Abstract
Methuselah (Mth) is a G protein-coupled receptor (GPCR) associated with longevity in Drosophila melanogaster. Previously, Stunted (Sun) was identified as a peptide agonist of Mth. Here, we identify two additional activators of Mth signaling: Drosophila Sex Peptide (SP) and a novel peptide (Serendipitous Peptide Activator of Mth, SPAM). Minimal functional sequences and key residues were identified from Sun and SPAM by studying truncation and alanine-scanning mutations. These peptide agonists share little sequence homology and illustrate the promiscuity of Mth for activation. mth mutants exhibit no defects in behaviors controlled by SP, casting doubt on the biological significance of Mth activation by any of these agonists, and illustrating the difficulty in applying in vitro studies to their relevance in vivo. Future studies of Mth ligands will help further our understanding of the functional interaction of agonists and GPCRs.
Keywords: alanine-scanning, GPCR, methuselah, peptide agonists, promiscuity
Introduction
The methuselah (mth) gene encodes a family B G protein-coupled receptor (GPCR) and is associated with longevity and stress resistance in Drosophila melanogaster.1 Through middle-age adulthood, the mth1 mutant is resistant to loss of the male germline stem cell population2 and exhibits more robust sensorimotor function,3 although lifespan extension is sensitive to laboratory conditions.3–5 Down-regulation of mth also affects synaptic transmission in the larval neuromuscular junction, a role that appears distinct from its effects on longevity.6 We previously isolated antagonists (RWR motif peptides) of Mth that extend lifespan when over-expressed in vivo.7 The interaction site and dynamics of peptide binding to Mth were modeled, describing how these antagonists interact with the Mth ectodomain.8 Further studies of activators and inhibitors of Mth signaling might lead to a better understanding of GPCR-agonist interactions.
Here, we identify and characterize two new peptide agonists of Mth: Drosophila Sex Peptide (SP) and Serendipitous Peptide Activator of Mth (SPAM). Agonists of Mth, including the previously identified Stunted (Sun) peptide,9 share minimal sequence homology, suggesting a remarkable promiscuity of Mth for activation. As mth mutants show no defects in SP-controlled behaviors, the physiological relevance of these interactions remains unclear. These peptides should provide new tools for probing the activation of class B GPCRs, a family associated with several human diseases.10
Results and Discussion
Characterization of a novel peptide agonist of Mth
Previously, a peptide derived from the N-terminus of Stunted (N-Sun) was identified as an agonist for Mth by screening fractionated Drosophila homogenates on HEK 293 cells stably expressing Mth and measuring intracellular calcium mobilization.9 We subsequently used mRNA display selection to identify novel RWR motif-containing peptides that bind with high affinity to Mth and act as inhibitors of Sun-mediated Mth activation.7 For two of the peptide antagonists, R8-01 and R8-12, we synthesized randomly scrambled mutants for use as negative controls. As expected, the scrambled R8-12 peptide exhibited no activity on Mth. Surprisingly, however, robust calcium mobilization was observed upon addition of the scrambled R8-01 peptide to Mth-expressing cells [Fig. 1(A)], whereas no activity was seen in nontransfected control cells. We subsequently named the R8-01 scrambled peptide, “Serendipitous Peptide Activator of Mth” (SPAM). SPAM has no homology with N-Sun and appears to be a more potent Mth agonist [EC50 of 2.5 μM compared with 11 μM for N-Sun, Fig. 1(B)]. SPAM was inhibited by Mth peptide antagonists, providing further evidence that activation is specific for Mth [Fig. 1(C)].
Figure 1.
