Abstract
Past experience is hypothesized to reduce computational demands in prefrontal cortex by providing bottom-up predictive information that informs subsequent stimulus–action mapping. The present fMRI study measured cortical activity reductions (‘neural priming’ / ‘repetition suppression’) during repeated stimulus classification to investigate the mechanisms through which learning from the past decreases demands on the prefrontal executive system. Manipulation of learning at three levels of representation—stimulus, decision, and response— revealed dissociable neural priming effects in distinct fronto-temporal regions, supporting a multi-process model of neural priming. Critically, three distinct patterns of neural priming were identified in lateral frontal cortex, indicating that frontal computational demands are reduced by three forms of learning: (a) cortical tuning of stimulus-specific representations, (b) retrieval of learned stimulus-decision mappings, and (c) retrieval of learned stimulus-response mappings. The topographic distribution of these neural priming effects suggests a rostro-caudal organization of executive function in lateral frontal cortex.
INTRODUCTION
Memory for the past provides predictive information that shapes current thought and action. The influence of past experience on present behavior often occurs implicitly—as exemplified by repetition priming—wherein reaction times and accuracy are facilitated when processing repeated compared to novel stimuli (Roediger & McDermott, 1993; Tulving & Schacter, 1990). At the neural level, repeated stimulus processing is associated with decreased neural activity (neural priming / repetition suppression) in brain regions engaged during initial stimulus processing (Gabrieli et al., 1996; Demb et al., 1995; Raichle et al., 1994; Squire et al., 1992; for review see Grill-Spector, Henson, & Martin, 2006; Henson, 2003). Within prefrontal cortex (PFC), neural priming is thought to reflect situations in which goal-relevant behavior is less dependent on ‘top-down’ executive control (Henson & Rugg, 2003; Badre & Wagner, 2002; Thompson-Schill, D’Esposito, & Kan, 1999). Specifically, because prefrontal control mechanisms are recruited in situations of uncertainty (Thompson-Schill & Botvinick, 2006; Miller & Cohen, 2001), neural priming in PFC may reflect the benefits of reduced uncertainty that emerge when learning from the past provides greater ‘bottom-up’ predictive information. While neural priming in PFC is generally regarded as reflecting decreased demands on executive control, the mechanism by which past experience confers prefrontal computational savings is still a matter of debate, being alternatively characterized as stemming from cortical tuning (Henson, 2003; Wiggs & Martin, 1998) or stimulus-response learning (Horner & Henson, 2008; Schacter, Wig, & Stevens, 2007; Dobbins, Schnyer, Verfaellie, & Schacter, 2004). The present functional MRI (fMRI) study aimed to delineate the nature of neural priming in PFC, with the goal of specifying the forms of learning that give rise to computational savings in distinct substrates of the prefrontal executive system.
For over a decade, the cortical tuning hypothesis has been the dominant account of neural priming effects. From this perspective, initial stimulus processing leads to the development of sparser or strengthened cortical representations, which decreases computational demands during subsequent stimulus processing (Grill-Spector et al., 2006; Henson, 2003; Wiggs & Martin, 1998). For example, fMRI studies of conceptual priming—i.e., facilitated access to stimulus meaning stemming from prior processing of stimulus meaning— have revealed activity reductions in left fronto-temporal regions involved in the representation (posterior middle temporal and fusiform cortical areas) and controlled retrieval (ventrolateral prefrontal cortex; VLPFC) of semantic knowledge (e.g., Gold, Balota, Kirchhoff, & Buckner, 2005; Buckner, et al., 1998; Wagner, Desmond, Demb, Glover, & Gabrieli, 1997; Demb et al., 1995). According to the cortical tuning hypothesis, prior stimulus processing during priming paradigms serves to tune cortical representations, such that conceptual, lexical, and perceptual features of the stimulus are more sharply or strongly represented. This ‘tuning’ of stimulus-specific representations is thought to enable subsequent stimulus processing to occur in a more ‘bottom-up’ manner, resulting in a concomitant reduction in demands on ‘top-down’ biasing from the PFC executive system (hence the accompanying neural priming in VLPFC).
The cortical tuning hypothesis has been challenged by recent data that indicate that neural and behavioral priming at least partially reflect the benefits of a different mechanism— response learning—wherein action selection is facilitated through the retrieval of a previous response that has become associated with a stimulus (Horner & Henson, 2008; Schnyer, Dobbins, Nicholls, Davis, & Verfaellie, 2007; Bunzeck, Schutze, & Duzel, 2006; Schnyer, Dobbins, Nicholls, Schacter, & Verfaellie, 2006; Dobbins et al., 2004; Logan, 1990). The response-learning hypothesis has its roots in instance theories of automaticity, in which the formation and automatic retrieval of stimulus-response associations provide a direct route to action, enabling the bypassing of slower, more deliberate processing stages (Logan, 1990; 1988). From this perspective, neural activity reductions in left fronto-temporal regions during repeated semantic classification of a stimulus mark a processing shift away from retrieval and analysis of stimulus-level conceptual features to direct response retrieval that is enabled by a learned stimulus-response association (Dobbins et al., 2004; Schacter, Dobbins, & Schnyer, 2004). Thus, neural priming within the PFC executive system may partially reflect the bypassing of VLPFC control processes that support controlled semantic retrieval.
The response-learning hypothesis is supported by two recent fMRI studies in which behavioral and neural priming were disrupted when subjects could not rely on learned ‘stimulus-response’ associations to make semantic classifications about repeated stimuli (Horner & Henson, 2008; Dobbins et al., 2004). For example, Dobbins et al. (2004) had subjects repeatedly classify visually presented objects (e.g., a picture of a HOUSE) according to one of two classification rules (either ‘Bigger than a shoebox?’ or ‘Smaller than a shoebox?’). In the key manipulation, the classification rule was either held constant across stimulus repetitions (e.g., ‘Bigger→’Bigger’) or was inverted across stimulus repetitions (e.g., ‘Bigger’→’Smaller’). Strikingly, the results revealed robust behavioral and neural priming when the classification rule was held constant, but disrupted behavioral priming and a concomitant reduction of neural priming in left VLPFC (~Brodmann’s areas [BA] 9/44 and 45) and elimination of neural priming in left fusiform cortex (~BA 19/37) when the rule was inverted. Importantly, because the targeted concept (e.g., HOUSE) was the same irrespective of the sign of the classification rule, Dobbins et al. argue that the disruption of behavioral and neural priming following rule inversion resulted from the need to re-engage in stimulus-level processing because subjects could no longer use learned ‘responses’ as an alternate route to action. Conversely, when ‘stimulus-response’ mappings were held constant, subjects could directly retrieve the learned ‘response’, thus bypassing stimulus-level processing mediated by left fronto-temporal regions (Horner & Henson, 2008; Dobbins et al., 2004; Schacter, Dobbins, & Schnyer, 2004).
While the response-learning hypothesis provides a fundamental challenge to accounts of PFC priming that focus exclusively on mechanisms of cortical tuning, extant data do not specify the nature of the acquired associative representations that give rise to neural priming. In particular, prior stimulus classification may result in the learning of a stimulus-decision association that relates the stimulus to a particular classification decision (e.g., HOUSE–‘Bigger’) or to a particular task (e.g., HOUSE–‘Bigger than?’); such learning would enable priming by facilitating subsequent decision selection (Schnyer et al., 2007; Logan, 1990). Alternatively, prior stimulus classification may result in the learning of a stimulus-response association (e.g., HOUSE–‘yes’ or HOUSE–‘left button press’) that facilitates subsequent response selection (Dobbins et al., 2004).
At the behavioral level, published data alternately suggest that the associations underlying ‘response-learning’ effects are between stimuli and abstract decisions (Schnyer, et al., 2007; Waszak, Hommel, & Allport, 2003; Logan, 1990) or between stimuli and responses (Dobbins et al., 2004; for discussion, see Hommel, 2007; Schacter et al., 2007). At the neural level, even less is known about the form of associative learning giving rise to neural priming because the experimental designs or analysis approaches in prior fMRI studies supporting the response-learning hypothesis were insufficient for distinguishing the effects of stimulus-decision learning from those of stimulus-response learning (Horner & Henson, 2008; Dobbins et al., 2004; Wagner, Koutstaal, Maril, Schacter, & Buckner, 2000; Thompson-Schill et al., 1999). For example, in the Dobbins et al. (2004) study, the design co-varied associative learning at multiple levels of representation, such that the observed neural priming could reflect the benefits of learned stimulus-decision mappings, learned stimulus-response mappings, or some combination of the two. Independent manipulation of repetition at the decision and the response levels is required to determine whether response-learning effects on neural priming stem from decision- versus response-related repetition (Schnyer et al., 2007) or, alternatively, whether these forms of repetition have unique and dissociable neural processing consequences.
