Abstract
Background
The pericarp color1 (p1) gene encodes for a myb-homologous protein that regulates the biosynthesis of brick-red flavonoid pigments called phlobahpenes. The pattern of pigmentation on the pericarp and cob glumes depends upon the allelic constitution at the p1 locus. p1 alleles have unique gene structure and copy number which have been proposed to influence the epigenetic regulation of tissue-specific gene expression. For example, the presence of tandem-repeats has been correlated with the suppression of pericarp pigmentation though a mechanism associated with increased DNA methylation.
Methodology/Principal Findings
Herein, we extensively characterize a p1 allele called P1-mosaic (P1-mm) that has mosaic pericarp and light pink or colorless cob glumes pigmentation. Relative to the P1-wr (white pericarp and red cob glumes), we show that the tandem repeats of P1-mm have a modified gene structure containing a reduced number of repeats. The P1-mm has reduced DNA methylation at a distal enhancer and elevated DNA methylation downstream of the transcription start site.
Conclusions/Significance
Mosaic gene expression occurs in many eukaryotes. Herein we use maize p1 gene as model system to provide further insight about the mechanisms that govern expression mosaicism. We suggest that the gene structure of P1-mm is modified in some of its tandem gene repeats. It is known that repeated genes are susceptible to chromatin-mediated regulation of gene expression. We discuss how the modification to the tandem repeats of P1-mm may have disrupted the epigenetic mechanisms that stably confer tissue-specific expression.
Introduction
Pericarp pigmentation in maize has important historical relevance in the field of genetics. For example, Gregor Mendel used pericarp pigmentation in maize to verify his work on inheritance ratios in peas [1], [2]. It was in 1869 when Mendel observed the F2 segregation from a hybrid cross between two Zea mays parents that had colorless and brick-red phlobaphene pigmentation on the pericarp. Decades later, R.A. Emerson showed through genetic linkage analysis that pigmentation on the pericarp and cob glumes was specified by pericarp color1 (p1) [3]. Alleles of p1 were classified using a two letter suffix system based on their pericarp and cob glumes pigmentation [4]. All stable combinations -P1-rr, P1-wr, P1-rw and p1-ww as well as two highly unstable p1 alleles were described (Figure 1A). The first, P1-vv, had variegated pericarp and cob glumes and was initially described by R.A. Emerson in 1914 [5], [6]. The second, P1-mosaic (P1-mm), had red mosaic pericarp sectors and colorless or very light cob glumes, and was first described by H.K. Hayes in 1917 [7]. Mosaic pericarp differs from variegated pericarp of P1-vv in that boundaries separating red and white regions are often unclear. Consequently, P1-mm was considered ‘less satisfactory’ for genetic studies [8]. Although the pigmentation of P1-mm is highly unstable, stocks that exhibit different levels of pigmentation including infrequent self-red revertant alleles that resembled the phenotype of P1-rr have been maintained [7], [9], [10], [11].
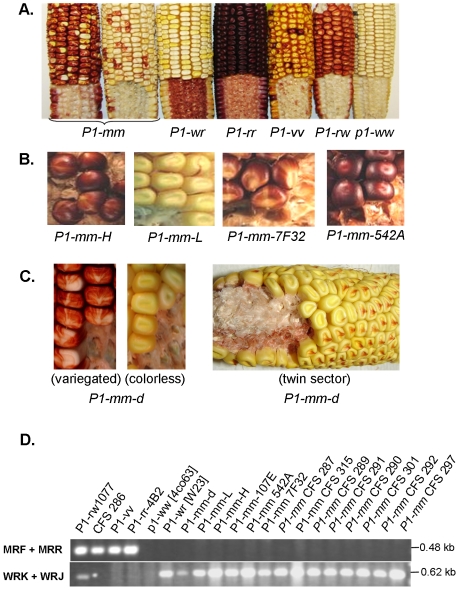
Figure 1. Phenotypic and molecular comparison of p1 alleles used in this study.
A. Diverse p1 alleles conferring different pericarp and cob glume pigmentation patterns. B. Representative ears of P1-mm stocks that differ with respect to their pigmentation. P1-mm-H exhibits a range of heavy variegated pericarp pigmentation and light red cob pigmentation; whereas P1-mm-L has light variegated pericarp pigmentation and light pink or colorless cob glume pigmentation. P1-mm-7F32 and P1-mm-542A are self-red revertant stocks of P1-mm derived from P1-mm-H. C. Representative ears from the P1-mm-d stock showing several possible pigmentation phenotypes. P1-wr-d ears with variegated and colorless pericarp pigmentation are shown. Also shown is an ear that displayed a red cob glumes sector in which the presence of cob pigmentation correlated with a stable “silk scar” type of pigmentation on the pericarp. D. PCR analysis to compare P1-mm alleles (see Table 1) with previously studied p1 alleles. Based on the amplification patterns, the P1-mm alleles could be classified as molecularly similar to P1-wr [W23].
These pioneering maize geneticists recognized the value of flavonoid pigmentation as genetic and phenotypic markers to study the tissue-specific expression manifested in the form of alleles [12]. Nearly a century later, the molecular basis for tissue-specific expression of p1 alleles is becoming clear. The major exception is P1-mm, which unlike P1-vv, has escaped molecular examination for all these years. The variegated pericarp and cob glumes of P1-vv was shown to be attributable to excisions of an Activator (Ac) transposable element from a P1-rr allele [13]. In fact, excisions and reinsertions of Ac have generated an allelic series containing insertions throughout the gene [14], [15], [16]. Depending on the location of Ac, unique pigmentation patterns on the pericarp and cob glumes were obtained. In one case, the transposition of Ac into a distal enhancer of P1-rr resulted in the complete loss of cob pigmentation [17]. Accordingly, the lack of cob pigmentation in the endogenous P1-rw allele has been attributed to the absence of this enhancer [18].
The basis for the pericarp pigmentation in P1-wr was examined by comparing its gene structure and DNA methylation levels with P1-rr. The P1-wr allele from the W23 inbred line was shown to have six tandemly-arranged gene copies, each containing a proximal promoter and coding sequence that share 99% sequence similarity with the single copy P1-rr allele [19]. Moreover, recent sequencing from the B73 inbred line has revealed a P1-wr allele with eleven similar tandemly-arranged copies [20]. In both cases, P1-wr is hypermethylated as compared with P1-rr, however, the presence of a dominant epigenetic modifier Unstable factor for orange1 (Ufo1) leads to a decrease in DNA methylation at P1-wr. The reduction in DNA methylation correlates with an increase in ectopic pericarp pigmentation [21].
A critical question pertains to how the copy numbers and structures of p1 alleles influence their epigenetic states. In order to address this, we set out to identify endogenous alleles that had variations of the copy number of P1-wr [W23]. It was suspected that P1-mm might have multiple copies because the gain of pericarp pigmentation in P1-wr plants in the presence of a dominant modifier Ufo1 can be variegated or mosaic. Examination of the gene structure revealed that P1-mm has a tandem repeat gene structure like P1-wr; however, it has a reduced copy number. We show that two gene copies are highly similar to P1-wr, while other two copies contain a structurally-distinct region in the second intron. Interestingly, P1-mm is also missing the tightly-linked paralogous pericarp color2 gene, which is located over 100 kb upstream from the p1 gene [22]. Since it is known that tandemly-repeated sequences are prone to epigenetic gene silencing, we investigated whether P1-mm has an altered DNA methylation state. We show here that, as compared to P1-wr, P1-mm has a distinct DNA methylation profile; a distal enhancer, shown to be important for pericarp and cob pigmentation [17], [23], [24], [25], is devoid of methylation in P1-mm. Conversely, the region immediately downstream from the transcription start site is hypermethylated in P1-mm. These opposing DNA methylation modifications suggest that both transcriptional enhancing and suppressing epigenetic mechanisms are responsible for the unique expression patterns of P1-mm. The fate of pigmentation would depend on the outcome of competition between these mechanisms.
