Abstract
Objective
Skeletal muscle blood flow during exercise is impaired in obesity. We tested the hypothesis that the attenuated vasodilation in skeletal muscle arterioles of obese Zucker rats (OZR) is due to altered KATP channel-mediated vasodilation.
Materials and Methods
KATP channel function was determined in isolated skeletal muscle arterioles in response to the KATP opener cromakalim (0.1–10 μM) during normal myogenic tone and α-adrenergic-mediated tone (0.1 μM phenylephrine). The spinotrapezius muscle was prepared and the vasodilatory responses to muscle stimulation or iloprost (0.028–2.8 μM) were observed before and after the application of the KATP inhibitor, glibenclamide (10 μM). Channel subunit expression was determined by using western blot analyses.
Results
Cromakalim concentration-response curves were shifted in OZR as compared to lean controls. OZR exhibited impaired functional and iloprost-induced vasodilation as compared to the lean controls. Glibenclamide inhibited the functional and iloprost-induced dilation in the lean rats with no effects in the obese animals. Channel subunit expression was similar in femoral arteries.
Conclusion
The impaired functional vasodilation in the OZR is associated with altered KATP channel sensitivity.
Keywords: vasodilation, arachidonic acid, microcirculation, exercise
The prevalence of obesity is increasing in the United States and worldwide [6, 34]. Associated with obesity are insulin resistance, hyperglycemia, dyslipidemia, a proinflammatory state, elevations in oxidative stress, and endothelial dysfunction. As such obesity is a major risk factor for a variety of cardiovascular diseases, including hypertension, coronary artery disease, and stroke, as well as metabolic conditions such as metabolic syndrome and type II diabetes [4]. In addition, obesity has been associated with an attenuated increase in muscle blood flow during exercise (i.e., functional hyperemia) in both humans [32, 37] and animal models, such as the obese Zucker rat (OZR) [12, 52, 53], although the exact mechanisms underlying this impairment are unclear.
One possible cause for the impaired functional hyperemia in obesity is endothelial dysfunction and the accompanying attenuated release of endothelium-derived regulators of blood flow [48]. The vasodilator prostaglandins, metabolites of arachidonic acid (AA) and the cyclooxygenase (COX) pathway, are important endothelium-derived regulators of arteriolar diameter and blood flow during muscle stimulation and exercise [17, 29, 33]. Prostacyclin (PGI2) is the primary prostaglandin released from endothelial cells and is considered to be involved in the regulation of skeletal muscle blood flow [44, 46]. During exercise, PGI2 levels increase, and inhibition of prostaglandin formation markedly attenuates muscle blood flow [38, 50, 51]. PGI2 results in a vasodilation through stimulation of the PGI2 receptor (IP) and activation of potassium channels, including ATP-sensitive potassium channels (KATP) [19, 21, 40]. Stimulation of the IP receptor, using the prostacyclin analog iloprost in the OZR, has been shown to be attenuated both in isolated arterioles from gracilis muscles [12] and in vivo preparations of the spinotrapezius muscle [53]. The mechanisms underlying the impaired IP-receptor-mediated vasodilation in the OZR are unclear. One possible mechanism underlying this impaired vasodilation is an alteration in the downstream effector mechanisms activated through IP-receptor-signaling mechanisms (i.e., KATP channels).
The aim of this study was to test the hypothesis that KATP-dependent responses are altered in the obese Zucker rat, and that these alterations are responsible for the impaired functional dilation. In addition, we determined if alterations in KATP channel subunit expression are responsible for the altered KATP responses.
MATERIALS AND METHODS
Animals
The experimental protocols for this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center. Male lean (LZR; n = 22; 325 ± 6 g) and obese (n = 24; 490 ± 9 g) Zucker rats (10–13 weeks old; Harlan, Inc., Indianapolis, IN) were used for these experiments, with some animals being used for both in vitro and molecular studies. All animals were housed two to three animals per cage at 22°C (12-hour light-dark cycle) with ad libitum access to food and water.
Isolated Arteriole Preparation
Rats were anesthetized with sodium pentobarbital (65 mg/kg, intraperitoneally [i.p.]) and the gracilis muscle was surgically dissected from the hind limb and secured in a Silastic-coated petri dish containing dissection solution [(in mM) 130 NaCl, 4 KCl, 1.2 MgSO4, 4 NaHCO3, 1.8 CaCl2, 10 HEPES, 1.18 KH2PO4, 0.03 EDTA, 6 glucose]. Animals were then euthanized by an overdose of sodium pentobarbital. Death was confirmed by a lack of a heartbeat and spontaneous breathing. Small feed arterioles were dissected free from the gracilis muscle, placed in a vessel chamber (Living Systems, Burlington, VT) containing dissection solution, cannulated with glass micropipettes, rinsed free of blood, and secured in place with nylon ties. Vessels were superfused (5 mL/min) with warmed (37°C), aerated (21% O2–5% CO2–balance N2) physiological saline solution [PSS; (in mM) 119 NaCl, 5.9 KCl, 25 NaHCO3, 1.18 NaH2PO4, 1.17 MgSO4, 2.5 CaCl2, 0.026 EDTA, 5.5 glucose]. Intraluminal pressure was slowly increased to 80 mmHg by using a column of PSS and monitored using an inline pressure transducer (Living Systems). Vessels were stretched to remove bends and allowed to equilibrate for a period of 30 minutes. Viability of the arteriole was assessed by using a single dose of the α1-adrenergic agonist, phenylephrine (PE; 10 μM), followed by a single dose of the endothelium-dependent vasodilator, acetylcholine (ACh; 10 μM). Arterioles then underwent a 15-minute washout period before concentration-response curves were performed. Passive diameter was determined at the end of each experiment by superfusing arterioles with Ca2+-free PSS [(in mM) 119 NaCl, 5.9 KCl, 25 NaHCO3, 1.18 NaH2PO4, 1.17 MgSO4, 2.5 EGTA, 5.5 glucose] for 30 minutes. The preparation was viewed on an inverted microscope (Nikon Diaphot 300, Tokyo, Japan) equipped with a Dage (Dage-MTI of MC, Inc. Michigan City, IN) camera. Images were collected by using MetaMorph 4.5 software (Molecular Devices, Downing, PA) with luminal diameter measured just prior to the administration of the next agonist concentration. The above chemicals and drugs were purchased from Sigma Chemicals (St. Louis, MO).
