Summary
The possible roles of mast cells in heath and disease have been a topic of interest for over one hundred and twenty five years. Many adaptive or pathological processes affecting the skin or other anatomical sites have been associated with morphological evidence of mast cell activation, and/or with changes in mast cell numbers or phenotype. Such observations, taken together with the known functions of the diverse mediators, cytokines and growth factors which can be secreted by mast cells, have suggested many potential functions for mast cells in health and disease. Definitively identifying the importance of mast cells in biological responses in humans is difficult. However, mutant mice which are profoundly mast cell-deficient, especially those which can undergo engraftment with wild type or genetically-altered mast cells, provide an opportunity to investigate the importance of mast cells, and specific mast cell functions or products, in various adaptive or pathological responses in mice. Such work has shown that mast cells can significantly influence multiple features of inflammatory or immune responses, through diverse effects that can either promote or, surprisingly, suppress, aspects of these responses. Through such functions, mast cells can significantly influence inflammation, tissue remodeling, host defense and homeostasis.
Keywords: Allergy, asthma, immunosuppression, IL-10, innate immunity
1. Introduction
Von Recklinghausen may have been the first to observe what later came to be called mast cells [1]. However, Paul Ehrlich provided the first description of some of the distinctive histochemical features of the cells that he shortly thereafter named “Mastzellen” (or “well fed cells”) [2]. Ehrlich also reported that human tissues affected by chronic inflammation exhibited striking increases in mast cell numbers [2].
The application of morphological approaches to the analysis of human and experimental animal tissues has resulted in the description of a long list of conditions in which mast cell numbers are increased, mast cell anatomical distribution or phenotype is altered, and/or mast cell activation is evident [3–8]. Moreover, the list of agents that can stimulate immunological or non-immunological activation of mast cells continues to grow [3, 4, 7–10] (Table 1), as does our understanding both of the diversity of actions that can be mediated by the secreted products of mast cells and of mast cell functions that can be expressed independently of the secretion of stored or newly synthesized products [3, 5, 8, 9, 11–13].
Table 1.
Mechanisms by which mast cells can be activateda
| Via immune receptors | |
| IgE | Via FcεRI; IgE can also bind to IgG receptors (FcγRII and FcγRIII), and to galectin-3, which are expressed on some mast cell-populations |
| IgG1 | Mouse: FcγRIII, human: FcγRI after treatment with IFN-γ |
| Ig-binding superantigens | Endogenous (e.g., protein Fv in HBV & HCV infections), bacterial (e.g., S. aureus Protein A, P. magnus protein L) or viral (e.g., HIV gp120) |
| Products of complement activation | |
| C3a, C5a, C3b, C4b | Via their respective receptors: C3aR, C5aR, CR3, CR4 |
| Ligands of toll-like receptors, e.g. | |
| Peptidoglycan | TLR2 |
| ds viral RNA, polyI:C | TLR3 |
| LPS | TLR4/CD14 |
| Flagellin | TLR5 (human mast cells) |
| ss viral RNA | TLR7 |
| CpG-DNA | TLR9 |
| Bacteria and their products, e.g. | |
| E. coli FimH | CD48 |
| Pseudomonas aeruginosa | Human mast cells |
| Virulence factors/toxins | e.g., C. difficile toxin A, cholera toxin, VacA (cytotoxin of H. pylori), streptolysin O |
| Viruses, e.g. | |
| Respiratory syncytial virus, influenza virus, Dengue virus, Sendai virus | Via TLR and possibly by other mechanisms |
| Parasites, e.g. | |
| Schistosoma mansoni | Activation by cercariae |
| Leishmania major | Activation by living promastigotes |
| Cytokines and inflammatory mediators, e.g.: | |
| Tryptase | Functional PAR2 has been shown in human skin mast cells and HMC-1 |
| SCF | Via c-Kit |
| IL-1, IL-12 | Secretion of selective mediators (in human mast cells) |
| TNF | In human mast cells |
| PGE2 | Secretion of selective mediators (in human mast cells, via EP2) or potentiation of IgE/Ag-induced mediator release (in mouse, via EP1/EP3) |
| LIGHT | Activation via LTβR in the presence of ionomycin or activated T cells |
| CD30 | Reverse signaling via CD30L induces selective secretion of chemokines |
| Adenosine | Increase of IgE/Ag-induced degranulation via A3R |
