Abstract
Background
The ecological factors contributing to the evolution of tropical vertebrate communities are still poorly understood. Primate communities of the tropical Americas have fewer folivorous but more frugivorous genera than tropical regions of the Old World and especially many more frugivorous genera than Madagascar. Reasons for this phenomenon are largely unexplored. We developed the hypothesis that Neotropical fruits have higher protein concentrations than fruits from Madagascar and that the higher representation of frugivorous genera in the Neotropics is linked to high protein concentrations in fruits. Low fruit protein concentrations in Madagascar would restrict the evolution of frugivores in Malagasy communities.
Methodology/Principal Findings
We reviewed the literature for nitrogen concentrations in fruits from the Neotropics and from Madagascar, and analyzed fruits from an additional six sites in the Neotropics and six sites in Madagascar. Fruits from the Neotropical sites contain significantly more nitrogen than fruits from the Madagascar sites. Nitrogen concentrations in New World fruits are above the concentrations to satisfy nitrogen requirements of primates, while they are at the lower end or below the concentrations to cover primate protein needs in Madagascar.
Conclusions/Significance
Fruits at most sites in the Neotropics contain enough protein to satisfy the protein needs of primates. Thus, selection pressure to develop new adaptations for foods that are difficult to digest (such as leaves) may have been lower in the Neotropics than in Madagascar. The low nitrogen concentrations in fruits from Madagascar may contribute to the almost complete absence of frugivorous primate species on this island.
Introduction
Primate communities of Madagascar are known for the paucity of frugivorous species. In contrast, the high representation of frugivores but under-representation of truly folivorous vertebrates in the Neotropics has been a long-standing enigma in ecology [1]–[3]. Neotropical primate communities (often used as proxy for mammal communities in general [1], [3], [4]), contain more frugivorous genera and species when compared to the Old World primate radiations of Africa/Asia, and these in turn have more frugivores than primate communities of Madagascar [5], [6]. Explanations for the different numbers of frugivores and folivores include phenological patterns of food resources and plant species diversity. First, it has been postulated for the Neotropics, that young leaves are rare at the time of year when fruit abundance is low. This makes it unlikely that species in the Americas can fall back on young leaves during times of fruit shortage [2] and does not favor the evolution of folivores. Second, food plant diversity, and in particular the regional species richness of figs as keystone fruit trees during times of food shortage, has been linked to the diversity of frugivores [7], [8]. Madagascar has very few species of figs and fruit production is erratic due to high climatic stochasticity [9]–[12]. Thus, both factors may contribute to the paucity of frugivores in Madagascar though the generalization of both hypotheses has been questioned and modified by analyses of extended datasets [13]–[15].
Here, we propose a supplementary hypothesis to explain the higher representation of frugivorous taxa in New World tropical communities compared to Madagascar. Protein, measured as nitrogen concentration, is assumed to be a limiting factor in many communities [16]. For primates, the biomass but not the diversity of folivores has been linked to the ratio of nitrogen to fiber in leaves of a given forest [17]–[19], indicating a strong effect of protein availability on folivorous primates, though the biologically most appropriate measures for protein availability in leaves are still being developed further [20], [21].
Our hypothesis is based on a somewhat different argument. The key assumption for the evolution of primate diversity postulates that primates evolved under the constraints of protein availability [22], [23]. According to this hypothesis, fruits, - although central food resources because they are easy to digest and protein digestion is not hindered by secondary plant components to the extent seen in leaves - , are not supposed to contain enough protein to satisfy the requirements of primates. Therefore, species were forced to add leaves (if large-bodied) or insects (if small-bodied) to satisfy their nitrogen needs [22]–[24], and supplement their diets with alternative food resources during times of fruit shortage [25].
There is no doubt that unusual environmental conditions and food shortage can cause famine and death in primates, and thus, lean seasons require special adaptations for survival [26]–[28]. But, large-bodied species store nutrients when they are available. This type of “capital breeding” seems to be favored when maternal investment is relatively low due to large body size (compared to the smaller species) and spread over longer periods of time [29]. Conversely, in most small primate species, reproductive success is linked primarily to food quality during the lush wet season when females give birth and lactate and infants are weaned (“income breeders”: Madagascar: [30]–[32]; New World: [33]). Nutrient availability during times of lactation and weaning would then represent a crucial factor for reproductive success [10], [30], [33], with adaptations to periods of food shortage potentially resulting in diversification [34]. Thus, once constraints imposed by seasonal fruit shortage had been solved through different adaptations [15], [35], primate communities could maintain more frugivorous taxa at sites where fruit protein concentrations were high enough to satisfy the protein needs of the lactating female and the infant during weaning. This would be relevant particularly in primate communities with a higher representation of small-bodied “income breeders”. Today, as well as in evolutionary times, primates of the Neotropics tend to be smaller than primates from Madagascar [6], [36]. Thus, the higher proportion of small frugivorous primate species in the Neotropics could have evolved if fruit protein content in the Neotropics would be above the primates' protein needs in the Neotropics, but below these requirements in Madagascar [24]. Based on this argument, we test the hypothesis that fruits in the Neotropics contain higher protein concentrations than fruits in Madagascar.
