Abstract
Local immunotherapies are under investigation for treatment of unresectable tumors and sites of solid tumor resection to prevent local recurrence. Successful local therapy could also theoretically elicit systemic immune responses against cancer. Here we explored the delivery of therapeutic dendritic cells (DCs), cytokines, or other immunostimulatory factors to tumors via the use of ‘self-gelling’ hydrogels based on the polysaccharide alginate, injected peritumorally around established melanoma lesions. Peritumoral injection of alginate matrices loaded with DCs and/or an interleukin-15 superagonist (IL-15SA) around 14-day established ova-expressing B16F0 murine melanoma tumors promoted immune cell accumulation in the peritumoral matrix, and matrix infiltration correlated with tumor infiltration by leukocytes. Single injections of IL-15SA-carrying gels concentrated the cytokine in the tumor site ~40-fold compared to systemic injection and enabled a majority of treated animals to suppress tumor growth for a week or more. Further, we found that single injections of alginate matrices loaded with IL-15SA and the Toll-like receptor ligand CpG or two injections of gels carrying IL-15SA alone could elicit comparable anti-tumor activity without the need for exogenous DCs. Thus, injectable alginate gels offer an attractive platform for local tumor immunotherapy and facilitates combinatorial treatments designed to promote immune responses locally at a tumor site while limiting systemic exposure to potent immunomodulatory factors.
Keywords: local tumor immunotherapy, IL-15 superagonist, dendritic cells, alginate, hydrogels, cancer vaccines
Introduction
Localized cancer therapies are utilized to treat unresectable tumors and/or for treating sites of surgical resection to combat local recurrence [1–3]. Immunotherapy treatments delivered directly at solid tumor sites have been explored with the goal of utilizing the tumor itself as a source of tumor antigens to generate a systemic immune response that could eliminate distal metastases. Most immunotherapy strategies seek to promote the robust infiltration of tumors with functional immune cells to promote tumor destruction, but defects in tumor vasculature, suppressive signals produced by tumor cells or co-opted tumor-resident immune cells, and rapid tumor growth can limit the accumulation of activated and competent immune cells [4, 5]. Recently, combinations of cytokine, chemotherapy, and/or immunostimulatory ligand treatments used to locally treat established tumors have shown promise in not only eliminating treated tumors but also generating systemic immunity [6–11]. However, such potent immunostimulatory regimens can elicit serious toxicity and may need to be coupled with methods to control delivery and limit systemic exposure [12, 13]. Prior work has demonstrated that controlled release of immunocytokines from gels or microparticles at a tumor site can enhance local immunotherapies, by sustaining the intratumoral concentration of these factors while reducing systemic exposure following a single injection [14–16]. Thus, the use of biomaterials to deliver local combinatorial immunotherapies may lead to further enhancements in the potency and safety of local immunotherapy.
We recently described an injectable gel formulation of the polysaccharide alginate, which can be loaded with exogenous immune cells, proteins, or immunoregulatory factors [17, 18]. Alginate has been studied extensively as a matrix for cell therapy and tissue engineering, and has been shown to be safe for use in humans [19–21]. We found that injection of alginate gels with embedded activated dendritic cells in healthy mice elicits a sustained infiltration of host T-cells and dendritic cells into the matrix, and that these matrices can release encapsulated cytokines over a period of 7–14 days [17, 18]. We hypothesized that a similar recruitment of lymphocytes and DCs to alginate gels surrounding established tumors could promote local antigen presentation, and provide a local reservoir of immune cells for tumor invasion. To test this concept, in the present study we surrounded established melanoma tumors with DC/cytokine/Toll-like receptor (TLR) ligand-loaded gels, and analyzed tumor growth and the recruitment of leukocytes to the tumor-engulfing gels and the tumors themselves. Because these gels were stable at least one month in vivo they were readily recovered post-treatment, and thus we also analyzed the composition of innate and adaptive immune cells recruited to both the gels and the tumor itself following peritumoral gel therapy.
Materials and Methods
Materials
Sterile alginates Pronova SLM20 (MW 75,000–220,000 g/mol, >50% M units) and Pronova SLG20 (MW 75,000–220,000 g/mol, >60% G units) were purchased from Novamatrix (FMC Biopolymers, Sandvika, Norway). Anti-mouse FITC-TCRβ, anti-mouse PE-I-Ab, anti-mouse APC-CD11c, anti-mouse APC-CD8α, anti-mouse APC-CD4, anti-moue FITC- and PE-NK1.1 were purchased from BD Biosciences (San Jose, CA). APC-tetramer ova-MHC I was from Beckman Coulter (Fullerton, CA), anti-mouse PE-CD8α antibody was from Invitrogen (San Diego, CA), and anti-mouse foxp3 staining kit was from eBioscience (San Diego, CA).
Isooctane was obtained from Mallinckrodt Baker (Phillipsburg, NJ). Calcium chloride dihydrate and alginate lyase were from Sigma-Aldrich (St. Louis, MO). CpG oligonucleotides with a phosphorothioate backbone (CpG 1826, sequence 5′-/5AmMC6/TCCATGACGTTCCTGACGTT-3′) were synthesized by Integrated DNA Technologies. Mouse IL-15 and mouse IL-15Rα-human Fc chimera recombinant proteins and ELISA detection kits were purchased from R&D Systems (Minneapolis, MN).
Animals and cells
Animals were cared for in the USDA-inspected MIT Animal Facility under federal, state, local and NIH guidelines for animal care. C57Bl/6 mice were obtained from the Jackson Laboratory. Bone marrow-derived dendritic cells were prepared following a modification of the procedure of Inaba [22] as previously reported [23]. DCs were activated/matured with 1μM CpG and pulsed with 1 μg/mL each of ova class I and ova class II peptides (Anaspec, San Jose, CA) for 18 hrs and washed 3X with PBS before use. B16F10 parental melanoma cells were obtained from American Type Culture Collection. B16-ova cells based on the B16F0 parental melanoma line were transfected with ovalbumin expressed as an MSCV vector with puromycin resistance, followed by an IRES and Ovalbumin-2A-green fluorescent protein (GFP). The ova expressed in these cells lacks the first 55 amino acids (deleting the secretion signal) and is soluble in the cytoplasm.
