by black arrow. (B) Palmitoylation of the NMDA receptor on the NR2A/2B subunit Cys cluster I ((I) palm-NR2) ensures proper surface delivery and increases the stability of NMDA receptors on the cell surface via tyrosine phosphorylation of tyrosine based internalization motifs (pY). In contrast, depalmitoylated forms of surface NMDA receptors are less stably associated with the plasma membrane. Palmitoylation is reversible between these two forms as shown by white arrows. (C) Palmitoylation on Cys cluster I ((I) palm-NR2) allows rapid constitutive internalization from surface in developing neurons as shown by slashed arrow.
Other NR2 subunits, NR2C and NR2D carry shorter C-terminal domains. NR2C, containing 16 intracellular cysteines, is the predominant NR2 subunit in cerebellum and has only one cysteine (Cys866) that shares homology to any of the palmitoylated cysteines in NR2A and NR2B (Supplemental Fig. S5). The expression of NR2D occurs mainly in the midbrain and spinal cord and peaks around postnatal day 7 and decreases thereafter (Monyer et al., 1994). The NR2D subunit has 12 intracellular cysteine residues and has two cysteines (Cys873 and Cys893) that are conserved at the corresponding sites with NR2A Cys848/Cys870 and NR2B Cys849/Cys871 in Cys cluster I. The amino acid sequence around NR2D Cys1168 and Cys1171 has low homology with first two cysteines in NR2A/2B Cys cluster II. It is possible that localization and trafficking of NR2C- or NR2D-containing NMDA receptors may also be regulated by palmitoylation of these partially conserved cysteines.
We previously reported that all AMPA receptor subunits (GluR1-4) are palmitoylated (Hayashi et al., 2005). The AMPA receptor subunits GluR1-4 also have two distinct palmitoylation sites, one in their TMD 2 region and one in their C-terminal domain. Similar to GODZ palmitoylation of cluster II in the NR2 subunits, the palmitoylation of TMD 2 by GODZ retains AMPA receptors in the Golgi apparatus. Depalmitoylation of the TMD 2 site is presumably a trigger for the receptor surface delivery. In contrast, C-terminal depalmitoylation of the AMPA receptor GluR1 subunit stabilizes the receptor surface expression through interacting molecules such as protein 4.1N. Amino acid sequences around these palmitoylation sites are not conserved between GluR1-4 and NR2A/2B. Interestingly, the trafficking of AMPA receptors and NMDA receptors both occur in a palmitoylation-dependent two-step manner (Supplemental Fig. S6). Namely, the Golgi localized PAT GODZ regulates GluR accumulation in Golgi apparatus through a palmitoylation site (TMD 2 in GluR1-4 or Cys cluster II in NR2A/2B), while palmitoylation of the C-terminal domain in AMPA receptors or Cys cluster I in NMDA receptors regulates the stability of the receptors on the surface plasma membrane. However, although palmitoylation of AMPA and NMDA receptors both have effects on receptor trafficking, the modulation of these two receptors would have distinct impacts on neuronal function. Modulation of AMPA receptor trafficking would directly affect the strength of synaptic transmission while modulation of NMDA receptor function would affect NMDA receptor dependent plasticity and activity dependent development.
Localization and trafficking of GluRs, as well as their ion channel properties, are regulated by post-translational protein modifications. The most well established post-translational protein modification of GluRs is phosphorylation. Previous studies have shown that channel properties and trafficking of NMDA receptors were regulated by phosphorylation of both NR1 and NR2 subunits (Collingridge et al., 2004). In this study, we revealed a novel regulatory mechanism of NMDA receptor trafficking by dual palmitoylation of NR2 subunits. Interestingly, NR2 palmitoylation is dynamically regulated in activity-dependent manner and palmitoylation of the NR2 subunits regulates NMDA receptor membrane trafficking. Palmitoylation of Cys cluster I regulates NMDA receptor internalization while palmitoylation of Cys cluster II retains the receptor in the Golgi apparatus. The dynamic regulation of NR2 palmitoylation by neuronal activity may profoundly affect the plasma membrane and synaptic localization of NMDA receptors critical for the regulation of NMDA receptor dependent plasticity and development.
