Abstract
Identification of tumor‐derived proteins in the circulation may allow for early detection of cancer and evaluation of therapeutic responses. To identify circulating tumor‐derived proteins, mice were immunized with concentrated culture medium conditioned by human breast cancer cells. Antibodies generated by hybridomas were screened against conditioned media from both normal epithelial cells and tumor cells. Antibody selectively reacting with tumor cell–conditioned media was further characterized. This led to the development of a monoclonal antibody (Alper‐p280) that reacts with a newly identified 280‐kDa secreted variant of human filamin‐A. Circulating filamin‐A was detected in patient plasma samples using Alper‐p280 in an ELISA assay. Human plasma samples from 134 patients with brain, breast, or ovarian cancer, 15 patients with active arthritis, and 76 healthy controls were analyzed. Filamin‐A protein levels in human cell lines and tissues were analyzed by western blotting, immunohistochemistry, and electron and confocal microscopy. Circulating filamin‐A was detected in the plasma of 109 of 143 patients with breast cancer and primary brain tumors. Plasma levels of filamin‐A showed 89.5% sensitivity (95% confidence interval [CI] = 0.67% to 0.99%) and 97.8% specificity (95% CI = 0.88% to 0.99%) for glioblastoma at a cut‐off of 21.0 ng/mL. Plasma levels of filamin‐A (>36.0 ng/mL) had 96.7% sensitivity (95% CI = 0.80% to 0.99%) and 67.8% specificity (95% CI = 0.54% to 0.79%) for metastatic breast cancer. Filamin‐A levels were increased in malignant breast or brain tissues, but not in normal control tissues. Filamin‐A localized to lysosomes in MDA.MB.231 breast cancer cells, but not in normal human mammary epithelial cells, suggesting that filamin‐A may undergo cancer‐specific processing. Plasma filamin‐A appears to be a specific and sensitive marker for patients with high‐grade astrocytoma or metastatic breast cancer. Additional novel cancer biomarkers have been identified and are being developed alongside Alper‐p280 for use in diagnosis of breast carcinoma and high‐grade astrocytoma, and for use in the evaluation of therapeutic responses. (Cancer Sci 2009; 100: 1748–1756)
Cancer patient blood often contains proteins useful in diagnosing and monitoring therapeutic responses. For instance, the lysosomal protein cathepsin B is increased in plasma of patients with metastatic cancers.( 1 , 2 , 3 ) CA19‐9 and carcinoembryonic antigen‐1 (CEA‐1) are biomarkers for colorectal, lung, pancreatic, and breast cancers.( 4 ) We have developed a novel method of antibody generation that identifies cancer biomarkers present in the plasma of cancer patients. Using this method, we previously generated a monoclonal antibody that specifically recognizes human epidermal growth factor receptor 2 (HER‐2) extracellular domain present in conditioned media of HER‐2 overexpressing breast cancer cell lines.( 5 ) Since our initial discovery of this soluble variant of HER‐2, clinical data indicate that serum levels of HER‐2 have prognostic significance with respect to disease‐free and overall survival in patients with breast cancer.( 6 ) Recent trials also suggest that monitoring serum levels of HER‐2 can be used to predict response to therapy and may permit early detection of disease progression which could allow for a more timely therapeutic intervention.( 7 , 8 , 9 )
Here, we provide evidence that filamin‐A is found in the plasma of patients with glioblastoma (GBM) or other malignant gliomas or breast cancer. A monoclonal antibody generated in mice immunized with concentrated conditioned media from the MDA.MB.231 human breast cancer cell line led to the identification of a 280‐kDa protein that was present only in tumor cell conditioned media, and not in media from normal mammary epithelial cells. Characterization of this antigen revealed that it is an unprocessed, secreted form of the cytoskeletal protein filamin‐A. Our studies indicate that tumor cells differentially process filamin‐A resulting in secretion of this high molecular weight soluble form of filamin‐A. We also determined that secreted filamin‐A is detectable in plasma samples obtained from patients with breast cancer or malignant glioma, but not in plasma samples obtained from control subjects.
Materials and Methods
Cell culture. MCF‐10A mammary epithelial cells; human breast carcinoma cell lines, MDA.MB.231, SK‐BR‐3, MCF‐7, ZR‐75‐1, BT‐20, BT‐474, BT‐549; human glioma cell lines, U118 MG, U251 MG, U87 MG, U138 MG, 59J, 59K; and an anaplastic astrocytoma cell line, Ln229, were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and were cultured according to the manufacturer's instructions. Normal human astrocytes and human mammary epithelial cells (HMEC) were purchased from Clonetics, Cambrex Bio Science (Walkersville, MD, USA) and cultured using the astrocyte and basal MGEM medium bullet kit (Clonetics, Cambrex Bio Science). The mouse myeloma cell line p3/X63‐Ag8U1 was kindly provided by the Pathology Division, National Cancer Research Institute (Tokyo, Japan) and cultured in RPMI‐1640 medium with 10% heat‐inactivated fetal calf serum (Hyclone Laboratories, Logan, UT, USA).
Monoclonal antibody production. MDA.MB.231 human breast carcinoma cells were grown in Iscove's modified essential medium (IMEM) supplemented with 5% fetal bovine serum (FBS). When cells reached 70% confluency, the media was changed to serum‐free IMEM media and cells were cultured for another day. The serum‐free culture supernatant was collected and concentrated 20–80 fold by ultracentrifugation under nitrogen using a Diaflo cell type 8200 (Amicon, Danvers, MA, USA) fitted with a Diaflo YM‐10 membrane (nominal Mr, cut‐off, 10 000; Amicon). The concentrates were sterilized with a 0.22‐µM Millipore filter unit and stored at –80°C. Prior to immunization, antigens were prepared using a patent‐pending method.
Fifteen BALB/c mice were divided into three groups and immunized by intraperitoneal injection using a patent‐pending method with the following inoculi three times weekly: Group 1, 200 µL FBS alone (n = 5); Group 2, 200 µL 20‐fold concentrated IMEM medium (n = 5); Group 3, 200 µL 20‐fold concentrated culture medium conditioned by MDA.MB.231 cells (n = 5). After 4 weeks, mice were boosted using the inoculation strategy described above. Three days later, sera obtained from the mice were tested by western blot analysis for the presence of antibodies that recognized proteins present in MDA.MB.231 breast cancer cell–conditioned media. Hybridomas were generated, as described previously,( 5 ) from a mouse producing sera that recognized a 280‐kDa protein present in MDA.MB.231 conditioned media, cloned by limited dilution, and then tested by western blot for the production of monoclonal antibody that specifically recognized p280 in the conditioned medium of MDA.MB.231 breast cancer cells. One clone was selected due to its high expression level of a p280‐specific monoclonal antibody. Following three rounds of limited dilution cloning, Alper‐p280 was selected as the lead monoclonal antibody for further characterization.