The response-learning hypothesis—and associated fMRI observations of a single pattern of neural priming in left VLPFC (Horner & Henson, 2008; Dobbins et al., 2004)—also challenge emerging theories of PFC executive function that posit that multiple forms of cognitive control are subserved by distinct subregions of lateral frontal cortex (Danker, Gunn, & Anderson, in press; Badre & D’Esposito, 2007; Badre & Wagner, 2007; Koechlin & Summerfield, 2007; Gold et al, 2006). In particular, initial fMRI evidence for the response-learning hypothesis suggests that multiple subregions of left VLPFC—inferior frontal pars opercularis and pars triangularis (Dobbins et al., 2004) and inferior frontal pars orbitalis and pars opercularis (Horner & Henson, 2008)—demonstrate neural priming because stimulus-level processing is bypassed in favor of decision or response retrieval. These findings appear to challenge Badre and Wagner’s (2007) two-process hypothesis of VLPFC functional organization, wherein left anterior VLPFC (pars orbitalis; area 47) biases processing in lateral and ventral temporal cortex in service of controlled retrieval of semantic knowledge, whereas left mid-VLPFC (pars triangularis; area 45) mediates selection between competing active representations in service of goal-directed action (Badre & Wagner, 2007; Badre & Wagner, 2006; Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005; Dobbins & Wagner, 2005; see also, Danker et al., in press; Gold, et al., 2006). Viewed from this perspective, past experience can produce learning at multiple levels of representations—conceptual, stimulus-decision, and/or stimulus-response—that differentially alters subsequent demands on distinct subcomponents of the prefrontal executive system. Resolution of this fundamental issue can only be derived through independent measures of neural priming that stems from stimulus-level learning, stimulus-decision learning, and stimulus-response learning.
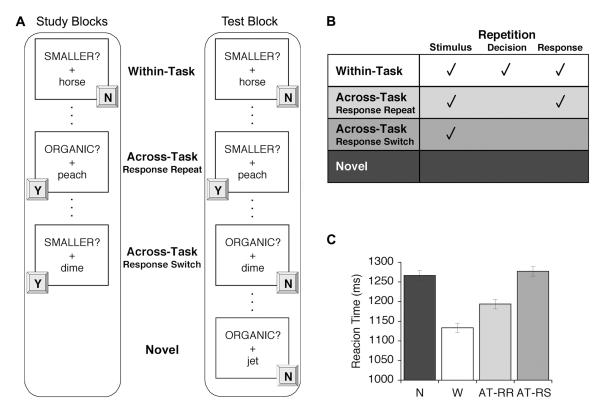
To understand how multiple forms of learning influence subsequent demands on distinct components of the prefrontal executive system, we conducted a fMRI study of priming that manipulated repetition at three levels: (a) stimulus repetition, (b) stimulus-decision repetition, and (c) stimulus-response repetition. Throughout the experiment, participants made one of two semantic classification decisions about words, making a yes/no response for each stimulus by pressing one of two keys (Figure 1A). On each trial, a stimulus appeared with a task cue (e.g., ‘SMALLER?’ or ‘ORGANIC?’) that indicated the decision to be made for that trial. During the study phase, stimuli were presented three times (Repeated items) over the course of three blocks (intermixed with novel items during the second and third block). Importantly, the task paired with each Repeated item was held constant across the three study repetitions, yielding primed items for which stimulus processing, stimulus-decision mappings, and stimulus-response mappings were repeated three times.
Figure 1.
Task schematic and behavioral measures of priming. (A) During study, each item was presented with the same decision cue three times, and subjects pressed one of two buttons to indicate a ‘yes’ (Y) or ‘no’ (N) response. At test, items presented at study were presented again either with the same cue (Within-Task) or a different cue (Across-Task). Of the Across-Task trials, half required the same response as at study (Across-Task Response-Repeat; AT-RR) and half required a different response (Across-Task Response-Switch; AT-RS). (B) The four test conditions differed according to repetition at the stimulus, stimulus-decision, and stimulus-response levels. (C) Test phase reaction times (restricted to correct trials) differed across conditions, revealing behavioral priming on Within-Task (W) and AT-RR trials compared to Novel (N) and AT-RS trials; priming was also greater on W than on AT-RR trials. In all figures, error bars reflect within-subject standard error.
During a subsequent critical test phase, all of the Repeated items were re-presented along with a set of Novel items using an event-related fMRI design. The level of repetition for each of the Repeated items was manipulated by varying the relationship between the tasks performed at study and test (Figure 1A). Half of the Repeated items were classified according to the same decision task as at study (Within-Task repetition), while half were now classified according to the other decision task (Across-Task repetition). Of the Across-Task items, half required the same response as at study (Response-Repeat), and half now required a different response (Response-Switch). In this manner, the level of repetition between study and test varied across conditions (Figure 1B): (a) Within-Task trials contained repetition at the stimulus, decision, and response levels; (b) Across-Task Response-Repeat (AT-RR) trials contained repetition at the stimulus and response levels; (c) Across-Task Response-Switch (AT-RS) trials contained repetition only at the stimulus level; and (d) Novel trials served as baseline items that did not contain any level of repetition. All analyses focused on the data from this critical test phase.
Given the independent manipulation of stimulus repetition, stimulus-decision repetition, and stimulus-response repetition, the experimental design allowed us to address two key questions. First, is the pattern of neural priming in lateral frontal cortex consistent across anatomical subregions or, alternatively, are dissociable patterns of neural priming present across distinct frontal subregions in a manner compatible with a multi-process model of prefrontal executive function? Second, do ‘response-learning’ effects on neural priming reflect stimulus-decision learning or stimulus-response learning, or are there distinct neural consequences of each form of learning on frontal computational demands? The answers to these questions directly bear on current theories of neural priming and of the functional organization of the prefrontal executive system.
METHODS
Participants
Twenty-six right-handed, native English speakers participated in the study (mean age = 22 yrs, range 18-35 yrs). Data from two additional participants was collected but excluded due to poor behavioral performance (>35% non-responses in one case and <65% accuracy across all conditions in the other case). Participants received $20/hr for participation, with the experiment lasting approximately 3 hrs. Participants were recruited from the Stanford University community and surrounding area, and gave informed consent in accordance with procedures approved by the Stanford University Institutional Review Board.
Stimuli
The stimulus set consisted of 512 nouns (mean word length = 6.2 letters; mean word frequency = 12.7/million). Half of the words referred to organic objects and half to inorganic objects. For both the organic and the inorganic words, half referred to objects smaller than a 13″ box and half to larger objects. The stimuli were divided into eight lists of 64 words, matched for mean word length and frequency, and containing 16 words from each of the organic/inorganic × smaller/larger crossings. For each participant, four of these lists served as Repeated items that were studied three times during encoding, two lists served as Novel items studied once during encoding, and two lists served as Novel items at test. Across participants, lists were counterbalanced across conditions.
Behavioral Procedure
The experiment proper consisted of three study blocks and a final test. FMRI data were collected during all blocks, with the present analyses focusing on data from the critical test block. Instructions and practice were given prior to the start of the experiment.
The same trial structure was maintained across study and test blocks. On each trial, a task cue and a target word were presented for 1 s followed by a 2-s fixation (‘+’). Task cues appeared in uppercase letters above a central fixation cross and indicated which of two semantic decisions was to be made for the target word that appeared below the cross in lowercase letters (Figure 1A). For the size decision task, the cue ‘SMALLER?’ or ‘LARGER?’ appeared, whereas the cue ‘ORGANIC?’ or ‘INORGANIC?’ appeared for the composition task. Each participant was presented with only one of the two possible cues for each classification task throughout the duration of the experiment (i.e., the same participant never encountered both ‘SMALLER?’ and ‘LARGER?’ trials, nor ‘ORGANIC?’ and ‘INORGANIC?’ trials). During each 3-s trial, participants were instructed to respond as quickly and accurately as possible by making a ‘yes’ or ‘no’ key press on a button box under their right middle or index fingers. The cue used for each task and the order of classification decisions were counterbalanced across participants. Within subjects, conditions were pseudo-randomized to ensure that the same cue or response did not repeat more than three times in a row. Trials were distributed in an event-related manner, separated in time by variable-duration null events (0–7.5s). During null events, participants indicated the direction of leftward or rightward pointing arrows by pressing the left or right key on the button box (Stark & Squire, 2001).