Tumor inoculation and alginate gel therapy
Except where noted otherwise, anesthetized C57Bl/6 mice were inoculated with 3×104 B16-ova cells s.c., which were allowed to establish for 14 days. IL-15 superagonist (IL-15SA) was prepared by incubating 5 μg IL-15 and 31.7 μg IL-15Ra/Fc (equimolar amounts) at 37°C in 15.9 μL PBS for 30 min. Matrix alginate (160 μL of 0.01 g/mL SLM20 alginate in sterile PBS at 4°C) was mixed with factors to be delivered (e.g., 2×106 dendritic cells, 36.7 μg IL-15 SA, and/or 80μg CpG in 150 μL gel) and kept on ice until injection. Calcium-loaded alginate microspheres were synthesized as previously described [17, 18]. Endotoxin levels in the alginate preparations were well below levels stimulatory for innate immune cells [24], as described previously [18]. The Calcium-loaded microspheres (~1×106) were mixed with the matrix alginate solution and 150 μL of the mixture immediately injected s.c., surrounding tumors. For i.p. injections, 36.7 μg of IL-15SA was injected in 200 μL of PBS, and for i.t. injections, the same amount of IL-15SA and 2 × 106 dendritic cells were injected into tumors in 30μL of PBS.
Flow cytometry analysis
Alginate gels, lymph nodes, and spleens recovered from treated animals were digested with 0.28 WÜ/mL Liberase Blendzyme 3 (Roche Applied Sciences, Indianapolis, IN) and 1 mg/mL of alginate lyase (Sigma) for 20 min at 37°C. Digested gels and tissues were passed through a 40 μm nylon mesh cell strainer (BD Falcon) with 9 mL complete RPMI medium. Recovered cells were resuspended in FACS buffer (1% BSA, 0.1% NaN3 in Hank’s balanced salt solution, pH 7.4) at 4°C, blocked with anti-CD16/32 antibody for 10 min, then stained with fluorescent antibodies for 20 min on ice, followed by 3 washes with FACS buffer and addition of 1.25μg/mL propidium iodide (PI) for viability assessment. For foxp3 staining, cells were fixed, permealized, and stained according to the manufacturer’s instructions. Stained cells were analyzed on a BD FACSCalibur flow cytometer. Enumeration of cellular infiltrates was performed by calibration of flow cytometer events to cell suspensions of known concentration, and cell frequencies determined from a live cell gate with low PI staining. Cell losses during the multiple wash/treatment steps of gel and tissue digestions were reproducible and were accounted for in the reported recovered cell numbers.
IL-15SA release in vitro and in vivo
Soluble IL-15Rα/Fc (15.9 μg, 2 mg/mL in PBS) was mixed with 80 μL of alginate matrix solution (0.01g/mL in PBS), and then gelled with 5×105 calcium-loaded alginate microspheres to crosslink the alginate in an epppendorf tube for 2 hrs at 37 °C. RPMI medium with 10% FCS was added (500 μL) and cytokine release was assessed by ELISA analysis quantifying IL-15Rα in the supernatant at staggered time points. The release of the IL-15SA was quantified in vivo by injecting alginate gels carrying 36.7 μg IL-15SA (31.7 μg IL-15Rα-Fc + 5 μg IL-15) around 14 day-old B16-ova tumors in C57Bl/6 mice. Gels and tumors were recovered from independent mice at staggered times, digested using the protocol described above, or by using T-Per Tissue Protein Extraction Reagent (Pierce, Rockford, IL) supplemented by Halt Protease Inhibitor Cocktail (Pierce) according to the manufacturer’s instructions. The digestion supernatants were collected and the amount of IL-15SA was quantified by using IL-15Rα ELISA kit or two-site IL-15Rα/IL-15 ELISA. IL-15 loss due to the digestion process was analyzed using control digestions using known quantities of IL-15SA and were accounted for in the analysis.
Statistical Analysis
All data are shown as mean±S.E. Comparisons of two experimental groups were performed using two-tailed Mann-Whitney tests. Comparisons of Kaplan-Meier survival curves were made using a log-rank test.
Results
T-cell recruitment to tumor-surrounding gels
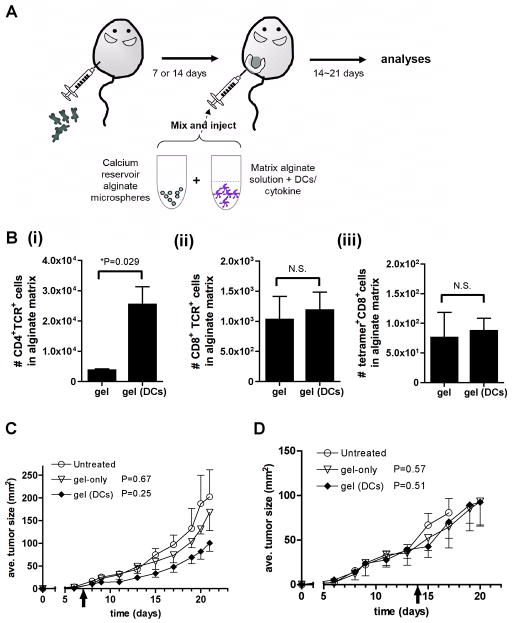
We recently developed an injectable self-gelling formulation of the polysaccharide alginate, obtained by mixing calcium-loaded alginate microspheres with an alginate solution just prior to injection [17, 18]. Upon injection, calcium ions from the microspheres and the surrounding interstitial fluid diffuse into the alginate solution and ionically crosslink the polysaccharide chains in situ, enabling gelation in < 60 min in vivo. The resulting soft gels retain more than 80% of their original mass for ~30 days in vivo, and can entrap co-injected cells or cytokines for slow release into the local environment [17, 18]. In our previous study in healthy animals [17], we showed that s.c. injection of alginate gels carrying activated, antigen-loaded dendritic cells (DCs) elicited priming of naïve CD8+ T-cells in the local draining lymph nodes (supported by migration of a small number of DCs from the gel to the lymph nodes), followed by trafficking of primed antigen-specific T-cells to the gels. To determine whether DCs delivered in alginate gels could similarly promote accumulation of antigen-specific T-cells at solid tumor sites, we analyzed the response of established melanoma tumors to locally-injected DC-carrying gels. We generated a model system where B16F0 melanoma cells were transfected with green fluorescent protein (GFP) and ovalbumin to enable visualization of antigen capture by dendritic cells and tracking of the immune response to a defined antigen, respectively. We first analyzed the response of established GFP/ova-expressing B16F0 (hereafter designated B16-ova) tumors to peritumoral injections of DCs in alginate gels. B16-ova cells were inoculated s.c. in C57Bl/6 recipients and allowed to establish for 7 or 14 days. Mice then received a single injection of alginate (150 μL) to surround tumors with gels carrying activated bone marrow-derived dendritic cells loaded with immunodominant class I- and class II-restricted ova peptides (Fig. 1A). Tumor growth was monitored and gels were recovered 14 days after injection for digestion and analysis of the local immune cell infiltrate accumulating at the tumor site. As shown in Fig. 1B, “empty” alginate gels injected around 14-day-old tumors contained few CD4+ or CD8+ T-cells. In contrast, gels carrying ova-pulsed, activated DCs elicited a substantial accumulation of CD4+ T-cells. However, in contrast to responses in healthy mice [17], DC-loaded gels did not recruit many CD8+ T-cells or endogenous ova-specific CD8+ T-cells to the peritumoral matrix (Fig. 1B). Further, the net therapeutic effect of this CD4+-rich T-cell recruitment to the tumor-surrounding gels was limited: DC-carrying gels elicited a minor suppression of 7-day established melanomas (which did not reach statistical significance) and were ineffective for treating 14-day established tumors (Fig. 1C). Thus, peritumoral gels carrying activated DCs supported the recruitment of CD4+ T-cells to the matrix, but attraction of CD8+ T-cells was poor and the effect on established tumor growth was limited.