Antibody characterization. Human cancer cell lines were seeded in tissue culture flasks at a density of 1 × 106 cells/mL. When the cells reached 70% confluency, the culture supernatant was collected, centrifuged at 864 g, and frozen at –80°C. Cell monolayers were washed with PBS, scraped, and centrifuged at 4°C. The cell pellets were lysed in cold RIPA‐buffer (50 mM Tris‐HCl [pH 7.4], 1% NP‐40, 0.25% Na‐deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and protease inhibitor [Complete Mini; Roche, Branchburg, NJ, USA]). Protein concentrations were determined with the Micro BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Proteins (500 µg/lane) were resolved by SDS‐PAGE using 6–8% SDS‐PAGE gels (Invitrogen, Carlsbad, CA, USA) and then transferred to PVDF membranes. Filamin‐A was detected with the following IgG mouse monoclonal antibodies: Alper‐p280 (Biosource International, Camarillo, CA, USA), and TI10 (Millipore, Billerica, MA, USA). To confirm equal protein loading, western blots were probed with anti‐actin monoclonal antibody 119 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bands were visualized with secondary HRP‐conjugated antibodies (Jackson ImmunoResearch, West Grove, PA, USA) and visualized using the enhanced ECL system (Amersham‐Pharmacia, Piscataway, NJ, USA). Developed blots were scanned with a ChemiImager 5500 (Alpha Innotech Corporation, San Leandro, CA, USA). Recombinant filamin‐A and an anti‐filamin‐A monoclonal antibody (Harvard MoAb 1‐6) which recognizes the C‐terminal of filamin‐A were kind gifts from Dr Thomas P. Stossel (Department of Medicine, Harvard Medical School, Boston, MA, USA). Calpain cleavage was conducted at room temperature for the indicated times. One‐mg calpain was used per 10 mL of culture supernatant. Calpain proteolysis was initiated by the addition of Ca2+ at a final concentration of 2 mM. Proteolysis was stopped by quenching with EGTA at a final concentration of 5 mM. Following calpain cleavage supernatants were resolved by SDS‐PAGE, transferred to nitrocellulose, and then western blotted using Alper‐p280. Blots were then reprobed with Harvard MoAb 1‐6 which recognizes the p100 C‐terminal region of filamin‐A.
Mass spectrometry. Protein bands from MDA.MB.231 cell culture supernatants corresponding to a molecular weight of 280 kDa were excised from 6% SDS‐PAGE gels and stored at –20°C. Subsequent sequence analysis was performed as previously described.( 10 ) Peptides from in‐gel trypsin digests were analyzed by Nano‐Spray LC‐MS/MS which is a mass spectrometry system that separates, then fragments, proteins into peptides that are characterized by the specific ion/mass ratio on an HPLC system (LC Packings, San Francisco, CA, USA) interfaced with a QSTAR mass spectrometer (Applied Biosystems, Foster City, CA, USA). MS/MS spectra were analyzed with the Mascot search engine (MatrixScience, London, UK) employing the SwissProt‐TrEMBL database and using Homo sapiens as a taxonomic restrictor.
Confocal laser scanning microscopy (LSM). Cells were seeded overnight in eight‐well glass chamber slides containing IMEM medium containing 10% FBS for the time periods indicated. Cells were fixed with ice cold methanol for 10 min and then permeabilized for 10 min with 0.1% Triton‐X100. Coverslips were washed with PBS, and then incubated for 30 min with either TI10 (Millipore) or Alper‐p280 anti‐filamin monoclonal antibodies in PBS with 0.1% gelatin and 10% normal donkey serum. Filamin‐A was then visualized with FITC‐labeled antimouse antibodies (Jackson ImmunoResearch). Actin was visualized by Texas Red‐phalloidin staining (Molecular Probes, Eugene, OR, USA). Slides were analyzed using a Zeiss LSM510 confocal microscope (Zeiss, Thornwood, NY, USA) equipped with ×63 and ×100/1.4NA objective lenses.
Lysosomal and endosomal staining was performed after culturing cells at 37°C for 48–72 h. The cells were then incubated either with Texas Red‐labeled Lysotracker (Molecular Probes) or Texas Red‐labeled Transferrin (Molecular Probes) for 20 min at 37°C and then washed with PBS. Immunofluorescent staining was performed as described above.
Immunohistochemistry. Paraffin sections of tissues were obtained from the Archives of Pathology at Georgetown University under an Institutional Review Board‐approved protocol for obtaining specimens for which there was no patient identifying data. The tissue sections were deparafinized and immunohistochemical staining for Filamin‐A was performed with DakoCytomation products (Dako, Carpinteria, CA, USA). Antigen retrieval was performed with EDTA pH 9.0 (Trilogy, Biocare Medical, Concord, CA, USA). The slides were blocked with 0.3% H2O2 for 15 min. Alper‐p280 monoclonal antibody was used at a concentration of 2 µg/mL and incubated for 1 h at room temperature. After washing, the slides were incubated with Envision plus anti‐mouse polymer secondary antibody for 30 min at room temperature. DAB plus was added for 5 min and the slides were counterstained with hematoxylin. The intensity of staining was scored as zero (no staining), 1+ (weak cytoplasmic staining in less than 10% of the cells), 2+ (moderate cytoplasmic staining in more than 10% of the cells) and 3+ (strong cytoplasmic staining in more than 10% of the cells). Only 2+ or 3+ staining was considered positive in this study.
Patients and sample collection. To analyze filamin‐A as a potential biomarker in patients with malignant central nervous system tumors, blood samples were collected from patients with primary brain tumors (World Health Organization [WHO] grade II–IV tumors) who underwent clinically indicated tumor resection. Blood samples and clinical information were collected as outlined in Institutional Review Board–approved protocols at the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (Bethesda, MD, USA) and the Cleveland Clinic Foundation (Cleveland, OH, USA).
Before resection, 10 mL of blood was collected into EDTA‐containing tubes and placed on ice immediately. Within 2 h of collection, blood samples were centrifuged at 1000 g for 20 min. The buffy coat and red blood cell layers were removed and the plasma was stored as 250–500‐µL aliquots at –70°C until analysis.
