Abstract
Crystal structures of Gαi (and closely related family member Gαt) reveal much of what we currently know about G protein structure, including changes which occur in Switch regions. Gαt exhibits a low rate of basal (uncatalyzed) nucleotide exchange and an ordered Switch II region in the GDP-bound state, unlike Gαi, which exhibits higher basal exchange and a disordered Switch II region in GαiGDP structures. Using purified Gαi and Gαt, we examined the intrinsic tryptophan fluorescence of these proteins, which reports conformational changes associated with activation and deactivation of Gα proteins. In addition to the expected enhancement in tryptophan fluorescence intensity, activation of GαGDP proteins was accompanied by a modest but notable red shift in tryptophan emission maxima. We identified a cation-π interaction between tryptophan and arginine residues in the Switch II of Gαi family proteins that mediates the observed red shift in emission maxima. Furthermore, amino-terminal myristoylation of Gαi resulted in a less polar environment for tryptophan residues in the GTPase domain, consistent with an interaction between the myristoylated amino terminus and the GTPase domain of Gα proteins. These results reveal unique insights into conformational changes which occur upon activation and deactivation of G proteins in solution.
Keywords: Gα, switch II, intrinsic fluorescence, cation-π, N-terminal myristoylation
Introduction
Activation of heterotrimeric G proteins coupled to 7-transmembrane receptors results in activated GαGTP and Gβγ subunits which activate downstream effectors. Hydrolysis of bound GTP to GDP facilitates re-association of GαGDP and Gβγ, a prerequisite for productive coupling to activated receptors. The conformational changes in Gα subunits that accompany nucleotide exchange and hydrolysis are sensed by three Switch regions: Switch I, II, and III. These regions adopt distinctive conformations in crystal structures of Gα proteins bound to GDP, GDP.Gβγ, GTPγS, and GDP-AlF4,1–9 facilitating interactions of Gα with Gβγ8,9 and a variety of effectors.10–14 Although much of what we know about Gα structure and function has been gleaned from crystal structures, the Switch II of Gαi which binds Gβγ8,9 is disordered in GαiGDP crystal structures in the absence of Gβγ, as is the N-terminus of isolated Gα proteins. Crystallographically disordered regions in proteins are often important interfaces for protein–protein interaction. The conformation of Switch II may be important for binding to not only Gβγ, but also other Gα effectors such as GIV, a recently identified GEF for Gαi proteins.15 Similarly, a peptide derived from the GoLoco region of RGS14 binds Switch II of GαiGDP16 to competitively inhibit Gβγ binding. Other Gα effectors which bind Switch II of Gα proteins in a conformationally sensitive manner include adenylyl cyclase (which binds activated Gαs)11 and the RGS domain of RhoGEF which binds activated Gα13.17
The Trp in Switch II (W211 in Gαi) reports conformational changes upon activation.20,21 The increase in emission intensity which accompanies the activation-dependent movement of the Switch II region into a hydrophobic pocket1–7 provides a convenient and reliable method to assess the functional integrity of purified proteins.22,23 Gαi contains two other Trp residues, W258 in the GTPase domain and W131 in the helical domain. Crystal structures show that the Trp in the helical domain of Gαi and Gαt proteins remains buried in both the inactive and active states,3,4,6 and NMR studies have shown it to be refractory to activation.24 A conformational change in a protein which relocates a Trp residue into a more solvent-excluded environment is typically accompanied by an increase in emission intensity (such as upon Gα activation) and a shift in emission maxima (emmax) to lower wavelengths (called a blue shift). However, in Gαi proteins, this activation-dependent enhancement in intensity is accompanied by a small but notable red shift in emmax (toward a higher wavelength). Investigation of this unexpected red shift revealed an activation-dependent cation-π interaction between Arg and Trp residues in Switch II as determined by steady-state fluorescence, and this finding was confirmed using site-directed mutagenesis. The relative differences in the Trp fluorescence of GαiGDP proteins before and after activation indicate an overall conformational similarity in solution between the Switch II region of Gαi and Gαt proteins.
Both Switch II and the N-terminus of Gα proteins participate in binding to Gβγ. In nature, the N-terminus of Gαi family proteins are permanently, cotranslationally myristoylated,18 which enhances Gα-Gβγ association,19 thus its conformation (along with that of Switch II) may influence the duration of Gβγ signaling. We also looked at the environment of Trp residues in myristoylated proteins, and found a myristoylation-dependent reduction in solvent exposure for Trp residues in the GTPase domain of Gαi proteins. Together these results shed light on conformational changes that occur in solution upon activation and deactivation of Gα subunits.
Results
There are three Trp residues in Gαi [Fig. 1(A)]: W211 and W258 in the GTPase domain, and a Trp in the helical domain, W131, which does not report activation-dependent changes.4,24 Because of the well-known ability of the Trp in Switch II of Gα proteins to report such changes, functional Gα proteins typically demonstrate a minimum 40% increase in Trp emission intensity upon activation with AlF4.25 AlF4 activates Gα proteins by formation of a GαGDP-AlF4 complex which mimics the pentavalent transition state for GTP hydrolysis. In this work, we investigated the activation-dependent red shift in Trp emmax in Gαi [Fig. 1(B)], which was also present after a prolonged incubation with GTPγS (not shown), as well as upon activation of Gαi HI [Fig. 1(C)], a Gαi protein lacking several solvent exposed cysteines which does not perturb function.26
Figure 1.