Fig. 1. Alginate gels carrying dendritic cells alone have minimal efficacy against established melanoma tumors.
(A) Schematic of therapeutic approach. (B–D) C57Bl/6 mice were inoculated with 5×104 B16-ova tumor cells, and 7 or 14 days later, mice received a single peritumoral injection of either empty self-gelling alginate matrices or alginate carrying 2×106 activated, ova peptide-loaded DCs. (B) Numbers of gel-infiltrating T-cells recovered 14 days after injections (n = 4 mice per group, bars: s.e.m.). (C, D) B16-ova tumor growth following treatment of 7-day- (C) or 14-day- (D) established tumors with alginate gels alone (▽) or alginate loaded with antigen-pulsed DCs (◆) compared to untreated tumors (○) (n = 4 mice per group). P values shown relative to untreated control. Data shown are representative from 1 of 2 independent experiments. (error bars: s.e.m.)
IL-15SA release from alginate gels
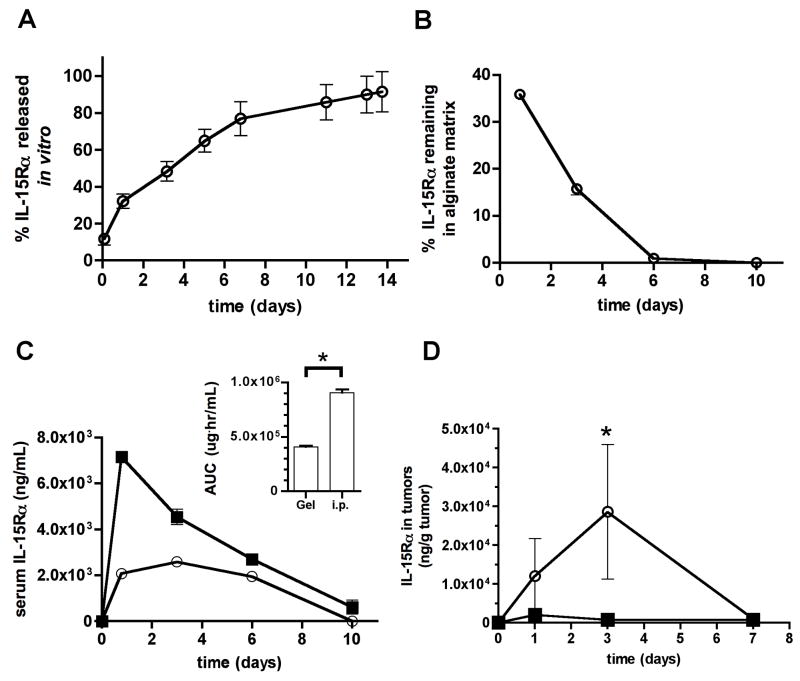
We hypothesized that the poor therapeutic effect of DC delivery in tumor-surrounding gels reflected insufficient recruitment of CD8+ T-cells (or other immune effector cells such as natural killer cells) to the matrices and/or suppression of recruited immune cell function at the tumor site. Thus, we next tested the ability of alginate gels to carry both DCs and immunocytokines, for slow release of signals that would support CD8+ T-cell accumulation and sustained effector functions in the tumor-local environment. Recent studies have demonstrated that interleukin-15 (IL-15) is capable of reverting the anergic state of tumor-infiltrating T-cells [25, 26]. In addition, it has been shown that mixtures of IL-15 with a recombinant soluble form of the IL-15 receptorα chain form long-lived complexes that function as an IL-15 superagonist (IL-15SA). These complexes potently expand CD8+ T-cell, natural killer (NK) cell, and NK T-cell populations in vivo [25, 27–29]. Motivated by these prior studies, we mixed pre-formed complexes of IL-15 and recombinant murine IL-15Rα-Fc fusion protein (referred to hereafter as IL-15SA) with alginate solutions prior to gelation, to obtain sustained release of this cytokine into the tumor environment. Self-gelled alginate released encapsulated IL-15SA over ~2 weeks in vitro (Fig. 2A). In vivo, IL-15SA-loaded alginate gels injected peritumorally around 14 day-old B16-ova tumors released cytokine for ~5–7 days (Fig. 2B). In agreement with prior studies of local cytokine delivery [14, 30], the level of cytokine detected in the systemic circulation following tumor-local gel release was substantially lower than levels achieved for the same total quantity of cytokine injected as a bolus i.p., particularly over the first 4 days following injection (Fig. 2C). In addition, local release of IL-15SA from peritumoral matrices led to peak concentrations of cytokine in the tumor on day 3 post injection that were ~40-fold greater than that achieved by systemic cytokine injection (Fig. 2D). Thus, IL-15SA delivered locally from peritumoral alginate ECMs lowered systemic exposure to the cytokine and greatly concentrated the dose achieved within the tumor itself compared to systemic injections.
Fig. 2. IL-15SA delivered via alginate matrices concentrates cytokine in the tumor and lowers systemic exposure relative to systemic injection.
(A) 16 μg IL-15Rα/Fc was mixed with 80 μL self-gelling alginate in vitro and release into medium at 37°C was measured over 2 weeks by ELISA. (Shown is 1 of 2 independent experiments). (B–D) 36.7 μg IL-15SA (5 μg IL-15 + 31.7 μg IL-15Rα/Fc, equimolar) was mixed with 150 μL self-gelling alginate and injected peritumorally around 14-day-old B16-ova tumors (○). For comparison, the same quantity of IL-15SA (in PBS) was injected i.p. as a separate group (■). Cytokine present in the gels (B), serum (C), and tumor itself (D) was monitored by ELISA over 7–10 days (n = 4 samples per time-point). Inset of (C) shows the integrated area under the curve quantifying total cytokine exposure in serum for gel vs. i.p. delivery of IL-15SA over the 10 day timecourse. * in C: P = 0.05 (one-tailed) compared to i.p. injection; * in D: P = 0.05 compared to i.p. injection. Data shown in B–D are from 1 of 2 independent experiments. (error bars: s.e.m.)