For analysis of filamin‐A as a biomarker in patients with breast cancer, blood was collected and archived at the Dana‐Farber/Harvard Cancer Center (Breast Cancer Blood Bank) under protocols approved by the Human Subjects Committee of the Partners HealthCare system (Boston, MA, USA). Patients with stage II, III, and IV breast cancers were selected for this study. Controls were obtained from healthy, cancer‐free women who donated blood to the Brigham and Women's Hospital Blood Bank (Boston, MA, USA). Breast cancer patient's blood was collected in sodium citrate tubes (Becton‐Dickinson, Franklin Lakes, NJ, USA) and processed according to the manufacturer's instructions. Plasma samples were aliquoted and stored at –80°C until analyzed.
Ovarian serous carcinoma (stage II–IV) patient blood samples (n = 20) were collected in sodium citrate tubes (Becton‐Dickinson) and were obtained from Brigham and Women's Hospital, Harvard Medical School. Blood samples were collected from 15 patients with rheumatoid arthritis (diagnosed based on revised American Rheumatism Association criteria for classification of rheumatoid arthritis) who were seen at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; National Institutes of Health, Bethesda, MD, USA) arthritis clinic.( 11 ) Blood was collected into EDTA‐containing tubes and placed immediately on ice until centrifugation at 1000 g for 20 min. Blood samples and clinical information were collected as part of Institutional Review Board–approved protocols at the NIAMS, National Institutes of Health.
Enzyme‐linked immunosorbent assay (ELISA). Soluble plasma filamin‐A levels were measured with a quantitative ELISA. The amount of filamin‐A was normalized to normal human plasma spiked with recombinant human filamin‐A (kind gift from Dr John H. Hartwig, Harvard Medical School) so that the quantitation was based on samples with consistent plasma protein concentrations. To determine the most suitable cut‐off level for distinguishing among healthy controls, non‐metastatic and metastatic breast, ovarian serous cancer, and brain cancer patients’ soluble plasma, filamin‐A levels were measured prior to decoding and correlating with the relevant clinical information. ELISA plates (Nalge Nunc International, Rochester, NY, USA) were coated with 100 µL/well randomized human plasma samples diluted 1:100 in PBS and incubated at 4°C overnight. The wells were washed with PBS once and then incubated at room temperature for 1 h with blocking buffer (5% BSA in PBS). After a wash with PBS, 100 µL/well of either TI10 antibody (45 µg/mL), or Alper‐p280 antibody (45 µg/mL) diluted in PBS, 1% BSA, and 0.01% Tween‐20 was added for 1 h. The wells were washed three times with 200 µL washing buffer/well (PBS, 0.03% Tween‐20) and then incubated at room temperature for 1 h in 100 µL/well secondary antibody, HRP‐donkey antimouse IgG (Jackson ImmunoResearch) at a dilution of 1:3000 in dilution buffer. After three washes, 100 µL/well Immunopure TMB substrate solution (Pierce) was added and the enzyme reaction was stopped by the addition of 100 µL/well 1 N H2SO4. Colorimetric analysis was performed with a 450 nanometer filter using an ELISA Reader.
Electron microscopy. Paraffin sections were double‐fixed in PBS‐buffered glutaraldehyde (2.5%) and osmium tetroxide (0.5%), dehydrated, and embedded into Spurr's epoxy resin. Ultra‐thin sections (90 nm) were made and double‐stained with uranyl acetate and lead citrate, and viewed in a Philips CM10 transmission electron microscope (Philips, Hillsboro, OR, USA). Tissue pieces were removed from a paraffin block, deparaffinated in xylene, placed in absolute ethanol, and embedded in LR White (SPI, West Chester, PA, USA). Ultra‐thin sections were mounted on 150‐mesh uncoated nickel grids. Grids were floated on blocking solution (PBS, 0.1% Tween‐20, 0.5% cold‐water fish gelatin [Ted Pella, Redding, CA, USA]) for 20 min, incubated for 1 h with the Alper‐p280 monoclonal antibody, rinsed in blocking buffer for 5 min, blocked with 2% rabbit serum, rinsed with blocking buffer, then incubated with 10 nm gold‐conjugated secondary goat antimouse antibody (Ted Pella), rinsed in PBS, and air dried. Sections were stained with aqueous uranyl acetate and examined with a Philips CM10 transmission electron microscope.
Statistical analyses. Clinical data were tested for normal distribution and equal variance and then experimental groups were compared to each other using two‐sided t‐tests. Statistical analysis was performed using Minitab software (Minitab, State College, PA, USA).
Results
Characterization of p280. To identify proteins derived from solid tumors that may serve as potential plasma biomarkers, we immunized mice with concentrated culture medium conditioned by MDA.MB.231 breast cancer cells. As controls, mice were immunized with FBS or concentrated cell culture media alone. Applying a novel screening method we previously developed,( 5 ) we observed a novel band with a molecular weight of 280 kDa (p280) only when conditioned medium of human MDA.MB.231 cells was blotted with antiserum obtained from mice injected with concentrated MDA.MB.231 conditioned medium (data not shown). Antibodies obtained from control mice injected with FBS and IMEM or commercially available normal mouse serum (NMS) did not cross react with the 280‐kDa band (data not shown). Hybridomas were generated, cloned by limited dilution, and then tested by western blot for the production of monoclonal antibody that specifically recognized p280 in conditioned medium of MDA.MB.231 breast cancer cells. Among the 384 clones evaluated, one clone was selected as the lead monoclonal antibody due to its high expression level of a p280‐specific monoclonal antibody (Fig. 1a). Following two additional rounds of limited dilution cloning, this clone was expanded and designated Alper‐p280. Alper‐p280 was identified as an IgG1κ subtype (data not shown).
Figure 1.