Experimental procedures
Primary neuron culture, HEK 293T cells and transfection
High-density cultured cortical neurons were prepared as previously described (Hayashi and Huganir, 2004). Cultured cortical neurons (106 cells per lane) were used between three and four weeks after plating for biochemical experiments. At indicated days in vitro (DIV), cultured cortical neurons on cover slips were used for GFP-NR2A/2B wild type or CS mutants transfection using Lipofectamine 2000. HEK 293T cells (107 cells per lane) were transfected with wild type and CS mutants of pcDNA1-NR2A, pRK5-NR2B and pRK5-NR1-1a or pRK5-GluR6 with or without pcDNA3-myc-GODZ and pcDNA3-myc-GODZ(DHHS) or pME18S-Fyn (CMV promoter) using a calcium phosphate method.
Analysis of palmitoylation
HEK 293T cells were transiently transfected with NR1/NR2 and Myc-tagged GODZ and metabolically labeled with [3H]palmitate (NEN, 1 mCi/ml) for 4–5 hrs, 2 days after transfection. Cells were lysed in RIPA buffer (containing (in mM) 10 Tris-HCl (pH 7.3), 150 NaCl, 1 EDTA, 1% Triton X-100, 0.1% SDS and 0.1% sodium deoxycholate, 10μg/ml aprotinin and 10 μg/ml leupeptin with 1 mM Na3VO4 and 1 mM NaF for tyrosine phosphorylation experiments) and immunoprecipitated with anti-NR2A (JH5524 and JH5525) and anti-NR2B (JH5522 and JH5523) antibodies. Immunoprecipitated samples and total cell lysates were separated by 7.5% SDS-PAGE followed by western blotting or X-ray film exposure, respectively. For fluorography, gels were fixed with 50% methanol, 10% acetic acid at room temperature for 30 min, treated with Amplify solution (Amersham) for 30 min, dried under vacuum and exposed to Hyperfilm-MP (Amersham) at −80°C. Protein expression was confirmed by western blotting with each antibody (anti-NR1 (JH4762), anti-Myc (9E10) antibodies). Anti-phosphotyrosine (PY20) antibody was purchased from Santa Cruz. Anti-NR2B pY1472 and anti-NR2B pY1252 antibodies were purchased from PhosphoSolutions. Anti-GODZ antibodies were purchased from Chemicon.
Cultured cortical neurons were metabolically labeled with [3H]palmitate (1 mCi/ml) for 24hrs. Neurons were lysed in RIPA buffer and immunoprecipitated with anti-GluR2/3 (JH4854), anti-NR2A or anti-NR2B antibodies. In the hydroxylamine treatment experiment, duplicate gels of labeled NR2A and NR2B, purified from cultured cortical neurons were treated with 1 M hydroxylamine (NH2OH, pH 7.0) or control 1 M Tris-HCl (pH 7.0) for 18 hrs at room temperature, followed by exposure to film as above. 10% of total immunoprecipitated NR2 was used for western blotting with anti-NR2 antibodies to confirm the immunoprecipitated NR2 and 90% was used for [3H]palmitate-labeled signal exposure. Data were representative of more than three experiments. NMDA receptor palmitoylation was also assessed using acyl-biotinyl exchange (Drisdel et al., 2006; Kang et al., 2008). The method of Wan et al. was followed, except that cultured cortical neurons were directly denatured in Lysis buffer (50 mM HEPES pH 7.0, 2% SDS, 1 mM EDTA plus protease inhibitor cocktail (PIC, Roche) containing 20 mM Methyl methanethiosulfonate to block free thiols) and acetone precipitation was used to move between steps. Following lysis, excess MMTS was removed by three sequential acetone precipitations and pellets were resuspended in buffer containing 4% (w/v) SDS (‘4SB’; Wan et al., 2007). Samples were diluted and incubated for 1h in either 0.7 M hydroxylamine pH 7.4 (to cleave thioester bonds) or 50 mM Tris pH 7.4. Three acetone precipitations were performed to remove hydroxylamine or Tris. Pellets were resuspended in 4SB, diluted in 50mM Tris pH 7.4 containing sulfhydryl-reactive (HPDP-) biotin and incubated for 1 h at room temperature. Three acetone precipitations were performed to remove unreacted HPDP-biotin and pellets were resuspended in lysis buffer without MMTS. SDS was diluted to 0.1% (w/v) and biotinylated proteins in the samples were affinity-purified using neutravidin-conjugated beads. Beta-mercaptoethanol (1% (v/v)) was used to cleave HPDP-biotin and release purified proteins from the beads. The released proteins in the supernatant were denatured in SDS sample buffer and processed for Western blotting with NR2A and NR2B antibodies.