Anti-tumor effects of Il-15SA-loaded alginate
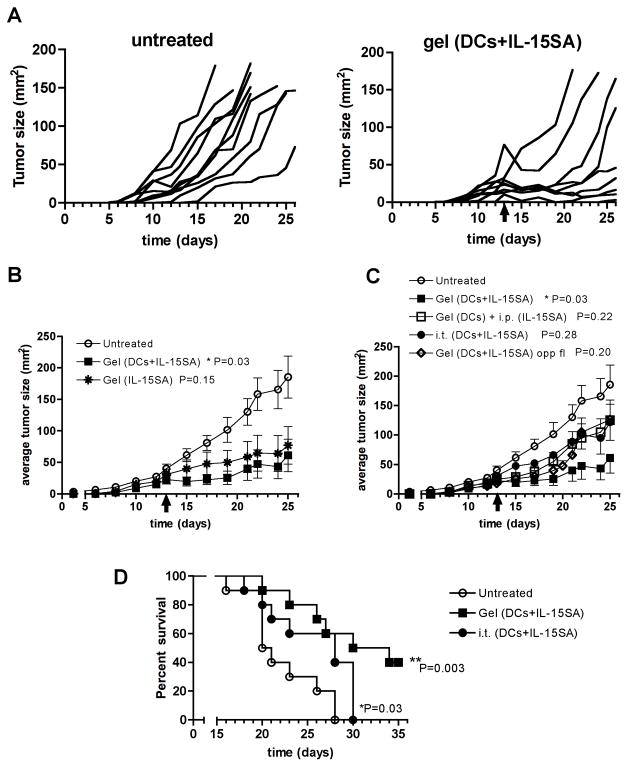
To test the therapeutic effect of combining DCs and IL-15SA in tumor-surrounding matrices, 14-day established B16-ova tumors were treated with a single peritumoral injection of alginate matrices carrying ova-pulsed dendritic cells and IL-15SA, and tumor growth was monitored for 21 days. Compared to untreated controls, a single peritumoral immunization with alginate carrying DCs and IL-15SA substantially retarded tumor growth in the majority of animals, and enabled mice with tumors smaller than ~50 mm2 at the time of treatment to control tumor growth for at least 1 week following gel injection (Fig. 3A). Larger tumors also occasionally showed substantial reductions in size transiently following injection of alginate ECMs (Fig. 3A). Surprisingly, comparison of mean tumor sizes in mice receiving gels with DCs+IL-15SA vs. IL-15SA alone showed that the cytokine played a dominant role in this combination treatment, with DCs providing only a minor enhancement of the anti-tumor response (Fig. 3B). Control of tumor growth required that IL-15SA was released locally from the peritumoral matrix, as gels carrying DCs and IL-15SA injected on the flank opposite tumors or delivery of IL-15SA via i.p. injection rather than locally from the gel had a much weaker anti-tumor effect than injection of the cytokine-loaded synthetic ECMs around tumors (Fig. 3C). Further, DCs and IL-15SA delivered in tumor-surrounding alginate gels were more effective than intratumoral (i.t.) injection of DCs and IL-15SA in the absence of the alginate matrix (Fig. 3C). Control of tumor growth elicited by DC/IL-15SA-carrying gels at the tumor site was reflected in improved survival compared to each of these alternative treatments (Fig. 3D and data not shown).
Fig. 3. Dendritic cells combined with IL-15SA in alginate matrices elicit prolonged control of tumor growth.
Established B16-ova tumors were left untreated or received peritumoral injections of alginate gels carrying antigen-pulsed DCs and/or IL-15SA (n = 10 per group). (A) Tumor growth curves are shown for untreated mice or mice treated with alginate carrying DCs+IL-15SA. (B) Average tumor growth for mice treated with alginate carrying DCs+IL-15SA (■) or IL-15SA alone in gels (✱), or left untreated (○). (C) Tumor growth for untreated mice (○) vs. mice treated with alginate carrying DCs+IL-15SA (■), intratumoral injection of DCs and IL-15SA in PBS (●), s.c. injection of alginate carrying DCs and IL-15SA on flank opposite from the tumor (◇), or peritumoral injection of alginate carrying DCs while IL-15SA is given i.p. (□). (D) Survival of mice treated with alginate carrying DCs+IL-15SA (■), intratumoral injection of DCs+IL-15SA (●), or left untreated (○). Shown are representative data from 1 of 2 independent experiments. (error bars: s.e.m.; P values: comparison to untreated controls)
To begin to understand how IL-15SA boosted the anti-tumor response elicited by tumor-surrounding alginate ECMs, we analyzed the impact of IL-15SA delivery on the recruitment of T cells at the tumor site following treatment of 14-day-established B16-ova tumors. As shown in Fig.s 4A-C, single peritumoral injections of alginate carrying DCs and IL-15SA increased the frequency but not the absolute number of CD4+ T-cells localizing in tumor-surrounding alginate matrices compared to gels carrying DCs alone, but did amplify accumulation of CD8+ T-cells by ~4-fold. Importantly, this CD8+ T-cell infiltrate included a population of tumor antigen-specific cells detected by ova peptide-H-2Kb MHC tetramer staining (Fig. 4D). Thus, colocalization of dendritic cells and IL-15SA in alginate gels around B16-ova tumors suppressed the growth of large established tumors for up to a week, and this anti-tumor response correlated with enhanced accumulation of CD8+ T-cells in the tumor-surrounding gels.
Fig. 4. Alginate gels carrying IL-15SA elicit accumulation of CD8+ T-cells in the matrix.