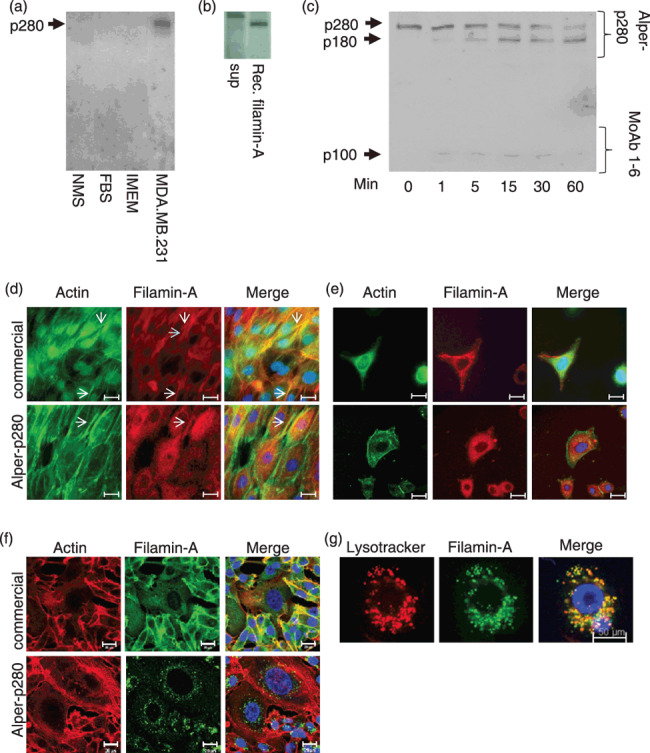
Characterization of p280. Alper‐p280 was used to western blot normal mouse serum (NMS), fetal bovine sera (FBS), concentrated Iscove's modified essential medium (IMEM), and culture medium conditioned by MDA.MB.231 cells (a). MDA.MB.231 conditioned media (sup) and recombinant filamin‐A were resolved by SDS‐PAGE followed by electrotransfer to nitrocellulose. Western blots were then probed using Alper‐p280 (b). MDA.MB.231 culture supernatant was subjected to calpain cleavage for the indicated times. Proteins were resolved by SDS‐PAGE, transferred to nitrocellulose, and then blotted using Alper‐p280 (c). Normal human mammary epithelial cells (HMEC) were fixed, permeabilized, and stained using rhodamine‐conjugated phalloidin (column 1, green), and filamin‐A using either a commercially available anti‐filamin‐A antibody (top middle panel, red) or Alper‐p280 (bottom middle panel, red). Far right hand panels are merged images. Scale bars = 20 µm (d). SKBR3 and MDA.MB.231 breast cancer cells were stained as described in (d). Scale bars = 20 µm (e,f). Lysosomes of MDA.MB.231 cells were labeled using lysotracker, fixed, and then permeabilized. Cells were stained with Alper‐p280 followed by Alexa488‐conjugated anti‐mouse secondary antibody. Scale bars = 50 µm (g). All data are representative of three independent experiments.
To identify the p280 antigen, concentrated culture medium conditioned by MDA.MB.231 cells was applied to two SDS‐PAGE gels. One gel was transferred and blotted with Alper‐p280; the second gel was stained with Coomassie blue and the band at 280 kDa was excised and analyzed by mass spectrometry. Amino acid sequence analysis performed by MS‐MS analysis identified seven peptides sequences from this protein band as identical to sequences found in human filamin‐A (Table 1). To confirm that filamin‐A was the antigen recognized by Alper‐p280, recombinant filamin‐A (p250 variant) was western blotted using the Alper‐p280 monoclonal antibody. Recombinant filamin‐A was detected by Alper‐p280 showing additional evidence that filamin‐A is the antigen recognized by Alper‐p280 (Fig. 1b). As a final test to confirm that Alper‐p280 recognizes filamin‐A, we tested to see if Alper‐p280 recognized filamin‐A subjected to calpain cleavage. As shown in Figure 1(c), Alper‐p280 recognized full‐length filamin‐A (p280), and the p180 calpain cleavage product. Alper‐p280 did not recognize the p100 C‐terminal fragment which was detected by the Harvard MoAb 1‐6 anti‐filamin‐A antibody which recognizes an epitope within the C‐terminal of filamin‐A (Fig. 1c, p100). These data confirm that filamin‐A is the antigen recognized by Alper‐p280. Filamin‐A immunoprecipitated from whole cell lysates using a commercially available antibody was also detected by Alper‐p280 (data not shown). We next tested Alper‐p280 by indirect immunofluorescence to determine if Alper‐p280 recognized actin‐associated filamin‐A. As a positive control for actin‐associated filamin‐A, one set of cover‐slips was stained with a commercially available anti‐filamin‐A antibody. Commercially available anti‐filamin‐A antibody detected filamin‐A associated with actin stress fibers (Fig. 1d, arrows). In contrast, Alper‐p280 showed a diffuse cytoplasmic staining with occasional colocalization with actin filaments (Fig. 1d, bottom row). We next compared the intracellular staining patterns in SKBR3 breast cancer cells using a commercially available anti‐filamin‐A antibody or Alper‐p280 (Fig. 1e). In SKBR3 cells, the commercial antibody recognized filamin‐A associated with the actin cytoskeleton (Fig. 1e, top panels). In contrast, Alper‐p280 had a punctate staining pattern suggesting it was staining a vesicular compartment (Fig. 1e, bottom panels). A similar difference in staining pattern was observed in MDA.MB.231 cells when the commercial anti‐filamin‐A antibody was compared to Alper‐p280. Similar to HMEC and SKBR3 cells, in MDA.MB.231 cells, the commercial antibody detected filamin‐A colocalized with actin stress fibers (Fig. 1f, top row). In contrast, punctate structures were detected by Alper‐p280 in MDA.MB.231 cells (Fig. 1f, bottom row). Filamin‐A has previously been reported to localize at lysosomes and late endosomes.( 12 , 13 ) Since MDA.MB.231 cells have previously been shown to aberrantly secrete lysosomal enzymes,( 14 ) we performed lysosomal and endosomal staining of MDA.MB.231 cells to identify which vesicular compartment filamin‐A associated. Greater than 90% colocalization between filamin‐A and lysotracker was observed in MDA.MB.231 cells indicating that in MDA.MB.231 cells, filamin‐A associates with a lysosomal compartment (Fig. 1g). Filamin‐A did not colocalize with the early endosomal marker transferrin (data not shown). These data suggest that Alper‐p280 recognizes an epitope of filamin‐A that distinguishes between filamin‐A found in normal cells and filamin‐A found in MDA.MB.231 breast cancer cells. These results led us to test clinical samples with Alper‐p280 to determine if filamin‐A may be a clinically relevant biomarker.
Table 1.