Results
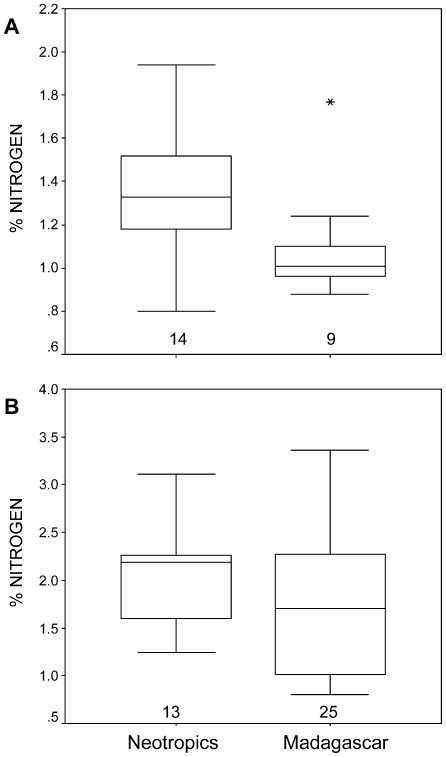
The nitrogen concentrations in ripe fruits were significantly lower at sites in Madagascar than in the Neotropics (Madagascar: 1.09±0.28%, n = 9 sites; Neotropics: 1.37±0.29%, n = 14 sites; MWU-test: z = 2.49, p = 0.011; Fig. 1; Table 1).
Figure 1. Average nitrogen concentration in fruits and primate vegetable foods in the Neotropics and in Madagascar.
(A) Average nitrogen concentrations in fruits. Number of sites listed along the x-axis (Table 1). (B) Average nitrogen concentrations of all vegetable food items consumed by primates in the Neotropics and in Madagascar. Number of studies listed on the x-axis (Table 3).
Table 1. Nitrogen concentrations of fruits at different sites in Madagascar and the Neotropics.
| Site | Country | % Nitrogen concentration in fruits (sample size) | Sampling | Source |
| Madagascar | ||||
| Anjamena (A) | Madagascar | 0.88 (25) | PF: Eulemur mongoz | [57] |
| Kirindy/CFPF-CS7 (B) | Madagascar | 1.10 (8) | PF: Propithecus verreauxi | Carrai unpubl. |
| Ranomafana (C) | Madagascar | 0.96 (6) | PF: Microcebus rufus | [58] |
| Sahamalaza (1) | Madagascar | 1.24±0.68 (67) | GS | Polowinsky & Schwitzer unpubl. |
| Kirindy/CFPF-N5 (2) | Madagascar | 1.04±0.42 (40) | GS | [59], Bollen et al. unpubl. |
| Ranomafana (3) | Madagascar | 1.01±0.40 (45) | PF: Propithecus edwardsi | Arrigo-Nelson unpubl. |
| Mandena (4) | Madagascar | 0.99±0.81 (71) | GS | [60], Lahann unpubl. |
| Sainte Luce (5) | Madagascar | 0.88± 0.39 (103) | GS | [61] |
| Berenty (6) | Madagascar | 1.77±0.75 (11) | PF: Microcebus griseorufus | [62] |
| Neotropics | ||||
| Los Tuxtlas (D) | Mexico | 1.34 (11) | PF: Alouatta palliata | [63] |
| Cockscomb Basin Wildlife Sanctuary (E) | Belize | 1.33 (16) | PF: Alouatta pigra | [64] |
| Barro Colorado Island (F) | Panama | 1.29 (8) | Several PF | [65] |
| Ilanos (G) | Venezuela | 1.12 (9) | PF: Alouatta seniculus | [24] |
| Lago Guri (H) | Venezuela | 1.47 (19) | PF: Pithecia pithecia + 3 fruits not eaten | [66] |
| Nouragues (I) | French Guiana | 0.79 (14) | GS | [67] |
| San Cayetano (J) | Argentina | 1.52 (2) | PF: Alouatta caraya | [68] |
| Mata Atlantica (K) | Brasil | 1.18 (22) | PF: Callicebus moloch | [69] |
| Lomas Barbudal (11) | Costa Rica | 1.33±0.75 (64) | PF: Cebus capuchinus | Vogel unpubl. |
| Tinigua National Park (12) | Columbia | 1.28±0.77 (53) | PF: Lagothrix lagotricha | Stevenson unpubl. |
| Yasuní National Park (13) | Ecuador | 1.59±0.79 (33) | PF + NPF: Alouatta seniculus | Derby unpubl. |
| Raleighvallen (14) | Suriname | 1.17±0.54 (13) | PF: Cebus apella | Boinski & Vogel unpubl. |
| Parque Estadual Carlos Botelho (15) | Brasil | 1.94±1.12 (4) | PF: Brachyteles arachnoides | Talebi unpubl. |
| Isla Brasilera (16) | Argentina | 1.77 (1) | PF: Alouatta caraya | Kowalewski unpubl. |
Letters and numbers listed in the first column refer to sites shown in Figure 2. Values of the published datasets (sites marked by letters in the left hand column and in Figure 2) are means or medians as listed in the original reference; sample size listed in brackets. For samples analyzed in the context of the present paper (sites marked by numbers) values are means and standard deviations. Nitrogen concentrations were recorded by general sampling (GS) of fruits from woody plant species available during the study period, or of fruits consumed by a specific primate species (PF) plus fruits not consumed by primates at the sites (NPF).
Sites are represented with different sample size. If the analyses were based on the individual plant species analyzed at each site, fruits from Madagascar contained 1.04±0.60% nitrogen (n = 334) while New World fruits contained on average 1.37±0.73% nitrogen (n = 168; MWU test: z = 5.08, p<0.001). Since the raw data are not available for most of the published studies, this analysis was restricted to the unpublished data marked with numbers in Table 1 and Figure 2 (six sites in the Neotropics and six sites in Madagascar).