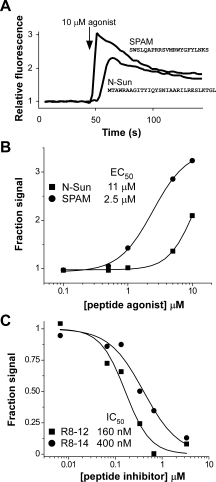
SPAM is a Mth agonist. (A) Application of 10 μM N-Sun or a scrambled variant of R8-01 (SPAM) at the indicated time results in mobilization of intracellular calcium and increased fluorescence in HEK-Mth cells. (B) Concentration dependence of Mth activation by N-Sun (▪) and SPAM (•) shows SPAM is a more potent agonist. (C) RWR motif peptides R8-12 (▪) and R8-14 (•), previously shown to inhibit N-Sun activation of Mth, also antagonize SPAM-mediated Mth signaling. The maximum fluorescence values after the addition of SPAM agonist (10 μM final) to HEK-Mth cells preincubated with varying concentrations of R8-12 or R8-14 are expressed as a fraction of the fluorescence observed in the absence of antagonists.
To identify motifs necessary for agonist activity, a series of 15-mer peptides for N-Sun and 12-mers for SPAM were synthesized and tested in the cell-based calcium mobilization assay. For N-Sun, the region of agonist activity was localized to the N-terminus, and the minimal active peptide sequence identified was AWRAAGITYIQYS [Fig. 2(A)]. For SPAM, the minimal functional peptide with high activity was LQAPRRSVMRW [Fig. 2(A)]. Since shorter peptides in the N-terminal region of N-Sun were not tested, it is possible that significantly shorter peptides could be derived from N-Sun that retain full activity.
Figure 2.
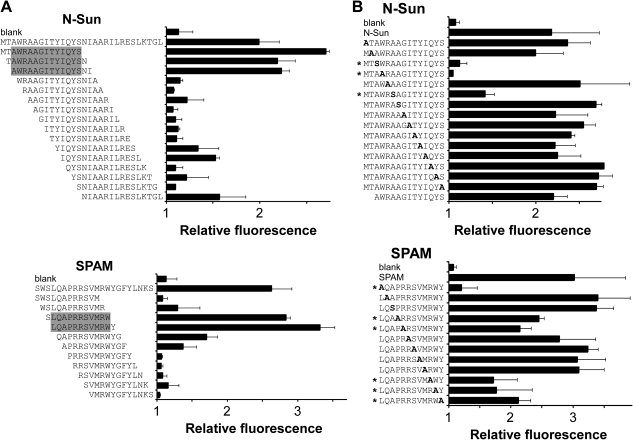
Identification of minimal sequences and critical residues for Mth activation. (A) A series of 15-mer peptides derived from N-Sun and 12-mer peptides derived from SPAM were tested for their ability to activate Mth in the cell-based calcium mobilization assay. Reported values (± s.d.) represent the maximum fluorescence achieved after the addition of peptide divided by the baseline average. The shaded region highlights the putative minimal peptide agonist. “Blank” is a negative control where buffer without peptide was added. (B) A series of alanine-scanning mutants were tested as in (A). Each residue in the minimal functional N-Sun and SPAM peptides was mutated to Ala and assayed. Wild-type Ala residues were mutated to Ser. All peptide sequences listed have an additional C-terminal glycine (not shown), and were tested at a concentration of 10 μM (N-Sun peptides) or 5 μM (SPAM peptides).
To identify the residues critical for Mth agonist activity, a series of alanine-scanning mutants for the minimal functional sequences of N-Sun and SPAM were assayed. These experiments revealed little overlap between the key residues in N-Sun and SPAM, except for the single Trp in each peptide, which was important for signaling [Fig. 2(B)]. Although N-Sun and SPAM differ greatly in their primary sequences, they may present the Trp residue similarly to the receptor during Mth activation. Further studies of the mutant peptides should determine the quantitative contributions of individual amino acids on agonist affinity and efficacy.
Sun is the ɛ subunit for the eukaryotic mitochondrial ATP synthase.11 Curiously, the functional 15-mer sequence of N-Sun for Mth activation corresponds well to the most conserved region of the peptide, in comparison with the ɛ subunit genes of other organisms including plants and mammals.12 The mth family of GPCRs, however, has thus far been found only in insects.13,14 Further studies will help to elucidate whether Sun functions both in and out of the mitochondria and verify its physiological interaction with Mth.