Results of mass spectrometry and protein identification
| Mass | Ion Score | Peptide | Start amino acid |
|---|---|---|---|
| 767.5519 | 59.68 | AEAGVPAEFSIWTR | 2251 |
| 715.5242 | 52.45 | AFGPGLQGGSAGSPAR | 1072 |
| 613.4237 | 9.51 | CAPGVVGPAEADIDFDIIRNDNDTFTVK | 810 |
| 576.4474 | 40.54 | DKGEYTLVVK | 2622 |
| 792.1157 | 3.57 | EAGAGGLAIAVEGPSKAEISFEDR | 2265 |
| 613.4276 | 56.6 | EATTEFSVDAR | 1273 |
| 832.0647 | 9.63 | FIPRENGVYLIDVK | 2392 |
As an initial test, Alper‐p280 was used to detect secreted filamin‐A from a series of breast cancer cell lines. Filamin‐A expression was highly elevated in the conditioned media of a diverse selection of human breast cancer cell lines, including MCF‐7, SK‐BR‐3, BT‐474, BT‐20, BT‐549, and ZR‐75‐1 (Fig. 2a). In contrast, secreted filamin‐A was not detected in the conditioned medium of normal HMECs or the non‐tumorigenic human mammary epithelial cells, MCF‐10A. We next used Alper‐p280 to determine if differences in filamin‐A could be detected between normal breast tissue and cancerous breast tissue. As shown in Figure 2(b), increased levels of filamin‐A were detected in breast cancer tissue samples (Fig. 2b, panel 3) as compared to normal breast tissue (Fig. 2b, panels 1 and 2). Of the 65 breast tissue samples analyzed, strong staining (2+ to 3+) was found in 85% of the invasive breast carcinoma samples of which 90% were invasive ductal carcinoma and the other 10% were invasive lobular carcinoma (Table 2). In contrast, only 30% of the ductal or lobular in situ carcinoma tissues were positive and 26% of benign breast disorder tissues, including hyperplasia, adenosis, and fibroadenoma cases, showed strong staining (2+, 3+) for filamin‐A. Normal breast tissue was negative in 85% of the cases. Increases in intracellular filamin‐A were evident in cytologically more atypical breast carcinoma samples as compared to normal breast tissue samples (Fig. 2b, panel 3, inset). Based on immunohistochemical staining, filamin‐A staining also appeared to be increased in the stroma of breast cancer tissue as compared to normal breast tissue. To confirm this observation, Alper‐p280 was used for immunogold staining of normal and cancerous breast tissue followed by analysis by electron microscopy. Alper‐p280 staining was higher in the stromal of breast cancer tissue (Fig. 2c, panel 2, arrow) as compared to normal breast tissue (Fig. 2c, panel 1). In addition, filamin‐A was detected within the lumen of vesicular compartments within the breast cancer tissue (Fig. 2c, panel 2, inset), but not in normal breast tissue (data not shown).
Figure 2.
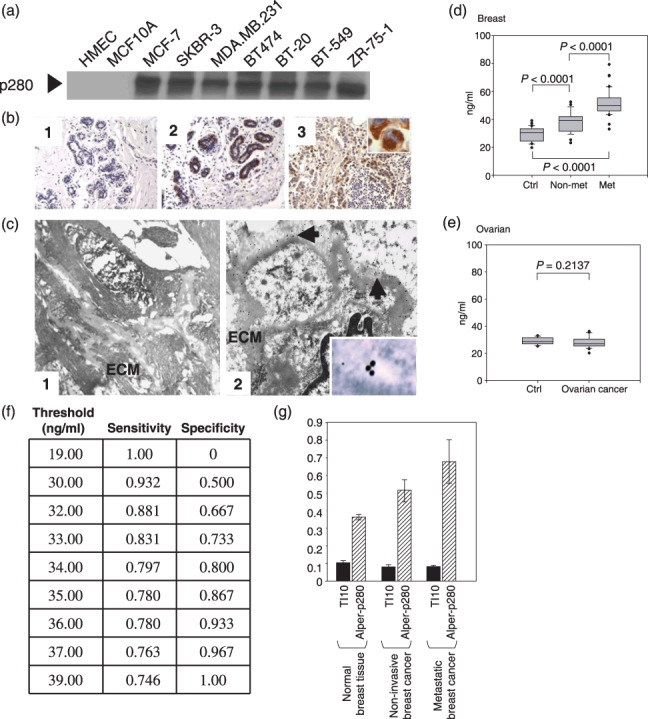
Filamin‐A protein levels are elevated in breast cancer cell lines, breast carcinoma tumor tissues, and in plasma collected from breast cancer patients. Alper‐p280 was used to western blot conditioned culture media of the indicated cell lines (a). Filamin‐A immunohistochemistry was done as described in the ‘Materials and Methods’. Two examples of Alper‐p280 staining in normal breast, one negative (panel 1) and one positive (panel 2), are shown. Alper‐p280 detected filamin‐A in invasive breast carcinoma (panel 3). The immunohistochemical staining for filamin‐A is granular and cytoplasmic (subcellular) (inset). Magnification, ×20 (b). Alper‐p280 was used to localize filamin‐A in ultra‐thin sections of normal breast tissue (panel 1) and non‐metastatic breast cancer (panel 2) using the immunogold labeling technique as described in the ‘Materials and Methods’ followed by electron microscopy. Filamin‐A was below the level of detection in normal breast tissue. In non‐metastatic breast carcinoma, filamin‐A was present in the extracellular matrix (arrows), and within the vacuolar space (inset). (c). Box plot analysis representing levels of the 280‐kDa form of filamin‐A in plasma detected by the Alper‐p280 antibody and measured by ELISA in plasma. Soluble filamin‐A levels were determined by ELISA in plasma samples from females without breast cancer (Ctrl), non‐metastatic breast cancer (non‐met), and metastatic breast cancer (met). Soluble filamin‐A plasma concentrations were determined by generating a standard curve using normal human plasma spiked with known amounts of recombinant human filamin‐A. P‐values were determined by comparison with controls by anova. Data are representative of four independent experiments performed in triplicate. All analyses were done under blinded conditions (d). ELISA analyses of soluble filamin‐A levels in plasma from patients with ovarian serous cancer (stage II–IV, n = 20) were conducted as described in panel D (e). Threshold chart is described in the text (f). An ELISA assay was used to compare the reactivity of TI10 and Alper‐p280 against plasma samples isolated from normal controls, patients with non‐metastatic breast carcinoma, and patients with metastatic breast carcinoma. Five samples from each group were analyzed in duplicate. Data are mean ± SD.
Table 2.