Results
Phenotypic Analysis of P1-mosaic
P1-mosaic (P1-mm) characteristically has a wide range of mosaic pericarp pigmentation and typically has very light or absent cob glumes pigmentation [4], [7], [8], [9], [11], [13], [26]. In agreement with previous studies, we found that P1-mm ears may have zones of entirely colorless kernels amongst the red variegated regions. The location of pericarp pigmentation was highly unpredictable and some ears completely lacked pigmentation (Figure 1C). Such an absence of pericarp pigmentation was unstable, as the progeny of ears with colorless pericarp expressed a range of variegated pericarp pigmentation. In the unstable stocks of P1-mm a relationship was evident between the pigmentation levels in different tissue types. For instance, the presence of pericarp pigmentation correlated with the presence of silk pigmentation. Similarly, the weak cob glumes pigmentation correlated with a low level of tassel glumes pigmentation. All of these tissues were pigmented in the self-red revertant P1-mm stocks (see Materials and Methods and Figure 1B). Interestingly, the P1-mm-542A revertant allele also had a striking red pigmentation on the leaf mid-rib that is commonly observed in P1-rr alleles. Additionally, the leaf mid-rib pigmentation is also present in some P1-wr plants that have been crossed with maize stocks carrying Ufo1. Like P1-wr Ufo1 plants, P1-mm-542A plants have a curvature to the stem, suggesting that the aberrant expression of p1 in leaves may cause pleiotropic developmental defects.
In the highly variegated stock called P1-mm-d, we identified some ears that had red sectors on the cob glumes (Figure 1C; see Materials & Methods for stock development). Interestingly, the red cob pigmentation sectors often laid below kernels that had a uniform “silk scar” pigmentation pattern on the pericarps. Thus, the uniform pericarp phenotype correlated with red cob pigmentation. Conversely, variegated or colorless pericarp pigmentation occurred above colorless or very light pink cob glumes. These results suggest that a common mechanism affects both pericarp and cob glumes pigmentation; albeit the suppression of pigmentation in cob glumes is usually more pronounced.
P1-mosaic Resembles P1-wr
R.A. Brink and other researchers have collected many different stocks with mosaic pericarp pigmentation (see Materials and Methods) [10]. These mosaic pericarp stocks may have unique origins, or may be similar due to trading amongst researchers or Native American groups [27] prior to entering Brink's collection. Thus, in order to determine if the unique stocks carrying mosaic pericarp have gene structures resembling other p1 alleles (P1-rw-1077, P1-rr-4B2, P1-vv, P1-wr [W23]), we developed an allele-specific PCR genotyping assay. For this assay, the MRF and MRR primer pair was selected because these primers are not present in the P1-wr [W23] sequence. In other functional reference p1 alleles: P1-vv, P1-rr-4B2, and P1-rw1077, the MRF and MRR primer pair results in a 0.48 kb product (Figure 1D). Additionally, the WRJ and WRK primer pair was chosen because it does not amplify the P1-vv and P1-rr-4B2 alleles. In P1-wr [W23] and P1-rw1077, WRJ and WRK yields a 0.62 kb band (Figure 1D). Remarkably, all available stocks carrying mosaic pericarp pigmentation (Table 1, Figure 1B–C) were found to have a P1-wr [W23]-like PCR amplification pattern (Figure 1D). Additionally, the self-red “RR” revertant stocks (P1-mm-542A, P1-mm-7F32, and P1-mm-CFS-315) derived from mosaic stocks (See Materials and Methods) also exhibited the ‘P1-wr’ amplification pattern. Thus, regardless of the origin, all P1-mm alleles carried the same PCR-amplification pattern that is present in P1-wr [W23].
Table 1. Description of various P1-mm alleles used in this study.
| Allele | Origin |
| P1-mm-107E | Unknown |
| P1-mm-L | Maintained light pigmentation though repeated self pollination |
| P1-mm-H | Maintained heavy pigmentation though repeated self pollination |
| P1-mm-542A “RRa” | Revertant stock derived from P1-mm-H |
| P1-mm-7F32 “RRa” | Revertant stock derived from P1-mm-H |
| P1-mm CFS 287b | Unknown |
| P1-mm CFS 315 “RR”a, b | Revertant stock derived from P1-mm CFS 287 |
| P1-mm CFS 289b | Possibly from San Juan, Pueblo, New Mexico |
| P1-mm CFS 291b | Unknown |
| P1-mm CFS 290b | U.S.A |
| P1-mm CFS 301b | Minnesota |
| P1-mm CFS 292b | Purchased from a store in Madison, WI |
| P1-mm CFS 297b | Tesuque, New Mexico |
| CFS286b | Misclassified as P1-mm; has phenotype and gene structure similar to P1-vv: Calea, Near Cuzco, Peru |
“RR” denotes that the allele was a stable revertant of P1-mm that had red pericarp and red cob glumes pigmentation.
Alleles' origins are based upon Brink's annotation in [10]. The P1-mm CFS 315 allele has been previously called P1-rr CFS 315. We report a different name, because it has an identical gene structure as P1-mm.
P1-mosaic and P1-wr Have Tandemly-Repeated Gene Structures
The PCR genotyping assay allowed us to ascertain sequence similarity between P1-mm and P1-wr. In order to explain the marked phenotypic differences between these two alleles, we further compared their gene structures by DNA gel blot analysis. Diagnostic bands revealed that P1-mm shares a tandem-repeat gene structure with P1-wr [W23] (Figure 2) [19]. For example, a 12.6 kb EcoRV band results from the cleavage of intron 2 EcoRV sites in adjacent copies. To verify the extent of the similarity between P1-mm and P1-wr, we sequenced the 5′ and 3′ ends of cDNA from three P1-mm alleles (P1-mm-H, P1-mm-L, and P1-mm-542A) (Figure S1). There was a perfect match to P1-wr [W23] sequence at the 5′ end and near perfect match at the 3′ end (We detected a possible base substitution for the P1-mm-542A allele at the 3′ end). Conversely, the sequence of P1-rr-4B2 is clearly unique at both regions, indicating that P1-wr and P1-mm indeed have common lineages. Though most of the P1-mm promoter remains to be sequenced, limited sequence data was generated while testing the DNA methylation for a 439 bp sequence at the p1 distal enhancer (genomic bisulfite sequencing data is presented later). Interestingly there was a 4 bp insertion in P1-mm (after position 1198 of P1-wr accession EF165349) in the distal enhancer of all 60 P1-mm clones analyzed. Since the multicopy P1-wr [W23] allele did not have this insertion, we may infer that P1-mm and P1-wr could have diverged from a common ancestor and underwent separate tandem duplication events. Alternatively, a recombination-based mechanism may have led to the presence of the 4 bp insertion in every P1-mm gene copy after a common tandem duplication event. All other p1 alleles with available sequences (P1-rr, P1-rw and P1-wr) also do not have the 4 bp insertion, making it unique to the sequence of P1-mm.
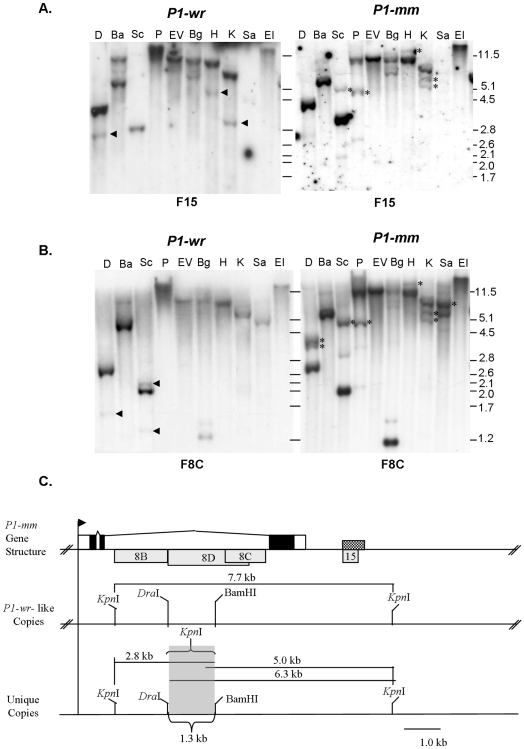
Figure 2. Structural comparison of P1-mosaic and P1-wr [W23].