Immunocytochemical experiments
Anti-GFP antibodies (JH4030) were raised against a GFP-fusion protein. Surface expression of GFP-NR2A and GFP-NR2B in neurons was selectively visualized as follows (Fig. 6, Fig. 8B–D, Supplemental Fig. S3A–C, F). Surface expressing GFP-NR2A or GFP-NR2B was labeled by incubating live neurons in media for 20 min at 10°C with anti-GFP antibodies. Then, neurons were washed twice with ice-cold PBS containing 4% sucrose for washout of the excess antibodies and then fixed with 4% paraformaldehyde, 4% sucrose in PBS (room temperature, 20 min). Fixed neurons were permeabilized with PBS containing 0.2% Triton X-100 (4°C, 15 min). Coverslips were blocked in 10% normal donkey serum in PBS at 4°C overnight. Then, remaining surface anti-GFP antibodies were visualized by staining with Alexa594-conjugated donkey anti-rabbit IgG secondary antibodies (Invitrogen), diluted in 10% normal donkey serum in PBS. Fluorescence images were acquired using a Photometrics CCD camera and quantified with Metamorph software (Universal Imaging Corporation, West Chester, PA). The fluorescence intensity averages of Alexa594-red and GFP-green signals in five dendritic regions in indicated numbers of different neurons were measured. Red fluorescence intensity indicative of surface receptors was divided by green fluorescence intensity to control for GFP-NR2A and GFP-NR2B protein expression level. Units of surface were measured as ratio of red/green fluorescence normalized to wild type controls. For Src family PTK-specific inhibitor treatment experiments (Fig. 6E–G, Supplemental Fig. S3B, D, E), transfected cultured cortical neurons were treated with PP2 (20 μM, Calbiochem), PP3 (20 μM, Calbiochem) or SU6656 (1 μM, Calbiochem) for 30 min at 37°C before antibody incubation. To visualize surface expression of endogenous NR2A, anti-NR2A N-terminus antibodies (JH6097) were raised against the synthetic peptide HDVTERELRNLWGPEC corresponding to amino acid 44-58 of NR2A (Fig. 8A and Supplemental Fig. S3D, E).
Internalized GFP-NR2B in neurons was selectively visualized as follows (Fig. 6E). Surface GFP-NR2B was labeled by incubating live neurons in media for 15 min at 10°C with anti-GFP antibodies. Then, neurons were incubated in media for 15 min at 37ºC. Treatment with PP2 (20 μM) or PP3 (20 μM) was done for 30 min during antibody incubation and recovery. After the incubation, neurons were washed and then fixed as mentioned above. The remaining surface anti-GFP antibodies were blocked with goat anti-rabbit IgG F(ab′)2 fragments (Jackson Immunoresearch Lab.) at room temperature for 2.5 hrs. Fixed neurons were permeabilized and then the internalized anti-GFP antibodies were visualized by staining with Alexa594-conjugated donkey anti-rabbit IgG secondary antibodies, diluted in 10% normal donkey serum in PBS. Fluorescence images were acquired as described above and the fluorescence intensity were measured. Internalization signals were divided by surface expression signals to control for GFP-NR2B protein expression level. Units of internalization were normalized to wild type.
GODZ effects on NR1/NR2 localization in transfected HEK 293T cells were visualized as follows (Fig. 7 and Supplemental Fig. S4). The cells on coverslips were washed twice with PBS containing 4% sucrose at 24 hours after transfection and then fixed with 4% paraformaldehyde, 4% sucrose in PBS (room temperature, 20 min). Fixed cells were permeabilized with PBS containing 0.2% Triton X-100 (4°C, 15 min). Coverslips were blocked in 10% normal donkey or goat serum in PBS at 4°C overnight, and then visualized using polyclonal anti-NR2A (JH5525), polyclonal anti-NR2B (JH5523), polyclonal anti-NR1 (JH4762), monoclonal anti-Myc (9E10) and monoclonal anti-GM130 (Pharmingen) antibodies, followed by incubation with Alexa594-conjugated donkey anti-rabbit IgG and Alexa488-conjugated goat anti-mouse IgG (Invitrogen) to visualize protein localization. The images in Fig. 7 and Supplemental Fig. S4 are representative of more than 20 transfected HEK 293T cells. All observed transfected cells showed similar patterns.