Identification of SP as a Mth agonist
The activation of Mth by SPAM, a non-physiological peptide with a randomly generated sequence, suggested that Mth has remarkably low ligand specificity. To further test this hypothesis, we challenged Mth-expressing HEK cells with other unrelated peptides and found that SP also elicited activation [Fig. 3(A)]. Agonist activity was eliminated when the SP sequence was scrambled or when the four C-terminal residues critical for SP-mediated behavioral effects15 were removed. SP did not induce calcium signaling in HEK 293 cells expressing the mth-like (mthl) receptors, mthl1, mthl2, mthl3, and mthl5, suggesting high specificity for Mth, although whether the mthl receptors are coupled to calcium mobilization in these cell lines is unknown.
Figure 3.
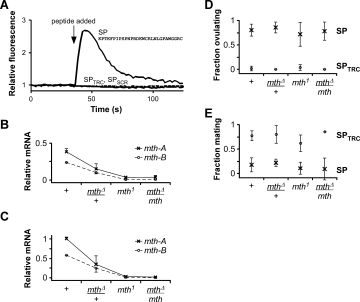
Drosophila Sex Peptide (SP) activates Mth. (A) Application of 10 μM SP induces mobilization of intracellular calcium and increased fluorescence in HEK 293 cells transiently transfected with mth-B. SPTRC (solid grey line), which lacks the C-terminal four residues of SP, and SPSCR (PWPKC NNGIP ARRGF KSLRT PPGCL PWDK, dashed line), a scrambled version of SP, do not activate Mth. (B, C) Relative transcript levels of the mth-A (×) and mth-B (○) splice variants in different mth mutant alleles from RNA isolated from (B) virgin female adults (1–2 day old) or (C) wandering 3rd instar larvae. Results are averages (± s.d.) from two independent collections of total RNA (biological replicates). (D, E) mth mutants exhibit no defects in SP-induced behaviors. (D) Injection of SP (×) into virgin females dramatically increases ovulation in the control and mutants of mth. (E) Injection of SP (×) into virgin females greatly reduces sexual receptivity to males. Injection of the negative control peptide, SPTRC (○), had a negligible effect on ovulation and mating. Behavioral results are averages (± s.d.) from two to seven independent experiments, each consisting of 6–25 flies.
To test the physiological relevance of the SP-Mth interaction, we generated a mth null mutant using FLP-FRT recombination,16 resulting in a line with a defined chromosomal deletion, mthΔ, encompassing all of the protein coding exons of mth. Homozygous mthΔ flies are embryonic lethal, which is consistent with published data suggesting that mth is an essential gene.1 Virgin female flies heterozygous for mthΔ showed a approximately two-fold reduction in mRNA transcript levels of both mth splice variants [Fig. 3(B)], supporting the view that mthΔ is a null allele. Previous studies have relied on a hypomorphic P-element insertion allele in the 3rd intron of mth. This homozygous insertion mutant (mth1) showed ∼10-fold and ∼25-fold reductions in mth-A and mth-B, respectively. Animals bearing mthΔ in trans to mth1 were viable and showed no obvious morphological or behavioral defects. Transcript levels in these flies were similar to levels in the homozygous mth1 mutant. qPCR analysis of total RNA from 3rd instar larvae showed similar ratios of mth transcript levels in all alleles [Fig. 3(C)], although mth mRNA levels were higher in larvae, consistent with previous microarray results.17
In Drosophila, mating strongly affects female behavior. This post-mating response, which includes stimulated ovulation and reduced sexual receptivity, is controlled by SP, a protein produced by the male accessory glands and transferred in the seminal fluid during copulation.15 We thus assayed behaviors associated with the postmating response in the mth alleles. Virgin female mutants exhibited normal ovulation and mating frequencies after SP injection [Figs. 3(D,E)]. The Sex Peptide Receptor (CG16752) is known to mediate the role of SP in triggering the postmating response.18 Hence, SP either does not interact with Mth in vivo, or the downstream effects are subtle and/or uncharacterized.