During each study block, participants classified 256 words—128 under the size task and 128 under the composition task. The same 256 words appeared in each of the three study blocks, with their order randomized within each study block. As stated above, for a given participant, the task cue associated with each word was held constant across the three study blocks. During both the second and third study blocks, 64 novel words were presented once along with the 256 repeatedly studied words.
During the critical final test block, the 256 repeatedly encountered words from the study blocks were represented along with 128 novel words that had not been previously presented. Of the 256 repeated words, half were presented with the same cue as at study, requiring the same classification and response as at study (Within-Task), whereas the other half were presented with the other task cue, requiring a decision switch from either a size-to-composition or a composition-to-size decision (Across-Task). Of the 128 Across-Task words, half required the same response as was previously appropriate during the study blocks (AT-RR), while the other half required a different response (AT-RS) (Figure 1).
fMRI Methods
Whole-brain imaging was collected on a 3.0T Signa MRI system (GE Medical Systems). Functional images were collected using a T2*-weighted two-dimensional gradient echo spiral-in/out pulse sequence (TR = 1.5 s; TE = 30 ms; 22 axial-oblique slices; 1 interleave; flip angle = 75°; FOV = 22 cm; 64 × 64 voxels; 5-mm through-plane) (Glover & Law, 2001). Eight discarded volumes (12 s) were collected at the beginning of each scan to allow for T1 stabilization. High-resolution T1-weighted (SPGR) anatomical images were collected for anatomical visualization. Head motion was restricted with a bite bar and padding surrounding the head.
Data were preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, London). Functional images were corrected for differences in slice acquisition timing followed by motion correction using sinc interpolation. Participants’ structural images were co-registered to their functional images and segmented into gray matter, white matter, and cerebrospinal fluid. The gray matter images were then stripped of any remaining skull and spatially normalized to a gray matter template image based on MNI stereotactic space. Normalization of the structural and functional images was based on this normalized gray matter image. Functional images were resampled into 3-mm cubic voxels and spatially smoothed with a Gaussian kernel (6-mm FWHM).
Statistical models were constructed with SPM2 under the assumptions of the general linear model. Correct and incorrect trials associated with each condition (Novel, Within-Task, AT-RR, AT-RS) were modeled as events. Motion parameters, linear trends, and session were all entered as nuisance covariates. Linear contrasts were used to obtain participant-specific estimates for each effect. These estimates were entered into a second-level analysis, treating subjects as a random effect, using a one-sample t-test against a contrast value of zero at each voxel. All statistical contrasts were restricted to correct trials. Voxel-based group effects in a priori expected frontal and temporal regions were considered significant if they (a) exceeded an uncorrected threshold of p < .001 and consisted of 5 or more contiguous voxels, and (b) survived small-volume correction.
Given numerous prior observations of neural priming in left VLPFC and premotor cortex, as well as in left posterior middle temporal and left fusiform cortical areas, during conceptual priming paradigms, we had specific a priori targeted regions of interest. Accordingly, voxel-level effects within these a priori predicted regions were small-volume corrected for multiple comparisons using anatomical masks drawn from a standard database (Anatomical Automatic Labeling [AAL]: http://www.cyceron.fr/freeware/) or from foci identified in prior published papers. For left VLPFC, a single mask was created by combining the three separate AAL masks for inferior frontal pars orbitalis, inferior frontal pars triangularis, and inferior frontal pars opercularis. For left premotor cortex, we used the AAL mask for precentral gyrus. Conservative masks for left posterior middle temporal cortex and left fusiform cortex were created by defining 24-mm diameter spheres centered at previously identified middle temporal cortex (MNI-converted coordinates: −54, −45, −4; Gold et al., 2006) and fusiform cortex (−45, −54, −24; Simons, Koutstaal, Prince, Wagner, & Schacter, 2003) peaks associated with conceptual priming.
Region of interest (ROI) analyses supplemented group-level voxel-based contrasts. ROIs included all significant voxels within a 6-mm radius of a maximum. Deconvolution of the BOLD signal within ROIs was performed using a finite impulse response function implemented with MarsBar (http:/marsbar.sourceforge.net). Integrated percent signal change associated with each condition was computed around the peak response plus and minus one TR (corresponding to 3–7.5 s post-trial onset). The resultant data were submitted to repeated-measures analyses of variance (ANOVA), using Huynh-Feldt correction where appropriate. All coordinates are reported in MNI space. For display purposes, FreeSurfer (CorTechs Labs, Inc.; Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999) was used to render group contrasts, overlaid on a surface representation of the MNI canonical brain (I. Kahn; http://spmsurfrend.sourceforge.net).
RESULTS
Behavioral Repetition Effects
The median reaction time (RT) was determined per condition, restricted to correct trials, and submitted to analysis. During the study phase, repetition priming was observed as faster RTs on Repeated compared to Novel trials, both after one repetition (F(1,25) = 40.04, p < .001) and after two repetitions (F(1,25) = 39.10, p < .001). During the critical test phase, the nature of repetition between study and test modulated behavioral priming (F(3,75) = 31.75, p < .001; Figure 1C). Specifically, compared to Novel trials, RTs were faster on both Within-Task and AT-RR trials (Fs(1,25) > 21.38, ps < .001), but not on AT-RS trials (F < 1). RTs for Within-Task were faster than for AT-RR trials (F(1,25) = 15.21, p < .001), revealing the benefits of stimulus-decision repetition during the Within-Task condition. Moreover, behavioral priming for AT-RR trials, while less than that for Within-Task trials, was also evident in faster RTs compared to AT-RS trials (F(1,25) = 17.50, p < .001). This AT-RR priming effect provides evidence that stimulus-response learning also can facilitate behavior even when stimulus-decision mappings change between study and test. Importantly, when Task (size/composition) was included as a factor, all of the above effects remained significant (ps < .05) and there was no effect of Task on the pattern of RTs across conditions (Task × Condition interaction, F < 1). The effect of Condition on accuracy at test was similar to that seen for RTs, with higher accuracy for Within-Task (92%) and AT-RR (94%) compared to both Novel (89%) and AT-RS (75%) trials (ps < .001).
Within-Task Repetition and Neural Priming
To validate our paradigm, we first contrasted BOLD activity on Novel and Within-Task trials (all fMRI analyses were restricted to correct trials). This comparison identified neural priming that could derive from repetition at any of the three manipulated levels of processing: stimulus, decision, or response. Consistent with previous studies of neural priming during repeated semantic classification (e.g., Wig, Grafton, Demos, & Kelley, 2005; Dobbins et al., 2004; Buckner, Koutstaal, Schacter, & Rosen, 2000; Wagner, Maril, & Schacter, 2000; Buckner et al., 1998; Gabrieli et al., 1996; Demb et al., 1995), activity reductions were observed in multiple left fronto-temporal cortical regions, including VLPFC, posterior middle temporal cortex, fusiform gyrus, and premotor cortex (p < .001, 5-voxel extent; all ps < .005, small-volume corrected) (Figure 2A). A qualitatively similar pattern of was observed when separately comparing Novel > Within-Task trials for each Task (size/composition), and an interaction analysis confirmed that neural priming in left fronto-temporal regions did not interact with Task. Accordingly, all subsequent fMRI analyses were collapsed across Task, thus affording greater power.
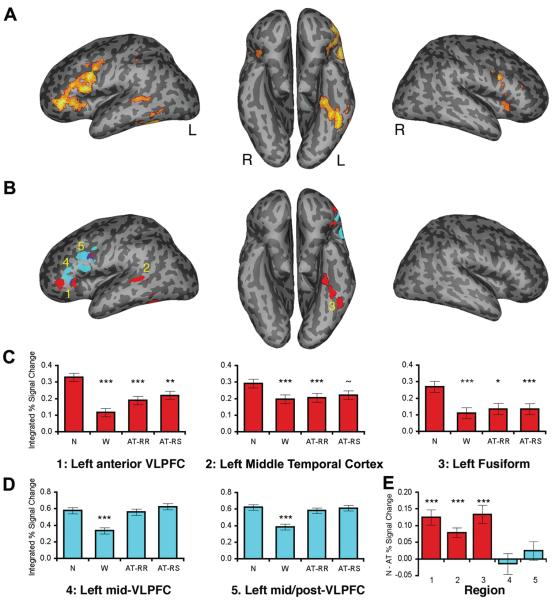
Figure 2.