Gel blots were prepared by digesting seedling leaf DNA with ten diagnostic restriction enzymes. Enzyme shown are: D, DraI; Ba, BamHI; Sc, ScaI; P, PstI; EV, EcoRV, Bg, BglII, H, HindIII; K, KpnI; Sa, SacI; EI, EcoRI. Blots were hybridized with p1 probes corresponding to the distal floral organ enhancer F15 (A) and intron 2 fragment F8C (B). The sizes of molecular weight marker bands are indicated in kilobase pairs to the right of blots. C. The P1-mm gene structure diagram is based upon the published sequence of P1-wr [W23] (Accession EF165349). The positions of exons are shown as rectangles and introns are represented by the connecting lines. The shaded regions of the rectangles represent coding sequence, whereas the unshaded regions represent the UTRs. The arrow represents the transcription start site. The hash marks indicate the positions of linked copies in the tandem gene array. The checkered box shows the position of the p1 distal enhancer, which is present in every gene copy. p1 probe fragments are indicated below the gene structure diagram as grey rectangles. Shown below the map of P1-wr are gene copies of P1-mm that are structurally similar (top) and unique (bottom) from P1-wr. The grey shaded box indicates the region of these copies that is shown to be structurally unique. The sizes of KpnI fragments are given in kilobase pairs.
P1-mosaic Is Missing Sequence Present in P1-wr and the Linked Paralogous pericarp color2 Gene
There are important differences between the gene structures of P1-mm and P1-wr, as indicated by other bands in Figure 2. Specific bands detected in P1-wr, were absent in P1-mm (see bands marked with arrowheads). For example, the distal floral organ enhancer probe fragment 15 did not detect 4.6 HindIII, 3.1 kb KpnI, 2.8 kb DraI and 1 kb-SalI fragments in P1-mm (Figure 2A). A 3.0 kb SalI band that is homologous with intron 2 probe 8B of P1-wr [W23] was also not detected in P1-mm (M. Robbins and S. Chopra, unpublished). Additionally, the p1 intron 2 probe F8C did not detect 2.1 and 1.3 kb ScaI bands or a 1.68 kb DraI band (Figure 2B). The 1.3 kb ScaI band and the 1.68 kb DraI band are derived from the linked pericarp color2 (p2) gene (Accession number AF210616.1), which is paralogous with p1. p2 is located over 100 kb upstream from the p1 gene and is expressed specifically in silk tissue along with p1 to yield a brownish pigmentation [22]. Since p2 is not present in P1-mm, the presence of silk pigmentation would depend solely on the level of p1 expression.
P1-mosaic Has Structurally Unique Copies Containing a Diverse Intron 2 Region
The absence of P1-wr-hybridizing sequence from P1-mm indicated that considerable structural differences exist between their respective gene structures. The region of the tandem array of P1-mm that is most structurally unique could be discerned based on the presence of several P1-mm-specific bands (indicated with asterisks on Figure 2). Particularly with DraI and BamHI digestions, the polymorphic region could be inferred. With intron 2 probe F8C, DraI produces two unique bands of approximately 3.8 and 4.0 kb size in P1-mm (Figure 2B). However, the BamHI banding pattern detected with probes F8C and F15 was similar in P1-wr and P1-mm (Figure 2 and data not shown). These results indicate that structural difference in P1-mm reside within a 1.3 kb region downstream from a DraI site and upstream from a BamHI in the large (4.6 kb) intron 2 (see grey-shaded box in Figure 2C). Gel blots carrying KpnI digested genomic DNA also indicate that there are structural differences at this location. For example, in P1-wr [W23], probes F15, F8B, and F8C are all contained within the same 7.7 kb KpnI fragment. However, in P1-mm, additional KpnI bands were observed: fragments F8C and F15 detect two bands of approximately 5.0 and 6.3 kb, whereas fragment F8B detects a single 2.8 kb band (Figure 2). This suggests that KpnI site(s) separate intron 2 fragments 8B and 8C in two unique P1-mm copies. One likely scenario is that these unique P1-mm copies could contain end deletions of the p1 tandem gene array. The absence of the upstream-linked p2 gene in P1-mm could be explained by an extensive deletion that also affects the 5′ end of the p1 tandem gene array. However, to accurately assemble the structure of the unique P1-mm copies, their sequencing will be required. It is important here to re-emphasize that the expected band sizes found in the tandem array of P1-wr are also found in P1-mm, meaning that some copies are structurally similar to P1-wr.
P1-mosaic Exhibits a Reduced Copy Number Relative to P1-wr
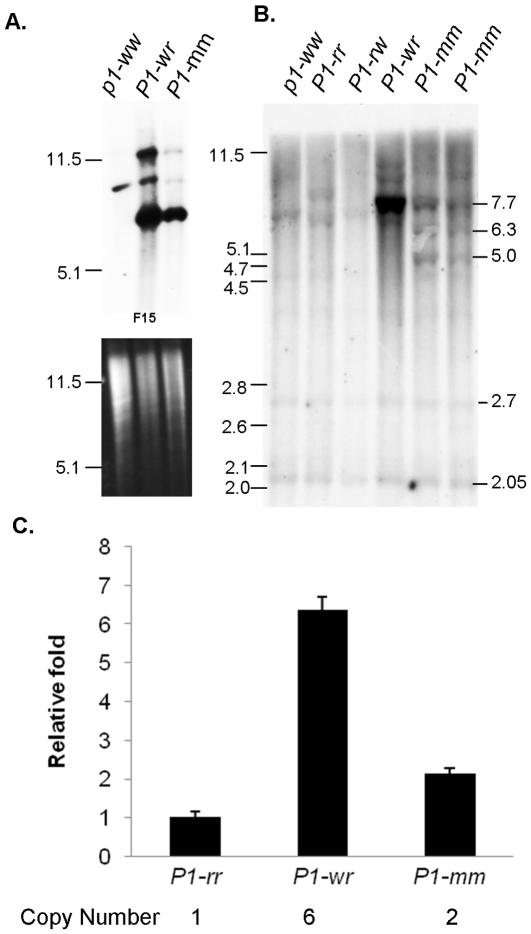
The missing p1 homologous bands in P1-mm indicated that some of the P1-wr gene structure is absent from P1-mm. Consequently, we considered the possibility that P1-mm may have a reduced copy number in its tandem gene array. To test this hypothesis, P1-wr [W23] and P1-mm genomic DNA were digested with BamHI and hybridized with the distal floral organ enhancer probe fragment 15. The signal intensity achieved from P1-mm was considerably less when compared with P1-wr [W23], indicating that the copy number of P1-mm was significantly lower than that of P1-wr (Figure 3A). The relative copy number of P1-mm and other p1 alleles was also compared on a gel blot carrying KpnI-digested DNA which was hybridized with intron 2 probe F8C (Figure 3B). The 2.7 and 2.05 kb bands can be considered as internal DNA loading controls, showing that all lanes had equal loading except the P1-rw lane, which has a reduced DNA loading. We inferred that the signal intensity in P1-mm of an expected 7.7 kb band was much reduced, as compared with P1-wr [W23]. However, the intensity was only slightly greater than the signal derived from the single copy P1-rr-4B2 allele. Consequently, we estimated that P1-mm has two intact gene copies. The KpnI restriction bands of 5.0 and 6.3 kb present in P1-mm and absent from P1-wr are derived from the two unique P1-mm gene copies. To verify the estimation of the copy number in P1-mm from Southern blot hybridization, real-time quantitative PCR (RT-qPCR) was performed using primers specific to p1 intron II probe F8B. P1-rr-4B2 was used as a reference genotype for single copy number. The RT-qPCR result showed a perfect match with Southern blot hybridization: P1-mm and P1-wr [W23] were identified to have two and six copies respectively (Figure 3C). This result also confirms the previous Southern blot-based estimation of P1-wr [W23] copy number [7].