In Vitro KATP Channel Stimulation
In the first set of experiments, gracilis arterioles from lean and obese Zucker rats (n = 6 for each group) were allowed to generate spontaneous myogenic tone. Vasodilatory responses to increasing cumulative concentrations of the KATP channel opener, cromakalim (0.01–10 μM; Sigma), were then determined. Following a second washout period, arterioles were treated with the KATP channel inhibitor, glibenclamide (10 μM; Sigma), for 30 minutes. The concentration-response curve to cromakalim was then repeated in the presence of glibenclamide. Glibenclamide was dissolved in dimethyl sulfoxide (DMSO) and diluted in PSS, with the final solution containing 0.04% DMSO. The vessels in the first experiment were under normal myogenic tone, which may not mimic the actual tone of the vessels in vivo, as they have been removed from any normal sympathetic stimulation. In order to mimic a modest increase in sympathetic tone, arterioles were constricted with phenylephrine in a second experiment. Arterioles from lean (n = 5) and obese (n = 6) rats were constricted with 0.1 μM PE prior to determining the vasodilatory response to cromakalim (0.01–10 μM). We have observed that this dose of PE causes a small decrease in diameter (~10%) that is similar in both lean and obese animals [31].
In Vivo Microcirculatory Surgical Preparation
In another set of experiments, the right spinotrapezius muscle was prepared for experimental observation, as previously described [1, 25]. In brief, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and the trachea was intubated. Animals spontaneously breathed a gas mixture containing 30% oxygen and 70% nitrogen. The left jugular vein was cannulated for the supplemental addition of anesthetic. At all times during the surgery and subsequent experiments, the spinotrapezius muscle was kept at in situ dimensions and continuously superfused with a PSS of the following composition [(in mM) 118.07 NaCl, 6.17 KCl, 2.55 CaCl2, 25 NaHCO3] and aerated with a 5% CO2–95% N2 gas mixture (pH = 7.4, 35°C). At the completion of the study, animals were euthanized by a cardiac injection of sodium pentobarbital. Death was confirmed by a lack of a heartbeat and spontaneous breathing.
In Vivo Arteriole Visualization and Muscle Stimulation
Animals were allowed to stabilize for 15–30 minutes after the completion of the surgical procedure. Segments of arteriolar arcades with similar diameters were selected for analysis. The microcirculation of the spinotrapezius muscle was transilluminated and observed with a Nikon microscope fitted with a 10× water immersion objective (numerical aperture = 0.30). The microscopic image was televised with a Dage closed-circuit television camera and displayed on a Sony monitor (Tokyo, Japan). The magnification of the image was ×660 from the tissue to the monitor screen. Vessel diameter was measured by using a Texas A&M video analyzer modified to function as a video micrometer. The resolution of this system was ±1 μm.
Two hooked silver-silver chloride electrodes (Grass Technologies, West Warwick, RI) were placed at each end of the spinotrapezius and connected to a Grass S44 stimulator. Diameters of the vessels were obtained in the resting muscle and immediately following two minutes of electrical stimulation (4–5 V, 1 Hz).
In Vivo Experimental Protocol
The right spinotrapezius muscle was prepared for the microcirculatory observation. Diameters of the arterioles were obtained in the resting muscle and immediately following two minutes of electrical muscle stimulation. After the arteriole had returned to its resting diameter, a concentration-response curve to the PGI2 analog, iloprost, was performed (0.028–2.8 μM; Cayman Chemical Company, Ann Arbor, MI). The superfusion solution was then replaced, and the arteriole was allowed to return to its resting diameter. The KATP channel inhibitor, glibenclamide (10 μM), was then added to the superfusion solution and remained in the superfusate until the end of the experiment. Following a 30-minute equilibration period, the muscle stimulation protocol and iloprost concentration-response curve were repeated. At the end of the experiment, adenosine (10 μM; Sigma) was added to the superfusion solution to determine the maximal luminal diameter.