Dissociable neural priming effects due to stimulus repetition and stimulus-decision repetition. (A) The contrast of Novel > Within-Task trials, rendered on an inflated MNI canonical surface, revealed neural priming effects in a priori predicted left fronto-temporal regions (p < .001, 5-voxel extent). (B) Stimulus-level repetition reductions (Novel > all Repeated, p < .001, 5-voxel extent; red) were observed in left anterior VLPFC, left middle temporal cortex, and left fusiform regions. By contrast, decision-level repetition reductions (AT-RR > Within-Task, p < .001, 5-voxel extent; cyan) were observed in left mid/post-VLPFC. (C) Data extracted from (1) left anterior VLPFC (−42, 33, −3), (2) left middle temporal cortex (−54, −42, 3), and (3) left fusiform (−45, −63, −24) ROIs identified in the Novel > all Repeated contrast confirmed that these regions demonstrated neural priming across all three repeated conditions. (D) Data extracted from (4) left mid-VLPFC (−51, 36, 12) and (5) left mid/post-VLPFC (−45, 15, 24) ROIs from the AT-RR > Within-Task contrast confirmed that these regions demonstrated decision-specific neural priming for Within-Task trials. [Note: For (C) and (D), neural priming relative to Novel trials is denoted ***p ≤ .005, **p < .01, *p < .05, ~p < .10]. (E) The regions associated with stimulus and stimulus-decision priming dissociated according to whether neural priming was observed on Across-Task trials (Novel – Across-Task; ***p < .005). [Note: The left post-VLPFC region identified in both the Novel > all Repeated and AT-RR > Within-Task contrasts (−45, 9, 21; (B) purple) showed a mixed decision/response pattern.]
Stimulus-Level Repetition Effects
Motivated by the two-process model of left VLPFC function (Badre and Wagner, 2007) and by the literature implicating left posterior middle temporal and fusiform cortical areas in semantic processing (e.g., Gold et al., 2006; Badre et al., 2005; Gold et al., 2005; Wheatley, Weisberg, Beauchamp, & Martin, 2005; Simons et al., 2003; Thompson-Schill et al., 1999; Hodges, Patterson, Oxbury, & Funnell, 1992), we hypothesized that stimulus-level learning would give rise to neural priming in regions associated with the representation of long-term conceptual knowledge (left posterior middle temporal cortex and fusiform) and the controlled retrieval of that knowledge (left anterior VLPFC; ~area 47, inferior frontal pars orbitalis). Analytically, neural regions sensitive to stimulus repetition were expected to show reduced activation for all repeated conditions relative to Novel trials (Figure 1B).
Consistent with these predictions, the contrast of Novel > all Repeated trials revealed neural priming in a left-lateralized fronto-temporal network that was a subset of that identified in the Novel > Within-Task contrast (Figure 2B, red; Table 1). Specifically, the Novel > all Repeated contrast identified neural priming in a priori predicted regions in left anterior VLPFC (~area 47: −42, 33, −3), left posterior middle temporal cortex (~BA 21: −54, −42, 3), and left fusiform cortex (~BA 37: −45, −63, −24) (p < .001, 5-voxel extent; all ps < .01, small-volume corrected). Confirmatory region-of-interest (ROI) analyses in each of these a priori predicted regions revealed neural priming during all of the repeated conditions, with significant activation reductions during both Within-Task and Across-Task trials compared to Novel trials (Fs(1,25) > 11.77, ps < .005) (Figure 2C). Moreover, neural priming was observed when separately comparing AT-RR and AT-RS trials to Novel trials: activation reductions during AT-RR were significant in all regions (Fs(1,25) > 6.61, ps < .05); similarly, the reductions during AT-RS trials were significant in left anterior VLPFC and left fusiform cortex (Fs(1,25) > 8.11, ps < .01), and approached significance in left posterior middle temporal cortex (F(1,25) = 3.13, p = .09) (Figure 2C). Collectively, these data provide compelling evidence for stimulus-level neural priming even in the absence of stimulus-decision or stimulus-response learning (i.e., there were significant activation reductions in these regions even on AT-RR and AT-RS trials).
Table 1.
A priori left frontal and temporal regions demonstrating neural priming effects.
| MNI Coordinates (x,y,z) | ~BA | |||
|---|---|---|---|---|
| Novel > all Repeated | ||||
| L anterior VLPFC | −42 | 33 | −3 | 47 |
| L anterior VLPFC | −45 | 42 | −9 | 47 |
| L posterior VLPFC | −45 | 9 | 21 | 44 |
| L middle temporal cortex | −54 | −42 | 3 | 21 |
| L fusiform gyrus | −45 | −63 | −24 | 37 |
| L fusiform gyrus | −39 | −48 | −27 | 37/20 |
| L fusiform gyrus | −33 | −33 | −21 | 37/20 |
| L fusiform gyrus | −33 | −33 | −30 | 37/20 |
| L fusiform gyrus | −45 | −57 | −12 | 37/19 |
| L fusiform gyrus | −45 | −51 | −15 | 37/19 |
| AT-RR > Within-Task | ||||
| L mid VLPFC | −51 | 36 | 12 | 45 |
| L mid VLPFC | −48 | 33 | 0 | 45 |
| L mid/post VLPFC | −45 | 15 | 24 | 45/44 |
| L mid/post VLPFC | −51 | 12 | 15 | 45/44 |
| AT-RS > AT-RR | ||||
| L dorsal premotor | −24 | 0 | 66 | 6 |
| L premotor | −39 | −6 | 54 | 6 |
| L ACC | −12 | 18 | 30 | 24 |
| Conjunction: Novel > Within-Task & AT-RS > AT-RR | ||||
| L premotor/post VLPFC | −39 | 0 | 39 | 6/44 |
| L pre-SMA | −9 | 9 | 51 | 6 |
Note: Main contrasts, p < .001, 5-voxel extent; conjunction analysis, p < .000025, 5-voxel extent.
In addition to the robust effects of stimulus-level learning, further analyses revealed that the magnitude of neural priming in the left anterior VLPFC ROI was also greater when decisions were repeated (Within-Task) than when decisions changed (Across-Task) (F(1,25) = 10.52, p < .005). Interestingly, this effect appeared to stem from the presence of a more sustained response in this region during the Across-Task relative to the Within-Task trials (Figure 3).
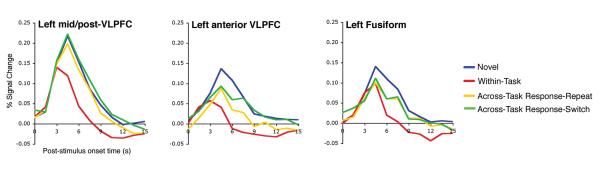
Figure 3.
Hemodynamic response functions (HRFs; in percent signal change) are plotted for each of the four conditions. Data are pooled across all clusters identified in the contrast of interest. For left mid/post-VLPFC, data are pooled across the mid-VLPFC and mid/post-VLPFC clusters (163 voxels) defined in the AT-RR > Within-Task contrast (Figure 2B, cyan voxels). For left anterior VLPFC, data are pooled across the anterior VLPFC clusters (33 voxels) defined in the Novel > Repeated contrast (Figure 2B, red voxels). For left fusiform, data are pooled across all fusiform clusters (86 voxels) identified in the Novel > Repeated contrast (Figure 2B, red voxels). The HRFs from anterior VLPFC and fusiform reveal a late divergence between Across-Task trials (AT-RR and AT-RR) and Within-Task trials. This divergence may reflect feedback from left mid/post-VLPFC onto these conceptual-level regions.
Stimulus-Decision Repetition Effects
We next sought to determine whether subregions of lateral frontal cortex demonstrate neural priming that uniquely stems from stimulus-decision learning. One possibility is that decision-specific neural priming would be observed in more caudal subregions of left VLPFC (~BA 45, inferior frontal pars triangularis; ~BA 44, inferior frontal pars opercularis), given the locus of prior rule-inversion effects (Dobbins et al., 2004; but see, Horner and Henson, 2008), as well as the two-process model of left VLPFC function that implicates left mid-VLPFC in goal-relevant selection (Badre et al., 2005; Dobbins & Wagner, 2005). Regions sensitive to stimulus-decision repetition were expected to show reduced activity specific to Within-Task trials, the only condition in which decisions were repeated from study to test (Figure 1B). We isolated the effects of decision-related repetition from those of response-related repetition by contrasting activity on Within-Task trials to that on AT-RR trials, as the only difference between these two trial types was the presence (Within-Task) vs. absence (AT-RR) of decision-level repetition.