Figure 3. DNA gel blot analysis showing the copy number and structure of P1-mm.
A. The copy number was inferred from BamHI digested DNA gel blots hybridized with the p1 distal floral organ enhancer fragment F15. The ethidium bromide stained gel indicates the relative DNA loading. Lanes are shown with names of alleles. B. Copy number was inferred from KpnI digested DNA gel blots hybridized with intron 2 probe fragment F8C. Molecular weight marker band sizes are shown to the left of the blots. The sizes of bands discussed in the text are indicated in kilobase pairs to the right of the blot. C. Real-time quantitative PCR was performed to estimate the copy number using P1-rr-4B2 as reference genotype (single copy). The relative fold is calculated using P1-rr-4B2 as a reference. Vertical lines indicate standard error (n = 3).
Diverse P1-mosiac Alleles Have Identical Gene Structures
The p1 gene structures for all P1-mosaic stocks in Table 1 were compared with one another. Diagnostic digestion with KpnI was used to analyze the alleles from R.A. Brink's collection using p1 probes 8C (intron II) and F15 (distal enhancer) [10]. The structures of other accessions (P1-mm-107E, P1-mm-d, P1-mm-H, P1-mm-L, P1-mm-7F32, and P1-mm-542A) were determined with different restriction enzymes. Interestingly, the structures of all P1-mm alleles were found to be identical. For simplicity sake, only the gene structure of one P1-mm allele is shown (Figure 2). Importantly, the stable self-red (i.e. “RR”) revertant alleles of P1-mm had the same gene structures as the unstable variegated alleles. This result strongly suggests that the revertant alleles did not arise by germinal transposon excisions from unstable P1-mm alleles. Therefore, transposon excision from P1-mm is not likely the cause of the mosaic pericarp pigmentation.
Major Maize Transposon Families Are Not Involved in the Regulation of P1-mm
In addition to the aforementioned gene structure analysis, several genetic tests support the assertion that the transposase activity of major maize transposon families is not involved in the regulation of P1-mm Crosses of P1-mm with an Ac- tester stock have determined that active Ac element(s) are not responsible for the phenotype of P1-mm [13]. Subsequently, it was reported that mosaic pericarp is still possible in the absence of active Spm elements [28], [29], [30]. A major class of maize transposable elements that had not been tested with respect to pericarp pigmentation of P1-mm belongs to the Mutator (Mu) family. In order to silence Mu elements, we crossed a homozygous P1-mm stock with a p1-ww stock heterozygous for Muk. Muk encodes an inverted repeat transcript that functions to silence the MuDR transposase which is required for the mobility of Mu elements [31], [32], [33]. F1 individuals were genotyped for the presence of Muk by PCR (see Methods). The range of mosaic pericarp pigmentation was similar for the 12 Muk and 12 wild type plants assayed, indicating that Mu activity does not affect the P1-mm phenotype (not shown).
DNA Methylation of P1-Mosaic Suggests the Involvement of an Epigenetic Mechanism
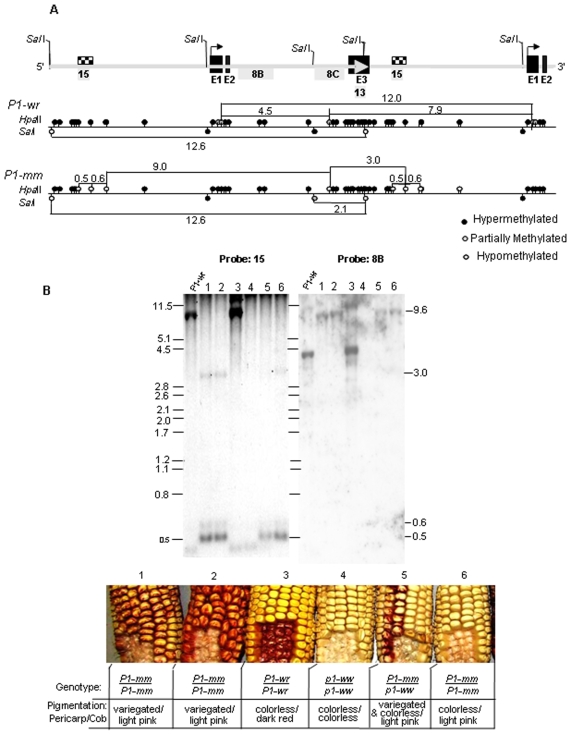
We showed that P1-mm alleles resemble the tandem-arrayed gene structure of P1-wr, albeit with structural differences in intron 2 in some copies. Additionally, we showed that the P1-mm has two rather than the six copies present in P1-wr [W23]. Given that P1-wr is hypermethylated relative to the single copy P1-rr allele [19], we considered the hypothesis that reduced copy number contributes to the presence of pericarp pigmentation. DNA gel blot analysis was used to construct a DNA methylation map comparing P1-mm and P1-wr [W23] (Figure 4A). With SalI, P1-wr [W23] produces a 12.6 kb band that extends the entire length of the gene. This is because two of the three SalI sites in P1-wr [W23] are hypermethylated. Interestingly, in P1-mm we detected an additional 2.1 kb band, which suggested that a SalI site in intron 2 (at position 10,310 of accession EF165349) was partially hypomethylated (Figure 4A). Considerable hypomethylation was also detected at the distal floral organ enhancer, as evidenced by diagnostic 500 bp and 600 bp F15-homolgous HpaII bands (Figure 4A, B). The presence of these small molecular weight bands in P1-mm was correlated with the complete absence of a 7.9 kb HpaII band. This result demonstrated that P1-mm copies are hypomethylated at the single HpaII site contained within the distal enhancer region. However, the presence of a probe F15 hybridizing 3.0 kb HpaII band in P1-mm suggests that the DNA methylation might be similar to P1-wr [W23] upstream from this distal enhancer (Figure 4A).
Figure 4. P1-mosaic has a highly unique DNA methylation pattern as compared to P1-wr.
For this analysis, the P1-mm-d allele was used. A. P1-wr [W23] was used as a template [19] to construct the DNA methylation map for P1-mm. The intron/exon structure of P1-wr is shown as a line diagram above the methylation maps. The large grey arrow shown on the line diagram represents the end of a copy in the tandem array. The bent arrow indicates the location of the transcription start site. Exons are abbreviated as E1, E2, and E3. The placement of p1 probes (grey shaded boxes) is shown immediately below the line diagram. DNA methylation maps are shown below the gene structure. On the DNA methylation maps, black circles indicate hypermethylated sites; grey circles indicate partially-methylated sites; non shaded circles represent hypomethylated sites. B. DNA methylation analysis of P1-mosaic sibling plants differing for pericarp pigmentation. Seedling leaf genomic DNA from plants of indicated genotypes was digested with the methylation-sensitive restriction enzyme HpaII and the resulting blot was sequentially hybridized with p1 probes corresponding to the distal floral enhancer fragment F15 and intron 2 fragment F8B. Ear phenotypes from plants used for DNA methylation analysis are shown below the blots. Genotype and phenotype information is also provided in the table below each ear. The methylation map showing P1-mm in A was constructed based on the results of these hybridizations. Since some P1-mm copies are structurally unique from P1-wr at a region in intron 2 (see Figure 2), the placement of fragments which span probe F8C were estimated based on the published sequence of P1-wr [W23].