Potassium Channel Subunit Protein Expression
Femoral arteries were collected from anesthetized lean and obese rats and snap frozen in liquid nitrogen, with arteries from two animals being pooled prior to protein extraction (n = 4 each group). Each sample was run in triplicate, and thoracic aortic samples were collected from lean animals to be used as positive controls. Vessels were homogenized in RIPA buffer containing a 1% protease inhibitor cocktail (Sigma). Samples were centrifuged at 14,000×g for five minutes at 4°C to remove insoluble debris. The supernatant was collected and sample protein concentrations were quantified by using the Bradford method. Equal amounts of protein were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (4–20% gradient Tris·HCl gels; Jule, Milford, CT) and transferred to a nitro-cellulose membrane (Bio-Rad, Hercules, CA). Blots were blocked for one hour in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) and incubated overnight at 4°C with a polyclonal antibody, either rabbit anti-Kir6.1 (1:500 dilution; Sigma) or goat anti-SUR-2B (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). For immunochemical labeling, blots were then incubated for one hour at room temperature with a fluorescent secondary antibody, either goat antirabbit or donkey antigoat IgG (1:5000 dilution; Rockland Immunochemicals, Inc., Gilbertsville, PA). Bands were detected using an Odyssey infrared imaging system (LI-COR Biosciences). Following potassium channel subunit detection, blots were washed in phosphate-buffered saline (PBS) containing 0.1% Tween 20 and incubated for one hour with mouse monoclonal antibody for β-actin (1:2000 dilution; Abcam, Cambridge, MA). For immunochemical labeling of βactin, blots were then incubated for one hour with donkey antimouse IgG (1:5000 dilution; Abcam, Cambridge, MA). Bands for potassium-channel subunits were quantified by densitometric analysis of scanned images using Odyssey 1.1 software (LI-COR Biosciences) and normalized to those of β-actin.
Statistics and Data Analysis
For the isolated vessel studies, luminal diameter changes in response to cromakalim are presented as an increase in arteriolar diameter from baseline diameter for arterioles with normal myogenic tone and from constricted diameter for arterioles constricted with 0.1 μM PE. For the in vivo studies, arteriolar diameters are presented as absolute values for functional vasodilation and as an increase in arteriolar diameter from baseline diameter for iloprost-induced vasodilation. Descriptive data were analyzed using a t-test. Individual concentration-response curves were compared using two-way analysis of variance (ANOVA), with two-way repeated measures ANOVA used to compare individual doses and effects of glibenclamide. A probability of p ≤ 0.05 was accepted as statistically significant for all comparisons. All data are presented as the means ± standard error.
RESULTS
In Vitro KATP Channel Stimulation
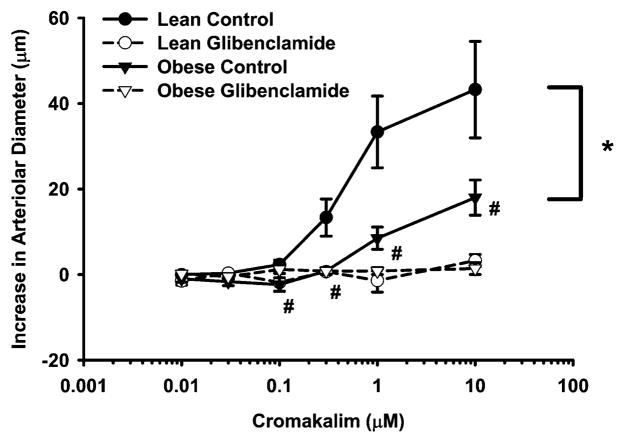
Figure 1 presents the dilation of gracilis arterioles with normal myogenic tone in response to administration of the KATP channel opener, cromakalim. Baseline diameters of gracilis arterioles were not significantly different between lean and obese groups (171 ± 9 vs. 173 ± 9 μm). Passive diameters of these vessels were also not significantly different between LZR (209 ± 6 μm) and OZR (193 ± 9 μm). The concentration-response curves to cromakalim were significantly different between lean and obese control groups (p < 0.001). The cromakalim-induced vasodilation was significantly different at 0.1–10-μM concentrations between lean and obese control groups (p = 0.035, p < 0.001, p = 0.001, and p = 0.005, respectively). Glibenclamide completely inhibited the cromakalim-induced vasodilation in both groups.
Figure 1.
Cromakalim-induced vasodilation of isolated gracilis arterioles from lean (n = 6) and obese (n = 6) Zucker rats before and after treatment with the KATP channel inhibitor, glibenclamide (10 μM). Values are the means ± standard error. *Significant difference between lean and obese control concentration-response curves. #Significant difference between lean and obese control groups at indicated cromakalim concentration.
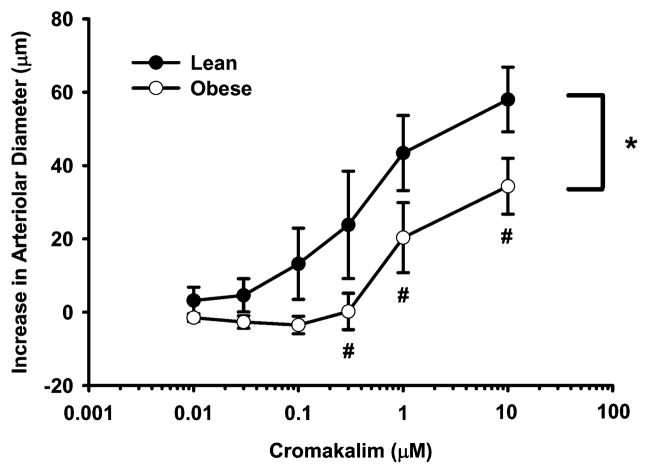
Figure 2 presents the cromakalim responses of gracilis arterioles constricted with 0.1 μM PE. This dose of PE increased the vessel tone to a similar degree in both lean and obese groups (7 ± 2 vs. 10 ± 4%) and constricted arteriole diameters were not different between lean and obese animals (150 ± 8 vs. 169 ± 12 μm). Passive diameters of these vessels were also not significantly different between LZR (216 ± 7 μm) and OZR (214 ± 7 μm). The concentration response curves to cromakalim of the arterioles constricted with PE were significantly different between lean and obese groups (p < 0.001). The cromakalim-induced vasodilation in the OZR was significantly different from the LZR at 0.3–10 μM concentrations (p = 0.035, p = 0.032, p = 0.028; respectively).