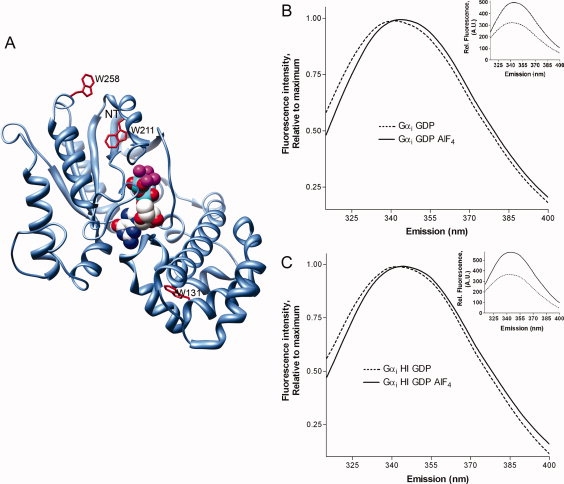
Intrinsic Trp fluorescence of Gαi. (A) Location of 3 native Trp residues (red) in GαiGDP-AlF4 (PDB 1GFI) with nucleotide rendered in space fill. NT, last resolved residue (33) in N-terminus. (B, C) Normalized emission of 400 nM Gαi (B, wt Gαi; C, Gαi HI), ex/em 280/310–400 nm, before (dotted) and after (solid) activation with AlF4. Control, inset: Gα proteins demonstrate ≥40% increase in intrinsic Trp fluorescence upon AlF4 activation (raw data).
To gain insight into the cause of the red shift, we sought to compare structures of Gαi before and after activation, however, GαiGDP is disordered in the Switch II region.7,27 Therefore, structures of the closely related Gαi family member, Gαt were examined, since Gαt shares a high sequence and structural homology with Gαi. Comparison of the Switch II conformation in GαtGDP and GαtGDP-AlF4 structures revealed an activation-dependent stacking of positively charged R204 (R208 in Gαi) over the π electrons of W207 (W211 in Gαi), reducing the distance between them by more than 2 Å [Fig. 2(A,B)]. A computational study of energetically favorable cation-π interactions determined that the CD and CZ atoms of Arg (δ-carbon and guanidinium carbon, respectively) are typically within 6 Å of the aromatic ring of Trp,28 as is the case here [Fig. 2(A)]. In Gα proteins, this interaction is aided by a network of interactions which contribute to the positioning of the Arg-Trp pair upon activation, including a salt bridge between this Arg and a Glu adjacent to Switch III [E241 in Gαt, Fig. 2(B), bottom panel], as well as arginine's interactions with glycine residues that contact bound nucleotide (Supporting Information Fig. 1). Structural studies show that activation tucks Switch II into a hydrophobic pocket, resulting in a closer proximity between the Arg and Trp, which may polarize Trp emission in the Switch II region. If so, this would explain the shift of the Trp emmax to higher, rather than lower wavelength.
Figure 2.
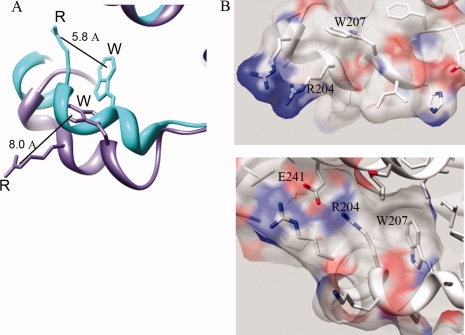
Activation-dependent increase in proximity between Arg and neighboring Trp in Switch II of Gαt. (A): Switch II Trp in Gαi family member Gαt is 2.2 Å closer to positively charged Arg upon activation, GαtGDP-AlF4 (PDB file 1TAD) (teal) overlay with GαtGDP (PDB file 1TAG) (purple). Note residues R204 and W207 in Gαt correspond to residues R208 and W211 in Gαi. (B, top panel): Switch II of GαtGDP shown with surface rendered. (B, bottom panel): Switch II tucks into protein upon activation, shown with surface of GαtGDP-AlF4 rendered.
Before ascertaining if the Switch II Arg was polarizing Trp emission in Gαi proteins, we first examined this proposal in Gαt, which is ordered in the Switch II region in both GDP- and GDP-AlF4-bound states. Unmyristoylated Gαt used in the structural determinations was prepared by treating Gαt obtained from rod outer segments with EndoLysC2 to cleave the myristoylated N-terminus of Gαt at residue 33, followed by gel filtration. After purification, the molecular weight of the truncated Gα protein was confirmed by SDS-PAGE [Fig. 3(A), ΔNT]; this protein retained the ability to report activation-dependent changes [Fig. 3(B), inset]. We scanned the emission of the unmyristoylated Gαt before and after activation, and found the emmax underwent a red shift [Fig. 3(B)] similar to the one observed for Gαi, consistent with a polarization of the Switch II Trp upon activation, linking structural changes in Gαt to shifts in emmax. In addition, comparison of unmyristoylated Gαi and Gαt proteins reveal a lower emmax for Gαt [Fig. 3(C)] regardless of activation, most likely due to the fact that Gαt has a Tyr instead of the more solvent exposed Trp at the position homologous to W258 in Gαi. Trp emission dominates protein spectra between 330 and 350 nm, while Tyr has an emmax at 303 nm. The emmax of Tyr is unaffected by changes in its environment, unlike Trp which demonstrates shifts in emmax in response to the macroenvironment and microenvironment of tryptophan's indole ring.
Figure 3.