| Endogenous peptides, e.g. | |
| Nerve growth factor, Substance P, CGRP and other neuropeptides, Endothelin-1 | Via respective G protein-coupled receptors on mast cells |
| Neurotensin | Via ETA |
| Antimicrobial peptides | e.g., β-defensin 2 and LL-37 |
| Venoms or venom components, e.g. | |
| Sarafotoxin 6b | Component of Atractaspis engaddensis venom, via ETA |
| Phospholipase A2 | Component of many different animal venoms |
| Mast cell degranulating peptide | And other components of honeybee venom |
| Numerous venoms or venom components | e.g., from scorpions, the platypus, frogs, ants, sea urchins, Portuguese man-of-war, etc. |
| Physical stimuli | |
| UV light, cold, heat, pressure, vibration | Possibly through direct and indirect effects on mast cells |
This is not a complete list. Receptors other than the ones mentioned also may be involved in the activation of certain mast cells by some of the listed agents. In some instances, the occurrence and extent, and biological significance, of mast cell activation via the listed receptors in vivo is not yet clear.
Reproduced, with the permission of the publisher, from reference [28]; please see that review for references for some of the stimuli listed in the table.
Such findings invite speculation about a extremely broad spectrum of potential mechanisms by which mast cells might influence the development, extent or duration of inflammatory and other biological responses [3, 4, 8–10, 13]. However, it can be quite challenging to prove that mast cells can perform such proposed functions in vivo, and even more difficult to assess the biological importance of such mast cell-dependent contributions.
In this article, we review some basic principles of mast cell biology and then describe one approach for investigating mast cell function in vivo: analyzing models of adaptive or pathological responses in c-kit mutant mast cell-deficient mice, the corresponding wild type mice, and c-kit mutant mice that have been selectively engrafted with wild type or genetically-altered mast cells. We then describe how this approach has been used to study the roles, and importance, of mast cells in some biological responses which are associated with inflammation and tissue remodeling, or that contribute to host defense or homeostasis.
2. Mast cell biology
2.1. Mast cell development, anatomical distribution and phenotype
Mast cells occur in virtually all vascularized tissues where they ordinarily reside in close proximity to blood vessels, nerves, smooth muscle cells, epithelial cells, mucus-producing glands and hair follicles; they can be especially numerous in anatomical sites that are directly exposed to the environment, including the skin, airways and gastrointestinal tract [3–5, 8, 14].
Mast cells arise from hematopoietic progenitor cells and mature mast cells ordinarily do not circulate in the blood; instead, mast cells acquire their mature phenotype locally in the tissues where they ultimately reside [14]. Moreover, several lines of evidence indicate that changes in mast cell numbers, tissue distribution and/or phenotypic characteristics can be regulated by growth factors such as the c-Kit ligand, stem cell factor (SCF), the main survival and developmental factor for mast cells, interleukin-3 (IL-3), and the T helper type 2 (Th2) cell-associated cytokines IL-4 and IL-9, and probably many other factors [3–5, 8, 14].
Variation in the phenotype of mast cells at different anatomical sites, or during the course of individual biological responses, has been called “mast cell heterogeneity” [8, 14–17]. The ability of the mast cell lineage to generate individual populations that differ in functional properties, mediator content and/or other aspects of phenotype probably confers on mast cells a potentially complex functional repertoire, in which both the ways the cell can be activated and the specific functions it can express can vary according to the requirements of the individual physiological, immunological, inflammatory, or other biological responses in which this cell participates.
2.2. Mast cell activation and secreted products
As a group, mast cells have the potential to secrete a wide variety of biologically active products upon exposure to many different immunological or non-immunological stimuli. Depending on the particular mechanism of mast cell activation and the strength of the stimulus, mast cells may release either distinct subsets of mediators and/or a large array of products [4, 5, 9, 18].