Conclusion
We have characterized two peptide agonists for Mth—SPAM and SP—that are of similar or higher potency than the previously identified Sun agonist, show specificity for Mth over Mth-like receptors, and are inhibited by the RWR motif antagonist peptides. The promiscuity of Mth casts doubt on the relevance of the agonists in vivo, and cautions against the broad interpretation of in vitro GPCR studies. However, despite the lack of support for physiological interactions of the agonists with Mth, recent studies of other promiscuous GPCRs with potential roles in nutrient-sensing and immune response20,21 hint at a possible function of Mth as a peptide sensor in vivo. The identification of a bona fide signaling partner for Mth, as well as the discovery of agonists for the related Mth-like receptors, will help elucidate the role of this GPCR family in Drosophila aging, health, and development.
Materials and Methods
Peptide synthesis
N-Sun (MTAWR AAGIT YIQYS NIAAR ILRES LKTGL) and SPAM (SWSLQ APRRS VMRWY GFYLN KS) were synthesized in-house on a 432A Synergy peptide synthesizer (Applied Biosystems, Foster City, CA) using standard Fmoc chemistry. Drosophila SP (KPTKF PIPSP NPRDK WCRLN LGPAW GGRC), a control peptide lacking the C-terminal four residues (SPTRC), and a scrambled version of SP (PWPKC NNGIP ARRGF KSLRT PPGCL PWDK, SPSCR) were synthesized by Bio-Synthesis, Inc. (Lewisville, TX) and provided on-resin. Synthetic peptides were cleaved from the resin and deprotected by agitation in trifluoroacetic acid (TFA):1,2-ethanediol:thioanisole (90:5:5) for 2 h at room temperature. Crude peptides were desalted by precipitation in methyltertbutyl ether. Peptides were purified by reversed-phase HPLC (C18, 250 × 10 mm, Grace Vydac, Hesperia, CA) on an aqueous acetonitrile/0.1% (v/v) TFA gradient. Peptide masses and the formation of the intramolecular disulfide bridge in SP were confirmed by MALDI-TOF mass spectrometry. Antagonist peptides, R8-01 (MNVSW GSFPS SWLQR YYLAK RR), R8-12 (MRLVW IVRSR HFGPR LRMA), and R8-14 (MAPRA VWIQR AIQAM FRLA), were obtained as described previously.7 All peptides were soluble in ddH2O or 1× PBS, and concentrations were determined by measuring absorbance at 280 nm.
Peptide truncation and alanine-scanning mutagenesis series were synthesized with C-terminal glycine residues by JPT Peptide Technologies GmbH (MicroScale Peptide Sets, Berlin, Germany). Crude, dried peptides were provided in 96-well plates at ∼50 nmol of full-length peptide per well. Peptides were reconstituted in 20 μL of dimethyl sulfoxide prior to their use in cell signaling assays.
Cell-based calcium signaling assay for Mth activation
Mth activation was measured by observing calcium mobilization essentially as described using HEK 293 cells stably expressing the B splice variant of mth (HEK-Mth cells) and the fluorescent calcium indicator, Fluo-4 AM (Molecular Probes, Eugene, OR).7 Data analysis and background subtraction were performed with Softmax Pro 4.8 (Molecular Devices, Sunnyvale, CA) and sigmoidal fits were calculated using Origin 6.0 Professional (OriginLab Corp., Northampton, MA). Assays of mth-B and mth-like (mthl) receptors were also performed using either transiently transfected (Lipofectamine, Invitrogen, Carlsbad, CA) or isogenic stable expression (Flp-In system, Invitrogen) HEK 293 cell lines generated with cDNA clones from the Drosophila Genomics Resource Center (mth-B, SD05804; mthl1, AT18671; mthl2, RH57551; mthl3, GM02553; and mthl5, RE31350). cDNA was cloned into the pcDNA3.1+ or the pcDNA5/FRT vectors (Invitrogen) for transient or Flp-In transfections, respectively, using sticky-end PCR19 with parental plasmid-specific primers.