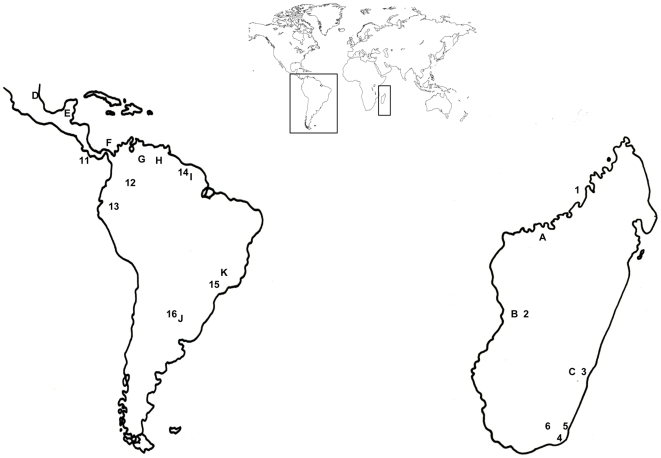
Figure 2. Sites for measures of fruit protein content (source of map: www.smithLifeScience.com/Tools.htm; free).
Site labels are listed in Table 1. Letters refer to studies published previously. Numbers refer to new and yet unpublished studies.
Given differences in fig availability between Madagascar and the Neotropics, it appeared possible that figs might play different roles in Madagascar and in the Neotropics. However, when figs were removed from the analyses, the two regions still differed significantly with average nitrogen contents of 1.04±0.61% in Madagascar (n = 322 samples) and of 1.40±0.76% in the Neotropics (n = 157 samples; MWU test: z = 5.19, p<0.001).
In contrast to the nitrogen concentrations of fruits, the nitrogen concentrations in the overall vegetable diet of primates (including leaves, exudates, flowers, fruits) did not differ between species of the Neotropis and of Madagascar (MWU-test: z = 1.28, p>0.05; Neotropics: mean nitrogen content of vegetable primate food: 2.05±0.57, n = 13 studies at 13 different sites and 9 different primate species; Madagascar: 1.74±0.73, n = 25 studies at 11 different sites and 23 different primate species; Fig. 1; Table 2).
Table 2. Mean nitrogen concentration of all vegetable food items consumed by various primate species in Madagascar and the Neotropics.
| Species | Site | Country | N | % Nitrogen | Source |
| Madagascar | |||||
| Avahi laniger | Ranomafana | Madagascar | 5 | 2.5 | [70] |
| Avahi meridionalis | Sainte Luce | Madagascar | 39 | 1.2 | Norscia unpubl. |
| Cheirogaleus major | Mandena | Madagascar | 77 | 0.9 | [60] |
| Cheirogaleus medius | Sainte Luce | Madagascar | 33 | 0.8 | [59] |
| Cheirogaleus medius | Mandena | Madagascar | 75 | 0.9 | [60] |
| Eulemur collaris | Sainte Luce | Madagascar | 100 | 1.0 | Donati unpubl. |
| Eulemur macaco | Ampasikely | Madagascar | 23 | 1.7 | [71] |
| Eulemur flavifrons | Sahamalaza | Madagascar | 88 | 1.6 | Polowinsky & Schwitzer unpubl. |
| Eulemur mongoz | Anjamena | Madagascar | 46 | 1.1 | [57] |
| Eulemur rufus | Kirindy | Madagascar | 20 | 1.0 | [59] |
| Hapalemur alaotrensis | Alaotra | Madagascar | 15 | 2.1 | [72] |
| Hapalemur aureus | Ranomafana | Madagascar | 63 | 3.2 | Tan unpubl. |
| Hapalemur griseus | Ranomafana | Madagascar | 40 | 3.4 | Tan unpubl. |
| Hapalemur merdidionalis | Mandena | Madagascar | 26 | 1.6 | Ralison unpubl. |
| Hapalemur simus | Ranomafana | Madagascar | 141 | 2.3 | Tan unpubl. |
| Indri indri | Mantadia | Madagascar | 10 | 1.7 | [73] |
| Lemur catta | Berenty | Madagascar | 28 | 2.4 | [74] |
| Lepilemur ruficaudatus | Kirindy N5 | Madagascar | 194 | 2.4 | [26], [75] |
| Microcebus griseorufus | Berenty | Madagascar | 25 | 1.5 | [62] |
| Microcebus murinus | Mandena | Madagascar | 77 | 0.9 | [60] |
| Microcebus rufus | Ranomafana | Madagascar | 12 | 0.9 | [76] |
| Propithecus diadema | Mantadia | Madagascar | 10 | 2.0 | [73] |
| Propithecus edwardsi | Ranomafana | Madagascar | 392 | 2.0 | Arrigo-Nelson unpubl. |
| Propithecus verreauxi | Kirindy CS7 | Madagascar | 246 | 2.2 | Carrai unpubl. |
| Propithecus verreauxi | Kirindy N5 | Madagascar | 14 | 2.3 | Ganzhorn unpubl. |
| Neotropics | |||||
| Alouatta caraya | San Cayetano | Argentina | 16 | 2.2 | [68] |
| Alouatta caraya | Isla Brasilera | Argentina | 30 | 2.8 | Kowalewski unpubl. |
| Alouatta palliata | Barro Colorado Island | Panama | 5 | 2.0 | [65] |
| Alouatta palliata | Los Tuxtlas | Mexico | 71 | 2.2 | [63], [77] |
| Alouatta pigra | Community Baboon Sancuary and Cockscomb Basin Wildlife Sanctuary | Belize | 124 | 3.1 | [64] |
| Alouatta seniculus | Yasuní NP | Ecuador | 124 | 2.3 | Derby unpubl. |
| Alouatta seniculus | Ilanos | Venezuela | 37 | 2.4 | [24] |
| Ateles geoffroyi | Barro Colorado Island | Panama | 4 | 1.9 | [65] |
| Brachyteles arachnoides | Parque Estadual Carlos Botelho | Brasil | 10 | 2.2 | Talebi unpubl. |
| Callicebus moloch | Mata Atlantica | Brasil | 32 | 1.6 | [69] |
| Cebus capuchinus | Barro Colorado Island | Panama | 3 | 1.2 | [65] |
| Cebus capuchinus | Barbudal | Costa Rica | 65 | 1.4 | Vogel unpubl. |
| Lagothrix lagothricha | Tinigua National Park | Columbia | 53 | 1.3 | [78] |
Discussion
On average, protein concentrations in the vegetable food of primates do not differ between regions. This indicates, that the protein requirements of species from different radiations are independent of their phylogenetic history, even though some of the strepsirhine primates of Madagascar can have reduced metabolic rates [37], [38]. In contrast to the overall vegetable food composition, fruits in the Neotropics and in Madagascar vary in their nitrogen concentrations. This pattern is not due to different sample size, since, despite the fact that fruits have been sampled most comprehensively in Madagascar, the larger number of samples per site does not lower the average protein concentration when compared to the fruits eaten most frequently (Table 3). Thus, the differences between regions are unlikely due to sampling artifacts.