Figure 2.
Cromakalim-induced vasodilation of isolated gracilis arterioles from lean (n = 5) and obese (n = 6) Zucker rats following constriction with 0.1 μM of phenyle-phrine. Values are the means ± standard error. *Significant difference between lean and obese concentration-response curves. #Significant difference between lean and obese groups at indicated cromakalim concentration.
Functional Vasodilation and In Vivo KATP Inhibition
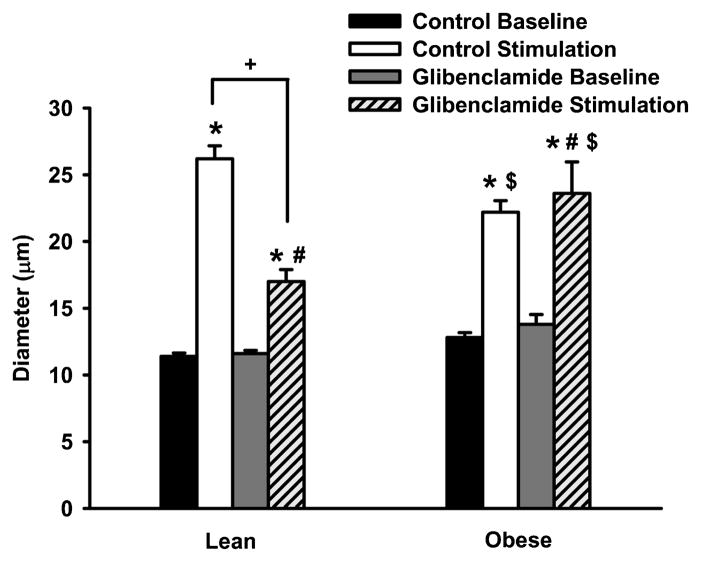
Figure 3 presents the functional vasodilation of spinotrapezius arterioles in LZR and OZR both in the presence and absence of glibenclamide. The basal diameters of lean (11 ± 1 μm; n = 5) and obese (13 ± 1 μm; n = 5) arterioles were not different between groups. In addition, the adenosine-induced dilation was not significantly different between groups. The muscle stimulation induced a greater increase in diameter in the lean Zucker rats as compared to the obese animals (p = 0.013). However, treatment with the KATP channel blocker, glibenclamide, only significantly inhibited the functional vasodilation in lean Zucker rats (p = 0.013) with no effect in the OZR. When expressed as a percent inhibition of the control response, approximately 65% of the functional vasodilation was attenuated by glibenclamide in the lean group, whereas vasodilation in the obese animals was not impaired by glibenclamide (p = 0.002).
Figure 3.
Basal diameter and muscle stimulation-induced diameter in spinotrapezius arterioles from lean (n = 6) and obese (n = 6) Zucker rats before and after treatment with 10 μM of glibenclamide. Values are the means ± standard error. *Significant difference from control baseline diameter. #Significant difference from glibenclamide baseline diameter. +Significant difference between control- and glibenclamide-stimulated diameters. $Significant difference between corresponding lean and obese measurements.
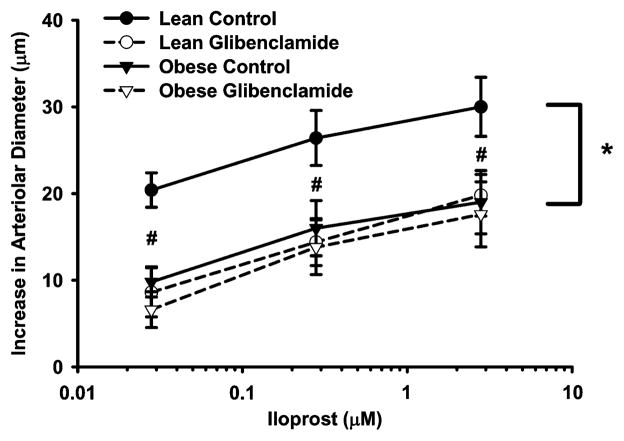
Figure 4 presents the iloprost-induced vasodilation of the spinotrapezius arterioles in LZR and OZR both in the presence and absence of glibenclamide. The iloprost-induced vasodilation was significantly different at all concentrations between lean and obese control groups (p = 0.004, p = 0.033, and p = 0.041, respectively). There was no difference between LZR and OZR glibenclamide groups. Glibenclamide attenuated the iloprost-induced vasodilation in the LZR group (p < 0.001), with no further impairment of the vasodilation in the OZR group.
Figure 4.
Iloprost-induced change in diameter in spino-trapezius arterioles from lean (n = 6) and obese (n = 6) Zucker rats before and after treatment with 10 μM of glibenclamide. Values are the means ± standard error. *Significant difference between lean and obese control concentration-response curves. #Significant difference between lean and obese control groups at indicated iloprost concentration.