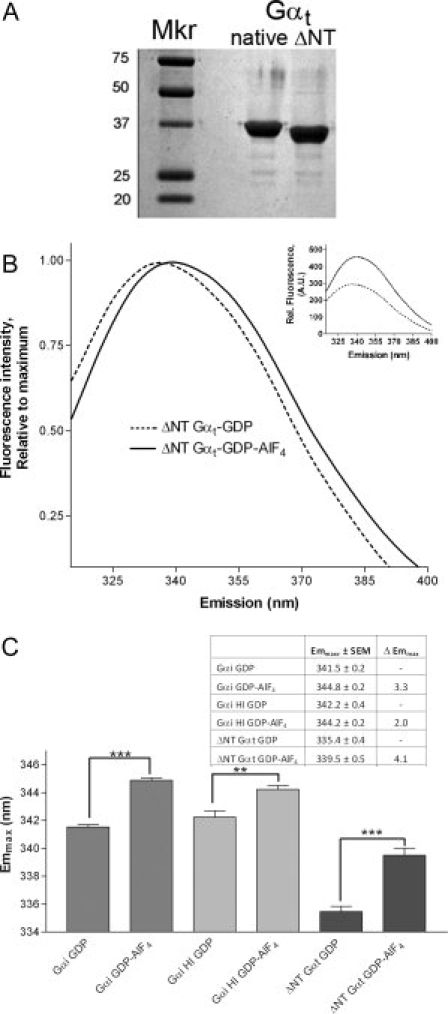
Trp fluorescence of unmyristoylated proteins. (A) Myristoylated Gαt (native) is enzymatically cleaved at the N-terminus to yield the unmyristoylated ΔNT Gαt protein used in structure determination.6 (B) Emission of 500 nM ΔNT-Gαt protein was scanned as above before (dotted line) and after (solid) activation with AlF4 as in Figure 1. Control, inset: ΔNT-Gαt demonstrated ≥40% increase in Trp fluorescence upon AlF4 activation. (C) Summary of emmax before and after activation for indicated Gα proteins (n ≥ 3, ±SEM; statistically significant changes noted by asterisk(s), ***P < 0.001, **P < 0.01). Dark gray bars, far left, Gαi; light gray, center, Gαi HI; black, far right, ΔNTGαt.
Given that the activation-dependent red shift observed in Gαi (Fig. 1) was recapitulated in Gαt (Fig. 3), and in light of the associated increase in proximity between Arg and Trp in Switch II of Gαt (Fig. 2), the red shift we observed in Gαi may indicate a similar geometry. If so, R208 and W211 of Gαi would be predicted to play critical roles. To investigate this, we examined the emmax of 4 Gαi HI proteins: W211C, R208C, R208L, and W258F, residues all located in the GTPase domain, where activation-dependent changes are known to occur. Gαi HI proteins were used in this study because they have been shown to tolerate cysteine mutations in the Switch II region29 and elsewhere throughout the protein.26,29,30 Residues W211 and R208 are part of the Switch II region of Gαi, and W258 is located on a more solvent exposed loop adjacent to Switch II in the GTPase domain [Fig. 1(A)].
Although the Gα proteins used in this study generally demonstrated a minimum of 40% increase in Trp intensity upon activation, mutation of residues implicated in the cation-π interaction precluded assessment of functionality by this method. Therefore, we used an extrinsic probe linked to GTPγS to confirm the ability of these proteins to exchange nucleotide. Uncatalyzed exchange of GDP for BD-GTPγS is reported by an increase in emission from BD-GTPγS that occurs upon its binding to Gαi subunits.31 Because Gαi subunits are known to bind to BD-GTPγS at a slower rate and with a lower affinity than unlabeled GTPγS,31 overall BD-GTPγS binding was measured after full exchange was allowed to occur. The indicated Gαi proteins showed the ability to exchange GDP for BD-GTPγS [Fig. 4(A), solid bars], which was eliminated by pre-boiling these proteins [Fig. 4(A), far right bars]. The mutant proteins were also monodisperse on gel filtration, exhibited retention time on gel filtration chromatography according to their predicted molecular weight, and migrated essentially the same as wild-type proteins on SDS PAGE, consistent with proper expression and folding of these proteins.
Figure 4.
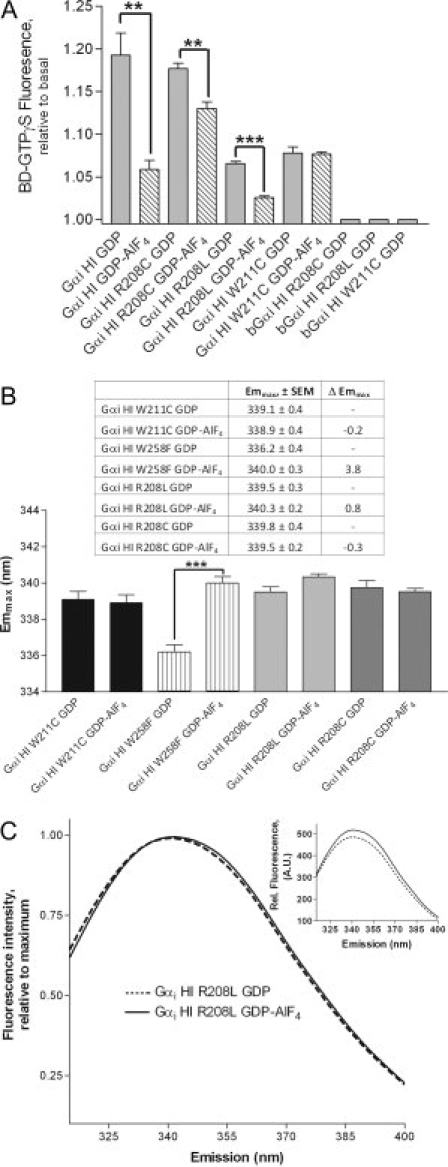
A: Extrinsic nucleotide exchange. Solid bars (controls): Gα proteins exchange GDP for BD-GTPγS, in contrast to heat-denatured Gα (boiled, last three bars). Striped bars: nucleotide exchange in Gα proteins that were preactivated by AlF4 before assay. B,C: Red shift is markedly reduced by mutation of either W211 or R208, unlike mutation of Trp outside of Switch II, W258 (A, B: n ≥ 3, ±SEM; **P < 0.01; ***P < 0.001).