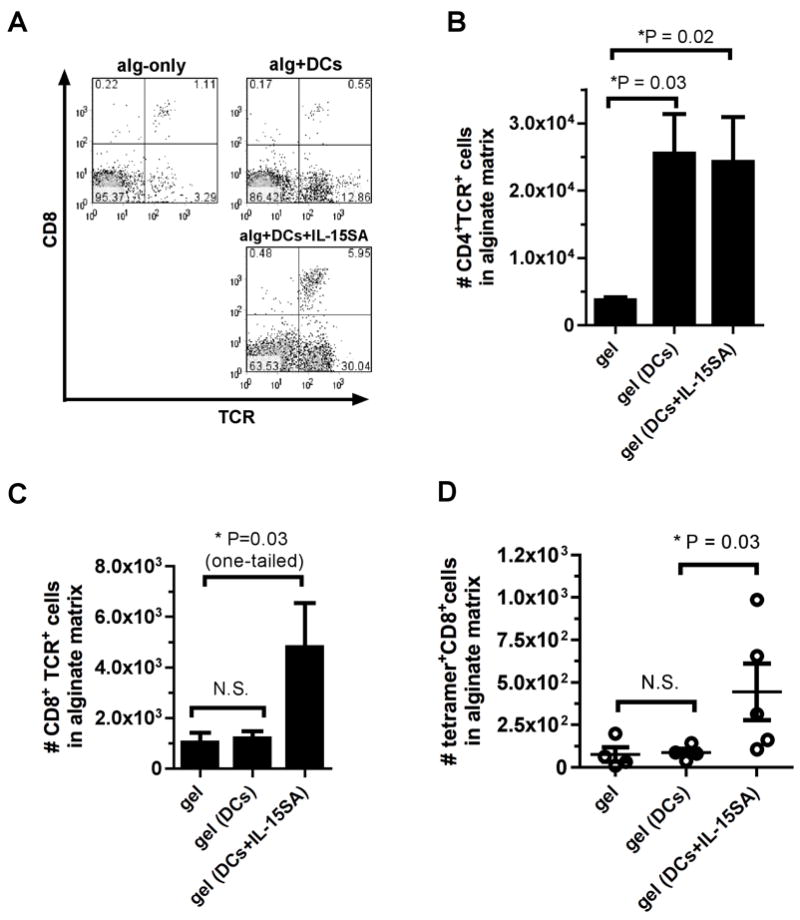
Established B16-ova tumors were treated on d14 by peritumoral injection of empty alginate gels or gels carrying antigen-pulsed DCs and/or IL-15SA (n = 4–5 per group). Gels were recovered on d28 for analysis. (A) Representative flow cytometry analyses (CD8 vs T cell receptor) of T-cell infiltration in gels. Mean numbers of CD4+ (B) CD8+ (C), and antigen-specific ova tetramer-stained T-cells recovered from gels (D) show increase in the number of CD8+ and antigen-specific T cells at the tumor microenvironment upon addition of IL-15SA. (error bars: s.e.m.)
Peritumoral delivery of IL-15SA and/or Toll-like receptor ligands from gels without dendritic cells
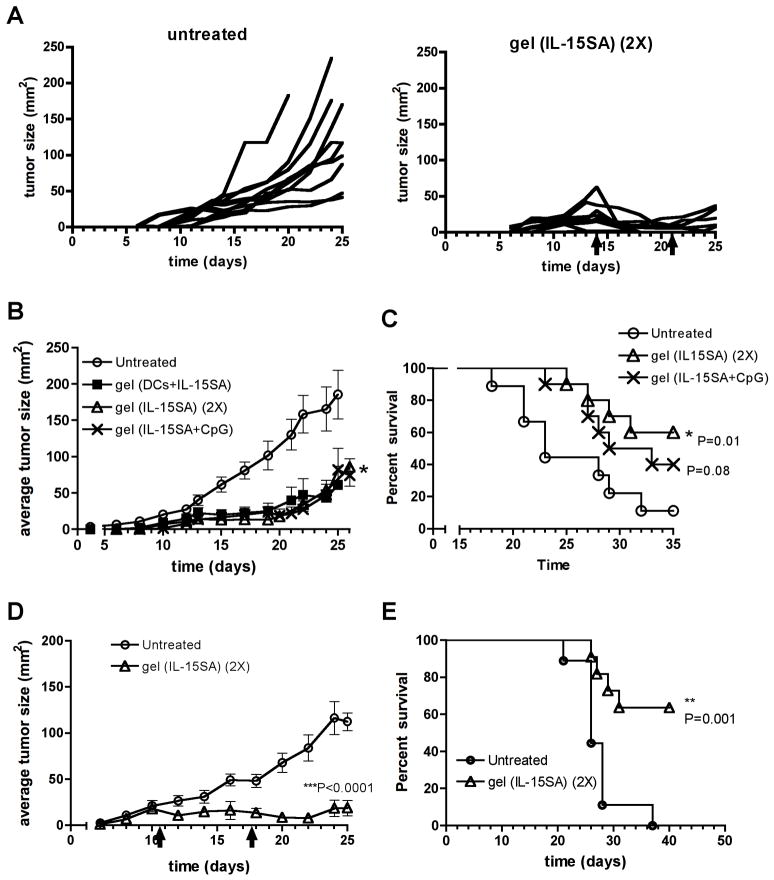
Because preparation of autologous DCs is a laborious and costly process for clinical implementation, we next tested whether cytokine or immunoregulatory factors alone could achieve results equivalent to tumors treated with DCs+IL-15SA. We tested two alternatives: first, providing two injections of alginate gel carrying IL-15SA alone, given peritumorally on days 14 and 21; second, giving a single injection of alginate carrying both IL-15SA and the Toll-like receptor-9 agonist CpG [9]. Testing IL-15SA alone was motivated by the strong anti-tumor effect elicited by IL-15SA alone following single injections (Fig. 3B), and we hypothesized that CpG might promote the activation and recruitment of local host dendritic cells [9, 31, 32], to replace the need for exogenous DCs in the gels. As shown in Fig. 5A, mice with day 14 B16-ova tumors receiving two injections of alginate loaded with IL-15SA impressively halted/regressed tumors compared to untreated animals. When compared in the same experiment, the average suppression of tumor growth seen for mice receiving a single injection of alginate carrying DCs+IL-15SA was equivalent to that obtained for a single injection of alginate gels carrying IL-15SA+CpG or two injections of alginate loaded with IL-15SA alone (Fig. 5B). This was also reflected in enhanced survival of mice treated with alginate carrying IL-15SA+CpG or two injections of alginate loaded with IL-15SA alone (Fig. 5C). Thus, optimal selection of immune-supporting factors for release from the peritumoral matrix enables gel-based delivery to provide prolonged control of tumor growth without the need for transfer of autologous DCs.
Fig. 5. Single peritumoral injections of alginate matrices carrying IL-15SA + CpG or two injections of IL-15SA-carrying gels are as effective as DC-carrying gels in controlling tumor growth.