Generation of a mth null line
To generate a defined chromosomal deletion between the P-element insertion lines flanking the mth gene, e03119 and d05374, we used FLP-FRT recombination, as described previously.16 Briefly, flies bearing heat-shock driven FLP recombinase and both the d05374 and e03119 P-element insertions in trans were heat-shocked to induce expression of FLP recombinase, resulting in the generation of chromosomal deletions that were identified by loss of a marker (eye color). Deletion lines generated in this way bear a new hybrid transposon combination containing fragments from both e03119 and d05374. Hence, the resulting lines were confirmed by genomic PCR with transposon-specific primers (RB3′-in: 5′-TGC ATT TGC CTT TCG CCT TAT and XP5′-in: 5′-AAT GAT TCG CAG TGG AAG GCT) that generate a fragment of known size across the newly formed hybrid element. The homozygous-lethal deletion line (mthΔ) was established and maintained over the TM6B balancer. Assays with mthΔ were performed by comparing heterozygotes (mthΔ/w1118) with transheterozygotes (mthΔ/mth1), where the mth1 mutant was extensively backcrossed (>10 generations) with the control line, w1118.
Quantitative real-time PCR
Total RNA from wandering 3rd instar larvae or virgin female adults (1–2 day old) was purified using TRIzol (Invitrogen), following the manufacturer's instructions. Approximately 10–20 animals were homogenized per replicate. Samples were treated with DNase I (Invitrogen), before being used for reverse transcription.
First-strand cDNA was synthesized with random nonamer primers in a final reaction volume of 20 μL. Total RNA (2 μg), primers (N9, 60 pmol), and dNTP (20 nmol each) were incubated at 65°C for 5 min followed by a quick chill on ice. Reverse-transcription buffer (1× final) and SUPERase-In (1 U/μL final, Applied Biosystems/Ambion, Austin, TX) were added and the reaction was incubated at room temperature for 2 min. Reverse transcriptase (Superscript II, 200 U, Invitrogen) was added and the reaction was incubated at 25°C for 10 min to initiate cDNA synthesis. Incubation temperature was ramped up and maintained at 42°C for 1 h prior to inactivating the reaction by heating at 70°C for 15 min. Negative control reactions were treated identically but did not contain reverse transcriptase.
Quantitative real-time PCR was performed on an iCycler iQ5 system (Bio-Rad, Hercules, CA) using iQ SYBR Green Supermix (Bio-Rad) with 1 μL of cDNA (100 ng RNA equivalent) and a final primer concentration of 0.25 μM in a total volume of 20 μL. Splice variants of mth were differentiated using exon-specific reverse primers (mthA-RP: 5′-CAC TGT TGT TTA CCT CCT CAC CCT and mthB-RP: 5′-TTC CCA CGG TAA TAC GAC TTG CCA) with a common forward primer (mthAB-FP: 5′-ACC AAA CTT GGG CCA ACG TCT TTC). Cycling conditions were 3 min at 95°C followed by 40 cycles of 95°C for 10 s (denaturation) and 60°C for 45 s (annealing and extension). Melting curves were generated after each qPCR run, and final PCR products appeared to be the correct size when analyzed by agarose gel electrophoresis. qPCR of Actin 88F (primers 5′-GAT CAC CAT TGG CAA CGA and 5′-TCT TGA TCT TGA TGG TCG) was performed as a positive control on all samples. To compare transcript levels across different life stages and/or age groups, qPCR results were normalized to input total RNA.
Behavioral assays
Peptides (∼200 nL of 240 μM in ddH2O) were injected into the thorax of CO2-anesthetized, 4- to 6-day-old virgin females. Flies recovered in food vials for ∼8 h and were subsequently assayed for the presence of an egg in the uterus by gently squeezing the tip of the abdomen with forceps. After 8–12 h, individual females were transferred without anesthesia to circular mating chambers (1 cm diameter, ∼0.6 cm height) containing 1 virgin, control (Canton-S) male and observed for 1–2 h. Courtship was confirmed for males in each chamber, and successful copulation was scored. Data from multiple independent experiments were averaged and shown ± s.d.
Acknowledgments
The authors thank the Bloomington Drosophila Stock Center for FRT-related fly lines (#7, 7758, and 8136), the Exelixis Collection at Harvard Medical School for P-element insertion lines (e03119 and d05374), the Drosophila Genomics Resource Center for cDNA clones, and X.-Y. Huang (Cornell University Weill Medical College) for the HEK-Mth cell line.