Table 3. Fruit selection of primates in relation to nitrogen concentrations; ns = not significant.
| Species | Site | Country | % Nitrogen in fruits eaten (sample size) | % Nitrogen in fruits not eaten (sample size) | Relationship between the frequency of consumption and nitrogen concentration | Source |
| Madagascar | ||||||
| Microcebus murinus | Mandena | Madagascar | 0.9 (61) | 0.8 (14) | ns | [60] |
| Cheirogaleus medius | Mandena | Madagascar | 0.9 (59) | 0.8 (16) | ns | [60] |
| C. medius | Kirindy/CFPF | Madagascar | 0.9 (23) | 1.0 (18) | ns | [59] |
| C. spp. | Sainte Luce | Madagascar | 0.8 (33) | 0.8 (64) | ns | [59] |
| C. major | Mandena | Madagascar | 0.9 (63) | 0.8 (14) | ns | [60] |
| Eulemur collaris | Sainte Luce | Madagascar | 0.9 (86) | 0.8 (18) | ns | [59], [79] |
| E. rufus | Kirindy/CFPF | Madagascar | 1.0 (20) | 0.9 (25) | ns | [59] |
| Neotropics | ||||||
| Lagothrix lagotricha | Tinigua National Park | Columbia | ns | [78], Stevenson, unpubl. | ||
| Cebus apella | Manu National Park | Brazil | Negative correlation between frequency of consumption and nitrogen concentration | [80] | ||
| Callicebus moloch | Mata Atlantica | Brazil | 1.2 (22) | ns | [69] | |
The protein requirements of primates are such that foods consumed should contain about 7–11% protein (equivalent to 1.1–1.8% nitrogen). These values include the consumption of leaves with secondary components inhibiting digestion [24], [39]. Even cattle and sheep with improved nitrogen digestion due to rumination avoid food with nitrogen contents below 1.1% [40]. Thus, fruits at sites in Madagascar with an average nitrogen concentration of 1.0–1.1% are on the lower end of nitrogen concentrations found in sampled fruits of the Neotropics and are below the nitrogen requirements for primates. In conjunction with environmental conditions which seem less predictable in Madagascar than in other parts of the world [11], [12], this might have contributed to the evolution of very few frugivorous and the high proportion of folivorous lemur species in Madagascar. Similarly, this might also explain the low representation of frugivorous bird species on Madagascar [1], [41].
The contemporary low representation of frugivorous lemur species is not an artifact of recent extinctions. Madagascar has lost its large vertebrate species (elephant birds, giant tortoises, pygmy hippopotamus, large lemurs) during the last millennium [42]. But except for two species of Pachylemur, none of these species seem to have relied on fruits as a staple diet [43].
In contrast to the situation in Madagascar, the average nitrogen concentrations of fruits in the New World are well within the nitrogen requirements of primates. Thus, the selection pressure to extend their diet beyond fruits seems to be lower in the Americas than it is in Madagascar.
The difference in nitrogen concentrations between regions may not appear to be biologically substantial (i.e.: 1.0–1.1% nitrogen in Madagascar and 1.4% in the Neotropics). Yet, assuming that animal-dispersed fruits evolved under the evolutionary pressure to attract dispersers without investing too much, the difference in nitrogen of around 0.3% between Madagascar and the Neotropics is likely to be quite relevant; from the plants' perspective, this small increment represents an increase in nitrogen investment (and nitrogen loss once the fruits are eaten) of about 30% between Madagascar and the New World fruits. Given that many trees are exhausted especially after mast fruiting [44], a 30% difference in the protein investments in fruits - and in a major food resource - must have profound consequences on the plant as well as on the consumer communities.
The hypothesis presented here should be considered as one of several constraining factors of evolutionary relevance. It is based on food quality and does neither consider quantitative aspects, nor does it take into account the need to match the animals' energy requirements (e.g., [45], [46]), specific mineral needs [47], or avoidance of plant secondary components [48]. However, these possibly confounding variables can not be separated as long as we do not have the means to measure the qualitative and quantitative availability of food, its individual and seasonal variation as well as seasonal or ontogenetic variation in ingestion and nutrient assimilation by the animals (e.g., [49], [50]).