(A) Tumor growth curves from individual mice shown for an independent experiment comparing untreated tumors vs. mice injected twice (day 14 and day 21) with alginate carrying IL-15SA. (B) Mean tumor size vs. time for mice receiving 3×104 B16-ova cells s.c. and left untreated (○) or receiving a single peritumoral injection on day 14 of alginate carrying 2×106 DCs and 36.7 μg IL-15SA (■, P = 0.04), a single injection of alginate carrying IL-15SA and 80 μg CpG (✖, P = 0.22), or receiving two injections of alginate carrying 36.7 μg IL-15SA on days 14 and 21 (△, P = 0.04) (n = 10 per group). (C) Survival of mice left untreated (○) or receiving a single peritumoral injection of alginate carrying IL-15SA and 80 μg CpG (✖), or receiving two injections of alginate each carrying 36.7 μg IL-15SA on days 14 and 21 (△). Data shown representative from 1 of 2 independent experiments. (D, E) Mice receiving 3×104 B16F10 tumor cells s.c. were left untreated (○) or were treated on days 11 and 18 with alginate gels carrying 36.7 μg IL-15SA (△). P-values relative to untreated controls. Data shown representative from 1 of 2 independent experiments. (error bars: s.e.m)
All of the experiments to this point involved melanoma tumor cells transduced with two different foreign antigens (ova and GFP), which could increase tumor immunogenicity and make the resulting tumors more readily rejected. We thus also tested whether 2 injections of alginate loaded with IL-15SA could halt the growth of unmanipulated B16F10 tumors, which are an accepted highly aggressive, poorly immunogenic melanoma. Because these parental B16 tumors grew more quickly than B16-ova tumors, they were treated with alginate on days 11 and 18 to treat them at comparable initial tumor sizes as in the experiments with B16-ova. As shown in Fig. 5D and E, treatment of B16F10 tumors with IL-15SA-loaded alginate gels also halted growth of B16 tumors and greatly enhanced survival of treated mice.
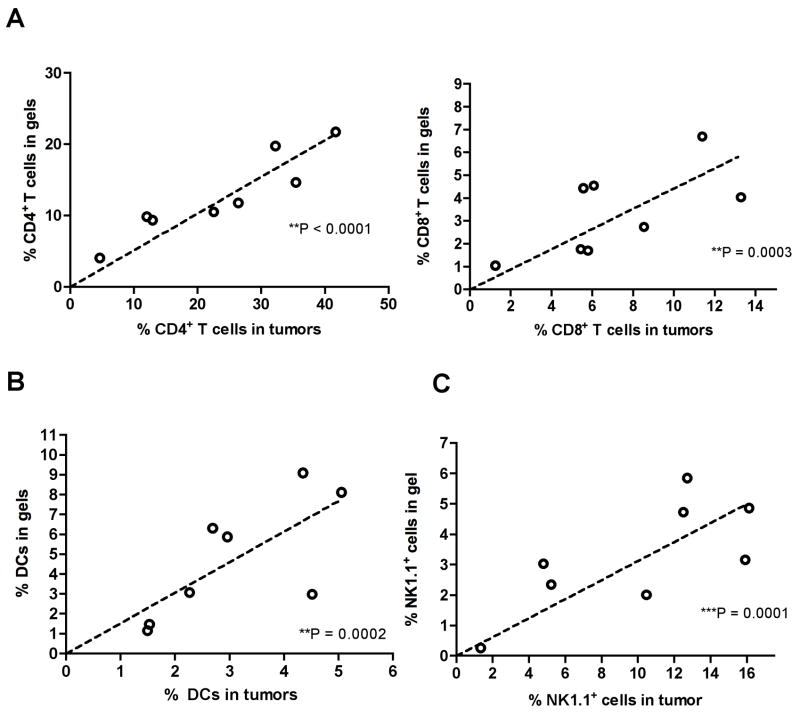
To understand the relationship between attraction of immune cells to the tumor-surrounding alginate gels and infiltration of the tumor itself, we next examined the cellular composition of the local immune response in gels and their associated tumors when 14-day-old B16-ova tumors were treated with two injections of IL-15SA-loaded alginate gels, analyzed 14 days after the first gel injection (d28). Enumerating the frequency of CD4+ and CD8+ T-cells in both the alginate matrix and tumors recovered from mice with a range of tumor burdens on day 28, we found a strong correlation between the frequencies of CD4+ and CD8+ T-cells accumulated in peritumoral matrices vs. within the tumor itself (Fig. 6A). In a similar manner, increased frequencies of CD11c+I-Ab+ dendritic cells (DCs) and NK1.1+ natural killer cells in the alginate gels coincided with increased frequencies of these cells within tumors (Fig. 6B, C). These results suggest that immune cell attraction to the peritumoral matrix either directly promoted infiltration of the tumor itself or served as a surrogate indicator of conditions favoring immune cell accumulation in tumors. As expected, infiltration of gels/tumors correlated with smaller final tumor sizes (data not shown).
Fig. 6. Immune cell accumulation in peritumoral alginate ECMs correlates with accumulation in tumors.
Established B16-ova tumors were treated with 2 injections of IL-15SA-loaded alginate as in Fig. 5. Gels and tumors were analyzed by flow cytometry and shown are pooled results for gels/tumors taken from the same mice on d28: (A) CD4+TCR+ and CD8+TCR+ T-cell frequencies; (B) CD11c+ cells; (C) NK1.1+ cells. (D) Correlation of T cell and NK cell infiltration of tumors with final tumor size. Shown is one of 3 independent experiments.