Since AlF4 binding to wild-type GαiGDP is known to reduce nucleotide exchange with GTP analogs due to additional stabilizing contacts introduced by binding AlF4,4,32 we next measured nucleotide exchange using Gα proteins pre-activated by AlF4 [Fig. 4(A), striped bars]. The stabilizing contacts introduced by AlF4 reduced binding of BD-GTPγS by Gαi HI as expected, and did so to a somewhat lesser extent in the R208 mutants, however mutation of W211 resulted in the complete loss of AlF4-mediated stabilization of bound GDP. This protein exchanged GDP for BD-GTPγS regardless of activation by AlF4 [Fig. 4(A), striped bars].
Mutation of either R208 or W211 attenuated the red shift upon activation [Fig. 4(B,C)], despite the ability of these proteins to exchange nucleotide [Fig. 4(A)]. Mutation of the same Switch II Trp in Gαo also prevents detection of activation by Trp fluorescence, without preventing activation-dependent conformational changes or the binding to labeled GTPγS.33 Both R208 and W211 are direct participants in the cation-π interaction and play a role in stabilization of the Switch II region upon activation through a combination of electrostatic, hydrophobic and van der Waals interactions. Mutation of W258 did not impair the activation-dependent red shift in emmax [Fig. 4(B)], and it resulted in a lower emmax than the parent GαiGDP HI protein [Fig. 4(B) vs. Fig. 3(C)], indicating a high degree of solvent exposure for this Trp. These studies were performed in the absence of Gβγ and receptor which contain numerous Trp residues that obscure detection of changes in Gα emission by increasing background signal.
Using Gαi proteins coexpressed with N-myristoyl transferase, we found that N-terminal myristoylation reduced the activation-dependent red shift in wild-type Gαi, and eliminated it in native Gαt [Fig. 5(A) vs. Fig. 3(C)], without impairing activation-dependent increases in intensity [Fig. 5(B), shown for Gαt]. Interestingly, myristoylation also reduced the emmax of GDP-bound Gαi proteins with a native Trp at position 258 [Fig. 5(C)], but did not have the same effect on Gα proteins lacking this or an equivalent Trp, Gαi HI W258F, and Gαt. Thus, W258 in the GTPase domain senses myristoylation in GDP-bound Gαi subunits. Myristoylation also decreases the emmax of activated, wild-type Gαi and Gαt [Fig. 5(D)], consistent with a myristoylation-dependent modulation of the cation-π interaction in these proteins, either directly or indirectly, however, the exact nature of the interaction has yet to be determined. Taken together, myristoylation has effects on both W258 and W211, which are located near the last resolved residues in the N-terminus of Gαi [Fig. 1(A)]. These data are in agreement with a previous model suggesting the myristoylated N-terminus of Gαi proteins interact with residues on the surface of Gαi,18,27 with myristoylation-dependent effects detected for Trp residues W211 and W258, located in and near the Switch II region in the GTPase domain of Gαi proteins.
Figure 5.
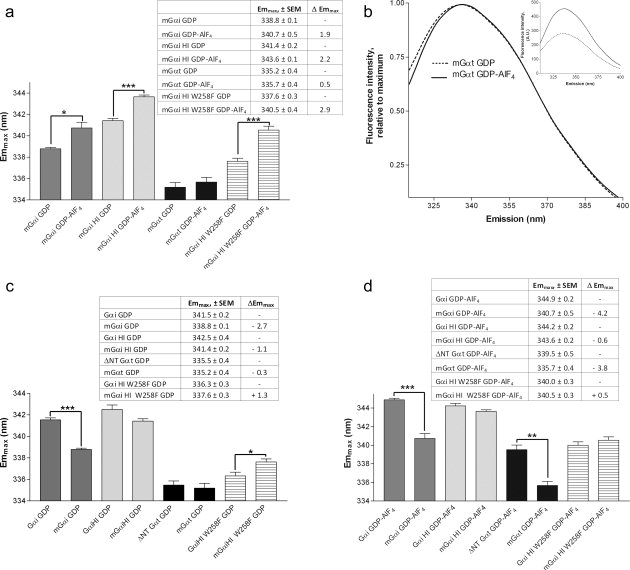
Effect of myristoylation on emmax. (A) Comparison of emmax of myristoylated proteins before and after activation. (B) Normalized spectra of Gαt (native, myristoylated) before and after activation with AlF4. Inset: raw data before and after activation. (C,D) Effect of myristoylation on emmax of GDP (C) and GDP-AlF4 bound proteins (D). (A, C, D: n ≥ 3, ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001).
These results suggest a model whereby upon GTP hydrolysis, GαiGDP adopts a conformation in the Switch II region [Fig. 6(A,B)] which resembles the ordered Switch II region seen in structures of GαtGDP [Fig. 6(B), purple]. The red shift upon activation provides information about the position of Gαi Switch II residues in the GDP-bound state relative to that in the GTP-bound state, indicating a qualitatively similar distancing of the Trp and Arg in the Switch II of GDP-bound Gαi and Gαt proteins after GTP hydrolysis has occurred. The activation-dependent cation-π interaction between R208 and W211 in Gαi (separated by one turn of the Switch II α-helix) is stabilized by a network of interactions, including a conserved pair of flexible glycines, G202 and G203 (Supporting Information Fig. 1), which allosterically link the Switch II region to activation-dependent changes in the nucleotide binding site. Computational analysis using the program CaPTURE28 estimates the strength of this interaction to be approximately −2.0 kcal/mol, which reflects both electrostatic and van der Waals contributions, resulting in an energetically relevant interaction between these residues.