The AT-RR > Within-Task contrast (Figure 2B, cyan; Table 1) revealed decision-related neural priming in left middle and posterior VLPFC (mid/post-VLPFC), corresponding to inferior frontal pars triangularis (~BA 45) and pars opercularis (~BA 44) (p < .001, 5-voxel extent; all ps < .01, small-volume corrected; similar left VLPFC foci were also identified when contrasting all Across-Task > Within-Task trials). ROI analyses confirmed the specificity of these activity decreases to Within-Task trials in both the rostral (~BA 45; −51, 36, 12) and caudal (~BA 45/44; −45, 15, 24) foci within mid-VLPFC. Specifically, in both ROIs, activity during Within-Task trials was significantly reduced compared to all other trial types (Novel, AT-RR, AT-RS; Fs(1,25) > 17.36, ps < .001), which, in turn, did not differ from each other (ps > .2) (Figure 2D). Importantly, activation in these regions during Across-Task trials did not differ from that during Novel trials (Fs < 1). Collectively, the Within-Task specificity of neural priming in left mid/post-VLPFC provides strong evidence for the contribution of stimulus-decision learning to neural repetition effects independent of stimulus-level and stimulus-response learning.
Importantly, these data suggest a functional dissociation between decision-specific repetition effects in left mid/post-VLPFC and stimulus-level repetition effects in left anterior VLPFC. This dissociation along the rostro-caudal axis of lateral PFC was confirmed by significant Region × Condition interactions between left anterior VLPFC and both the left mid-VLPFC and the left mid/post-VLPFC foci (F(3,75) = 8.08, p < .001 and F(3,75) = 4.74, p < .01, respectively). Similarly, dissociations were observed between these left mid/post-VLPFC regions and the left middle temporal cortex and fusiform regions that were sensitive to stimulus repetition (Fs(3,75) > 4.97, ps < .005). As all regions demonstrated repetition-related reductions during Within-Task trials, these across-region dissociations reflect the presence of significant Across-Task neural priming in left anterior VLPFC, posterior middle temporal cortex, and fusiform cortex and the absence of such effects in left mid/post-VLPFC (Figure 2E).
Stimulus-Response Repetition Effects
While the preceding analyses revealed dissociable neural priming effects following stimulus and stimulus-decision repetition, they do not address the possible additional contributions of stimulus-response learning to neural priming. One possibility is that response-level priming would be observed in caudal frontal regions, at or near premotor cortex, that have been previously associated with response selection processes that mediate the mapping of stimuli to actions (Badre & D’Esposito, 2007; Koechlin, Ody, & Kouneiher, 2003; Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002). Analytically, to investigate this possibility we contrasted activity for the two Across-Task conditions, as these conditions only differed with respect to whether responses were repeated (AT-RR) or switched (AT-RS) from study to test (Figure 1B).
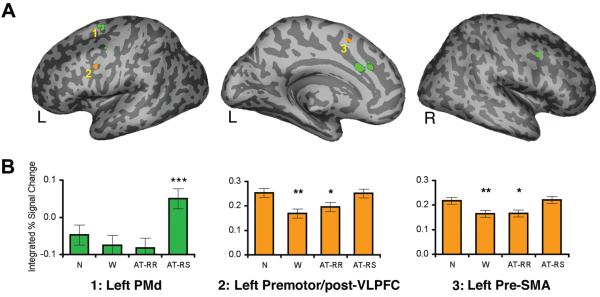
The AT-RS > AT-RR contrast (Figure 4A, green; Table 1) revealed effects in left premotor cortex (~BA 6), left anterior cingulate cortex (ACC: ~BA 24), right dorsal premotor cortex (PMd: ~BA 6), and right orbitofrontal cortex (~BA 47/11) (p < .001, 5-voxel extent). ROI analyses confirmed the presence of activity reductions for AT-RR compared AT-RS trials in all of these regions (Fs(1,25) > 10.68, ps < .005). Interestingly, this AT-RS vs. AT-RR difference was not consistently driven by a reduction in activity on AT-RR trials, as some regions demonstrated an increase on AT-RS trials relative to Novel trials. In particular, the most superior region of left PMd (~BA 6: −24, 0, 66) displayed greater activity during AT-RS trials than during all other trial types (Fs(1,25) > 10.57, ps < .005), which in turn did not differ from each other (Figure 4B; Fs < 1). This increased activity during AT-RS trials may reflect situations in which primed responses are no longer valid and must be overridden (i.e., response competition).
Figure 4.
Neural effects of stimulus-response repetition. (A) The contrast of AT-RS > AT-RR (p < .001, 5 voxel-extent; green) and the conjunction of AT-RS > AT-RR and Novel > Within-Task (conjoint p < .000025, 5-voxel extent; orange) rendered on an inflated MNI canonical surface. (B) A ‘response competition’ effect was observed in (1) left dorsal premotor (PMd; −24, 0, 66), wherein activity for AT-RS trials was greater than all other trial types (ps < .005). A ‘response facilitation’ effect was observed in both (2) left premotor/post-VLPFC (−39, 0, 39) and (3) left pre-supplementary motor area (pre-SMA; −9, 9, 51), wherein activity was reduced for both conditions in which responses repeated (AT-RR and Within-Task) relative to Novel and AT-RS trials. [Note that for panel B, neural priming relative to Novel trials is denoted ***p < .005, **p < .01, *p ≤ .05].
To explore possible response facilitation effects common to the Within-Task and AT-RR trials that may have been obscured by the lower power inherent in the AT-RS > AT-RR contrast (due to these conditions containing the fewest number of trials), we computed a conjunction analysis across the orthogonal contrasts of AT-RS > AT-RR and Novel > Within-Task (both at p < .005, yielding a conjoint p < .000025, 5-voxel extent). Note that the one factor manipulation common to these contrasts was whether the stimulus-response mappings were novel (AT-RS and Novel conditions) or repeated (AT-RR and Within-Task conditions). This analysis revealed response facilitation effects in two regions—left premotor/posterior VLPFC (~BA 6/44: −39, 0, 39; p <.05, small-volume corrected) and left pre-supplementary motor area (pre-SMA; ~BA 6: −9, 9, 51) (Figure 4A, orange; Table 1). Confirmatory ROI analyses demonstrated that activity in these regions was reduced for both Within-Task and AT-RR trials compared to Novel and AT-RS trials (Fs(1,25) > 4.07, ps ≤ .05; Figure 4C). Moreover, activity did not differ between Within-Task and AT-RR trials (ps > .25), nor between Novel and AT-RS trials (Fs < 1).
Collectively, these analyses revealed two types of response-level influences on neural activity—response facilitation in left premotor/post-VLPFC and left pre-SMA that occurs when learned responses are compatible with current goal-relevant responses, and response competition in left PMd that occurs when learned responses are no longer appropriate. Significant Region × Condition interactions were present between left PMd and both left premotor/post-VLPFC (F(3,75) = 3.12, p < .05) and pre-SMA (F(3,75) = 3.85, p < .05).
Importantly, Region × Condition interactions dissociated the caudal frontal regions demonstrating response-level effects (left PMd, premotor/post-VLPFC, and pre-SMA) from (a) the left mid/post-VLPFC regions demonstrating decision-level priming (Fs(3,75) > 7.79, ps < .001), and (b) the left anterior VLPFC regions demonstrating stimulus-level priming (Fs(3,75) > 5.25, ps < .005). Together, these three dissociable neural priming effects along the rostro-caudal axis of lateral frontal cortex reveal that multiple forms of learning serve to reduce demands on distinct components of the frontal executive system.
Brain-Behavior Correlations
Across subjects, the neural priming effect (i.e., difference in integrated % signal change) in each of the eight primary regions of interest (left anterior VLPFC, left posterior middle temporal cortex, left fusiform cortex, left mid-VLPFC, left mid/post-VLPFC, left PMd, left premotor/post-VLPFC, and left pre-SMA) was entered into a multiple regression to examine the relationship to behavioral priming (i.e., difference in RT). Multiple regressions computed on Novel – Within-Task, AT-RR – Within-Task, and AT-RS – AT-RR failed to reveal significant brain-behavioral relationships (ps > .05). Separate analyses of the relationship between neural priming in each ROI and behavioral priming revealed only one notable correlation. Specifically, the magnitude of RT slowing on AT-RS compared to AT-RR trials positively correlated with the magnitude of increased neural activity on AT-RS compared to AT-RR trials in left PMd (r = .48, p < .05). However, this correlation did not survive correction for multiple comparisons (8 regions, p = .11).
DISCUSSION
Learning from past goal-directed behavior can alter the underlying neural computations supporting subsequent goal-directed action. The present data demonstrate that learning can occur at multiple representational levels—stimulus, stimulus-decision, and stimulus-response— with each giving rise to neural priming in distinct regions of lateral frontal cortex. Three novel findings bear on the cortical tuning and response-learning hypotheses of neural priming, and illuminate the multi-component nature of the frontal executive system.