References
- 1.Lin Y-J, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 2.Wallenfang MR, Nayak R, Dinardo S. Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell. 2006;5:297–304. doi: 10.1111/j.1474-9726.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 3.Petrosyan A, Hsieh I-H, Saberi K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant methuselah. Behav Genet. 2007;37:585–594. doi: 10.1007/s10519-007-9159-y. [DOI] [PubMed] [Google Scholar]
- 4.Baldal EA, Baktawar W, Brakefield PM, Zwaan BJ. Methuselah life history in a variety of conditions, implications for the use of mutants in longevity research. Exp Gerontol. 2006;41:1126–1135. doi: 10.1016/j.exger.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Mockett RJ, Sohal RS. Temperature-dependent trade-offs between longevity and fertility in the Drosophila mutant, methuselah. Exp Gerontol. 2006;41:566–573. doi: 10.1016/j.exger.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Song W, Ranjan R, Dawson-Scully K, Bronk P, Marin L, Seroude L, Lin Y-J, Nie Z, Atwood HL, Benzer S, Zinsmaier KE. Presynaptic regulation of neurotransmission in Drosophila by the G protein-coupled receptor methuselah. Neuron. 2002;36:105–119. doi: 10.1016/s0896-6273(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 7.Ja WW, West AP, Delker SL, Bjorkman PJ, Benzer S, Roberts RW. Extension of Drosophila melanogaster life span with a GPCR peptide inhibitor. Nat Chem Biol. 2007;3:415–419. doi: 10.1038/nchembio.2007.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heo J, Ja WW, Benzer S, Goddard WA. The predicted binding site and dynamics of peptide inhibitors to the Methuselah GPCR from Drosophila melanogaster. Biochemistry. 2008;47:12740–12749. doi: 10.1021/bi801335p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvejic S, Zhu Z, Felice SJ, Berman Y, Huang X-Y. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nat Cell Biol. 2004;6:540–546. doi: 10.1038/ncb1133. [DOI] [PubMed] [Google Scholar]
- 10.Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001;2:reviews3013.1–reviews3013.10. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidd T, Abu-Shumays R, Katzen A, Sisson JC, Jiménez G, Pinchin S, Sullivan W, Ish-Horowicz D. The ɛ-subunit of mitochondrial ATP synthase is required for normal spindle orientation during the Drosophila embryonic divisions. Genetics. 2005;170:697–708. doi: 10.1534/genetics.104.037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu Q, Yu L, Zhang P, Zhang M, Zhang H, Jiang J, Chen C, Zhao S. Cloning, characterization and mapping of the human ATP5E gene, identification of pseudogene ATP5EP1, and definition of the ATP5E motif. Biochem J. 2000;347:17–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Brody T, Cravchik A. Drosophila melanogaster G protein-coupled receptors. J Cell Biol. 2000;150:F83–F88. doi: 10.1083/jcb.150.2.f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 15.Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, Deal-Herr ME, Grant D, Marcinko M, Miyazaki WY, Robertson S, Shaw KJ, Tabios M, Vysotskaia V, Zhao L, Andrade RS, Edgar KA, Howie E, Killpack K, Milash B, Norton A, Thao D, Whittaker K, Winner MA, Friedman L, Margolis J, Singer MA, Kopczynski C, Curtis D, Kaufman TC, Plowman GD, Duyk G, Francis-Lang HL. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- 17.Arbeitman MN, Furlong EE, Imam F, Johnson E, Null BH, Baker BS, Krasnow MA, Scott MP, Davis RW, White KP. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297:2270–2275. doi: 10.1126/science.1072152. [DOI] [PubMed] [Google Scholar]
- 18.Yapici N, Kim Y-J, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 19.Zeng G. Sticky-end PCR: new method for subcloning. Biotechniques. 1998;25:206–208. doi: 10.2144/98252bm05. [DOI] [PubMed] [Google Scholar]
- 20.Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Bräuner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]