Figure 6.
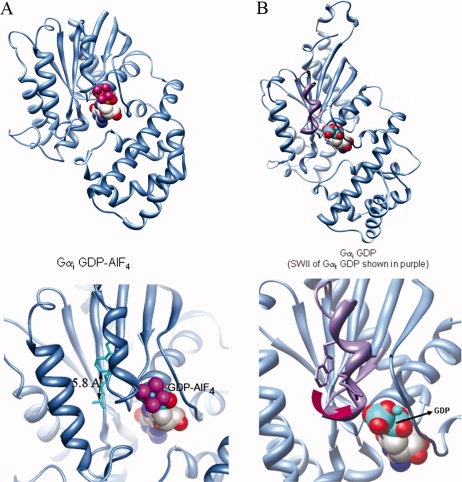
Model. (A) Crystal structures of activated Gαi (PDB file 1GFI, top panel, overview; bottom panel, Switch II region enlarged, nucleotide shown in spacefill) demonstrate that the Trp π electrons are within 6 Å from the positively charged Arg in the Switch II helix. (B) Ordered regions of GαiGDP (PDB file 1BOF) shown in blue, Switch II region of GαtGDP in purple (from structural alignment of PDB file 1TAG and 1BOF), and nucleotide shown in spacefill. Top, overview; bottom panel, enlarged, depicting spatial separation of 8 Å or more between the Switch II Arg and Trp after GTP hydrolysis has occurred.
Discussion
This study provides insights into the Switch II conformation of GαiGDP in solution. Crystal structures of Gα proteins demonstrate that upon activation, the Switch II tryptophan moves into a more solvent-protected environment, resulting in enhanced Trp emission intensity, and a small but unexpected red shift in emmax. This was originally noted without elaboration in one of the first reports of Gα protein isolation.23 We used a combination of mutational and biochemical approaches to identify the cause of this shift, an activation-dependent cation-π interaction, mediated by Arg and Trp in the Switch II helix of Gαi and Gαt proteins. The homology between Gαi and Gαt was used as a link between this shift and Switch II conformation, as the Switch II of GαtGDP is ordered in crystal structures, unlike GαiGDP. These solution studies indicate a relatively similar orientation of Arg and Trp in Switch II of Gαi and Gαt proteins both before and after activation.
A recent study indicated that almost 40% of energetically relevant cation-π interactions involve an Arg-Trp pair,28 and many of these help stabilize α-helices, as in the case of Gα proteins. The majority position Trp's aromatic ring 6 Å or less from the CD and CZ atoms of Arg, over which the charge is distributed.28 In Gαi, the CZ of R208 moves to within 6 Å of the aromatic ring of W211 upon activation, with the CD of R208 within 5 Å of the ring, polarizing Trp emission. In Gαt, this results in a 2.2 Å reduction in the distance between the Arg-Trp pair. Similarly, in the protein ricin, ligand binding results in a 1.7 Å reduction between its Arg-Trp pair and a 3–4 nm red shift.34 Common cation-π interactions35,36 position the cation parallel to the plane of tryptophan's ring of p-orbitals.37 Energetically relevant Arg-Trp interactions are estimated at −2.9 ± 1.4 kcal/mol,28 which includes the −2.0 kcal/mol calculated for the Arg-Trp pair in Gαi, in agreement with a proposed cation-π interaction suggested from comparison of Gαβγi and GαiGTPγS structures.36 Van der Waals and hydrophobic contacts along the cation side chain contribute to the favorable energetics of the Arg-Trp pair, along with charge delocalization which maximizes Arg's interaction with Trp's indole ring. In addition, Arg often forms conventional hydrogen bonds with other residues while simultaneously interacting with Trp,38–40 as seen in the activation-dependent interaction of R208 with E245 just next to Switch III, which allosterically links changes in nucleotide binding to Switch III conformation in Gαi proteins.
Residues R208 and W211, as well as G202 and G203, are highly conserved among G proteins. Mutation of W211 abrogates the stabilizing influence normally imparted by formation of a GDP-AlF4 complex. The structure of GαiGDP-AlF4 shows that W211 is hydrogen bonded to G202, with G203 making hydrogen bonds to AlF4 and R208, thus allosterically linking Switch II conformation to nucleotide binding. The flexibility of these conserved glycines may also facilitate Q204's interactions with the catalytic water molecule during GTP hydrolysis, which together with R178, Q171 and T181 help orient this water toward the developing positive charge on the γ-phosphate.4 In our study, GTPγS binding also resulted in red-shifted Trp emission, as interactions that the backbone amide hydrogens of G202 and G203 also make with the γ phosphate help bring R208 and W211 within range to polarize W211 emission. Altogether, a concerted network of allosteric interactions between these conserved residues and others in the GTPase domain may drive Switch II conformation during GTP binding and hydrolysis.