First, the present functional neuroanatomic dissociations provide strong evidence that neural priming due to cortical tuning of stimulus-level representations co-occurs with neural priming stemming from stimulus-outcome associative learning. Specifically, while retrieved stimulus-decision and stimulus-response associations make important, and unique, contributions to neural priming in lateral and medial frontal regions, our data indicate that these additional routes to action do not circumvent the processing of stimulus-specific representations in inferior and lateral temporal cortex. Rather, neural priming was observed in left fusiform gyrus (see also, Horner and Henson, 2008) and posterior middle temporal cortex even in the absence of decision or response repetition, indicating that these effects cannot be attributed to bypassed stimulus-level processing. Second, the present data reveal that stimulus-level learning also contributes to neural priming in left anterior VLPFC, providing novel evidence that multiple forms of learning serve to decrease demands on lateral PFC executive processes. Specifically, the present data are the first, to our knowledge, to demonstrate that cortical tuning and stimulus-outcome learning reduce computational demands in dissociable regions along the rostro-caudal axis of left VLPFC. Finally, the present data provide new evidence that stimulus-decision and stimulus-response learning result in distinct neural priming effects in lateral frontal cortex, and further demonstrate that the retrieval of learned responses can yield both facilitative and competitive effects within lateral and medial frontal areas. Collectively, these novel findings highlight the multi-process nature of neural priming during semantic classification, wherein mechanisms of cortical tuning, stimulus-decision learning, and stimulus-response learning operate in tandem to drive experience-dependent modulations of neural activity.
Cortical Tuning of Stimulus-Level Representations
Relative to novel stimuli, neural priming was observed in left anterior VLPFC, fusiform, and posterior middle temporal regions for all repeated stimuli (Figure 2B-C), providing evidence that significant repetition-related activity reductions can occur even in the absence of stimulus-decision or stimulus-response repetition. Of particular note was the presence of significant neural priming in left anterior VLPFC during Across-Task trials, which stands in contrast to recent proposals that neural priming in VLPFC reflects stimulus-outcome learning (Horner & Henson, 2008; Dobbins et al., 2004). Because alternate pathways to action were not available on AT-RR trials (no stimulus-decision repetition) nor on AT-RS trials (no stimulus-decision or stimulus-response repetition), the observed VLPFC neural priming on Across-Task trials—as well as such priming in left middle temporal and fusiform areas—cannot reflect bypassed processing of stimulus-level representations in favor of decision or response retrieval. As such, these data suggest that neural priming in these structures may stem from cortical tuning.
Extensive behavioral data indicate that prior stimulus processing can result in priming at multiple levels of stimulus representation—perceptual, lexical, and conceptual (e.g., Roediger & McDermott, 1993). Because our paradigm was designed to delineate stimulus-level effects from stimulus-decision and stimulus-response effects, it does not permit specification of whether the stimulus-level effects observed in left fronto-temporal regions reflect the tuning of conceptual, lexical, and/or perceptual representations. However, we note that the localization of the observed stimulus-level effects corresponds with a rich literature implicating these regions in the representation (left posterior middle temporal and fusiform) and controlled retrieval (left anterior VLPFC) of semantic information, suggesting that these neural priming effects reflect facilitation at the conceptual level (Badre & Wagner, 2007; Martin, 2007). In particular, stimulus-level neural priming in left fusiform and posterior middle temporal cortex is consistent with an experience-dependent tuning of stimulus-specific conceptual representations that facilitates subsequent processing within these regions during repeated access to stimulus meaning (Simons et al., 2003; Koutstaal et al., 2001). This tuning of posterior neocortical representations may constitute a form of experience-dependent prediction, resulting in increased ‘bottom-up’ retrieval of relevant conceptual information. In this manner, posterior neocortical tuning may serve to reduce demands on the prefrontal executive system, such that the concomitant neural priming in left anterior VLPFC reflects a decreased reliance on, or increased efficacy of, ‘top-down’ signals that control semantic retrieval as the recovery of conceptual information becomes more automatic (Badre et al., 2005; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001). This interpretation complements other evidence documenting (a) a functional coupling between left anterior VLPFC and these left temporal cortical areas during conceptual processing (Bokde, Tagamets, Friedman, & Horwitz, 2001), (b) a necessary role of left rostral VLPFC in establishing neural priming in left posterior temporal cortex (Wig et al., 2005), and (c) a priming-related increase in neural synchrony between left VLPFC and left posterior temporal cortex (Ghuman, Bar, Dobbins, & Schnyer, 2008). Future studies that are specifically designed to test this fronto-temporal interactive model may shed further light on how left anterior VLPFC neural priming relates to experience-dependent changes in posterior temporal cortex.
While our theoretical focus is on delineating how learning relates to neural priming in the frontal executive system, as noted above, the present pattern of neural priming in left fusiform cortex stands in contrast to that observed by Dobbins et al. (2004). Specifically, whereas Dobbins et al. revealed a response-learning effect in left fusiform cortex, here the predominant fusiform priming pattern was consistent with a cortical tuning effect. Indeed, in the present study, multiple foci in left fusiform cortex—extending from posterior lateral (~BA 37/19) to anterior medial regions (~BA 37/20) (Figure 2B; Table 1)—demonstrated significantly reduced activation in all three repeated conditions (Within, AT-RR, AT-RS) and no significant differences between these primed conditions (with one exception — −45, −51, −15 — where the dominant effect of cortical tuning was accompanied by a modest, but significant (p < .05), difference between AT-RS vs. Within trials). Recently, Horner and Henson (2008) reported a similar pattern of generalized neural priming in left fusiform, raising questions about the extent of ‘response-learning’ effects in fusiform gyrus.
The divergence between the present data and those of Dobbins et al. (2004) may reflect functional heterogeneity within left ventral temporal cortex, as the present left fusiform foci do not appear to overlap with the left fusiform region observed by Dobbins and colleagues (~BA 19/37; −24, −57, −15), but rather overlap with left fusiform regions previously implicated in semantic processing (Simons et al., 2003; Buckner et al., 2000; Thompson-Schill et al., 1999). Thus, some subregions in left fusiform may demonstrate neural priming associated with stimulus-outcome learning (we also observed a focal left fusiform region identified in the Across-Task > Within-Task contrast [7 voxels with peak at −48, −54, −21] that showed a stimulus-decision effect), whereas other subregions may demonstrate neural priming associated with cortical tuning (the dominant pattern observed herein). A critical objective for future investigation is to determine what differentiates these two classes of left fusiform subregions (e.g., do they differentially subserve perceptual vs. conceptual processing?; Simons et al., 2003). Regardless, the present data provide novel evidence that neural priming in left fusiform, posterior middle temporal, and anterior VLPFC can reflect stimulus-level changes, arguing against the proposal that the processing of stimulus-level representations is bypassed in favor of stimulus-outcome retrieval (c.f., Horner & Henson, 2008; Dobbins et al., 2004).
Decision-Specific Priming in Left VLPFC
Neural priming in left mid and posterior VLPFC is greater when the same semantic task or decision rule is performed during initial and repeated stimulus processing relative to when the task or rule is changed (Horner & Henson, 2008; Dobbins et al., 2004; Wagner, Koutstaal, et al., 2000; Thompson-Schill et al., 1999). Because decision and response repetition were not independently manipulated in prior studies, it has remained unclear whether this neural priming effect reflects a processing benefit stemming from retrieval of learned stimulus-decision or stimulus-response mappings. Delineating the level at which ‘response learning’ operates has implications for theories of neural priming (Schacter et al., 2007) and automaticity (Logan, 1990), as well as models of prefrontal executive control (Badre & Wagner, 2007; Fuster, 2001).
The present experimental design isolated the contributions of decision learning from response learning, enabling the first test of whether neural priming in left VLPFC reflects reduced demands on stimulus-decision or stimulus-response mapping. Strikingly, in left mid/post-VLPFC regions that appeared to fall at or near the left VLPFC regions previously reported to be sensitive to some aspect of ‘stimulus-response’ learning (Horner & Henson, 2008; Dobbins et al., 2004), we observed neural priming that was selective to the one condition in which stimulus-decision (or stimulus-task) mappings were repeated (i.e., Within-Task trials). Accordingly, the present data provide novel evidence that experience-dependent activity reductions in left mid/post-VLPFC reflect processing benefits that stem from stimulus-decision learning, rather than from stimulus-response learning or stimulus-level priming.