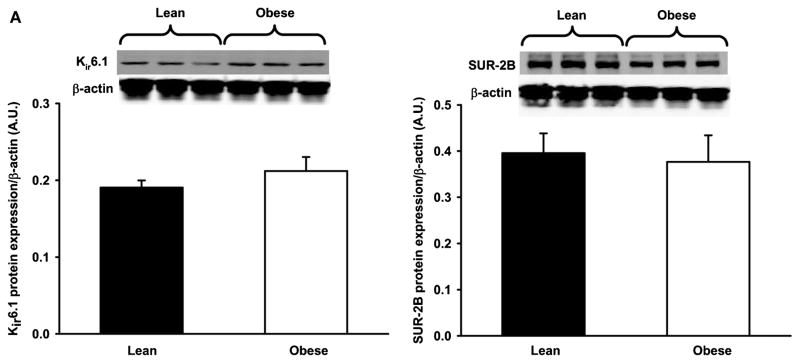
KATP Subunit Protein Expression
The potassium channel subunit expression in femoral arteries from LZR and OZR is shown in Figure 5. Neither expression of the α-subunit (Kir6.1) nor expression of the β-subunit (SUR-2B) were significantly different between groups.
Figure 5.
KATPα (A) and KATPβ (B) subunit expression in femoral arteries from lean (n = 4) and obese (n = 4) Zucker rats. Values are the means ± standard error. Protein levels were normalized to β-actin. There was no significant difference between groups.
DISCUSSION
The major findings of this study are that 1) KATP-mediated vasodilation of skeletal muscle arterioles is impaired in obese Zucker rats both under normal myogenic conditions (Fig. 1) and following increases in α-adrenergic-mediated tone (Fig. 2); 2) functional vasodilation in the LZR is dependent on KATP channel activation but not in the OZR (Fig. 3); 3) iloprost-induced vasodilation of skeletal muscle arterioles in the LZR is dependent on KATP channel activation but not in the OZR (Fig. 4); and 4) potassium channel subunit expression is similar between groups in the larger arteries (Fig. 5). These findings support our hypothesis that the impaired functional hyperemia in the obese Zucker rat may be due to alterations in the function of the KATP channels that mediate PGI2-mediated vasodilation. However, the mechanism underlying the impaired KATP channel response is apparently not due to a decrease in channel number, but rather due to decreased channel sensitivity.
The OZR is an animal model of obesity and the metabolic syndrome, exhibiting hyperphagia due to nonfunctional leptin receptors, as well as insulin resistance, dyslipidemia, endothelial dysfunction, and elevations in oxidative stress [14, 35, 52]. In association with its metabolic abnormalities, obese patients [32, 36, 37] and animals [12, 52, 53] exhibit impaired functional hyperemia. Previous studies from our laboratory, using the spinotrapezius preparation, have shown that the OZR exhibits blunted functional vasodilatory responses, as well as impaired vasodilation in response to acetylcholine, arachidonic acid, iloprost, and sodium nitroprusside [52, 53]. These studies correlate with in vitro and in vivo studies from ours and other laboratories, in which the altered functional hyperemia in the OZR has been linked to altered vasodilatory mechanisms [9, 11], enhanced α-adrenergic constriction [9, 31], and structural remodeling of the microcirculation [10, 12, 13]. However, the mechanism(s) underlying this impaired blood-flow response during exercise remain unclear.
Arachidonic acid metabolites are important regulators of skeletal muscle blood flow, as inhibition of prostaglandin formation via cyclooxygenase inhibition has been shown to significantly attenuate vasodilatory responses to exercise [24, 51]. The predominant prostaglandin synthesized from endothelial cells is prostacyclin [44, 45], and PGI2 levels have been shown to increase during exercise in humans [38, 49, 50]. While decreases in PGI2 synthesis due to alterations in AA metabolism could potentially explain the attenuated AA-induced vasodilation, the OZR also exhibits impaired iloprost-induced vasodilation as compared to lean animals [12, 53], suggesting that alterations in downstream effector mechanisms could play a role in the attenuated vasodilation. In the present study, we examined whether alterations in KATP channels may play a role in the altered vasodilation seen in the OZR skeletal muscle vasculature. These channels contribute to the membrane potential of vascular smooth-muscle cells (VSMCs) [7, 20], and endogenous vasodilators, such as NO and PGI2, can activate these channels and thus induce potassium efflux and membrane hyperpolarization [19, 21, 41]. This hyperpolarization of VSMCs, in turn, causes the closure of voltage-gated calcium channels, a decrease in intracellular calcium concentration, and vasodilation [8, 20, 42]. To our knowledge, alterations in KATP channel-induced vasodilation have not been extensively studied in the obese Zucker vasculature.
IP-receptor stimulation results in vasodilation through the activation of potassium channels, namely, KATP and large-conductance calcium-activated potassium channels (BK) [19, 21, 40]. Our present study shows that glibenclamide inhibits the functional dilation in lean Zucker rats (Fig. 3), suggesting that under normal conditions, the KATP channels are important in mediating the functional vasodilation. This result is consistent with our previous findings in hamster cremaster muscles that ATP-sensitive potassium channels play a role in the functional dilationof different sizes of arterioles [17, 39]. In one study, vasodilation of first- and third-order arterioles in response to 1-μM cromakalim administration was significantly inhibited following 10-μM glibenclamide application. Further, functional vasodilation in these first- and third-order arterioles was significantly attenuated following glibenclamide application [39]. In a second study, fourth-order arterioles were also found to exhibit impaired functional vasodilation following glibenclamide application [17]. Therefore, we tested the hypothesis that the impaired functional dilation observed in the OZR is due, at least in part, to KATP channel dysfunction. The present study shows that inhibition of KATP channels with glibenclamide failed to further blunt the functional dilation in obese Zucker rats, suggesting the presence of KATP dysfunction, which may contribute to the impaired functional dilation. Moreover, glibenclamide inhibited the iloprost-induced dilation in lean rats to the same level observed in the OZR with glibenclamide having no effect in the OZR (Figure 4). These results suggest that the impairment of iloprost-induced dilation in OZR is mainly due to an altered KATP function. Taken together, since PGI2 production is important in mediating the functional vasodilation, the present study suggests that a blunted PGI2 response due to KATP channel dysfunction may be responsible for the impaired functional dilation in OZR.