Despite structural similarities between Gαi and Gαt, the magnitude of the red shift differs somewhat between these proteins. In unmyristoylated Gα proteins, the relatively larger red shift for Gαt is likely due to an additional more solvent exposed Trp present in Gαi proteins, W258, that is absent in Gαt. Mutation of W258 in Gαi HI reduces its emmax and results in a Gαt-like emission profile, due to the location of W258 on a solvent exposed loop of Gαi, and indicating a qualitatively similar environment for Switch II tryptophans in Gαt and Gαi proteins in solution. The conformation of Switch II after GTP hydrolysis (before Gβγ binding) may be important in regulation of basal signaling, evidenced by a peptide that binds Switch II and peels it away from the core of Gαi-GDP, increasing nucleotide exchange.41 We recently determined the structure of a Gαi protein with mutations near the receptor-binding carboxy terminus which displayed elevated nucleotide exchange and altered Switch II conformation,32 consistent with an allosteric linkage between receptor binding, Switch II conformation and nucleotide exchange.
Not reflected in any Gα structure is N-terminal myristoylation, which has been shown in some systems to act as a myristoyl switch,42,43 with effects on spatial and temporal regulation of signaling44 in the visual system. Our previous work suggested an immobile and solvent excluded environment for myristoylated N-terminal residues even in the absence of Gβγ, using a series of extrinsic probes linked to specific N-terminal residues in Gαi HI proteins.18 The current study has uncovered an effect of N-terminal myristoylation on Trp residues far removed in sequence from the N-terminus, suggesting an interaction of the myristoylated N-terminus with or near Trp residues in the GTPase domain. Mutation of W258 to Phe eliminates its contribution to overall Trp emission, but the preservation of a hydrophobic residue at this position likely does not impair any potential interactions that residue 258 may make with the myristoylated N-terminus. Although myristoylated proteins exhibited the requisite activation-dependent increases in Trp intensity upon activation, indicative of the movement of the Switch II Trp into a hydrophobic pocket, the associated red shifts were reduced by myristoylation of Gαi and eliminated in myristoylated Gαt, indicating a myristoylation-dependent modulation of the cation-π interaction. Together these results shed light on the environment of the Switch II region of Gαi proteins before and after activation, and suggest a conformationally sensitive effect of N-terminal myristoylation on residues in the GTPase domain of Gα proteins, consistent with an intramolecular binding site for the myristoylated N-terminus of Gαi family proteins.
Materials and Methods
Materials
GDP and GTPγS were purchased from Sigma-Aldrich (Milwaukee, WI). BODIPY FL-GTPγS, thioester, was purchased from Invitrogen (Madison, WI). The endoproteinase EndoLysC was purchased from Calbiochem (San Diego, CA). All other reagents and chemicals were of the highest available purity.
Preparation of Gα proteins
Gαt was obtained and prepared as described previously.2 Gαil and Gαil HI were used as is or as a basis for site-specific mutagenesis in selected residues. Construction, expression, and purification of Gαil and Gαil HI proteins (unmyristoylated and myristoylated) were performed as described previously.18,26 Briefly, a parent Gαil HI lacking solvent-exposed cysteines which was previously shown to have properties similar to wild type26 was generated with an expression vector encoding rat Gαi1, with the following amino acids substituted: (C66A-C214S-C305S-C325A-C351I), a hexahistidine tag inserted between amino acid residues M119 and T120, and the Switch II mutants described previously have an alanine at residue 3, which does not perturb function.26,29 Mutations were introduced using the QuickChange system (Stratagene, La Jolla, CA); DNA sequencing confirmed all mutations. Proteins were expressed with N-myristoyl transferase to obtain myristoylated protein, and the same protein expressed in the absence of the N-myristoyl transferase vector to produce unmyristoylated proteins. Proteins were expressed and purified as detailed previously18 in E. coli BL21-Gold (DE3) with or without a N-myristoyl transferase (NMT) vector pbb131, which also encodes kanamycin resistance for selection purposes (NMT vector generously provided by M. Linder, Washington University). Coomassie staining of urea SDS PAGE gels45 demonstrate Gαi proteins are fully myristoylated18 when coexpressed with the NMT vector. Purified proteins were stored at −80°C in buffer A containing 50 mM Tris, 100 mM NaCl, 2 mM MgCl2, 10 μM GDP, pH 7.5, and 10% (v/v) glycerol; wild-type Gα containing native, solvent-exposed cysteines was additionally supplemented with 5 mM β-mercaptoethanol or 1 mM DTT before storage at −80°C. After purification, all proteins used in this study were greater than 85% pure, as estimated by Coomassie staining of SDS-PAGE gels.
Intrinsic Trp fluorescence
Proteins without mutations in Switch II demonstrated ≥40% increase in intrinsic Trp emission upon AlF4 activation, relative to emission in the GDP-bound state, as previously described.20 Briefly, Gα proteins (200 nM) in 50 mM Tris, 100 mM NaCl, 1 mM MgCl2, pH 7.5 containing 10 μM GDP were monitored by excitation/emission 280/340 nm before and after the addition of AlF4 (10 mM NaF and 50 μM AlCl3) using a Varian Cary Eclipse Fluorometer. For proteins with mutations in Switch II region which impair the ability to report activation-dependent changes via intrinsic fluorescence, an extrinsic fluorescence using BD-GTPγS was used to assess ability to undergo activation-dependent changes (see below).
Nucleotide exchange of GDP and GDP-AlF4 as measured by BODIPY-GTPγS fluorescence
Basal nucleotide exchange was measured as fold-increase in emission of 1 μM BODIPY-GTPγS (BD-GTPγS, λex = 490 nm, λem = 515 nm) in buffer containing 50 mM Tris, 100 mM NaCl, 1 mM MgCl2, 10 μM GDP, pH 7.5, at 21°C, in the presence and absence of 75 μM AlF4 before and 60 min after the addition of Gαi subunits (200 nM) to the cuvette containing BD-GTPγS. Boiled proteins (b) were heated at 95°C for 5 min before analysis.