Summary of immunohistochemical analysis of invasive or in situ breast carcinomas and benign breast tissue using Alper‐p280 anti‐filamin‐A monoclonal antibody. Filamin‐A is expressed mostly on invasive carcinomas
| Sample classification | Number of cases | 3+ | 2+ | 1+ or negative |
|---|---|---|---|---|
| Benign/normal | 35 | 1 | 8 | 26 |
| DCIS/LCIS | 10 | 1 | 2 | 7 |
| Invasive carcinoma (DCIS, n = 18; LCIS, n = 2) | 20 | 7 | 10 | 3 |
DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Since Alper‐p280 recognized a secreted variant of filamin‐A in culture media of breast cancer cells, and was present in the stromal of breast carcinoma tissues, we next determined if secreted filamin‐A was detectable in plasma from breast cancer patients. Filamin‐A levels were detected in plasma by ELISA as described in the ‘Materials and Methods’. Samples were obtained prior to treatment from 30 patients with stage II or III ductal breast carcinoma, from 30 patients with stage IV metastatic ductal breast carcinoma, and from 30 healthy, sex‐matched controls of similar age (see Table 2). Overall, filamin‐A levels were significantly elevated (P < 0.0001) in the plasma taken from patients with non‐metastatic ductal breast carcinoma and metastatic breast cancer as compared to plasma obtained from healthy subjects (Fig. 2d). Furthermore, we found significantly higher plasma levels of filamin‐A in patients with metastatic breast carcinoma compared to those with locoregional disease (P < 0.0001) (Fig. 2d). To determine whether filamin‐A can be considered a specific marker for breast cancer, we examined 20 patients with stage II–IV ovarian serous cancer and found no difference in plasma filamin‐A levels compared to healthy age‐matched female controls (P = 0.2137) (Fig. 2e). We also found no difference between filamin‐A immunohistochemical staining in normal ovarian tissue as compared to ovarian serous carcinoma samples (data not shown). We next evaluated the sensitivity and specificity of the breast cancer patient ELISA data. At a threshold value of 36 ng/mL, the sensitivity was 96.7% (i.e. 96.7% of the breast cancer patient samples had assay values above the threshold value), and the specificity was 67.8% (i.e. 67.8% of the controls had assay values below the threshold value) (Fig. 2f). Specific associations with tumor grade, histology, estrogen receptor, progesterone receptor, and HER‐2 receptors were examined in this cohort, but no correlations were found (data not shown). Since filamin‐A associates with actin, we also western blotted the samples using actin antibody and no actin was detected, which suggests that filamin‐A release is specific and is not due to cell death or related to the secretion of other cytoskeletal proteins (data not shown). We next compared a commercially available anti‐filamin‐A antibody with Alper‐p280 antibody to determine if Alper‐p280 uniquely detected secreted filamin‐A in plasma isolated from either patients with breast carcinoma or from normal controls (Fig. 2g). The commercially available antibody failed to detect increased levels of filamin‐A in the plasma of patients with breast carcinoma. In contrast, increases in secreted filamin‐A were detected by Alper‐p280. Taken together, these data indicate that secreted filamin‐A is increased in plasma isolated from patients with breast cancer.
We then determined whether secreted filamin‐A may be a candidate biomarker for other solid tumors. We initially screened conditioned media from normal astrocytes and several brain tumor cell lines. As shown in Figure 3(a), low amounts were detected in the conditioned media of astrocytes. In contrast, brain tumor cell lines secreted filamin‐A at levels approximately four‐times higher than was observed in conditioned media from normal astrocytes. We also compared cellular levels of filamin‐A from whole cell extracts of astrocytes and brain tumor cell lines (Fig. 3b). Filamin‐A levels differed between cell lines; however, differences in protein levels did not correlate with the aggressiveness of the brain tumor that the cancer cell lines were derived from.
Figure 3.
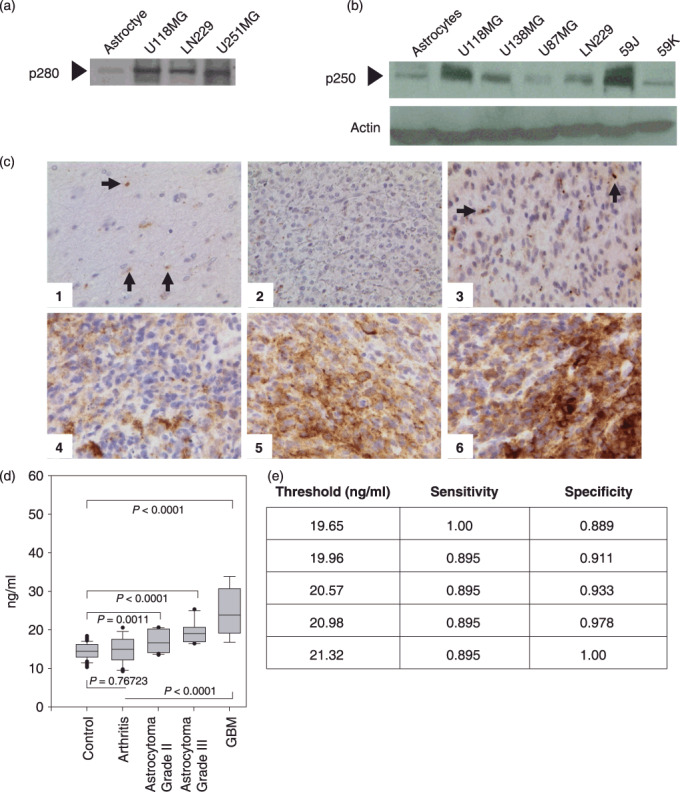
Evaluation of filamin‐A levels in human brain cell lines, brain tissue, and plasma samples. Alper p280 was used to western blot conditioned culture media of the indicated cell lines (a). Alper‐p280 was used to evaluate cellular filamin‐A (p250) levels from the indicated whole cell extracts. Actin western blot analysis was done as a control for equal loading (b). Immunohistochemical analysis on formalin‐fixed paraffin‐embedded sections of representative brain lesions using Alper‐p280 antibody against filamin‐A. Negative staining in normal brain tissue (1) and World Health Organization grade 2 oligodendroglioma (2); 2+ cytoplasmic (subcellular) staining in occasional neoplastic cells in grade 2 astrocytoma (3) and 3+ cytoplasmic (subcellular) staining in neoplastic cells in grade IV glioblastoma multiforme (4), (5), and (6). Magnification, ×400. Arrows, filamin‐A (c). Box plot analysis representing levels of the 280‐kDa form of filamin‐A in plasma detected by the Alper‐p280 antibody and measured by ELISA in plasma. Soluble filamin‐A levels were determined by ELISA from plasma samples obtained from normal controls, patients with arthritis, astrocytomas, or glioblastoma (GBM). Soluble filamin‐A plasma concentrations were determined by generating a standard curve using normal human plasma spiked with known amounts of recombinant human filamin‐A. P‐values were determined by comparison with controls by anova. Data are representative of four independent experiments performed in triplicate. All analyses were done under blinded conditions (d). Threshold Chart (e).