The stimulus-decision effect in left mid/post-VLPFC functionally dissociated from the stimulus-level effect in left anterior VLPFC (Figure 2E). This dissociation is consistent with Badre and Wagner’s (2007) two-process model of left VLPFC executive function, and demonstrates that different forms of learning can reduce processing demands in distinct regions of prefrontal cortex. Specifically, while neural priming in left anterior VLPFC likely reflects reduced demands on controlled semantic retrieval, neural priming in left mid/post-VLPFC likely reflects reduced demands on selection of an appropriate classification decision. That is, when a stimulus is already associated with a goal-relevant decision, two sources of evidence may favor this decision during subsequent stimulus classification—namely, retrieved semantic knowledge supporting the decision and retrieved stimulus-decision associative knowledge. The availability of this second source of learned evidence further favors activation of the relevant decision, thus reducing decision-level uncertainty and demands on left mid/post-VLPFC selection processes (Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997).
This interpretation of decision-level priming in left mid/post-VLPFC is in accordance with research associating activity in this region with interference resolution during task switching (e.g., Badre and Wagner, 2006; Braver, Reynolds, & Donaldson, 2003; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000). During task-switching paradigms, the ability to switch from one task to a second is argued to require configuration of the present task set as well as resolution of proactive interference from the previous task set (Wylie & Allport, 2000; Rogers & Monsell, 1995; Allport, Styles, & Hsieh, 1994). Interference on switch trials can be triggered by ‘task set priming’, wherein a bivalent stimulus serves to reactivate the stimulus-task rule mappings formed during the preceding trial (Brown, Lehmann & Poboka, 2006; Waszak, Hommel & Allport, 2004; Waszak et al., 2003; Sohn & Anderson, 2003). Recent fMRI data indicate that the magnitude of left mid-VLPFC activation on switch trials tracks the magnitude of decision-level interference, but not response-level interference (Badre & Wagner, 2006). When viewed from this perspective, the present stimulus-decision neural priming effect in left mid-VLPFC may reflect the benefit of reduced decision-level conflict due to facilitative priming from the learned stimulus-decision association. Moreover, while the task-switching literature has focused primarily on how left mid-VLPFC resolves conflict following short-term learning (e.g., Badre & Wagner, 2006; see also, Badre & Wagner, 2005; Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998), the present data demonstrate that long-term stimulus-decision associative memory can also alter demands on this form of cognitive control (Thompson-Schill et al., 1999).
The present findings suggest that a re-interpretation of previously reported task-specific and across-task PFC neural priming effects is necessary (Wagner, Koutstaal, et al., 2000; for related behavioral effects, see Thompson-Schill & Gabrieli, 1999; Vriezen, Moscovitch, & Bellos, 1995). For example, a prior fMRI study of within-task (semantic→semantic) and across-task (perceptual→semantic) repetition observed (a) reduced activation in left mid-to-anterior VLPFC (~BA 45/47) only during the within-task condition, and (b) reduced activation in left post-VLPFC/premotor (~BA 44/6) during both within- and across-task repetition (Wagner, Koutstaal, et al., 2000). While the task-specific effect in left rostral PFC was originally interpreted as revealing that neural priming in this region depends on having previously accessed semantic knowledge—an interpretation that remains viable for left anterior VLPFC (~area 47)—the present data suggest that the absence of an across-task effect in left mid-VLPFC (~BA 45) was early evidence that stimulus-decision learning drives neural priming in this region. Moreover, whereas the across-task effect in left caudal PFC was originally argued to reflect priming at the lexical/phonological level, because the stimulus-response mappings were held constant on 50% of the across-task trials, this effect is compatible with the present observation that neural priming in caudal PFC stems from stimulus-response learning (see below).
Another intriguing aspect of the present data is that they raise the possibility of interactive effects across different levels of the prefrontal executive system (Botvinick, 2007; Koechlin & Summerfield, 2007). In particular, while neural priming in left anterior VLPFC was significantly modulated by stimulus-level learning (Figure 2B-C), the magnitude of neural priming in this region was also significantly greater when decisions were repeated (Within-Task) than when decisions changed (Across-Task). One possibility is that this decision-level effect in anterior VLPFC reflects partial voluming due to variable functional-anatomic overlap across subjects. Alternatively, and more interestingly, this finding might reveal feedback from decision-level processing regions onto conceptual processing regions that occurs when direct retrieval of a goal-relevant stimulus-decision mapping is unavailable as a source of evidence for decision selection (i.e., on Across-Task trials). This feedback from decision-level to conceptual-level executive processing regions may up-regulate the conceptual executive system that biases recovery of semantic knowledge that is relevant to decision selection. Consistent with this interpretation, whereas all repeated conditions demonstrated neural priming in left anterior VLPFC and left fusiform when the BOLD response was measured from 3.0–7.5 s post-stimulus onset (i.e., surrounding the peak of the response), the Across-Task conditions also showed a more sustained response extending into the 7.5–10.5 s period (Figure 3). This late separation between Across-Task and Within-Task trials may reflect the greater decision uncertainty inherent in the former condition, such that feedback from the decision level temporally-extends engagement of processing at the conceptual level. While this interpretation is speculative and awaits testing on data with higher temporal resolution, this proposal is compatible with interactive models of cognitive control (Botvinick, 2007; Koechlin & Summerfield, 2007) and may offer an account for previously reported outcome-level influences on neural priming in left anterior VLPFC (Horner & Henson, 2008).
Response-Level Repetition and Neural Priming
The possible contributions of response repetition to neural priming during semantic classification, independent of decision-related repetition, have been largely unexplored. By isolating effects of response repetition, the present data provide novel evidence that stimulus-response learning modulates subsequent demands on the frontal executive system. Specifically, when learned responses remained goal-appropriate, regardless of whether stimulus-decision mappings were held constant (Within-Task) or switched (AT-RR), neural priming was observed in caudal portions of lateral frontal cortex (left premotor/post-VLPFC) and medial frontal cortex (left pre-SMA). This finding provides evidence that neural priming in these regions reflects the benefits of facilitated response selection—that is, when retrieval of a learned stimulus-response association provides additional evidence in favor of the goal-relevant response, demands on caudal frontal executive processes that subserve response selection decrease (Simmonds, Pekar, & Mostofsky, 2008; Badre & D’Esposito, 2007; Picton et al., 2007; Koechlin & Jubault, 2006; Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999). Moreover, the response-learning effect in left premotor/post-VLPFC functionally dissociated from (a) the decision-level effect observed in left mid/post-VLPFC regions, and (b) the stimulus-level effect observed in left anterior VLPFC. Thus, the present data revealed a functional triple dissociation in lateral frontal cortex indicating that multiple types of learning yield distinct computational savings along the rostro-caudal extent of left frontal cortex.
In addition to the facilitation that stems from retrieving learned goal-relevant stimulus-response mappings, the current results also revealed a processing cost when retrieved responses conflict with the current goal-relevant action. Specifically, neural activity increased in left PMd when responses switched from study to test (AT-RS) compared to when they did not (all other conditions). This activity increase may reflect a form of ‘negative priming’ (Waszak & Hommel, 2007; Waszak et al., 2003; Thompson-Schill et al., 1999), wherein a retrieved stimulus-response association conflicts with the goal-relevant response, causing response competition. Evidence for response competition was also evident in subjects’ accuracy scores, which were significantly reduced on AT-RS compared to all other conditions. While it is unclear why competition effects were selectively observed at the response level—in theory, one might also predict such effects at the decision level—these effects nevertheless suggest that additional mechanisms are required to override prepotent response tendencies or that left PMd represents active response representations. In support of the former conclusion, prior studies have associated activity in left premotor cortex with inhibitory control (Watanabe et al., 2002; Kiefer, Marzinzik, Weisbrod, Scherg, & Spitzer, 1998). Together with the response facilitation effect in left premotor/post-VLPFC, these data suggest that the dorsal and ventral subregions of premotor cortex make distinct contributions to action selection (Hoshi & Tanji, 2007).
Extending Hierarchical Models of Executive Control
The present dissociable neural priming effects in lateral frontal cortex lend support to models of cognitive control that posit that hierarchically-organized executive functions depend on the rostro-caudal axis of lateral frontal cortex (Badre & D’Esposito, 2007; Koechlin & Summerfield, 2007; Koechlin & Jubault, 2006; Fuster, 2001). By this view, regions proximal to motor cortex select “acts and programs of movement” closest to response output while increasingly ‘higher’ levels of representation that select “plans” and “concepts” map to progressively more anterior regions of lateral PFC (Fuster, 2001). Consistent with this perspective, the present neural priming data revealed conceptual-level effects localized to anterior VLPFC, decision-level effects localized to mid/post-VLPFC, and response-level effects localized to premotor/post-VLPFC. These findings suggest that the hierarchical organization of lateral frontal cortex is not restricted to dorsal frontal structures; rather, a parallel hierarchy may extend from premotor through ventrolateral PFC structures. Future studies are required to systematically test whether responses along this ventrolateral pathway obey hierarchical principles.