The effect of Fyn on NMDA receptor endocytosis in transfected HEK 293T cells was visualized as follows (Fig. 5C). Surface expressing NR1 was labeled by incubating transfected HEK 293T in media for one hour at 4°C with rabbit polyclonal anti-NR1 N-terminus antibodies (JH2590). Then, cells were incubated in media for 15 min at 37ºC. After the incubation, cells were washed twice with PBS containing 4% sucrose for washout of the excess antibodies and then fixed as above. The remaining surface anti-NR1 antibodies were blocked with goat anti-rabbit IgG F(ab′)2 fragments (Jackson Immunoresearch Lab.) at room temperature for 2.5 hrs. Fixed neurons were permeabilized and blocked. Then, total expression of NR1 was labeled with mouse monoclonal anti-NR1 antibody (s3c11). Primary antibodies were visualized by staining with Alexa594-conjugated donkey anti-rabbit IgG and Alexa488-conjugated goat anti-mouse IgG diluted in 10% normal donkey serum in PBS as secondary antibodies. Fluorescence images were acquired as described above and the fluorescence intensity was measured.
Supplementary Material
Supplemental Figure S1
Association of NR1 and NR2 CS mutants and Fyn-mediated tyrosine phosphorylation of NR2A 4CS and NR2B 5CS
(A) Coimmunoprecipitation of NR2A wild type and NR2A CS mutants (3CS and 4CS) with NR1 in lysates from transfected HEK 293T cells. Immunoprecipitation (IP) with anti-NR1 antibodies and western blotting (WB) with anti-NR1 or anti-NR2A antibodies were shown (top). Expression of each protein was confirmed by western blotting (WB) (bottom). (B) Coimmunoprecipitation of NR2B wild type and NR2B CS mutants (3CS and 5CS) with NR1 in lysates from transfected HEK 293T cells. Immunoprecipitation (IP) with anti-NR1 antibodies and western blotting (WB) with anti-NR1 or anti-NR2B antibodies were shown (top). Expression of each protein was confirmed by western blotting (WB) (bottom). (C) Fyn-mediated tyrosine phosphorylation of NR2A in NR1/NR2A and NR1/NR2A 4CS receptors. HEK 293T cells were cotransfected with NR2A or NR2A 4CS and NR1 in the presence or absence of Fyn. Transfected cells were solubilized and then immunoprecipitated with anti-NR2A antibodies, followed by probing with phosphotyrosine antibody (PY20) (upper top panel). Immunoprecipitation (IP, upper bottom) and total protein expression (cell lysate) of each protein (lower panels) were confirmed by western blotting (WB). (D) Fyn-mediated tyrosine phosphorylation of NR2B in NR1/NR2B and NR1/NR2B 5CS receptors. HEK 293T cells were cotransfected with NR2B or NR2B 5CS and NR1 in the presence or absence of Fyn. Transfected cells were solubilized and then immunoprecipitated with anti-NR2B antibodies, followed by probing with phosphotyrosine antibody (PY20) (upper top panel). IP of NR2B (top), site-specific tyrosine phosphorylation (NR2BpY1472 and NR2BpY1252, middle) and total protein expression (cell lysate) of each protein (bottom) were confirmed by WB.
Supplemental Figure S2
Analysis of GODZ-mediated palmitoylation
(A) Effect of exogenous palmitoyl acyl transferase GODZ on GluR6 palmitoylation in transfected HEK 293T cells. GluR6 was transfected into HEK 293T cells with or without myc-GODZ. Immunoprecipitation (IP) and exposure of metabolically labeled [3H]palmitate signals (upper top) or IP and Western blotting (WB) with anti-GluR6 antibodies were shown (lower top). Expression of GluR6 and myc-tagged GODZ protein was confirmed by Western blotting (WB) (bottom). (B) GODZ expression in vivo. Expression of endogenous GODZ in neurons. Protein expression of GODZ in adult mouse whole brain lysate (positive control) and 11 DIV or 22 DIV cultured cortical neurons was confirmed by Western blotting (WB) with anti-GODZ antibodies. 50 g total proteins were loaded in each lane.