Next, we used the Alper‐p280 antibody to perform immunohistochemical staining of 79 human brain tissue samples (Table 3). Strong (3+, 2+) filamin‐A expression was observed in 23 out of 61 GBM (38%). Only one of six low‐grade (WHO grade II) gliomas (17%) exhibited 2+ or 3+ staining. Samples (n = 12) from normal temporal neocortex or from temporal neocortex with gliosis (for example, tissue with gliotic change after trauma, in which reactive, but non‐neoplastic astrocytes were readily identified) were examined and no staining was identified. Representative examples of tissues examined are presented in Figure 3(c). We next screened for plasma levels of soluble filamin‐A in control groups that included healthy adult volunteers (n = 19) and patients with arthritis (n = 15). Patients with active disease (pain and swollen joints) as well as well‐controlled disease under active treatment (the mean erthryocyte sedimentation rate was 45.5) were compared with 19 patients with GBM. Soluble filamin‐A levels were not significantly different between healthy controls and arthritis patients (P = 0.75723) (Fig. 3d). In contrast, significantly higher levels of filamin‐A were detected in plasma samples of patients with GBM tumors as compared to control plasma samples (P < 0.0001 and P < 0.0001, two‐sided t‐test).
Table 3.
Summary of immunohistochemical analysis of brain tumors and benign brain tissue using Alper‐p280 anti‐filamin‐A monoclonal antibody. Filamin‐A is expressed mostly on high grade gliomas
| Sample classification | Number of cases | 3+ | 2+ | 1+ or negative |
|---|---|---|---|---|
| Benign/normal | 12 | 0 | 0 | 12 |
| Low‐grade including oligodenroglioma | 6 | 0 | 1 | 5 |
| Glioblastoma | 61 | 7 | 16 | 38 |
In patients with WHO grade II–IV glial neoplasms (n = 49), levels of filamin‐A were significantly elevated and highest in patients with GBM. We found the lowest levels in patients with low grade astrocytomas (n = 15, WHO grade II), and intermediate levels in patients with astrocyomas (n = 15, WHO grade III). Filamin‐A levels between grade II and grade III astrocytomas were not significantly different (P < 0.08). However, patients with GBM tumors had filamin‐A levels that were significantly higher than patients with grade II and grade III astrocytomas (P < 0.002, and P < 0.01, respectively). We additionally screened patients with grade II and III oligodendrogliomas (n = 10 total, 5 of each grade) for secreted filamin‐A and found significantly higher levels of filamin‐A in plasma from patients with grade II or III oligodendrogliomas as compared to plasma of control groups (n = 19) (P < 0.0001) (data not shown). Based on evaluation of the sensitivity and specificity of the brain cancer ELISA data, at a threshold value of 20.98 ng/mL, the sensitivity was 89.5%, and the specificity was 97.8% (Fig. 3e).
Discussion
Using a patent‐pending approach we had previously employed to identify a secreted variant of HER‐2,( 5 ) we generated a monoclonal antibody (Alper‐p280) that recognizes a 280‐kDa variant of filamin‐A which is uniquely secreted by breast and brain tumor cells. The secreted variant of filamin‐A was detected by western blot in conditioned media of breast and glioblastoma cancer cell lines, and by western blot analysis of plasma obtained from patients with either breast cancer or malignant gliomas. Filamin‐A was also detected in the extracellular matrix of breast carcinoma tissue samples, but not in normal breast tissue samples. Immunohistochemical analyses comparing normal breast and brain tissues to breast carcinoma and a variety of gliomas suggest a positive correlation between filamin‐A levels and tumor grade.
Filamin‐A is a ‘filamentous’ dimer that co‐localizes with actin stress fibers and thus ties actin‐filament networks to plasma membrane receptors and acts as a scaffold for intracellular signaling cascades.( 12 , 15 , 16 , 17 , 18 , 19 , 20 ) In addition to its role in actin cross‐linking at the cell cortex, filamin‐A also functions in vesicular trafficking.( 21 , 22 , 23 , 24 ) For instance, in melanoma cells that lack filamin‐A, receptor and ion channel levels are altered due to changes in filamin‐A mediated trafficking.( 15 ) Filamin‐A has also been reported to function in trafficking of the prostate‐specific membrane antigen to the recycling endocytic compartment.( 24 ) Filamin‐A has previously been reported to function in sorting internalized furin to late endosomes and lysosomes.( 13 ) Our data indicate that in MDA.MB.231 cells, filamin‐A localizes to lysosomes. We hypothesize that secreted filamin‐A may be endocytosed and then trafficked to lysosomes where it is cleaved to the 250‐kDa variant.
Compared to other solid tumors, biomarkers for use in early diagnosis and therapeutic monitoring of primary brain tumors, such as gliomas, are conspicuously absent. Given this, identification of brain cancer biomarkers is urgently needed. Our initial study of a heterogeneous series of WHO grade II–IV astrocytic and oligodendroglial tumors provides evidence that filamin‐A levels are increased in the plasma of patients with gliomas and that filamin‐A levels correlate with the pathological grade. Filamin‐A levels were also increased in brain cancer tissues as compared normal/benign brain tissues. When taken together, these data suggest that filamin‐A could be a biomarker for primary brain tumors such as astrocytic and oligodendroglial neoplasms. While the results of our initial studies are promising, there are limitations. First, the results of our studies are based on a small sample size. Second, longitudinal data is lacking so we are unable to determine if secreted filamin‐A levels decrease in response to decreased tumor burden. Plans are in place to conduct a large scale study of plasma from patients with a variety of gliomas and control subjects to validate whether secreted filamin‐A meets the criteria of being a brain tumor biomarker.