Materials and Methods
Database
We compiled data on the nitrogen concentrations of ripe fruits in forests of Madagascar and in the New World from the literature and supplemented the data with additional analyses of fruits from six sites in the Neotropics and six sites in Madagascar. Non-forest habitats were not considered. We used nitrogen concentrations as measured by the Kjeldahl procedure rather than crude protein in this comparison, because different conversion factors from nitrogen to crude protein have been suggested [51]–[53]. As other measures of protein concentrations, such as ninhydrin, Biorad, or amino acids can not be transformed to nitrogen concentrations using a simple transformation factor, only studies reporting total nitrogen measured with the Kjeldahl method were used in the present analysis. Few ripe fruits contain digestion inhibitors such as frequently found in leaves. Thus, there was no need to control for these digestion-inhibiting components [54], [20]. Except for the samples collected by Polowinsky and Schwitzer (unpubl.), all other samples collected by the authors for the present paper (listed in Table 1) were analyzed with the same equipment and procedure in the labs of JUG [55].
Community-wide data on the chemical composition of fruits are scant. However, of 10 published primatological studies addressing protein selection in fruits in Madagascar and the Neotropics (and several others from Africa, not considered here; plus several unpublished studies from Madagascar and the Neotropics), none found a positive significant difference in nitrogen concentrations between those fruits eaten and not eaten by the primates under study. One study that did report a significant difference, reported a negative correlation between consumption and protein concentrations (Table 3). Therefore, we consider the fruits consumed by primates as a conservative representative sample of the nitrogen concentrations for all fruits available at each site. Thus, we use three types of data to characterize fruit nitrogen content of a given site: (1) comprehensive sampling of all fruits obtained during a study, (2) fruits eaten by primates, and (3) fruits eaten and not eaten by primates if non-eaten fruits had been collected for comparisons (Table 1).
To control for possible physiological differences between primate radiations (such as between the lemurs of Madagascar and haplorhine primates in the Neotropics), we investigated whether the nitrogen content of all dietary plant components (fruits, leaves, flowers, seeds) differed between primate radiations. According to our hypothesis, the diet of the species of the different primate radiations should not differ in the nitrogen concentrations of their overall diet, but fruits in the New World should have higher nitrogen concentrations than in Madagascar.
Statistical Analysis
Since data deviated from normality, we applied non-parametric tests for the comparisons. Tests were run with SPSS 9.0 [56].
Acknowledgments
For assistance with the chemical analyses and preparation of the manuscript we are grateful to Irene Tomaschewski, Ulrike Walbaum, Klaus Rupp, Sabine Baumann and Monika Hänel.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Association Européenne pour l'Etude et la Conservation des Lémuriens (AEECL), the Belgian Fund for Scientific Research, Flanders, Conservation International's Primate Action Fund, the Deutsche Forschungsgemeinschaft, the Deutscher Akademischer Austauschdienst, Earthwatch Institute, the German Primate Center, the Italian Ministry for Scientific Research (MURST), the J. William Fulbright Foundation, the Margot Marsh Biodiversity Foundation, the National Science Foundation, National Geographic, and St. Louis Zoo. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fleming TH, Breitwisch R, Whitesides GH. Patterns of tropical vertebrate frugivore diversity. Annual Review Ecology and Systematics. 1987;18:91–109. [Google Scholar]
- 2.Terborgh J, van Schaik CP. Convergence vs. nonconvergence in primate communities. In: Gee JHR, Giller PS, editors. Organization of Communities. Oxford: Blackwell; 1987. pp. 205–226. [Google Scholar]
- 3.Willig MR, Kauffman DM, Stevens RD. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu Rev Ecol Syst. 2003;34:273–309. [Google Scholar]
- 4.Emmons LH. Of mice and monkeys: primates as predictors of mammal community richness. In: Fleagle JF, Janson C, Reed K, editors. Primate Communities. Cambridge: Cambridge University Press; 1999. pp. 171–188. [Google Scholar]
- 5.Fleagle JG, Reed KE. Comparing primate communities: A multivariate approach. Journal of Human Evolution. 1996;30:489–510. [Google Scholar]
- 6.Kappeler PM, Heymann EW. Nonconvergence in the evolution of primate life history and socio-ecology. Biological Journal Linnean Society. 1996;59:297–326. [Google Scholar]
- 7.Terborgh J. Keystone plant resources in the tropical forest. In: Soulé ME, editor. Conservation Biology. Sunderland: Sinauer; 1986. pp. 330–344. [Google Scholar]
- 8.Kissling WD, Rahbek C, Böhning-Gaese K. Food plant diversity as broad-scale determinant of avian frugivore richness. Proceedings of the Royal Society of London, B. 2007;274:799–808. doi: 10.1098/rspb.2006.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman SM, Ganzhorn JU. Rarity of figs (Ficus) on Madagascar and its relationship to a depauperate frugivore community. Revue d'Ecologie. 1997;52:321–329. [Google Scholar]
- 10.Ganzhorn JU, Wright PC, Ratsimbazafy J. Primate communities: Madagascar. In: Fleagle JG, Janson C, Reed KE, editors. Primate Communities. Cambridge: Cambridge University Press; 1999. pp. 75–89. [Google Scholar]
- 11.Wright PC. Lemur traits and Madagascar ecology: Coping with an island environment. Yearbook of Physical Anthropology. 1999;42:31–72. doi: 10.1002/(sici)1096-8644(1999)110:29+<31::aid-ajpa3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Dewar RE, Richard AF. Evolution in the hypervariable environment of Madagascar. Proceedings of the National Academy of Science USA. 2007;104:13723–13727. doi: 10.1073/pnas.0704346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautier-Hion A, Michaloud G. Are figs always keystone resources for tropical frugivorous vertebrates? A test in Gabon. Ecology. 1989;70:1826–1833. [Google Scholar]