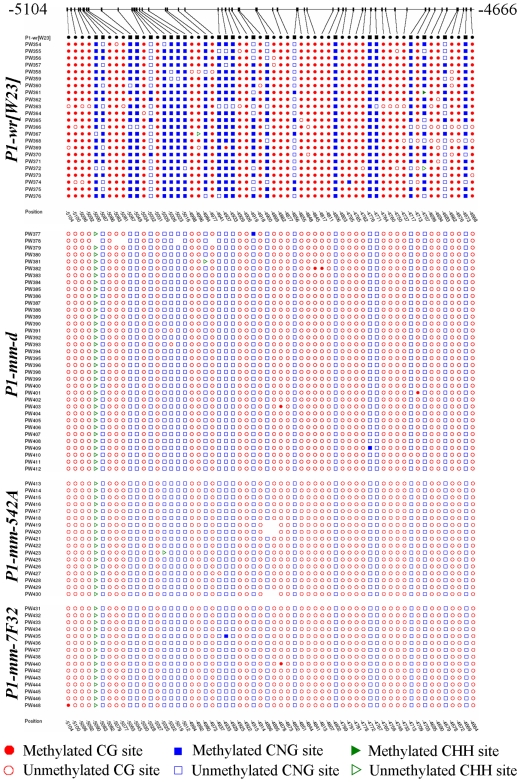
To further resolve methylation status of individual cytosine residues, we performed genomic bisulfite sequencing of a 439 bp region of the p1 distal enhancer (positions -5104 to -4666 of EF165349) (Figure 5). This region has been previously shown to house enhancer elements that are important for p1-induced pericarp and cob pigmentation [21], [34]. In agreement with our previous observations (Chopra 2003; Sekhon 2007), P1-wr [W23] has high levels of CG and CNG methylation in the region as indicated by predominantly filled circles and squares, respectively. Interestingly, the distal enhancer region of mosaic and self-red revertant P1-mm alleles was essentially devoid of DNA methylation in CG and CNG contexts. Such an absence of DNA methylation at the distal enhancer region was also observed for P1-rr, which shows uniformly red pericarp and cob pigmentation (R. Sekhon and S. Chopra, unpublished). Finally, CNN methylation levels in the distal enhancer region were negligible for all the alleles tested (data not shown). Overall, these results show that the P1-mm alleles are uniformly hypomethylated and that the mosaic (P1-mm-d) and self-red reverent (P1-mm-7F32 and P1-mm-542A) alleles do not differ for their methylation across the distal enhancer region.
Figure 5. The distal floral enhancer of mosaic and self-red revertant alleles of P1-mm has negligible levels of DNA methylation when compared to P1-wr [W23].
The DNA methylation of a 439 bp fragment located at the 3′ end of a distal floral organ enhancer was analyzed by genomic bisulfite sequencing. The length of this fragment is 443 bp for P1-mm alleles due to a 4 bp insertion (See Materials and Methods for details). The location of this distal enhancer is shown as a checkered box on the gene structure diagram in Figure 4A. Seedling leaf genomic DNA from P1-wr [W23], P1-mm-d and the self-red revertant alleles P1-mm-7F32 and P1-mm-542A was used for this assay. Methylation status of cytosines in CG (circles) and CNG (squares) context in individual clones of the p1 alleles is shown. One triangle present in three P1-mm alleles represents a CNN site that was created due to the 4 bp insertion in this region. The rest of the CNN sites are not shown as they completely lacked methylation in P1-wr [W23] and the three P1-mm alleles. [50]
As compared with P1-wr, P1-mm has a striking increase in DNA methylation downstream from the transcription start site (Figure 4A). This is evidenced by the absence of a 4.5 kb HpaII band that is homologous with intron 2 probe fragment 8B (Figure 4B). The 4.5 kb band size results from digestion of a HpaII site downstream from the transcription start. In P1-mm the 4.5 kb band is replaced by a 9 kb fragment (Figure 4B). The 9 kb fragment can be explained by increased DNA methylation at HpaII site(s) downstream from the transcription start site (Figure 4A). The 9 kb band size also takes into account the absence of DNA methylation at the distal enhancer, as previously discussed. The F8C probe region of intron 2 in P1-mm contains a large number of low intensity HpaII bands that were not placed on the methylation map (M. Robbins and S. Chopra unpublished). This result was expected, since the F8C probe is adjacent to the region where we have identified structural differences between P1-mm and P1-wr (see Figure 2C). The HpaII sites producing these bands are predicted to reside in the structurally unique copies of P1-mm. We found that the intron 2 region, like the distal enhancer region, exhibited no difference in the HpaII banding pattern between mosaic and revertant P1-mm stocks using p1 probe F8C.
In summary, DNA methylation did not correlate with establishment or maintenance of pigmentation levels in P1-mm and was thus not predictive of the level of phlobaphene pigmentation. The same DNA methylation pattern was present for individuals with variegated, colorless, and self-red revertant pericarp (Figure 4B). It is, however, important to stress that altered DNA methylation in P1-mm, as compared with P1-wr [W23], is predictive of modified chromatin packaging [35]. A highly interesting pattern was observed: P1-mm is devoid of methylation at a distal floral enhancer and is hypermethylated downstream from the transcription start site.
Discussion
Phlobaphene Pigmentation Is Correlated with the Copy Number of p1 Alleles
Tandem repeats have been linked with heterochromatin-associated gene silencing [36]. Transgenes that incorporate as arrays of multiple copies are especially prone to variegation associated with their silencing [37]. Such Repeat Induced Gene Silencing (RIGS) of transgenes in plants involves a homologous DNA pairing mechanism that correlates with DNA methylation levels [38]. The pairing of homologous DNA in ‘RIGS’ parallels that described for Position Effect Variegation (PEV) in Drosophila [39]. In PEV, the variable placement of a marker gene in euchromatin or heterochromatin dictates its expression [40]. Positioning in heterochromatin is enhanced by the presence of multiple gene copies, and an increased level of variegated silencing is thought to involve pairing interactions between copies [41], [42], [43], [44]. Such preferential pairing interactions between homologous transgenes have also been observed in Arabidopsis by fluorescent chromatin tagging experiments [45]. Pairing may be accomplished by proteins binding to identical sites in linked copies, which subsequently form homodimers that strengthen chromatin association [36], [38], [39], [46]. In P1-wr, the multiple tandemly-arranged copies may similarly associate to direct heterochromatin formation. Evidence that supports this copy paring hypothesis in P1-wr was presented for a Mu transposon insertion allele called P1-wr-mum6 that contains a Mu1 transposon in the 5′UTR of one of the P1-wr gene copies [47]. P1-wr-mum6 exhibits ectopic pericarp pigmentation that was shown to arise from the increased expression of gene copies that were not interrupted by the transposon. Furthermore, the stable gain of pericarp expression in P1-wr-mum6 correlated with the hypomethylation of a distal floral organ enhancer. Thus, disruption of a single P1-wr gene copy can lead to the loss of suppression of other wildtype copies, which suggests for a possible association between these gene copies.
In opposition to longstanding dogma in the P1-mm literature, our genetic and molecular data suggest that the characteristic mosaic pigmentation of P1-mm arises by a mechanism that does not involve active transposon insertions or excisions. Rather, the unique gene structure of P1-mm itself may influence its variable expression states. We showed that both P1-mm and P1-wr have tandemly-repeated gene copies; however, P1-mm had a significantly lower copy number and was missing p1 homologous sequences that are predicted to be linked. Interestingly, we found that the upstream linked p2 gene which, is a paralog of p1 that is exclusively expressed in silk [22], is not present in P1-mm. The absence of p2 suggests a deletion 5′ at the p1 gene in P1-mm. Such deletion(s) may have also affected the tandem array of P1-mm since two gene copies of P1-mm were found to be structurally unique from P1-wr at an intron 2 region. These unique copies in P1-mm may be truncated versions of previously functional copies that could have been required for stable tissue-specific expression patterns. It is possible that the unique P1-mm copies could be a source of aberrant transcripts that initiate siRNA-based silencing in a variable fashion to yield the mosaic pigmentation patterns. For example, a truncated copy of the tandemly repeated barley Mlo gene produces siRNA that functions to block the expression from wild type copies [48].
The location of the structural difference in the intron 2 region of P1-mm may be critical for governing its phenotype. Importantly, the intron 2 region contains a 168 bp regulatory sequence that has been implicated in the specification of cob pigmentation in P1-wr [49]. The DNA hypermethylation of this 168 bp sequence was correlated with the loss of cob pigmentation in an epiallele of P1-wr called P1-wr*. Therefore, the disruption of this region in P1-mm may lead to suppression of cob pigmentation that is typical of most P1-mm ears. Our P1-mm phenotypic association data also showed that the absence of cob pigmentation was strictly related with the mosaic suppression of pericarp pigmentation. Thus, in addition to cob pigmentation, the structural difference in the intron 2 region could have engendered colorless sectors on P1-mm pericarp. The mosaic pericarp pigmentation in P1-mm may reflect variable activity of a suppression mechanism associated with the presence of multiple gene copies.