Structural analysis/depictions
Computational determination of the energetics of the cation-π interaction in 1GFI was determined using the CaPTURE program.28 Molecular graphics images were produced using the UCSF Chimera package.46,47 Graphs and statistical analysis were performed using GraphPad Prism 4.0, GraphPad Software, San Diego, California.
Acknowledgments
The authors acknowledge Prof. Al Beth for insightful comments and Rachel Beavins for careful reading of this manuscript. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at UCSF.
Glossary
Abbreviations:
- AU
arbitrary units
- BD-GTPγS
BODIPY-GTPγS
- emmax
maximal emission wavelength
- ex/em
excitation/emission
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- Gi
family of G proteins coupled to inhibition of adenylyl cyclase
- Gs
family of G proteins coupled to activation of adenylyl cyclase
- Gt
G protein of the rod outer segment, transducin
- GTP
guanosine triphosphate
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- Gα
α subunits of G proteins
- Gβγ
βγ dimer of heterotrimeric G proteins which binds Gα
- HI
Gαil isoform lacking several solvent-exposed cysteines
- N-terminus
amino terminus
- SEM
standard error of mean
- wt
wild-type.
References
- 1.Sprang SR. G Protein Mechanisms: Insights from Structural Analysis. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 2.Noel JP, Hamm HE, Sigler PB. The 2.2 Å crystal structure of transducin-α complexed with GTPγS. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- 3.Lambright DG, Noel JP, Hamm HE, Sigler PB. Structural determinants for activation of the α-subunit of a heterotrimeric G protein. Nature. 1994;369:621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- 4.Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Structures of Active Conformations of Giα1 and the Mechanism of GTP Hydrolysis. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 5.Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Tertiary and quaternary structural changes in Giα1 induced by GTP hydrolysis. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 6.Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB. GTPase mechanism of Gproteins from the 1.7-Å crystal structure of transducin α•GDP•AIF4−. Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
- 7.Coleman DE, Sprang SR. Crystal structures of the G protein Giα1 complexed with GDP and Mg2+: a crystallographic titration experiment. Biochemistry. 1998;37:14376–14385. doi: 10.1021/bi9810306. [DOI] [PubMed] [Google Scholar]
- 8.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 9.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83:1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 10.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4−-activated Giα1: stabilization of the transition state for GTP hydrolysis. Cell. 1997;89:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 11.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα·GTPγS. Science. 1997;278:1907–1916. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 12.Sterne-Marr R, Tesmer JJ, Day PW, Stracquatanio RP, Cilente JA, O'connor KE, Pronin AN, Benovic JL, Wedegaertner PB. G protein-coupled receptor Kinase 2/G alpha q/11 interaction. A novel surface on a regulator of G protein signaling homology domain for binding G alpha subunits. J Biol Chem. 2003;278:6050–6058. doi: 10.1074/jbc.M208787200. [DOI] [PubMed] [Google Scholar]
- 13.Slep KC, Kercher MA, He W, Cowan CW, Wensel TG, Sigler PB. Structural determinants for regulation of phosphodiesterase by a G protein at 2.0 Å. Nature. 2001;409:1071–1077. doi: 10.1038/35059138. [DOI] [PubMed] [Google Scholar]
- 14.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJ. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc Natl Acad Sci USA. 2009;106:3178–3183. doi: 10.1073/pnas.0900294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimple RJ, Kimple ME, Betts L, Sondek J, Siderovski DP. Structural determinants for GoLoco-induced inhibition of nucleotide release by Gα subunits. Nature. 2002;416:878–881. doi: 10.1038/416878a. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Singer WD, Sternweis PC, Sprang SR. Structure of the p115RhoGEF rgRGS domain-Gα13/i1 chimera complex suggests convergent evolution of a GTPase activator. Nat Struct Mol Biol. 2005;12:191–197. doi: 10.1038/nsmb888. [DOI] [PubMed] [Google Scholar]
- 18.Preininger AM, Van Eps N, Yu NJ, Medkova M, Hubbell WL, Hamm HE. The myristoylated amino terminus of Gαi1 plays a critical role in the structure and function of Gαi1 subunits in solution. Biochemistry. 2003;42:7931–7941. doi: 10.1021/bi0345438. [DOI] [PubMed] [Google Scholar]
- 19.Linder ME, Pang IH, Duronio RJ, Gordon JI, Sternweis PC, Gilman AG. Lipid modifications of G protein subunits. Myristoylation of Goα increases its affinity for βγ. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- 20.Mazzoni MR, Hamm HE. Tryptophan207 is involved in the GTP-dependent conformational switch in the alpha subunit of the G protein transducchymotryptic digestion patterns of the GTP gamma S and GDP-bound forms. J Protein Chem. 1993;12:215–221. doi: 10.1007/BF01026043. [DOI] [PubMed] [Google Scholar]