- 14.Heymann EW. Can phenology explain the scarcity of folivory in New World primates? American Journal of Primatology. 2001;55:171–175. doi: 10.1002/ajp.1050. [DOI] [PubMed] [Google Scholar]
- 15.Brockman DK, van Schaik CP. Cambridge: Cambridge University Press; 2005. Primate Seasonality: Studies of Living and Extinct Human and Non-human Primates. [Google Scholar]
- 16.White TCR. Berlin: Springer-Verlag; 1993. The Inadequate Environment: Nitrogen and the Abundance of Animals. [Google Scholar]
- 17.Oates JF, Whitesides GH, Davies AG, Waterman PG, Green SM, et al. Determinants of variation in tropical forest primate biomass: New evidence from West Africa. Ecology. 1990;71:328–343. [Google Scholar]
- 18.Ganzhorn JU. Leaf chemistry and the biomass of folivorous primates in tropical forests. Oecologia (Berlin) 1992;91:540–547. doi: 10.1007/BF00650329. [DOI] [PubMed] [Google Scholar]
- 19.Chapman CA, Chapman LJ, Naughton-Treves L, Lawes MJ, McDowell LR. Predicting folivorous primate abundance: Validation of a nutritional model. American Journal of Primatology. 2004;65:55–69. doi: 10.1002/ajp.20006. [DOI] [PubMed] [Google Scholar]
- 20.DeGabriel JL, Wallis IR, Moore BD, Foley WJ. A simple, integrative assay to quantify nutritional quality of browses for herbivores. Oecologia (Berlin) 2008;156:107–116. doi: 10.1007/s00442-008-0960-y. [DOI] [PubMed] [Google Scholar]
- 21.Felton AM, Felton A, Lindenmayer DB, Foley WJ. Nutritional goals of wild primates. Functional Ecology. 2009;23:70–78. [Google Scholar]
- 22.Milton K. Factors influencing leaf choice by howler monkeys: A test of some hypotheses of food selection by generalist herbivores. American Naturalist. 1979;114:362–378. [Google Scholar]
- 23.Kay RF. On the use of anatomical features to infer foraging behavior in extinct primates. In: Rodman RS, Cant JGH, editors. Adaptation for Foraging in Non-human Primates. New York: Columbia University Press; 1984. pp. 21–53. [Google Scholar]
- 24.Oftedal OT. The nutritional consequences of foraging in primates: The relationship of nutrient intake to nutrient requirements. Philosophical Transaction of the Royal Society London B. 1991;334:161–170. doi: 10.1098/rstb.1991.0105. [DOI] [PubMed] [Google Scholar]
- 25.van Schaik CP, Madden R, Ganzhorn JU. Seasonality and primate communities. In: Brockman DK, van Schaik CP, editors. Seasonality in Primates: Studies of Living and Extinct Human and Non-human Primates. Cambridge: Cambridge University Press; 2005. pp. 445–463. [Google Scholar]
- 26.Foster RB. Famine on Barro Colorado Island. In: Leigh EG Jr., Stanley AS, Windsor DM, editors. The Ecology of a Tropical Forest. Washington D.C.: Smithsonian Institution Press; 1982. pp. 201–212. [Google Scholar]
- 27.Milton K. Annual mortality patterns of a mammal community in central Panama. Journal of Tropical Ecology. 1990;6:493–499. [Google Scholar]
- 28.Gould L, Sussman RW, Sauther ML. Natural disasters and primate populations: The effects of a 2-year drought on a naturally occurring population of Ring-Tailed Lemurs (Lemur catta) in Southwestern Madagascar. International Journal of Primatology. 1999;20:69–84. [Google Scholar]
- 29.Janson C, Verdolin J. Seasonality of primate births in relation to climate. In: Brockman DK, van Schaik CP, editors. Tropical Fruits and Frugivores: The Search for Strong Interactors. Cambridge: Cambridge University Press; 2005. pp. 307–350. [Google Scholar]
- 30.Ganzhorn JU. Distribution of a folivorous lemur in relation to seasonally varying food resources: Integrating quantitative and qualitative aspects of food characteristics. Oecologia (Berlin) 2002;131:427–435. doi: 10.1007/s00442-002-0891-y. [DOI] [PubMed] [Google Scholar]
- 31.Richard AF, Dewar RE, Schwartz M, Ratsirarson J. Mass change, environmental variability and female fertility in wild Propithecus verreauxi. Journal of Human Evolution. 2000;39:381–391. doi: 10.1006/jhev.2000.0427. [DOI] [PubMed] [Google Scholar]
- 32.Wright PC, Razafindratsita VR, Pochron ST, Jernvall J. The key to Madagascar frugivores. In: Dew JL, Boubli JP, editors. Tropical Fruits and Frugivores: The Search for Strong Indicators. Dordrecht: Springer; 2005. pp. 121–138. [Google Scholar]
- 33.Stevenson PR. Potential keystone plant species for the frugivore community at Tinigua Park, Colombia. In: Dew JL, Boubli JP, editors. Tropical Fruits and Frugivores: The Search for Strong Interactors. Dordrecht: Springer; 2005. pp. 37–57. [Google Scholar]
- 34.Terborgh J. New Jersey: Princeton University Press, Princeton; 1983. Five New World primates. [Google Scholar]
- 35.Wright PC. Behavioral and ecological comparisons of Neotropical and Malagasy primates. In: Kinzey WG, editor. New World Primates: Ecology, Evolution, and Behavior. New York: Aldine de Gruiter; 1997. pp. 127–141. [Google Scholar]
- 36.Fleagle JG, Reed KE. Phylogenetic and temporal perspectives on primate ecology. In: Fleagle JG, Janson C, Reed KE, editors. Primate Communities. Cambridge: Cambridge University Press; 1999. pp. 92–115. [Google Scholar]
- 37.McNab BK. The influence of food habits on the energetics of eutherian mammals. Ecological Monographs. 1986;56:1–19. [Google Scholar]
- 38.Dausmann K . An (opposable) thumbs-up for hibernation - hypometabolism in primates. In: Masters J, Gamba M, Génin F, editors. Leaping Ahead: Advances in Prosimian Biology. New York: Springer Science + Business Media.(in press); [Google Scholar]
- 39.Conklin-Brittain NL, Wrangham RW, Hunt KD. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. II. Macronutrients. International Journal of Primatology. 1998;19:971–998. [Google Scholar]
- 40.FAO. Guidelines: land evaluation for extensive grazing. FAO. 1991.