Supplemental Figure S3
Effects of the C-terminal palmitoylation and Src family PTK specific inhibitors on the steady state surface expression of NR2A
(A) Ratio of surface to total GFP expression of GFP-NR2A at 22 DIV (WT and 3CS, n = 13 neurons respectively) *p < 0.05 compared with WT. (B) Effects of treatment with Src family PTK specific inhibitor PP2 (20 μM) and its inactive analog PP3 (20 μM) for 30 min on steady state surface expression of GFP-NR2A WT and GFP-NR2A 3CS in transfected cultured cortical neurons were examined. Ratio of surface to total expression of GFP-NR2A at 22 DIV (four bars (WT+PP3, WT+PP2, 3CS+PP3, 3CS+PP2), n = 12 neurons respectively, F = 6.463, p < 0.001, ANOVA). ***p < 0.001 compared with PP3 treatment. (C) Ratio of surface to total expression of GFP-NR2A at 22 DIV (WT and 4CS, n = 13 neurons respectively) *p < 0.05 compared with WT. (D) The effect of PP2 (20 μM) or the inactive structural analog PP3 (20 μM) on surface expression of endogenous NR2A in cultured cortical neurons at 22 DIV (n = 12 neurons respectively). Cultured cortical neurons were treated with PP2 or PP3 for 30 min and surface expressions of endogenous NR2A were quantified. (E) The effect of SU6656 (1 μM) or DMSO vehicle control on surface expression of endogenous NR2A in cultured cortical neurons at 22 DIV (n = 12 neurons respectively). Cultured cortical neurons were treated with SU6656 or DMSO for 30 min and surface expressions of endogenous NR2A were quantified. Student t-test. ***p < 0.001 compared with control. (F) Effect of myc-GODZ or myc-GODZ(DHHS) inactive mutant on surface expression of GFP-NR2A in transfected cultured cortical neurons at 22 DIV. Ratio of surface to total expression of GFP-NR2A at 22 DIV (WT and 4CS, n = 16 neurons respectively). *p < 0.05 compared with WT. Student t-test. Error bars indicate the sem.
Supplemental Figure S4
GODZ-mediated accumulation of NR1, NR2A 3CS and NR2B 3CS in Golgi apparatus
(A) Colocalization of NR1 with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2A with myc-GODZ (upper panels) and with NR1 and NR2A with myc-GODZ(DHHS) (lower panels). (B) Colocalization of NR1 with GODZ in HEK 293T cells cotransfected with NR1 and NR2A with myc-GODZ (upper panels) and with NR1 and NR2A with myc-GODZ(DHHS) (lower panels). (C) Colocalization of NR2A 3CS with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2A 3CS with or without myc-GODZ or myc-GODZ(DHHS). (D) Colocalization of NR2A 3CS with GODZ in HEK 293T cells cotransfected with NR1 and NR2A 3CS with myc-GODZ or myc-GODZ(DHHS). (E) Colocalization of NR2B 3CS with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2B 3CS with or without myc-GODZ or myc-GODZ(DHHS). (F) Colocalization of NR2B 3CS with GODZ in HEK 293T cells cotransfected with NR1 and NR2B 3CS with myc-GODZ or myc-GODZ(DHHS).
Supplemental Figure S5
Sequence alignment of NR2 subunits NR2A-D in their Cys cluster I and II
Amino acids around NR2A, NR2B, NR2C and NR2D Cys cluster I (top) and Cys cluster II (bottom) are shown. Transmembrane domain (TMD) 4 is underlined. Dotted box indicates AP-2 binding motif (YXXΦ).
Supplemental Figure S6
Palmitoylation-dependent two-step trafficking mechanism of glutamate receptors
Accumulation of NMDA receptors (black) and AMPA receptors (blue) in Golgi apparatus is regulated by GODZ-mediated palmitoylation of the receptors, respectively. GODZ increases palmitoylation of NR2A/2B on the Cys cluster II and that of GluR1-4 on the TMD 2 site. Depalmitoylation of these sites may regulate release of receptors from the Golgi apparatus for surface delivery.
Surface expression of the AMPA receptor is not influenced by palmitoylation of the AMPA receptor on the C-terminal site. In contrast to palmitoylation of the AMPA receptor, palmitoylation of the NMDA receptor on the Cys cluster I controls the steady state surface expression of the NMDA receptor.
The depalmitoylated form of surface NMDA receptors is constitutively internalized. Similarly, the palmitoylated form of surface AMPA receptors is more susceptible to AMPA and NMDA induced internalization.
Acknowledgments
We are grateful to Dr. V. Anggono for valuable discussion, encouragement and critical reading of manuscript, Ms. M. Coulter for antibody preparation and Ms. K. Searcy-Goldberg and Lisa Hamm for excellent Administrative assistance. GODZ cDNAs were special gifts from Dr. M. Mishina. This work was supported by research grants from HHMI and NIMH.