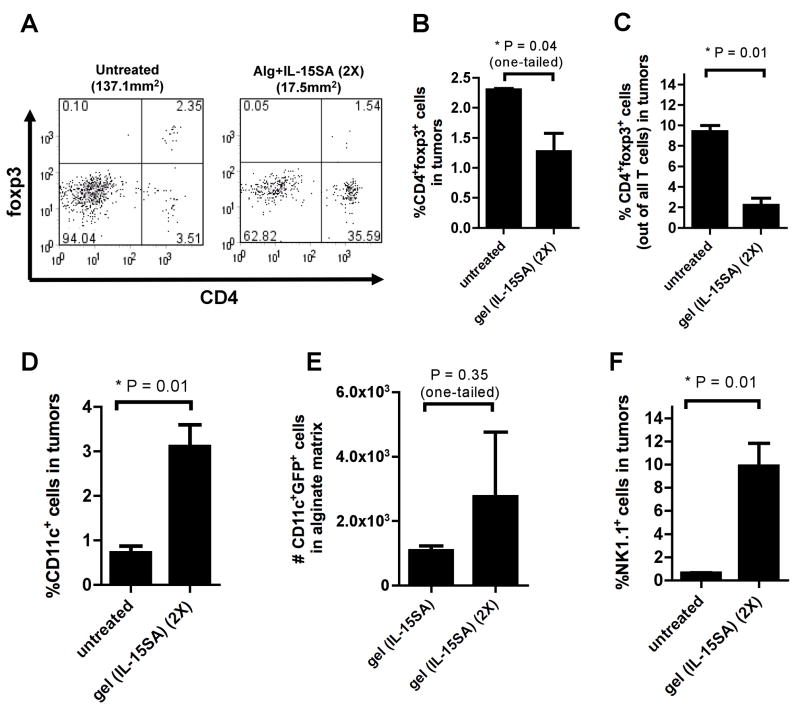
Finally, we extended our previous analysis of cellular infiltrates accumulating in the tumor-surrounding gels for the case of mice that received two injections of IL-15SA-loaded alginate, and examined regulatory T-cells and innate immune cells accumulating in the gels and tumors. Untreated tumors were infiltrated by low frequencies of CD4+ cells, and 30–50% of these cells were foxp3+, a marker of immunosuppressive regulatory T-cells (Fig. 7A). In response to IL-15SA/gel therapy, both the frequency of foxp3+ cells (Fig. 7B) and the ratio of foxp3+ T-cells to total T-cells [33] within tumors (Fig. 7C) were significantly reduced. In addition, the frequencies of intratumoral NK cells and DCs were increased 16- and 4-fold respectively for IL-15SA/gel-treated tumors compared to untreated controls (Fig. 7D, F). Treated mice showed a trend (which did not reach statistical significance) of increased numbers of GFP+ host DCs infiltrating the alginate gels, suggesting that active acquisition of tumor antigen was occurring in the peritumoral matrices (Fig. 7E). Thus, immunotherapy with IL-15SA-loaded gels enhanced CD8+ T-cell recruitment to the tumor site, lowered the relative frequency of regulatory T-cells, and enhanced the frequency of innate immune cells, all factors expected to augment the anti-tumor response.
Fig. 7. NK cells and DCs accumulate at tumor sites treated with IL-15SA/alginate gels, while frequencies of Tregs in the tumor are reduced.
B16-ova-tumor-bearing mice were left untreated or received two injections of IL-15SA-loaded alginate on days 14 and 21 as in Fig. 5. On day 28, tumors and gels were recovered and frequencies of CD4+foxp3+ cells, CD11c+ DCs, and NK1.1+ natural killer cells were determined. (A) Representative flow cytometry plots showing CD4 and foxp3 stainings. Bar graphs: the frequencies of CD4+foxp3+ cells out of all live lymphocytes (A) and those out of all T cells (B); the frequencies of CD11c+ DCs (D), and NK1.1+ natural killer cells in tumors (F). (E) Numbers of gel-infiltrating host DCs that were GFP+, suggesting active acquisition of tumor antigen in the peritumoral matrices. (error bars: s.e.m.)
Discussion
Immune responses to tumors are limited by a number of factors developing systemically and at the tumor site directly. Two limitations in the anti-tumor immune response are insufficient recruitment of T-cells to tumors [34–36] and loss of T-cell and innate effector cell functions in the tumor microenvironment, either due to suppressive factors produced in the tumor site or via the action of regulatory T-cells [37–39]. Complementary to many traditional immunotherapy treatments being studied to overcome these challenges, biomaterials are beginning to be explored as delivery vehicles to alter/augment anti-tumor immune responses, both for parenteral delivery of anti-tumor vaccines [40, 41] and for delivery of immunotherapeutics directly at tumor sites [6–11].
In an attempt to both stimulate T-cells and overcome immunosuppression at the tumor site, we investigated local tumor immunotherapy achieved by engulfing established tumors with injectable synthetic ECMs carrying antigen-pulsed dendritic cells or immunoregulatory cytokines. The use of the biopolymer alginate in these studies was motivated by its known biocompatibility and extensive track record in cell transplantation and tissue engineering applications [19–21, 42]. We previously demonstrated an injectable self-gelling formulation of alginate obtained by mixing calcium-alginate microspheres with an alginate solution, which crosslinked to form a soft gel in vivo via redistribution of calcium from the microspheres into the surrounding alginate solution and diffusion of calcium from the interstitial fluid into the alginate matrix [17, 18]. Alginate gels loaded with activated dendritic cells and injected s.c. in healthy mice elicited robust recruitment of T-cells to the gels, and DCs loaded with specific peptide antigens recruited antigen-specific T-cells into the gels [17].
Here we explored use of this synthetic ECM as a carrier for tumor-local delivery of dendritic cells and immunoregulatory factors, particularly IL-15 in a superagonist form. DCs have been examined in preclinical and clinical studies as possible cell-based cancer therapeutics capable of breaking tolerance to tumors [43–45], while IL-15 has been reported to trigger proliferation of anergic tumor-infiltrating T-cells [26] and revive effector functions of tumor-resident T-cells [25]. Superagonist IL-15 formed by complexing IL-15 with a recombinant soluble form of its high-affinity IL-15Rα chain has been shown to expand CD8+ T-cells, NK cells, and NK T-cells in vivo more effectively than IL-15 alone [27, 28], and repeated systemic injections of IL-15SA have been shown to slow tumor growth in vivo [25]. IL-15 can also induce differentiation of monocytes into Langerhans Cell-like IL-15 DCs [46], and plays a role in maximizing APC function [47]. We hypothesized that by surrounding tumors with DC/IL-15SA-loaded gels, leukocyte accumulation would be elevated and IL-15SA would be concentrated at the tumor site over several days, while systemic exposure to the cytokine would be reduced.
Prophylactic immunization in murine models has often been found to elicit protective immunity that can reject subsequent tumor challenges, but elimination of solid tumors that have grown for a week or more and have an established blood supply (the situation of most relevance to human cancer therapy) is a much more difficult challenge for immunotherapy strategies. Using a melanoma model, we found that activated, antigen-loaded DCs alone in peritumoral alginate ECMs were unable to slow established melanoma tumor growth. In contrast to gels carrying DCs alone, DCs combined with IL-15SA in gels elicited anti-tumor responses that could control established melanomas for up to a week following a single injection, and enhanced survival of treated animals (Fig. 3). This optimal therapeutic response from a single injection was achieved only when IL-15SA was provided locally at the tumor site and slow-released from the tumor-surrounding matrix. Notably, exogenous DCs could be excluded from the therapy by either providing repeated IL-15SA/gel injections or by combining IL-15SA with Toll-like receptor ligands for slow release in the tumor environment, substantially simplifying this approach from a clinical standpoint.