Competing Transcriptional Enhancing and Suppressing Epigenetic Mechanisms May Be Responsible for the Unique Expression Patterns of P1-mm
Nearly 30 years ago, Drew Schwartz proposed a presetting and erasure model for the expression of pigmentation in P1-vv [50]. Pigmentation in the kernel gown was immediately preset to a suppressed state; however the suppression on the kernel crown and cob glumes was not set until the subsequent generation. The suppressed state of the kernel gown in P1-vv, could usually be erased to a P1-rr state if progeny were sown from kernels exhibiting full crown pigmentation. Based on these observations, Schwartz hypothesized that the p1 gene contains distinct cis-regulatory regions for kernel crown, kernel gown, and cob glumes pigmentation. We now know that such temporal, non heritable and tissue-specific differences in expression can all be explained by changes to the epigenetic state of a gene. More recent studies have begun to shed light on how the epigenetic state of specific regulatory regions of p1 correlates with allelic expression differences.
Herein, we found that DNA methylation levels did not correlate with pericarp pigmentation levels of P1-mm. We showed that P1-mm plants with pigmented, colorless, or self-red revertant pericarps exhibited no difference in DNA methylation. However, the extensive DNA methylation differences between P1-wr and P1-mm would be predicted to affect their respective chromatin structures. In other words, the DNA methylation status of P1-mm may report for relatively unstable chromatin structure. Relative to P1-wr, P1-mm was shown to be both hypermethylated and hypomethylated, depending on which region was assayed. The DNA of P1-mm was hypermethylated downstream from the transcription start site, but was devoid of methylation at a distal floral organ enhancer. The opposing DNA methylation modifications at these distinct regulatory regions may respectively direct the placement of P1-mm in euchromatin and heterochromatin. Thus, for unstable P1-mm alleles, epigenetic enhancing and suppressing mechanisms may compete to dictate the outcome of gene expression and pigmentation.
The aforementioned hypothesis for the competition between cis regulatory regions is strengthened when the regions exhibiting DNA methylation differences in P1-mm are taken into account. In several studies, elevated P1-wr expression that results in pericarp pigmentation is correlated with the hypomethylation at a distal floral organ enhancer. For example, several P1-wr alleles that have pericarp pigmentation confined to the kernel gown are partially hypomethylated at this enhancer [10], [51], [52]. Additionally, both P1-wr-mum6 and P1-wr Ufo1 plants that have ectopic pericarp pigmentation also exhibit partial hypomethylation in this region [21], [47]. Thus, DNA hypomethylation at the distal floral organ enhancer has been suggested to be conducive to pericarp pigmentation. It was therefore interesting to discover that the substantial hypomethylation of the distal floral organ enhancer did not always correlate with the presence of variegated pericarp pigmentation in P1-mm. Such variability in pigmentation was not explainable until other regions of P1-mm were examined for DNA methylation. The DNA hypermethylation downstream from the transcription start site in P1-mm parallels results found for the P1-wr* epiallele [49]. In both P1-wr* and P1-mm this hypermethylation was correlated with the suppression of cob pigmentation. However, in P1-mm, we showed that the suppression of cob pigmentation was correlated with the instability of pericarp pigmentation. Thus, the hypermethylation downstream from the transcription start site may have a suppressing affect on both pericarp and cob glumes pigmentation.
Hypermethylation has typically been associated with heterochromatin and transcriptional suppression, whereas hypomethylation is associated with euchromatin and transcriptional activation [53]. In this model, the hypomethylation at the distal floral organ enhancer would report for the involvement of a mechanism that induces euchromatin formation and gene expression. Meanwhile, the hypermethylation downstream from the transcription start site would suggest for the involvement of a mechanism that induces heterochromatin formation and transcriptional suppression. The interplay between the unique gene structure and DNA methylation may have rendered P1-mm a rather recalcitrant target for the heterochromatin machinery. However, it is likely that chromatin states of P1-mm can become more stable though the continued selection of either heavy of light pigmentation levels in self pollinated progeny. For example, the P1-mm-H allele with dark mosaic pigmentation would be expected to have a more relaxed chromatin structure than the P1-mm-L allele that produces light mosaic pigmentation. The self-red revertant alleles may be the outcome of infrequent events, in which the chromatin structure changed so that the aforementioned suppression mechanism is no longer active. We conclude that mosaic pericarp pigmentation of P1-mm is likely induced by the absence or modification of some of its tandemly-repeated gene copies that were present in a p1 ancestral allele. Such structural modifications in P1-mm may have led to the variable activity of the epigenetic silencing mechanism that in P1-wr stably regulates the suppression in pericarp tissue. This study paves the way for the future cloning of P1-mm and the further dissection of the epigenetic mechanism that governs its unique expression patterns.
Materials and Methods
P1-mosaic Stocks
The mosaic and self-red revertant stocks of P1-mm were introgressed into p1-ww [4co63] by R.A. Brink [10], [13]. Subsequently, the Brink's collection was maintained by self pollination at the Maize Genetics Cooperation - Stock Center (University of Illinois, Urbana-Champaign). The Brink's stocks are of unique origins (Table 1) with the exception of P1-mm-CFS-315, which was derived from the mosaic P1-mm-CFS-287 allele. In this study, we used P1-mm stocks generously given to us by Dr. Thomas Peterson (Iowa State University). These stocks include unstable heavy (P1-mm-H) and light (P1-mm-L) mosaic stocks that were generated by repeatedly selecting for high and low pigmentation levels in self pollinated progenies (Figure 1B). Additionally, we obtained from Dr. Peterson stable P1-rr-like revertants from P1-mm-H. These revertants called P1-mm-7F32 and P1-mm-542A had red pericarp and red cob glumes (Figure 1B). The P1-mm-7F32 stock had red patterned appearance, whereas the P1-mm-542A had uniformly red pericarps and cob glumes. An additional P1-mm stock, called P1-mm-d (derivative), was developed by crossing P1-mm-H and P1-wr plants. Resulting F2 generation plants were crossed with p1-ww [4co63] and the ensuing progeny was self pollinated. From these crosses, P1-mm-d, P1-wr, and p1-ww [4co63] segregants were molecularly examined.
Generation of P1-mosaic Stock with Inactive Mutator Elements
The effect of Mutator (Mu) transposon activity on P1-mm variegation was tested by crossing a homozygous P1-mm stock with a p1-ww stock that was heterozygous for Mu killer (Muk) [31], [32], [33]. The presence of Muk was determined by PCR assay available at http://plantbio.berkeley.edu/~mukiller/using.html
DNA Gel Blot Analysis
Seedling leaf genomic DNA was prepared using a modified CTAB method [54]. PCR genotyping was performed using standard conditions with primers listed in Table S1. Restriction digestion was achieved by using enzymes, reagents and protocols from Promega (Madison, WI). Restricted genomic DNA was fractionated on 0.8% agarose gels and subsequently transferred to Nylon membranes. Membranes were prehybridized for 15 h and hybridized for 15 h at 65°C in buffer containing NaCl (1 M), SDS (1%), Tris-HCl (10 mM) and 0.25 mg/ml salmon sperm DNA [15]. The DNA probes used for hybridizations were labeled with [α-32P]dCTP through random priming using Prime-It® RmT Random Primer Labeling Kit (Stratagene, La Jolla, CA). Blots were stripped of previous probe by boiling in 0.1% SDS before they were reused. The relative copy number of P1-mm was estimated by comparing its signal intensity with that of other p1 alleles for which copy number is known [19]. The methylation-sensitive restriction enzymes HpaII, and SalI were used to characterize the DNA methylation status of the p1 gene at several regions.
cDNA Sequence Analysis
Pericarp tissue was collected 18 days after pollination and 500 mg was used for RNA extraction. Total RNA was isolated using a standard Trizol-based extraction protocol (Invitrogen Corporation, Carlsbad, CA). 25 µg of total RNA was subsequently incubated with DNAseI according to the manufacturer's directions (Invitrogen Corporation, Carlsbad, CA). First-strand cDNA was synthesized using the SuperScript® III First-Strand Synthesis System (Invitrogen Corporation, Carlsbad, CA). The reverse transcription reaction was done by using an oligo(dT) adaptor primer and 5 µg of DNAseI-treated total RNA. The cDNA was used as a template for PCR reactions with gene specific primers listed in Table S1. PCR products were cloned into a PT7-Blue cloning vector (Novagen) and sequenced. Sequences were aligned using the ClustalW2 web-based program.
p1 Probe Fragments
The p1 probe fragments F8B, F8C, F13, and F15 used in this study have previously been described [19], [49], [55]. The p1 probe fragment 8D was purified from the PCR product amplified using the WR8F and WR7R primers (Table S1).