Locus of Learning and Relation to Behavioral Priming
The present response-level and decision-level neural priming effects raise the question as to the locus of such stimulus-outcome learning. One possibility is that stimulus-outcome learning reflects the development of long-term representations within PFC (e.g., Wood and Grafman, 2003). Alternatively, frontal neural priming effects may reflect changes in long-range interactions between PFC and posterior structures, as evidenced by enhanced interregional neural synchrony with repeated stimulus processing (Ghuman et al., 2008; Schacter et al., 2007). Finally, recent neuropsychological data also indicate that stimulus-outcome priming effects at least partially depend on medial temporal lobe (MTL) mechanisms that support associative memory, as patients with MTL damage demonstrate stimulus-level but not stimulus-outcome behavioral priming (Schnyer et al., 2006). An important issue for future research is to determine whether the MTL is necessary for stimulus-decision priming, stimulus-response priming, or both.
The multi-process nature of the observed repetition effects also suggests that a complex relationship may exist between neural priming and behavioral priming. For example, while we observed significant neural priming on AT-RS trials in left anterior VLPFC and left fusiform regions (Figures 2C and 3), we did not observe significant behavioral priming on AT-RS compared to Novel trials. One possibility is that this behavioral pattern indicates a lack of stimulus-level learning. However, the observed stimulus-level neural priming effects would appear to argue against such an interpretation. Alternatively, it is possible, and even more likely, that RTs are influenced by multiple processes, some facilitative and others disruptive. From this view, the absence of a behavioral priming effect in the AT-RS condition may reflect the effects of stimulus-response conflict that offset any stimulus-level facilitation. At the neural level, AT-RS trials were associated with both stimulus-level neural priming in multiple regions as well as stimulus-response ‘negative’ neural priming in left PMd, lending support for this latter interpretation. More broadly, the multi-process nature of priming may make one-to-one mappings between specific neural priming effects and changes in RT difficult, possibly accounting for why significant correlations between the magnitudes of neural priming and behavioral priming were not observed (but see, Horner and Henson, 2008; Dobbins et al., 2004; Maccotta and Buckner, 2004).
Conclusion
Computational demands in the present are affected by predictive evidence derived from the past. The current study provides novel evidence that the past provides predictive evidence at multiple levels—stimulus, decision, and response—that are likely to be relevant to present stimulus-to-action mapping. This predictive evidence reduces computational demands along the rostro-caudal axis of lateral PFC, revealing a topographically organized prefrontal executive system that reaps the benefits of distinct forms of experience-dependent learning.
Acknowledgments
Supported by the National Institute of Mental Health (5R01MH080309), National Science Foundation (BCS–0401641), and Alfred P. Sloan Foundation. The authors are grateful to David Badre and Brice Kuhl for fruitful discussions of the data presented herein.
REFERENCES
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and performance. MIT Press; Cambridge: 1994. pp. 421–52. [Google Scholar]
- Badre D, D’Esposito M. Functional Magnetic Resonance Imaging Evidence for a Hierarchical Organization of the Prefrontal Cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Semantic retrieval, mnemonic control, and prefrontal cortex. Behavioral and Cognitive Neuroscience Reviews. 2002;1:206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cerebral Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proceedings of the National Academy of Sciences U S A. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Multilevel structure in behaviour and in the brain: a model of Fuster’s hierarchy. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2007;362:1615–1626. doi: 10.1098/rstb.2007.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124(Pt 10):2074–86. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Schutze H, Duzel E. Category-specific organization of prefrontal response-facilitation during priming. Neuropsychologia. 2006;44:1765–1776. doi: 10.1016/j.neuropsychologia.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–26. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brown S, Lehmann C, Poboka D. A critical test of the failure-to-engage theory of task switching. Psychonomic Bulletin & Review. 2006;13:152–9. doi: 10.3758/bf03193827. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis I: Segmentation and Surface Reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Danker JF, Gunn P, Anderson JR. A rational account of memory predicts left prefrontal activation during controlled retrieval. Cerebral Cortex. doi: 10.1093/cercor/bhn027. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, et al. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature. 2004;428:316–319. doi: 10.1038/nature02400. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Research. Cognitive Brain Research. 2000;9:103–9. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical Surface-Based Analysis II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, et al. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, et al. Dissociation of automatic and strategic lexical-semantics: functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. Journal of Neuroscience. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from FMRI adaptation. Cerebral Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Proceedings of the National Academy of Sciences U S A. 2008;105:8405–9. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD FMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hommel BJ. Feature integration across perception and action: event files affect response choice. Psychological Research. 2007;71:42–63. doi: 10.1007/s00426-005-0035-1. [DOI] [PubMed] [Google Scholar]
- Horner AJ, Henson RN. Priming, response learning and repetition suppression. Neuropsychologia. 2008;46:1979–1991. doi: 10.1016/j.neuropsychologia.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Current Opinion in Neurobiology. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences U S A. 1998;95:8410–3. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer M, Marzinzik F, Weisbrod M, Scherg M, Spitzer M. The time course of brain activations during response inhibition: evidence from event-related potentials in a go/no go task. Neuroreport. 1998;9:765–770. doi: 10.1097/00001756-199803090-00037. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca’s area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends in Cognitive Sciences. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, et al. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Logan GD. Automaticity, resources, and memory: theoretical controversies and practical implications. Human Factors. 1988;30:583–598. doi: 10.1177/001872088803000504. [DOI] [PubMed] [Google Scholar]
- Logan GD. Repetition priming and automaticity: Common underlying mechanisms? Cognitive Psychology. 1990;22:1–35. [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. Journal of Cognitive Neuroscience. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, et al. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Raichle MA, Feiz JA, Videen TO, MacLeod AMK, Pardo AMK, et al. Practice-related changes in human functional anatomy during non-motor learning. Cerebral Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Roediger HL, III, McDermott KB. Implicit memory in normal human subjects. In: Spinnler H, Boller F, Boller F, Grafman J, editors. Handbook of Neuropsychology. Elsevier; Amsterdam: 1993. pp. 63–131. [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Peterson SE, Videen TO, et al. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proceedings of the National Academy of Sciences U S A. 1992;89:1837–41. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Dobbins IG, Schnyer DM. Specificity of priming: a cognitive neuroscience perspective. Nature Reviews Neuroscience. 2004;5:853–862. doi: 10.1038/nrn1534. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Current Opinion in Neurobiology. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Davis S, Verfaellie M, et al. Item to decision mapping in rapid response learning. Memory & Cognition. 2007;35:1472–1482. doi: 10.3758/bf03193617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Schacter D, Verfaellie M. Rapid response learning in amnesia: delineating associative learning components in repetition priming. Neuropsychologia. 2006;44:140–149. doi: 10.1016/j.neuropsychologia.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Anderson JR. Stimulus-related priming during task switching. Memory & Cognition. 2003;31:775–80. doi: 10.3758/bf03196115. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences U S A. 2001;98:12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Botvinick MM. Resolving conflict: a response to Martin and Cheng (2006) Psychonomic Bulletin & Review. 2006;13:402–408. doi: 10.3758/bf03193860. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences U S A. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Gabrieli JD. Priming of visual and functional knowledge on a semantic classification task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:41–53. doi: 10.1037//0278-7393.25.1.41. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Vriezen ER, Moscovitch M, Bellos SA. Priming effects in semantic classification tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:933–946. [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic repetition priming for verbal and pictorial knowledge, A functional MRI study of left inferior prefrontal cortex. Journal of Cognitive Neuroscience. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cerebral Cortex. 2000;10:1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: When priming hinders new episodic learning. Journal of Cognitive Neuroscience. 2000;12(S2):52–60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B. The costs and benefits of cross-task priming. Memory & Cognition. 2007;35:1175–86. doi: 10.3758/bf03193487. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A. Task-switching and long-term priming: role of episodic stimulus-task bindings in task-shift costs. Cognitive Psychology. 2003;46:361–413. doi: 10.1016/s0010-0285(02)00520-0. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. Journal of Cognitive Neuroscience. 2005;17:1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nature Neuroscience. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8:227–233. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A. Semantic generalization of stimulus-task bindings. Psychonomic Bulletin and Review. 2004;11:1027–33. doi: 10.3758/bf03196732. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, et al. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nature Reviews Neuroscience. 2003;4:139–47. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of “switch costs”. Psychological Research. 2000;63:212–33. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]