Our data indicate that increased filamin‐A secretion occurs in breast and primary brain tumors, but not in ovarian cancers. The mechanistic explanation for these data is not currently known. Studies by others indicate that intracellular filamin‐A levels are increased in some cancers, whereas in other cancers filamin‐A protein levels are reduced as compared to normal samples. For instance, intracellular filamin‐A levels were found to be decreased in prostate cancer and bladder cancer, but increased in lung cancer.( 25 , 26 , 27 ) Secreted protein profiles likely differ between cancers. As more complete secretomes are generated for various cancers, analyses comparing secretomes of different cancers will enable the development of diagnostic kits that are cancer specific. In summary, the results of this study suggest that circulating filamin‐A could be used as a diagnostic and prognostic cancer‐specific marker in breast and primary brain tumors.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institute Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA. The authors thank Dianne Hirsch, PhD, for critical reading and editing of the manuscript. The authors thank Ken Yamaguchi, MD, for scientific discussions and support during the early stages of project development.
References
- 1. Kageshita T, Yoshii A, Kimura T et al . Biochemical and immunohistochemical analysis of cathepsins B, H, L and D in human melanocytic tumours. Arch Dermatol Res 1995; 287: 266–72. [DOI] [PubMed] [Google Scholar]
- 2. Kos J, Stabuc B, Schweiger A et al . Cathepsins B, H, and L and their inhibitors stefin A and cytostatin C in sera of melanoma patients. Clin Cancer Res 1997; 3: 1815–22. [PubMed] [Google Scholar]
- 3. Poole AR, Tiltman KJ, Recklies AD et al . Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature 1978; 273: 545–7. [DOI] [PubMed] [Google Scholar]
- 4. Iwanicki‐Caron I, Di Fiore F, Roque I et al . Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J Clin Oncol 2008; 26: 3681–6. [DOI] [PubMed] [Google Scholar]
- 5. Alper Ö, Yamaguchi K, Hitomi J et al . The presence of c‐erbB‐2 gene product‐related protein in culture medium conditioned by breast cancer cell line SK‐BR‐3. Cell Growth Differ 1990; 12: 591–9. [PubMed] [Google Scholar]
- 6. Lüftner D, Lüke C, Possinger K. Serum HER‐2/neu in the management of breast cancer patients. Clin Biochem 2003; 36: 233–40. [DOI] [PubMed] [Google Scholar]
- 7. Carney WP, Neumann R, Lipton A et al . Potential clinical utility of serum HER‐2/neu oncoprotein concentrations in patients with breast cancer. Cancer Clin Chem 2003; 49: 1579–98. [DOI] [PubMed] [Google Scholar]
- 8. Pupa SM, Tagliabue E, Ménard S et al . HER‐2: a biomarker at the crossroads of breast cancer immunotherapy and molecular medicine. J Cell Physiol 2005; 205: 10–8. [DOI] [PubMed] [Google Scholar]
- 9. Pichon MF, Hacene K, Guepratte S et al . Serum HER‐2 extracellular domain (ECD) before the first metastasis in 128 breast cancer patients. Clin Lab 2004; 50: 163–70. [PubMed] [Google Scholar]
- 10. Stone KL, Williams KR. Enzymatic digestion of proteins and HPLC peptide isolation. In: Matsudaira P, ed. A Practical Guide to Protein and Peptide Purification for Microsequencing. San Diego, CA: Academic Press, 1993. [Google Scholar]
- 11. Arnett FC, Edworthy SM, Bloch DA et al . The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1987; 31: 315–24. [DOI] [PubMed] [Google Scholar]
- 12. Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signaling. Nat Cell Biol 2004; 6: 1043–8. [DOI] [PubMed] [Google Scholar]
- 13. Liu G, Thomas L, Warren RA et al . Cytoskeletal protein ABP‐280 directs the intracellular trafficking of furin and modulates proprotein processing in the endocytic pathway. J Cell Biol 1997; 139: 1719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heylen N, Vincent LM, Devos V et al . Fibroblasts capture cathepsin D secreted by breast cancer cells: possible role in the regulation of the invasive process. Int J Oncol 2002; 20: 761–7. [DOI] [PubMed] [Google Scholar]
- 15. Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin‐binding protein filamin. J Neurosci 2000; 20: 8736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marti A, Luo Z, Cunningham C et al . Actin‐binding protein‐280 binds the stress‐activated protein kinase (SAPK) activator SEK‐1 and is required for tumor necrosis factor‐alpha activation of SAPK in melanoma cells. J Biol Chem 1997; 272: 2620–8. [DOI] [PubMed] [Google Scholar]
- 17. Ohta Y, Suzuki N, Nakamura S et al . The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci U S A 1999; 96: 2122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharma CP, Ezzell RM, Arnaout MA. Direct interaction of filamin (ABD) with the β‐2 integrin subunit CD18. J Immunol 1995; 154: 3461–70. [PubMed] [Google Scholar]
- 19. Calderwood DA, Huttenlocher A, Kiosses WB et al . Increased filamin binding to β‐integrin cytoplasmic domains inhibits cell migration. Nature Cell Biol 2001; 3: 1060–8. [DOI] [PubMed] [Google Scholar]
- 20. Sasaki A, Masuda Y, Ohta Y et al . Filamin associates with Smads and regulates transforming growth factor‐β signaling. J Biol Chem 2001; 276: 17871–7. [DOI] [PubMed] [Google Scholar]
- 21. Stossel TP, Condeelis J, Cooley L et al . Filamins as integrators of cell mechanics and signaling. Nat Rev Mol Cell Biol 2001; 2: 138–45. [DOI] [PubMed] [Google Scholar]
- 22. Onoprishvili I, Andria ML, Kramer HK et al . Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharm 2003; 64: 1092–100. [DOI] [PubMed] [Google Scholar]
- 23. Thelin WR, Chen Y, Gentzsch M et al . Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J Clin Invest 2007; 117: 364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anilkumar G, Rajasekaran SA, Wang S et al . Prostate‐specific membrane antigen association with filamin A modulates its internalization and NAALDase activity. Cancer Res 2003; 63: 2645–8. [PubMed] [Google Scholar]
- 25. Lin JF, Xu J, Tian HY et al . Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer 2007; 121: 2596–605. [DOI] [PubMed] [Google Scholar]
- 26. Smith SC, Oxford G, Baras AS et al . Expression of ral GTPases, their effectors, and activators in human bladder cancer. Clin Cancer Res 2007; 13: 3803–13. [DOI] [PubMed] [Google Scholar]
- 27. Keshamouni VG, Michailidis G, Grasso CS et al . Differential protein expression profiling by iTRAQ‐2DLC‐MS/MS of lung cancer cells undergoing epithelial‐mesenchymal transition reveals migratory/invasive phenotype. J Proteome Res 2006; 5: 1143–54. [DOI] [PubMed] [Google Scholar]