Genomic Bisulfite Sequencing
Two plants each of three P1-mm alleles and P1-wr [W23] were subjected to genomic bisulfite sequencing. Eight micrograms seedling leaf DNA from individual plants was restricted with SspI and ScaI and purified with phenol-chloroform before treating with sodium bisulfite [49], [56]. The upper strand of the sodium bisulfite treated DNA was amplified using specially designed PCR primers [47] to yield a 439 bp band from the p1 distal enhancer (positions -5104 to -4666 of EF165349). The length of the region studied in P1-mm alleles (P1-mm-d, P1-mm-542A, and P1-mm-7F32) is 443 bp due to a four bp insertion (at position -5088) in these alleles. Furthermore, due to the insertion, a CNG site becomes a CNN site in these alleles. PCR products were gel-purified and cloned with a TOPO TA cloning kit (Invitrogen Corporation, Carlsbad, CA) and sequenced using vector primers.
Real-Time Quantitative PCR
Genomic DNA was quantified using NanoDrop (NanoDrop 1000, Thermo Scientific) and diluted to the same concentration (100 ng/µl) for Real-Time PCR use according to the NanoDrop measurements. The DNA was subsequently run on agarose gel to verify its quality and concentration. RT-qPCR was performed in the ABI7500 fast real-time PCR system, using SYBR Green I (Roche, Indianapolis, IN) as the detection system and the default program: 10 minutes of pre-incubation at 95°C followed by 40 cycles for 15 seconds at 95°C and one minute at 60°C. Each RT-qPCR reaction was carried out in 20 µl volumes containing 100 ng of genomic DNA from each genotype, 1 µM RTF8B-1 primers and 2X SYBR Green I master mix. Real-time PCR oligonucleotide primers which located on the p1 F8B fragment are designed to amplify a 60 bp fragment (positions 8299–8359 of EF165349) using Primer Express 3.0 (Applied Biosystems). The ΔCt was calculated as follows ΔCt = Ct (P1-rr-4B2, P1-wr or P1-mm)-Ct (P1-rr) for each genotype. P1-rr served as a reference genotype because it is known to have one gene copy. Hence, the results for copy number are expressed in N-fold (N = 2−ΔCt) changes relative to P1-rr-4B2. Standard errors were calculated using three independent preparations of genomic DNA and three technical replicates for each genomic DNA preparation.
Supporting Information
Alignment of 5′ and 3′ cDNA sequences obtained form three P1-mm alleles with known sequences of P1-wr, P1-rr, and p2.
(0.04 MB DOC)
Sequences of PCR primers used in this study.
(0.04 MB DOC)
Acknowledgments
We would like to thank Dr. Thomas Peterson, Iowa State University and the Maize Genetics Cooperation - Stock Center for the generous gift of P1-mm stocks. We thank Dr. Marty Sachs for sharing his phenotypic observation of P1-mm alleles.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by an NSF award MCB-0619330, a Hatch 4154 award to SC, and dissertation award from the College of Agricultural Sciences and Research Assistantships from the Department of Crop and Soil Sciences and Plant Biology Graduate Program to MLS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Coe EH., Jr The origins of maize genetics. Nat Rev Genet. 2001;2:898–905. doi: 10.1038/35098524. [DOI] [PubMed] [Google Scholar]
- 2.Correns CG. Mendel's Regel uber das Verhalten der Nachkommenschaft der Rassenbastarde. Berichte Deut Bot Ges. 1900;18:158–168. [Google Scholar]
- 3.Emerson RA. Genetic correlation and spurious allelomorphism in maize. Ann Rept Nebraska Agric Exp Sta. 1911;24:59–90. [Google Scholar]
- 4.Anderson EG. Pericarp studies in maize: II. The allelomorphism of a series of factors for pericarp color. Genetics. 1924;9:442–453. doi: 10.1093/genetics/9.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emerson RA. The inheritance of a recurring somatic variation in variegated ears of maize. Am Nat. 1914;48:87–115. [Google Scholar]
- 6.Emerson RA. Genetic studies of variegated pericarp in maize. Genetics. 1917;2:1–35. doi: 10.1093/genetics/2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes HK. Inheritance of a Mosaic Pericarp Pattern Color of Maize. Genetics. 1917;2:261–281. doi: 10.1093/genetics/2.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EG, Ter Louw AL. Description of Mosaic Pericarp Color in Maize. Mich Acad Sci Arts and Letters Papers. 1929;9:5–9. [Google Scholar]
- 9.Eyster WH. Mosaic Pericarp in Maize. Genetics. 1925;10:179–196. doi: 10.1093/genetics/10.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brink RA, Styles ED. A collection of pericarp factors. Maize Genetics Cooperation Newsletter. 1966;40:149–160. [Google Scholar]
- 11.Suto T. Genetic analysis of a mosaic pericarp in maize, with special reference to the genetic change of the color pattern induced by the possiby unequal crossing-over in a small regoin containing the P-locus and its neighbors of chromosome I. Journal of the Faculty of Science, Hokkaido University. 1952;6:68–150. [Google Scholar]
- 12.Fedoroff N. Marcus Rhoades and transposition. Genetics. 1998;150:957–961. doi: 10.1093/genetics/150.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barclay PC, Brink RA. The Relation between Modulator and Activator in Maize. Proc Natl Acad Sci U S A. 1954;40:1118–1126. doi: 10.1073/pnas.40.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Athma P, Grotewold E, Peterson T. Insertional mutagenesis of the maize P gene by intragenic transposition of Ac. Genetics. 1992;131:199–209. doi: 10.1093/genetics/131.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athma P, Peterson T. Ac induces homologous recombination at the maize P locus. Genetics. 1991;128:163–173. doi: 10.1093/genetics/128.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grotewold E, Athma P, Peterson T. A possible hot spot for Ac insertion in the maize P gene. Mol Gen Genet. 1991;230:329–331. doi: 10.1007/BF00290684. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Peterson T. Comparisons of maize pericarp color1 alleles reveal paralogous gene recombination and an organ-specific enhancer region. Plant Cell. 2005;17:903–914. doi: 10.1105/tpc.104.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang F, Peterson T. Gene conversion between direct noncoding repeats promotes genetic and phenotypic diversity at a regulatory locus of Zea mays (L.). Genetics. 2006;174:753–762. doi: 10.1534/genetics.105.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra S, Athma P, Li XG, Peterson T. A maize Myb homolog is encoded by a multicopy gene complex. Mol Gen Genet. 1998;260:372–380. doi: 10.1007/s004380050906. [DOI] [PubMed] [Google Scholar]
- 20.Goettel W, Messing J. Change of gene structure and function by non-homologous end-joining, homologous recombination, and transposition of DNA. PLoS Genet. 2009;5:e1000516. doi: 10.1371/journal.pgen.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chopra S, Cocciolone SM, Bushman S, Sangar V, McMullen MD, et al. The maize Unstable factor for orange1 is a dominant epigenetic modifier of a tissue specifically silent allele of pericarp color1. Genetics. 2003;163:1135–1146. doi: 10.1093/genetics/163.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Chopra S, Peterson T. A segmental gene duplication generated differentially expressed myb-homologous genes in maize. Plant Cell. 2000;12:2311–2322. doi: 10.1105/tpc.12.12.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidorenko LV, Li X, Cocciolone SM, Chopra S, Tagliani L, et al. Complex structure of a maize Myb gene promoter: functional analysis in transgenic plants. Plant J. 2000;22:471–482. doi: 10.1046/j.1365-313x.2000.00750.x. [DOI] [PubMed] [Google Scholar]