- 21.Faurobert E, Otto-Bruc A, Chardin P, Chabre M. Tryptophan W207 in transducin T alpha is the fluorescence sensor of the G protein activation switch and is involved in the effector binding. EMBO J. 1993;12:4191–4198. doi: 10.1002/j.1460-2075.1993.tb06103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bigay J, Deterre P, Pfister C, Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985;191:181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- 23.Higashijima T, Ferguson KM, Sternweis PC, Ross EM, Smigel MD, Gilman AG. The effect of activating ligands on the intrinsic fluorescence of guanine nucleotide-binding regulatory proteins. J Biol Chem. 1987;262:752–756. [PubMed] [Google Scholar]
- 24.Abdulaev NG, Ngo T, Zhang C, Dinh A, Brabazon DM, Ridge KD, Marino JP. Heterotrimeric G-protein α-subunit adopts a “Preactivated” conformation when associated with βγ-subunits. J Biol Chem. 2005;280:38071–38080. doi: 10.1074/jbc.M505259200. [DOI] [PubMed] [Google Scholar]
- 25.Higashijima T, Ferguson KM, Smigel MD, Gilman AG. The effect of GTP and Mg2+ on the GTPase activity and the fluorescent properties of Go. J Biol Chem. 1987;262:757–761. [PubMed] [Google Scholar]
- 26.Medkova M, Preininger AM, Yu NJ, Hubbell WL, Hamm HE. Conformational changes in the amino-terminal helix of the G Protein αi1 following dissociation from Gβγ subunit and activation. Biochemistry. 2002;41:9962–9972. doi: 10.1021/bi0255726. [DOI] [PubMed] [Google Scholar]
- 27.Sprang SR, Chen Z, Du X. Structural basis of effector regulation and signal termination in heterotrimeric galpha proteins. Adv Protein Chem. 2007;74:1–65. doi: 10.1016/S0065-3233(07)74001-9. [DOI] [PubMed] [Google Scholar]
- 28.Gallivan JP, Dougherty DA. Cation-pi interactions in structural biology. Proc Natl Acad Sci USA. 1999;96:9459–9464. doi: 10.1073/pnas.96.17.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eps N, Oldham WM, Hamm HE, Hubbell WL. Structural and dynamical changes in an alpha-subunit of a heterotrimeric G protein along the activation pathway. Proc Natl Acad Sci USA. 2006;103:16194–16199. doi: 10.1073/pnas.0607972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol. 2006;13:772–777. doi: 10.1038/nsmb1129. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran S, Cerione RA. Stabilization of an intermediate activation state for transducin by a fluorescent GTP analogue. Biochemistry. 2004;43:8778–8786. doi: 10.1021/bi0362774. [DOI] [PubMed] [Google Scholar]
- 32.Preininger A, Funk M, Meier S, Oldham W, Johnston C, Adhikary S, Kimple A, Siderovski D, Hamm H, Iverson T. Helix dipole movement and conformational variability contribute to allosteric GDP release in Gi subunits. Biochemistry. 2009;48:2630–2642. doi: 10.1021/bi801853a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan KL, Remmers AE, Neubig RR. Roles of G(o)alpha tryptophans in GTP hydrolysis, GDP release, and fluorescence signals. Biochemistry. 1998;37:837–843. doi: 10.1021/bi972122i. [DOI] [PubMed] [Google Scholar]
- 34.Carra JH, Mchugh CA, Mulligan S, Machiesky LM, Soares AS, Millard CB. Fragment-based identification of determinants of conformational and spectroscopic change at the ricin active site. BMC Struct Biol. 2007;7:72. doi: 10.1186/1472-6807-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zacharias N, Dougherty DA. Cation-pi interactions in ligand recognition and catalysis. Trends Pharmacol Sci. 2002;23:281–287. doi: 10.1016/s0165-6147(02)02027-8. [DOI] [PubMed] [Google Scholar]
- 36.Neuwald AF. Galpha Gbetagamma dissociation may be due to retraction of a buried lysine and disruption of an aromatic cluster by a GTP-sensing Arg Trp pair. Protein Sci. 2007;16:2570–2577. doi: 10.1110/ps.073098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougherty DA. Cation-pi interactions involving aromatic amino acids. J Nutr. 2007;137:1504S–1508S. doi: 10.1093/jn/137.6.1504S. discussion. [DOI] [PubMed] [Google Scholar]
- 38.Ma JC, Dougherty DA. The cation-pi Interaction. Chem Rev. 1997;97:1303–1324. doi: 10.1021/cr9603744. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell JB, Nandi CL, Mcdonald IK, Thornton JM, Price SL. Amino/aromatic interactions in proteins: is the evidence stacked against hydrogen bonding? J Mol Biol. 1994;239:315–331. doi: 10.1006/jmbi.1994.1370. [DOI] [PubMed] [Google Scholar]
- 40.Flocco MM, Mowbray SL. Planar stacking interactions of arginine and aromatic side-chains in proteins. J Mol Biol. 1994;235:709–717. doi: 10.1006/jmbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- 41.Johnston CA, Willard FS, Jezyk MR, Fredericks Z, Bodor ET, Jones MB, Blaesius R, Watts VJ, Harden TK, Sondek J, Ramer JK, Siderovski DP. Structure of Gαi1 bound to a GDP-selective peptide provides insight into guanine nucleotide exchange. Structure. 2005;13:1069–1080. doi: 10.1016/j.str.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- 44.Kerov V, Rubin WW, Natochin M, Melling NA, Burns ME, Artemyev NO. N-terminal fatty acylation of transducin profoundly influences its localization and the kinetics of photoresponse in rods. J Neurosci. 2007;27:10270–10277. doi: 10.1523/JNEUROSCI.2494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mumby SM, Linder ME. Myristoylation of G-protein alpha subunits. Methods Enzymol. 1994;237:254–268. doi: 10.1016/s0076-6879(94)37067-2. [DOI] [PubMed] [Google Scholar]
- 46.Sanner MF, Duncan BS, Carrillo CJ, Olson AJ. Integrating computation and visualization for biomolecular analysis: an example using python and AVS. Pac Symp Biocomput. 1999:401–412. doi: 10.1142/9789814447300_0039. [DOI] [PubMed] [Google Scholar]
- 47.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]