Footnotes
Under a licensing agreement between Millipore, Inc. and The Johns Hopkins University, Dr. Huganir is entitled to a share of royalty received by the University on sales of products described in this article. Dr. Huganir is a paid consultant to Millipore, Inc. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, Fukaya M, Yagi T, Mishina M, Watanabe M, Sakimura K. NMDA receptor GluRepsilon/NR2 subunits are essential for postsynaptic localization and protein stability of GluRzeta1/NR1 subunit. J Neurosci. 2004;24:7292–7304. doi: 10.1523/JNEUROSCI.1261-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaluf S, Mulvihill ER, McIlhinney RA. The metabotropic glutamate receptor mGluR4, but not mGluR1 alpha, is palmitoylated when expressed in BHK cells. J Neurochem. 1995;64:1548–1555. doi: 10.1046/j.1471-4159.1995.64041548.x. [DOI] [PubMed] [Google Scholar]
- Bijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Bizzozero OA. Chemical analysis of acylation sites and species. Methods Enzymol. 1995;250:361–379. doi: 10.1016/0076-6879(95)50085-5. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Cavara NA, Hollmann M. Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol. 2008;38:16–26. doi: 10.1007/s12035-008-8029-9. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE 2004. 2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Dietrich LE, Gurezka R, Veit M, Ungermann C. The SNARE Ykt6 mediates protein palmitoylation during an early stage of homotypic vacuole fusion. Embo J. 2004;23:45–53. doi: 10.1038/sj.emboj.7600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LE, Ungermann C. On the mechanism of protein palmitoylation. EMBO Rep. 2004;5:1053–1057. doi: 10.1038/sj.embor.7400277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Drisdel RC, Alexander JK, Sayeed A, Green WN. Assays of protein palmitoylation. Methods. 2006;40:127–134. doi: 10.1016/j.ymeth.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Manzana E, Green WN. The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J Neurosci. 2004;24:10502–10510. doi: 10.1523/JNEUROSCI.3315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini Ael D, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 Palmitoylating Enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Huganir RL. Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J Neurosci. 2004;24:6152–6160. doi: 10.1523/JNEUROSCI.0799-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, Metzler M, Mullard A, Haigh B, Gauthier-Campbell C, Gutekunst CA, et al. Huntingtin-Interacting Protein HIP14 Is a Palmitoyl Transferase Involved in Palmitoylation and Trafficking of Multiple Neuronal Proteins. Neuron. 2004;44:977–986. doi: 10.1016/j.neuron.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J Biol Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, et al. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. Embo J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng GY, George SR, Zastawny RL, Caron M, Bouvier M, Dennis M, O’Dowd BF. Human serotonin1B receptor expression in Sf9 cells: phosphorylation, palmitoylation, and adenylyl cyclase inhibition. Biochemistry. 1993;32:11727–11733. doi: 10.1021/bi00094a032. [DOI] [PubMed] [Google Scholar]
- Ng GY, Mouillac B, George SR, Caron M, Dennis M, Bouvier M, O’Dowd BF. Desensitization, phosphorylation and palmitoylation of the human dopamine D1 receptor. Eur J Pharmacol. 1994a;267:7–19. doi: 10.1016/0922-4106(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, Caron M, Dennis M, Brann MR, George SR. Phosphorylation and palmitoylation of the human D2L dopamine receptor in Sf9 cells. J Neurochem. 1994b;63:1589–1595. doi: 10.1046/j.1471-4159.1994.63051589.x. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Kihara A, Sano T, Igarashi Y. Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta. 2006;1761:474–483. doi: 10.1016/j.bbalip.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Pickering DS, Taverna FA, Salter MW, Hampson DR. Palmitoylation of the GluR6 kainate receptor. Proc Natl Acad Sci U S A. 1995;92:12090–12094. doi: 10.1073/pnas.92.26.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponimaskin EG, Heine M, Joubert L, Sebben M, Bickmeyer U, Richter DW, Dumuis A. The 5-hydroxytryptamine(4a) receptor is palmitoylated at two different sites, and acylation is critically involved in regulation of receptor constitutive activity. J Biol Chem. 2002;277:2534–2546. doi: 10.1074/jbc.M106529200. [DOI] [PubMed] [Google Scholar]