- 41.Langrand O. New Haven: Yale University Press; 1990. Guide to the Birds of Madagascar. [Google Scholar]
- 42.Burney DA, Pigott Burney L, Godfrey LR, Jungers WL, Goodman SM, et al. A chronology for late prehistoric Madagascar. Journal of Human Evolution. 2004;47:25–63. doi: 10.1016/j.jhevol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Godfrey LR, Semprebon GM, Jungers WL, Sutherland MR, Simons EL, et al. Dental use wear in extinct lemurs: evidence of diet and niche differentiation. Journal of Human Evolution. 2004;47:145–169. doi: 10.1016/j.jhevol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Kelly D. The evolutionary ecology of mast seeding. Trends in Ecology and Evolution. 1994;9:465–470. doi: 10.1016/0169-5347(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 45.Lambert JE. Primate nutritional ecology: feeding biology and diet at ecological and evolutionary scales. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in perspective. Oxford: Oxford University Press; 2007. pp. 482–495. [Google Scholar]
- 46.Strier KB. Boston: Allyn and Bacon; 2007. Primate behavioral ecology. [Google Scholar]
- 47.O'Brien TG, Kinnard MF, Dierenfeld ES, Conklin-Brittain NL, Wrangham RW, et al. What's so special about figs? Nature. 1998;392:668. [Google Scholar]
- 48.Freeland WJ, Janzen DH. Strategies in herbivory by mammals: The role of plant secondary compounds. American Naturalist. 1974;108:269–289. [Google Scholar]
- 49.Chapman CA, Chapman LJ, Rode KD, Hauck EM, McDowell LR. Variation in nutritional value of primate foods: among trees,time periods and areas. International Journal of Primatology. 2003;24:317–333. [Google Scholar]
- 50.Rothman JM, Dierenfeld ES, Hintz HF, Pell AN. Nutritional quality of gorilla diets: Consequences of age, sex, and season. Oecologia (Berlin) 2008;155:111–122. doi: 10.1007/s00442-007-0901-1. [DOI] [PubMed] [Google Scholar]
- 51.Milton K, Dintzis FR. Nitrogen-to-protein conversion factors for tropical plant samples. Biotropica. 1981;13:177–181. [Google Scholar]
- 52.Conklin-Brittain NL, Dierenfeld ES, Wrangham RW, Norconk M, Silver SC. Chemical protein analysis: A comparison of Kjeldahl crude protein and total ninhydrin protein from wild, tropical vegetation. Journal of Chemical Ecology. 1999;25:2601–2622. [Google Scholar]
- 53.Levey DJ, Bissell HA, O'Keefe SF. Conversion of nitrogen to protein and amino acids in wild fruits. Journal of Chemical Ecology. 2000;26:1749–1763. [Google Scholar]
- 54.Wrangham RW, Conklin-Brittain NL, Hunt KD. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. International Journal of Primatology. 1998;19:949–970. [Google Scholar]
- 55.Bollen A, van Elsacker L, Ganzhorn JU. Tree dispersal strategies in the littoral forest of Sainte Luce (SE-Madagascar). Oecologia (Berlin) 2004;139:604–616. doi: 10.1007/s00442-004-1544-0. [DOI] [PubMed] [Google Scholar]
- 56.SPSS . Chicago: SPSS Inc; 1999. SPSS Base 9.0 User's Guide. [Google Scholar]
- 57.Curtis DJ. Diet and nutrition in wild Mongoose Lemurs (Eulemur mongoz) and their implications for the evolution of female dominance and small group size in lemurs. American Journal of Physical Anthropology. 2004;124:234–247. doi: 10.1002/ajpa.10268. [DOI] [PubMed] [Google Scholar]
- 58.Atsalis S. Diet of the brown mouse lemur (Microcebus rufus) in Ranomafana National Park. International Journal of Primatology. 1999;20:193–229. doi: 10.1002/(SICI)1098-2345(200005)51:1<61::AID-AJP5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Bollen A, Donati G, Fietz J, Schwab D, Ramanamanjato J-B, et al. An intersite comparison on fruit characteristics in Madagascar: Evidence for selection pressure through abiotic constraints rather than through co-evolution. In: Dew JL, Boubli JP, editors. Tropical Fruits and Frugivores: The Search for Strong Interactors. Dordrecht: Springer; 2005. pp. 93–119. [Google Scholar]
- 60.Lahann P. Feeding ecology and seed dispersal of sympatric cheirogaleid lemurs (Microcebus murinus, Cheirogaleus medius, Cheirogaleus major) in the littoral rainforest of south-east Madagascar Journal of Zoology, London. 2007;271:88–98. [Google Scholar]
- 61.Bollen A. Fruit characteristics: fruit selection, animal seed dispersal and conservation matters in the Sainte Luce forests. In: Ganzhorn JU, Goodman SM, Vincelette M, editors. Biodiversity, Ecology, and Conservation of Littoral Ecosystems in the Region of Tolagnaro (Fort Dauphin), Southeastern Madagascar. Washington, D.C.: Smithsonian Institution; 2007. pp. 127–145. [Google Scholar]