- 24.Sidorenko LV, Peterson T. Transgene-induced silencing identifies sequences involved in the establishment of paramutation of the maize p1 gene. Plant Cell. 2001;13:319–335. doi: 10.1105/tpc.13.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekhon RS, Chopra S. Progressive Loss of DNA Methylation Releases Epigenetic Gene Silencing From a Tandemly Repeated Maize Myb Gene. Genetics. 2009;181:81–91. doi: 10.1534/genetics.108.097170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson RA. A Zygotic Lethal in Chromosome 1 of Maize and Its Linkage with Neighboring Genes. Genetics. 1939;24:368–384. doi: 10.1093/genetics/24.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckler ES, Gaut BS, McMullen MD. Molecular and functional diversity of maize. Curr Opin Plant Biol. 2006;9:172–176. doi: 10.1016/j.pbi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Brawn RI. A test for Spm control of mosaic pericarp. Maize Genetics Cooperation Newsletter. 1966;40:92–94. [Google Scholar]
- 29.Brawn Spm regulation of Diffuse and mosaic pericarp. 1131967;41 [Google Scholar]
- 30.Nelson OE. Mosaic pericarp does not result from an Spm insertion. Maize Genetics Cooperation Newsletter. 1991;65:85–86. [Google Scholar]
- 31.Slotkin RK, Freeling M, Lisch D. Mu killer causes the heritable inactivation of the Mutator family of transposable elements in Zea mays. Genetics. 2003;165:781–797. doi: 10.1093/genetics/165.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slotkin RK, Freeling M, Lisch D. Heritable transposon silencing initiated by a naturally occurring transposon inverted duplication. Nat Genet. 2005;37:641–644. doi: 10.1038/ng1576. [DOI] [PubMed] [Google Scholar]
- 33.Lisch D, Carey CC, Dorweiler JE, Chandler VL. A mutation that prevents paramutation in maize also reverses Mutator transposon methylation and silencing. Proc Natl Acad Sci U S A. 2002;99:6130–6135. doi: 10.1073/pnas.052152199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Peterson T. Comparisons of maize pericarp color1 alleles aeveal paralogous gene recombination and an organ-specific enhancer region. Plant Cell. 2005;17:903–914. doi: 10.1105/tpc.104.029660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Hall TC, Holmes-Davis R. Plant chromatin: development and gene control. Bioessays. 2002;24:234–243. doi: 10.1002/bies.10055. [DOI] [PubMed] [Google Scholar]
- 36.Bender J. Cytosine methylation of repeated sequences in eukaryotes: the role of DNA pairing. Trends Biochem Sci. 1998;23:252–256. doi: 10.1016/s0968-0004(98)01225-0. [DOI] [PubMed] [Google Scholar]
- 37.Henikoff S. Conspiracy of silence among repeated transgenes. Bioessays. 1998;20:532–535. doi: 10.1002/(SICI)1521-1878(199807)20:7<532::AID-BIES3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 38.Assaad FF, Tucker KL, Signer ER. Epigenetic repeat-induced gene silencing (RIGS) in Arabidopsis. Plant Mol Biol. 1993;22:1067–1085. doi: 10.1007/BF00028978. [DOI] [PubMed] [Google Scholar]
- 39.Ye F, Signer ER. RIGS (repeat-induced gene silencing) in Arabidopsis is transcriptional and alters chromatin configuration. Proc Natl Acad Sci U S A. 1996;93:10881–10886. doi: 10.1073/pnas.93.20.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schotta G, Ebert A, Dorn R, Reuter G. Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin Cell Dev Biol. 2003;14:67–75. doi: 10.1016/s1084-9521(02)00138-6. [DOI] [PubMed] [Google Scholar]
- 41.Sabl JF, Henikoff S. Copy number and orientation determine the susceptibility of a gene to silencing by nearby heterochromatin in Drosophila. Genetics. 1996;142:447–458. doi: 10.1093/genetics/142.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandler VL. Poetry of b1 paramutation: cis- and trans-chromatin communication. Cold Spring Harb Symp Quant Biol. 2004;69:355–361. doi: 10.1101/sqb.2004.69.355. [DOI] [PubMed] [Google Scholar]
- 43.Matzke M, Mette MF, Jakowitsch J, Kanno T, Moscone EA, et al. A test for transvection in plants: DNA pairing may lead to trans-activation or silencing of complex heteroalleles in tobacco. Genetics. 2001;158:451–461. doi: 10.1093/genetics/158.1.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant-Downton RT, Dickinson HG. Plants, pairing and phenotypes–two's company? Trends Genet. 2004;20:188–195. doi: 10.1016/j.tig.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Pecinka A, Meister A, Schubert I, Lam E. DNA hypomethylation reduces homologous pairing of inserted tandem repeat arrays in somatic nuclei of Arabidopsis thaliana. Plant J. 2005;44:531–540. doi: 10.1111/j.1365-313X.2005.02546.x. [DOI] [PubMed] [Google Scholar]
- 46.Bender J. Chromatin-based silencing mechanisms. Curr Opin Plant Biol. 2004;7:521–526. doi: 10.1016/j.pbi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Robbins ML, Sekhon RS, Meeley R, Chopra S. A Mutator Transposon Insertion Is Associated With Ectopic Expression of a Tandemly Repeated Multicopy Myb Gene pericarp color1 of Maize. Genetics. 2008;178:1859–1874. doi: 10.1534/genetics.107.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piffanelli P, Ramsay L, Waugh R, Benabdelmouna A, D'Hont A, et al. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nature. 2004;430:887–891. doi: 10.1038/nature02781. [DOI] [PubMed] [Google Scholar]
- 49.Sekhon RS, Peterson T, Chopra S. Epigenetic modifications of distinct sequences of the p1 regulatory gene specify tissue-specific expression patterns in maize. Genetics. 2007;175:1059–1070. doi: 10.1534/genetics.106.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz D. Tissue-specific regulation of gene function: Presetting and erasure. Proc Natl Acad Sci U S A. 1982;79:5991–5992. doi: 10.1073/pnas.79.19.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cocciolone SM, Chopra S, Flint-Garcia SA, McMullen MD, Peterson T. Tissue-specific patterns of a maize Myb transcription factor are epigenetically regulated. Plant J. 2001;27:467–478. doi: 10.1046/j.1365-313x.2001.01124.x. [DOI] [PubMed] [Google Scholar]
- 52.Robbins ML, Peterson T, Chopra S. Identification and characterizations of P1-wr epialleles in maize that show a gain of pericarp function. Maize Genet Coop Newslett. 2008;82:28–30. [Google Scholar]
- 53.Gehring M, Henikoff S. DNA methylation dynamics in plant genomes. Biochim Biophys Acta. 2007;1769:276–286. doi: 10.1016/j.bbaexp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lechelt C, Peterson T, Laird A, Chen J, Dellaporta SL, et al. Isolation and molecular analysis of the maize P locus. Mol Gen Genet. 1989;219:225–234. doi: 10.1007/BF00261181. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen SE, Sakai H, Finnegan EJ, Cao X, Meyerowitz EM. Ectopic hypermethylation of flower-specific genes in Arabidopsis. Curr Biol. 2000;10:179–186. doi: 10.1016/s0960-9822(00)00324-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of 5′ and 3′ cDNA sequences obtained form three P1-mm alleles with known sequences of P1-wr, P1-rr, and p2.
(0.04 MB DOC)
Sequences of PCR primers used in this study.
(0.04 MB DOC)