To further determine whether or not there is an impaired KATP channel function in OZR, we compared the vasodilatory responses between lean and obese rats to direct stimulation of KATP channels, using the KATP channel opener cromakalim. We observed a shift in the concentration-response curve of gracilis arterioles from obese animals (Figure 1), suggesting a decreased activity of VSMC KATP channels in OZR. This result is in opposition to a study by Frisbee in which isolated LZR and OZR gracilis arterioles had similar concentration-response curves to the KATP channel opener, aprikalim [11]. In that study, slope coefficients were measured in response to increasing concentrations of aprikalim, with the maximum concentration being 1 μM. However, in our study, we used concentrations of cromakalim of up to 10 μM. Thus, the difference in KATP-mediated vasodilations between Frisbee’s study and our study could have been due to the differences in the concentrations of the drugs or possibly even a difference in sensitivity to the channel openers themselves. Studies of streptozotocin-induced diabetic animals, a model of type I diabetes, have shown alterations in KATP responses during stimulation with cromakalim and in the aorta [23] and cerebral circulation [27, 28]. The intramuscular arterioles from the animals in our study were of similar size and baseline diameter; thus, the shift in the vasodilatory-response curve of the OZR vessels indicates that the sensitivity of KATP channels in the vascular smooth muscle is diminished in the obese animals. As previously stated, these arterioles were under normal myogenic tone, which may not mimic the actual tone of the vessels in vivo, as they have been removed from any normal sympathetic stimulation. In order to mimic a modest increase in sympathetic tone, a second experiment was performed in which arterioles were constricted with 0.1 μM of PE prior to the cromakalim administration (Figure 2). The cromakalim concentration-response curve was also shifted in the OZR PE-constricted vessels, again indicating that the KATP channels are less sensitive in the obese animals. This apparent decrease in KATP channel sensitivity in the obese Zucker rat skeletal muscle arterioles suggests that obesity, via an unknown mechanism, alters the sensitivity of the channels.
One way in which in vitro channel sensitivity could be altered is through a decrease in overall channel number. Thus, we determined whether expression levels of the two subunits of the KATP channels, the pore-forming α-subunit (Kir6.1) and the regulatory sulfonylurea receptor β-subunit (SUR-2B), were diminished in the obese Zucker vasculature. Western blot analysis of the femoral arteries (Figure 5) indicates that the channel subunit expression is not altered in the large conduit arteries that perfuse the hind-limb vasculure. However, these results may not correlate directly with what is seen in the intramuscular arteriolar network, where small arteries and arterioles play a more critical role in determining vascular resistance and blood flow. For instance, in other vascular beds, ion channels have been shown to exhibit substantial heterogeneity between larger conduit vessels and smaller resistance vessels [3, 43]. These results suggest that although the channels are less sensitive in the obese vessels, the overall channel number is not decreased. Interpretation of these results is difficult, as these channels have not been extensively studied in our model and other studies have found mixed results. As previously mentioned, KATP channels have been linked to impaired relaxation in other vascular beds in type I diabetic animals. However, these studies did not investigate whether the impaired vasodilations were due to decreases in channel sensitivity or number. Further complicating the interpretation of these data is an apparent temporal component to KATP channel dysfunction in diabetic models [47]. Early diabetic states have been linked to an enhanced KATP channel response [18], with advanced diabetic states exhibiting diminished KATP activation [2]. To our knowledge, expression levels of KATP channels have not been previously characterized in the lean and obese Zucker rat skeletal muscle vasculature. Although the mechanisms are unclear, the blunted KATP channel-mediated vasodilation without a decrease in channel numbers still suggests an impairment of channel sensitivity in this animal model of obesity. Further molecular studies are needed to examine potassium channel subunit expression in the intramuscular arteriolar network and the apparent decrease in KATP channel sensitivity exhibited in the OZR.
In the present study, the inhibition of KATP channels fails to completely block the functional dilation in the lean Zucker rats (Figure 3). However, Figure 1 shows that glibenclamide completely blocked the vasodilatory response to the KATP channel opener, cromakalim. Therefore, the remaining functional vasodilation after the administration of glibenclamide suggests an involvement of some other mechanism(s) or pathways in mediating the hyperemic responses. For example, the blockade of adenosine receptors has been reported to decrease blood flow in exercising humans [30], and the circulating levels of adenosine triphosphate (ATP) have been shown to increase in an exercise intensity-dependent manner [15]. There is also evidence that the increase in interstitial potassium concentration surrounding the vasculature in response to muscle contraction results in a vasodilation through the activation of smooth-muscle Kir channels [22, 26]. Additionally, several studies suggest a role for the conduction of a hyperpolarization through gap junctions to the underlying smooth muscle [16]. Moreover, the present study cannot exclude the possibility that other endothelium-derived hyperpolarizing factor(s) are involved in the functional vasodilatory response. Although PGI2-mediated vasodilation has been shown to involve KATP channels [5], it is not clear whether or not other endogenous vasodilators, such as nitric oxide or adenosine, also activate KATP channels during muscle contraction, and the role of KATP channel activation in functional vasodilation in the rat spinotrapezius muscle is not clear. Alternatively, the remaining dilation may be a compensatory effect in response to the inhibition of KATP channels by yet to be determined factor(s). However, the present study shows that the remaining functional dilation after KATP inhibition is higher in the OZR as compared to the LZR, suggesting a compensation mechanism, which most likely occurs in response to the chronic inhibition of KATP. Further studies are needed to determine the mechanisms responsible for the remaining functional vasodilation in LZR and the enhanced dilation in the presence of KATP inhibition in OZR.