As a first step toward understanding how gel-mediated cytokine/DC delivery enhanced the anti-tumor immune response, we sought to analyze the composition of immune cells accumulating in gels and tumors. To this end, alginate matrices simultaneously served as stable surrogate ECMs surrounding the tumor site, facilitating characterization of the immune response developing following tumor cell inoculation and immunotherapy. We found that DCs alone in peritumoral alginate ECMs attracted CD4+ T-cells to tumor sites but elicited weak CD8+ T-cell recruitment and had a very limited therapeutic effect on established B16-ova tumors. However, IL-15SA delivery in the presence or absence of DCs increased the numbers of CD8+ T-cells within alginate matrices (Fig. 5B and data not shown), and increasing peritumoral accumulation of T-cells correlated with increased frequencies of lymphocytes within tumors (Fig. 6). In prior studies where IL-15SA was used as a stand-alone therapy for treatment of pancreatic tumors by repeated systemic injection, the enhanced anti-tumor response appeared to be driven by rescued CD8+ T-cells already resident with tumors rather than by recruitment of new T-cells to the tumor site [25]. Here we found that when IL-15SA was released locally at the tumor site over several days from the alginate matrix (alone or with DCs), the cytokine promoted accumulation of T-cells in both the peritumoral matrix and tumor, and reduced the relative frequency of foxp3+ Tregs. Whether these accumulating T-cells were expanding lymphocytes already resident in tumors at the time of treatment or represented cells recruited to the site following gel therapy remains to be determined.
Conclusions
The ultimate goal of local immunotherapy is the generation of a systemic immune response capable of eliminating disseminated tumors and distant metastases following treatment of an accessible tumor site, and several recent reports provide evidence that this may be possible. The studies reported here suggest that injectable alginate matrices may be useful materials for tumor-local delivery of IL-15SA or other immunostimulatory factors, while importantly providing a means to lower systemic exposure and frequency of dosing of these potent and potentially toxic factors. The ability of tumor-surrounding gels to concentrate immunoregulatory factors in neighboring tumors over several days should simultaneously maximize the therapeutic effect on nascent immune responses generated directly at tumor sites. Successful local immune responses might enable the transformation of treated tumors from growing malignancies into in situ vaccines that trigger systemic immunity.
Acknowledgments
This work was supported by the Defense Advanced Research Projects agency (contract # W81XWH-04-C-0139 to D.J.I.), the NIH (EB007280 to D.J.I.; U54-CA126515 and U54-CA112967 to R.O.H.), and the National Science Foundation (award 0348259 to D.J.I.). D.J.I. and R.O.H. are investigators of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gimbel MI, Delman KA, Zager JS. Therapy for unresectable recurrent and in-transit extremity melanoma. Cancer Control. 2008;15(3):225–232. doi: 10.1177/107327480801500305. [DOI] [PubMed] [Google Scholar]
- 2.Crittenden MR, Thanarajasingam U, Vile RG, Gough MJ. Intratumoral immunotherapy: using the tumour against itself. Immunology. 2005;114(1):11–22. doi: 10.1111/j.1365-2567.2004.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joosten J, Jager G, Oyen W, Wobbes T, Ruers T. Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. Eur J Surg Oncol. 2005;31(10):1152–1159. doi: 10.1016/j.ejso.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Herber DL, Nagaraj S, Djeu JY, Gabrilovich DI. Mechanism and therapeutic reversal of immune suppression in cancer. Cancer Res. 2007;67(11):5067–5069. doi: 10.1158/0008-5472.CAN-07-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 6.Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, et al. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol. 2008;20(11):1467–1479. doi: 10.1093/intimm/dxn104. [DOI] [PubMed] [Google Scholar]
- 7.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399–415. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 8.Furumoto K, Soares L, Engleman EG, Merad M. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113(5):774–783. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179(4):2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 10.Nierkens S, den Brok MH, Sutmuller RP, Grauer OM, Bennink E, Morgan ME, et al. In vivo colocalization of antigen and CpG [corrected] within dendritic cells is associated with the efficacy of cancer immunotherapy. Cancer Res. 2008;68(13):5390–5396. doi: 10.1158/0008-5472.CAN-07-6023. [DOI] [PubMed] [Google Scholar]
- 11.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, et al. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182(9):5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 12.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10(2):187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 13.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25(7):876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 14.Hanes J, Sills A, Zhao Z, Suh KW, Tyler B, DiMeco F, et al. Controlled local delivery of interleukin-2 by biodegradable polymers protects animals from experimental brain tumors and liver tumors. Pharm Res. 2001;18(7):899–906. doi: 10.1023/a:1010963307097. [DOI] [PubMed] [Google Scholar]
- 15.Bos GW, Jacobs JJ, Koten JW, Van Tomme S, Veldhuis T, van Nostrum CF, et al. In situ crosslinked biodegradable hydrogels loaded with IL-2 are effective tools for local IL-2 therapy. Eur J Pharm Sci. 2004;21(4):561–567. doi: 10.1016/j.ejps.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, Bankert RB. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60(14):3832–3837. [PubMed] [Google Scholar]
- 17.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29(27):3671–3682. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Hori Y, Winans AM, Irvine DJ. Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater. 2009;5(4):969–982. doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343(8903):950–951. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 20.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: technique and early results. Ann Thorac Surg. 1998;65(6):1540–1544. doi: 10.1016/s0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 21.Hasse C, Klock G, Schlosser A, Zimmermann U, Rothmund M. Parathyroid allotransplantation without immunosuppression. Lancet. 1997;350(9087):1296–1297. doi: 10.1016/S0140-6736(05)62473-7. [DOI] [PubMed] [Google Scholar]
- 22.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stachowiak AN, Wang Y, Huang YC, Irvine DJ. Homeostatic lymphoid chemokines synergize with adhesion ligands to trigger T and B lymphocyte chemokinesis. J Immunol. 2006;177(4):2340–2348. doi: 10.4049/jimmunol.177.4.2340. [DOI] [PubMed] [Google Scholar]
- 24.Jotwani R, Pulendran B, Agrawal S, Cutler CW. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol. 2003;33(11):2980–2986. doi: 10.1002/eji.200324392. [DOI] [PubMed] [Google Scholar]
- 25.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68(8):2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 26.Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12(3):335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 27.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180(4):2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103(24):9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177(9):6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 31.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65(8):3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 32.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66(14):7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 33.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205(9):2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175(9):6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 36.Ryschich E, Schmidt J, Hammerling GJ, Klar E, Ganss R. Transformation of the microvascular system during multistage tumorigenesis. Int J Cancer. 2002;97(6):719–725. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 37.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19(2):217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 40.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8(2):151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heit A, Schmitz F, Haas T, Busch DH, Wagner H. Antigen co-encapsulated with adjuvants efficiently drive protective T cell immunity. Eur J Immunol. 2007;37(8):2063–2074. doi: 10.1002/eji.200737169. [DOI] [PubMed] [Google Scholar]
- 42.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6(8):623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 43.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117(5):1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 46.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194(7):1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2001;2(12):1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]