- Ponimaskin EG, Schmidt MF, Heine M, Bickmeyer U, Richter DW. 5-Hydroxytryptamine 4(a) receptor expressed in Sf9 cells is palmitoylated in an agonist-dependent manner. Biochem J. 2001;353:627–634. doi: 10.1042/0264-6021:3530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Platano D, Olcese R, Costantin JL, Stefani E, Birnbaumer L. Unique regulatory properties of the type 2a Ca2+ channel beta subunit caused by palmitoylation. Proc Natl Acad Sci U S A. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JW, Catterall WA. Palmitylation, sulfation, and glycosylation of the alpha subunit of the sodium channel. Role of post-translational modifications in channel assembly. J Biol Chem. 1987;262:13713–13723. [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J Neurosci. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH. The TiPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol Sci. 1993;14:297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Stephenson FA. Subunit characterization of NMDA receptors. Curr Drug Targets. 2001;2:233–239. doi: 10.2174/1389450013348461. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymond LA, Huganir RL. Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res. 1999;33:49–78. doi: 10.1016/s1040-7952(99)80005-6. [DOI] [PubMed] [Google Scholar]
- Uemura T, Mori H, Mishina M. Isolation and characterization of Golgi apparatus-specific GODZ with the DHHC zinc finger domain. Biochem Biophys Res Commun. 2002;296:492–496. doi: 10.1016/s0006-291x(02)00900-2. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003a;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Sans N, Standley S, Prybylowski K, Petralia RS. Early events in the trafficking of N-methyl-D-aspartate (NMDA) receptors. Biochem Soc Trans. 2003b;31:885–888. doi: 10.1042/bst0310885. [DOI] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1
Association of NR1 and NR2 CS mutants and Fyn-mediated tyrosine phosphorylation of NR2A 4CS and NR2B 5CS
(A) Coimmunoprecipitation of NR2A wild type and NR2A CS mutants (3CS and 4CS) with NR1 in lysates from transfected HEK 293T cells. Immunoprecipitation (IP) with anti-NR1 antibodies and western blotting (WB) with anti-NR1 or anti-NR2A antibodies were shown (top). Expression of each protein was confirmed by western blotting (WB) (bottom). (B) Coimmunoprecipitation of NR2B wild type and NR2B CS mutants (3CS and 5CS) with NR1 in lysates from transfected HEK 293T cells. Immunoprecipitation (IP) with anti-NR1 antibodies and western blotting (WB) with anti-NR1 or anti-NR2B antibodies were shown (top). Expression of each protein was confirmed by western blotting (WB) (bottom). (C) Fyn-mediated tyrosine phosphorylation of NR2A in NR1/NR2A and NR1/NR2A 4CS receptors. HEK 293T cells were cotransfected with NR2A or NR2A 4CS and NR1 in the presence or absence of Fyn. Transfected cells were solubilized and then immunoprecipitated with anti-NR2A antibodies, followed by probing with phosphotyrosine antibody (PY20) (upper top panel). Immunoprecipitation (IP, upper bottom) and total protein expression (cell lysate) of each protein (lower panels) were confirmed by western blotting (WB). (D) Fyn-mediated tyrosine phosphorylation of NR2B in NR1/NR2B and NR1/NR2B 5CS receptors. HEK 293T cells were cotransfected with NR2B or NR2B 5CS and NR1 in the presence or absence of Fyn. Transfected cells were solubilized and then immunoprecipitated with anti-NR2B antibodies, followed by probing with phosphotyrosine antibody (PY20) (upper top panel). IP of NR2B (top), site-specific tyrosine phosphorylation (NR2BpY1472 and NR2BpY1252, middle) and total protein expression (cell lysate) of each protein (bottom) were confirmed by WB.
Supplemental Figure S2
Analysis of GODZ-mediated palmitoylation
(A) Effect of exogenous palmitoyl acyl transferase GODZ on GluR6 palmitoylation in transfected HEK 293T cells. GluR6 was transfected into HEK 293T cells with or without myc-GODZ. Immunoprecipitation (IP) and exposure of metabolically labeled [3H]palmitate signals (upper top) or IP and Western blotting (WB) with anti-GluR6 antibodies were shown (lower top). Expression of GluR6 and myc-tagged GODZ protein was confirmed by Western blotting (WB) (bottom). (B) GODZ expression in vivo. Expression of endogenous GODZ in neurons. Protein expression of GODZ in adult mouse whole brain lysate (positive control) and 11 DIV or 22 DIV cultured cortical neurons was confirmed by Western blotting (WB) with anti-GODZ antibodies. 50 g total proteins were loaded in each lane.