CONCLUSION
In summary, the results of the present study indicate that a decrease in KATP channel sensitivity in the OZR skeletal muscle vasculature may be a mechanism underlying the impaired vasodilation in response to exercise. These findings represent the first observations suggesting that, in obesity, the KATP channels involved in PGI2-induced vasodilation during functional hyperemia may be less active. This decreased sensitivity of KATP channels would potentially limit muscle blood flow during exercise, a treatment option known to improve glucose, lipid, and weight control. Further investigation is needed to determine the mechanisms by which obesity can induce alterations in KATP function and thus impair functional hyperemia.
Acknowledgments
BL Hodnett and LX contributed equally to this article and both should be considered as first authors. This work was supported by an AHA BGIA (LX), AHA Predoctoral Fellowship (BH), NIH HL51971, and a UMC Intramural Grant.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schöenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol. 2000;279:H3131–H3137. doi: 10.1152/ajpheart.2000.279.6.H3131. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard J-F, Dumont EC, Lamontagne D. Modification of vasodilator response in streptozotocin-induced diabetic rat. Can J Physiol Pharmacol. 1999;77:980–985. [PubMed] [Google Scholar]
- 3.Bowles DK, Hu Q, Laughlin MH, Sturek M. Heterogeneity of L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol. 1997;273:H2083–H2089. doi: 10.1152/ajpheart.1997.273.4.H2083. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA. Risks of obesity. Endocrinol Metab Clin North Am. 2003;32:787–804. doi: 10.1016/s0889-8529(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 5.Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. [Accessed 1 November 2007];National Health and Nutrition Examination Survey. 2006 Available at: http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm.
- 7.Clapp LH, Gurney AM. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 1992;262:H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- 8.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 9.Frisbee JC. Enhanced arteriolar α-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 2004;97:764–772. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- 10.Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12:383–392. doi: 10.1080/10739680590960241. [DOI] [PubMed] [Google Scholar]
- 11.Frisbee JC. Impaired dilation of skeletal muscle microvessels to reduced oxygen tension in diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol. 2001;281:H1568–H1574. doi: 10.1152/ajpheart.2001.281.4.H1568. [DOI] [PubMed] [Google Scholar]
- 12.Frisbee JC. Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1124–R1134. doi: 10.1152/ajpregu.00239.2003. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2003;285:H104–H111. doi: 10.1152/ajpheart.00118.2003. [DOI] [PubMed] [Google Scholar]
- 14.Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;283:H2160–H2168. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 16.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer LW, Ligon AL, Hester RL. Differential inhibition of functional dilation of small arterioles by indomethacin and glibenclamide. Hypertension. 2001;37:599–603. doi: 10.1161/01.hyp.37.2.599. [DOI] [PubMed] [Google Scholar]
- 18.Ikenaga H, Bast JP, Fallet RW, Carmines PK. Exaggerated impact of ATP-sensitive K+ channels on afferent arteriolar diameter in diabetes mellitus. J Am Soc Nephrol. 2000;11:1199–1207. doi: 10.1681/asn.v1171199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson WF. Arteriolar tone is determined by activity of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1993;265:H1797–H1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- 20.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson WF, Konig A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1993;264:H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- 22.Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- 23.Kamata K, Miyata N, Kasuya Y. Functional changes in potassium channels in aortas from rats with streptozotocin-induced diabetes. Eur J Pharmacol. 1989;166:319–323. doi: 10.1016/0014-2999(89)90076-9. [DOI] [PubMed] [Google Scholar]
- 24.Kilbom A, Wennmalm A. Endogenous prostaglandins as regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol (Lond) 1976;257:109–121. doi: 10.1113/jphysiol.1976.sp011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lash JM, Bohlen HG. Perivascular and tissue PO2 in contracting rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 1987;252:H1192–H1202. doi: 10.1152/ajpheart.1987.252.6.H1192. [DOI] [PubMed] [Google Scholar]
- 26.Lott MEJ, Hogeman CS, Vickery L, Kunselman AR, Sinoway LI, MacLean DA. Effects of dynamic exercise on mean blood velocity and muscle interstitial metabolite responses in humans. Am J Physiol Heart Circ Physiol. 2001;281:H1734–H1741. doi: 10.1152/ajpheart.2001.281.4.H1734. [DOI] [PubMed] [Google Scholar]
- 27.Mayhan WG. Effect of diabetes mellitus on response of the basilar artery to activation of ATP-sensitive potassium channels. Brain Res. 1994;636:35–39. doi: 10.1016/0006-8993(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 28.Mayhan WG, Faraci FM. Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1993;265:H152–H157. doi: 10.1152/ajpheart.1993.265.1.H152. [DOI] [PubMed] [Google Scholar]