- 62.Génin FGS, Masters JC, Ganzhorn JU . Gummivory in cheirogaleids: primitive retention or adaptation to hypervariable environments? In: Burrows A, Nash LT, editors. Evolution of Exudativory in Primates. New York: Springer.(in press); [Google Scholar]
- 63.Estrada A, Coates-Estrada R. Frugivory in Howling monkeys (Alouatta palliata) at Los Tuxtlas: Dispersal and fate of seeds. In: Estrada A, Fleming TH, editors. Frugivores and Seed Dispersal. Dordrecht: Dr. W. Junk Publishers; 1986. pp. 93–104. [Google Scholar]
- 64.Silver SC, Ostro LET, Yeager CP, Dierenfeld ES. Phytochemical and mineral components of food consumed by black howler monkeys (Alouatta pigra) at two sites in Belize. Zoo Biology. 2000;19:95–109. [Google Scholar]
- 65.Hladik CM, Hladik A, Gueguen L, Mercier M, Viroben G, et al. Le régime alimentaire des Primates de l'Ile de Barro-Colorado (Panama). Résultats des analyses quantitatives. Folia Primatologica. 1971;16:85–122. doi: 10.1159/000155393. [DOI] [PubMed] [Google Scholar]
- 66.Norconk MA, Conklin-Brittain NL. Variation on frugivory: The diet of Venezuelan White-faced sakis. International Journal of Primatology. 2004;25:1–26. [Google Scholar]
- 67.Simmen B, Sabatier D. Diets of some French Guianan primates: Food composition and food choices. International Journal of Primatology. 1996;17:661–693. [Google Scholar]
- 68.Zunino GE. Hábitat, dieta y actividad del mono aullador negro (Alouatta caraya) en el noreste de la Argentina. Boletín Primatológico Latinoamericano. 1989;1:75–97. [Google Scholar]
- 69.Heiduck S. Food choice in Masked Titi Monkeys (Callicebus personatus melanochir): selectivity or opportunism? International Journal of Primatology. 1997;18:487–502. [Google Scholar]
- 70.Faulkner AL, Lehman SM. Feeding patterns in a small-bodied nocturnal folivore (Avahi laniger) and the influence of leaf chemistry: A preliminary study. Folia Primatologica. 2006;77:218–228. doi: 10.1159/000091231. [DOI] [PubMed] [Google Scholar]
- 71.Simmen B, Bayart F, Marez A, Hladik A. Diet, nutritional ecology, and birth season of Eulemur macaco in an anthropogenic forest in Madagascar. International Journal of Primatology. 2007;28:1253–1266. [Google Scholar]
- 72.Mutschler T. Folivory in a small-bodied lemur: The nutrition of the Alaotran gentle lemur (Hapalemur griseus alaotrensis). In: Rakotosamimanana B, Rasamimanana H, Ganzhorn JU, Goodman SM, editors. New Directions in Lemur Studies. New York: Kluwer Academic / Plenum Press; 1999. pp. 221–239. [Google Scholar]
- 73.Powzyk JA, Mowry CB. Dietary and feeding differences between sympatric Propithecus diadema diadema and Indri indri. International Journal of Primatology. 2003;24:1143–1162. [Google Scholar]
- 74.Rasamimanana B. 2004. La dominance des femelles makis (Lemur catta) : quelles stratégies énergétiques et quelle qualité de ressources dans la réserve de Berenty, au sud de Madagascar ? Muséum National d'Histoire Naturelle, Paris.
- 75.Ganzhorn JU, Pietsch T, Fietz J, Gross S, Schmid J, et al. Selection of food and ranging behaviour in a sexually monomorphic folivorous lemur: Lepilemur ruficaudatus. Journal of Zoology, London. 2004;263:393–399. [Google Scholar]
- 76.Atsalis S. A Natural History of the Brown Mouse Lemur. Pearson Prentice Hall 2007 [Google Scholar]
- 77.Estrada A, Coates-Estrada R. Fruit eating and seed dispersal by howling monkeys Alouatta palliata in the tropical rain forest of Los Tuxtlas. American Journal of Primatology. 1984;6:77–91. doi: 10.1002/ajp.1350060202. [DOI] [PubMed] [Google Scholar]
- 78.Stevenson PR. Fruit choice by Woolly Monkeys (Lagothrix lagotricha) in Tinigua National Park, Columbia. International Journal of Primatology. 2004;25:367–381. doi: 10.1002/ajp.1350320205. [DOI] [PubMed] [Google Scholar]
- 79.Donati G, Bollen A, Borgognini-Tarli SM, Ganzhorn JU. Feeding over the 24-hour cycle: dietary flexibility of cathemeral collared lemurs (Eulemur collaris). Behavioral Ecology and Sociobiology. 2007;61:1237–1251. [Google Scholar]
- 80.Janson CH, Stiles EW, White DW. Selection of plant fruiting traits by brown capuchin monkeys: a multivariate approach. In: Estrada A, Fleming TH, editors. Frugivores and Seed Dispersal. Dordrecht: Dr. W. Junk Publishers; 1986. pp. 83–92. [Google Scholar]