Supplemental Figure S3
Effects of the C-terminal palmitoylation and Src family PTK specific inhibitors on the steady state surface expression of NR2A
(A) Ratio of surface to total GFP expression of GFP-NR2A at 22 DIV (WT and 3CS, n = 13 neurons respectively) *p < 0.05 compared with WT. (B) Effects of treatment with Src family PTK specific inhibitor PP2 (20 μM) and its inactive analog PP3 (20 μM) for 30 min on steady state surface expression of GFP-NR2A WT and GFP-NR2A 3CS in transfected cultured cortical neurons were examined. Ratio of surface to total expression of GFP-NR2A at 22 DIV (four bars (WT+PP3, WT+PP2, 3CS+PP3, 3CS+PP2), n = 12 neurons respectively, F = 6.463, p < 0.001, ANOVA). ***p < 0.001 compared with PP3 treatment. (C) Ratio of surface to total expression of GFP-NR2A at 22 DIV (WT and 4CS, n = 13 neurons respectively) *p < 0.05 compared with WT. (D) The effect of PP2 (20 μM) or the inactive structural analog PP3 (20 μM) on surface expression of endogenous NR2A in cultured cortical neurons at 22 DIV (n = 12 neurons respectively). Cultured cortical neurons were treated with PP2 or PP3 for 30 min and surface expressions of endogenous NR2A were quantified. (E) The effect of SU6656 (1 μM) or DMSO vehicle control on surface expression of endogenous NR2A in cultured cortical neurons at 22 DIV (n = 12 neurons respectively). Cultured cortical neurons were treated with SU6656 or DMSO for 30 min and surface expressions of endogenous NR2A were quantified. Student t-test. ***p < 0.001 compared with control. (F) Effect of myc-GODZ or myc-GODZ(DHHS) inactive mutant on surface expression of GFP-NR2A in transfected cultured cortical neurons at 22 DIV. Ratio of surface to total expression of GFP-NR2A at 22 DIV (WT and 4CS, n = 16 neurons respectively). *p < 0.05 compared with WT. Student t-test. Error bars indicate the sem.
Supplemental Figure S4
GODZ-mediated accumulation of NR1, NR2A 3CS and NR2B 3CS in Golgi apparatus
(A) Colocalization of NR1 with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2A with myc-GODZ (upper panels) and with NR1 and NR2A with myc-GODZ(DHHS) (lower panels). (B) Colocalization of NR1 with GODZ in HEK 293T cells cotransfected with NR1 and NR2A with myc-GODZ (upper panels) and with NR1 and NR2A with myc-GODZ(DHHS) (lower panels). (C) Colocalization of NR2A 3CS with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2A 3CS with or without myc-GODZ or myc-GODZ(DHHS). (D) Colocalization of NR2A 3CS with GODZ in HEK 293T cells cotransfected with NR1 and NR2A 3CS with myc-GODZ or myc-GODZ(DHHS). (E) Colocalization of NR2B 3CS with Golgi-apparatus marker GM-130 in HEK 293T cells cotransfected with NR1 and NR2B 3CS with or without myc-GODZ or myc-GODZ(DHHS). (F) Colocalization of NR2B 3CS with GODZ in HEK 293T cells cotransfected with NR1 and NR2B 3CS with myc-GODZ or myc-GODZ(DHHS).
Supplemental Figure S5
Sequence alignment of NR2 subunits NR2A-D in their Cys cluster I and II
Amino acids around NR2A, NR2B, NR2C and NR2D Cys cluster I (top) and Cys cluster II (bottom) are shown. Transmembrane domain (TMD) 4 is underlined. Dotted box indicates AP-2 binding motif (YXXΦ).
Supplemental Figure S6
Palmitoylation-dependent two-step trafficking mechanism of glutamate receptors
Accumulation of NMDA receptors (black) and AMPA receptors (blue) in Golgi apparatus is regulated by GODZ-mediated palmitoylation of the receptors, respectively. GODZ increases palmitoylation of NR2A/2B on the Cys cluster II and that of GluR1-4 on the TMD 2 site. Depalmitoylation of these sites may regulate release of receptors from the Golgi apparatus for surface delivery.
Surface expression of the AMPA receptor is not influenced by palmitoylation of the AMPA receptor on the C-terminal site. In contrast to palmitoylation of the AMPA receptor, palmitoylation of the NMDA receptor on the Cys cluster I controls the steady state surface expression of the NMDA receptor.
The depalmitoylated form of surface NMDA receptors is constitutively internalized. Similarly, the palmitoylated form of surface AMPA receptors is more susceptible to AMPA and NMDA induced internalization.