- 29.McKay MK, Gardner AL, Boyd D, Hester RL. Influence of venular prostaglandin release on arteriolar diameter during functional hyperemia. Hypertension. 1998;31:213–217. doi: 10.1161/01.hyp.31.1.213. [DOI] [PubMed] [Google Scholar]
- 30.Murrant CL, Sarelius IH. Coupling of muscle metabolism and muscle blood flow in capillary units during contraction. Acta Physiologica Scandinavica. 2000;168:531–541. doi: 10.1046/j.1365-201x.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 31.Naik JS, Xiang L, Hester RL. Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R154–R161. doi: 10.1152/ajpregu.00245.2005. [DOI] [PubMed] [Google Scholar]
- 32.Negrao CE, Trombetta IC, Batalha LT, Ribeiro MM, Rondon MUPB, Tinucci T, Forjaz CLM, Barretto ACP, Halpern A, Villares SMF. Muscle metaboreflex control is diminished in normotensive obese women. Am J Physiol Heart Circ Physiol. 2001;281:H469–H475. doi: 10.1152/ajpheart.2001.281.2.H469. [DOI] [PubMed] [Google Scholar]
- 33.Nuttle LC, Ligon AL, Farrell KR, Hester RL. Inhibition of phospholipase A2 attenuates functional hyperemia in the hamster cremaster muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1289–H1294. doi: 10.1152/ajpheart.1999.276.4.H1289. [DOI] [PubMed] [Google Scholar]
- 34.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 35.Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R522–R530. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro MM, Silva AG, Santos NS, Guazzelle I, Matos LNJ, Trombetta IC, Halpern A, Negrao CE, Villares SMF. Diet and exercise training restore blood pressure and vasodilatory responses during physiological maneuvers in obese children. Circulation. 2005;111:1915–1923. doi: 10.1161/01.CIR.0000161959.04675.5A. [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro MM, Trombetta IC, Batalha LT, Rondon MUPB, Forjaz CLM, Barretto ACP, Villares SMF, Negrao CE. Muscle sympathetic nerve activity and hemodynamic alterations in middle-aged obese women. Braz J Med Biol Res. 2001;34:475–478. doi: 10.1590/s0100-879x2001000400006. [DOI] [PubMed] [Google Scholar]
- 38.Ritter JM, Barrow SE, Blair IA, Dollery CT. Release of prostacyclin in vivo and its role in man. Lancet. 1983;1:317–319. doi: 10.1016/s0140-6736(83)91626-4. [DOI] [PubMed] [Google Scholar]
- 39.Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 1996;270:H1649–H1654. doi: 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- 40.Schubert R, Serebryakov VN, Mewes H, Hopp HH. Iloprost dilates rat small arteries: role of KATP-and KCa-channel activation by cAMP-dependent protein kinase. Am J Physiol Heart Circ Physiol. 1997;272:H1147–H1156. doi: 10.1152/ajpheart.1997.272.3.H1147. [DOI] [PubMed] [Google Scholar]
- 41.Siegel G, Carl A, Adler A, Stock G. Effect of the prostacyclin analogue iloprost on K+ permeability in the smooth muscle cells of the canine carotid artery. Eicosanoids. 1989;2:213–222. [PubMed] [Google Scholar]
- 42.Siegel G, Emden J, Wenzel K, Mironneau J, Stock G. Potassium channel activation in vascular smooth muscle. Adv Exp Med Biol. 1992;311:53–72. doi: 10.1007/978-1-4615-3362-7_5. [DOI] [PubMed] [Google Scholar]
- 43.Smirnov SV, Beck R, Tammaro P, Ishii T, Aaronson PI. Electrophysiologically distinct smooth muscle cell subtypes in rat conduit and resistance pulmonary arteries. J Physiol. 2002;538:867–878. doi: 10.1113/jphysiol.2001.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith WL. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Ann Rev Physiol. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- 45.Smith WL. Prostanoid biosynthesis and mechanisms of action. Am J Physiol Renal Physiol. 1992;263:F181–F191. doi: 10.1152/ajprenal.1992.263.2.F181. [DOI] [PubMed] [Google Scholar]
- 46.Smith WL, Marnett LJ, DeWitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Ther. 1991;49:153–179. doi: 10.1016/0163-7258(91)90054-p. [DOI] [PubMed] [Google Scholar]
- 47.Sobey CG. Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol. 2001;21:28–38. doi: 10.1161/01.atv.21.1.28. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vesterqvist O, Green K. Development of a GC-MS method for quantitation of 2,3-dinor-6-keto-PGF1α and determination of the urinary excretion rates in healthy humans under normal conditions and following drugs. Prostaglandins. 1984;28:139–154. doi: 10.1016/0090-6980(84)90121-7. [DOI] [PubMed] [Google Scholar]
- 50.Wennmalm A, FitzGerald GA. Excretion of prostacyclin and thromboxane A2 metabolites during leg exercise in humans. Am J Physiol Heart Circ Physiol. 1988;255:H15–H18. doi: 10.1152/ajpheart.1988.255.1.H15. [DOI] [PubMed] [Google Scholar]
- 51.Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol Heart Circ Physiol. 1993;265:H171–H175. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]
- 52.Xiang L, Naik J, Hester RL. Exercise-induced increase in skeletal muscle vasodilatory responses in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R987–R991. doi: 10.1152/ajpregu.00702.2004. [DOI] [PubMed] [Google Scholar]
- 53.Xiang L, Naik JS, Hodnett BL, Hester RL. Altered arachidonic acid metabolism impairs functional vasodilation in metabolic syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;290:R134–R138. doi: 10.1152/ajpregu.00295.2005. [DOI] [PubMed] [Google Scholar]