The best studied mechanism of mast cell activation is that induced by the crosslinking of FcεRI, the high affinity receptor for IgE, e.g. with IgE and specific bi- or multi-valent antigen (Table 1). IgE can also bind to the IgG receptors FcγRII and FcγRIII, and to galectin-3, which are expressed on some mast cell populations, but IgE is thought to influence mast cell function mainly through its interaction with FcεRI [4, 5, 18, 19]. In mice, mast cells can also be activated to degranulate and release mediators in response to antigen- and IgG1-dependent signaling through FcγRIII [5, 20–22].
However, activation through Fc receptors is only one of many potential mechanisms of mast cell activation (Table 1). For example, mast cells can be activated directly by pathogens, including bacteria, viruses and parasites, as well as by many soluble products derived from these pathogens. Microbial products can activate mast cells directly via toll-like receptors or receptors for bacterial toxins, or indirectly, e.g., by activating the complement system that then can generate products that interact with appropriate receptors expressed by mast cells [9, 23, 24]. Furthermore, mast cells can be activated by a variety of endogenous and exogenous peptides, cytokines and other inflammatory mediators [4, 5, 10, 25, 26].
Upon appropriate activation, mast cells can release a diverse array of potent biologically active products (which sometimes are called “mediators”). These mediators can classified as: 1) preformed mediators, that are stored in the cells’ prominent cytoplasmic granules; including histamine (and in rodents, serotonin), proteoglycans, and proteases such as chymase, tryptase and carboxypeptidase A; 2) de novo synthesized lipid mediators, e.g., metabolites of arachidonic acid via either the cyclooxygenase (e.g., PGD2) or lipoxygenase (e.g., LTC4) pathways; and 3) a large number of cytokines, chemokines and growth factors [3, 4, 8, 11, 12].
Many of these products are regarded as “pro-inflammatory” in that, individually or in concert, they can elicit features of inflammation that are observed in settings of acute and/or chronic inflammation, e.g., vasodilatation, plasma extravasation and the recruitment and activation of granulocytes (i.e., neutrophils, eosinophils and basophils), T cells, B cells, dendritic cells and monocytes [4, 8, 9, 27, 28]. Mast cell products also can promote changes in the tissues that are affected by such inflammatory responses, including the local degradation or remodelling of structural elements of the tissues, which can be associated with significant changes in the function of the affected organs [29–31].
However, some of the products which might be released by mast cells during individual biological responses can suppress features of these responses. IL-10 and TGF-β, for example, each of which is known to have anti-inflammatory or immunosuppressive properties, are expressed by mast cells and can be released upon their appropriate activation [4, 32–34]. It therefore can be difficult to predict, in a particular biological response, whether the net effect of mast cell participation will be to promote or to suppress the response (or perhaps to have both effects, but at different stages of the response).
3. C-kit mutant mast cell-deficient and ‘mast cell knock-in’ mice
Although in vitro studies of mast cells can be very useful for investigating mechanisms by which mast cells might influence adaptive immunity or other biological responses, it is important to attempt to assess the in vivo relevance and biological importance of such in vitro observations. c-kit mutant mice that virtually lack mast cell populations can be employed to assess the importance of mast cells in regulating the expression of biological responses in vivo.
The most commonly used model for such studies has been the (WB/ReJ × C57BL/6J)F1-KitW/W-v, or WBB6F1-KitW/W-v, mouse [35–37] (Fig. 1). However, C57BL/6-KitW-sh/W-sh mice are now gaining popularity as an alternative model [38–40]. Although each of these c-kit mutant mice is profoundly mast cell-deficient and also virtually lacks melanocytes and interstitial cells of Cajal, the double dose of Wsh mutations in C57BL6-KitW-sh/W-sh mice results in fewer, or less severe, phenotypic abnormalities than are observed in KitW/W-v mice; for example, unlike WBB6F1-KitW/W-v mice, C57BL/6-KitW-sh/W-sh mast cell-deficient mice are not anemic and are fertile, and therefore can easily be crossed with mice carrying other defined genetic abnormalities of interest [5, 38–40].
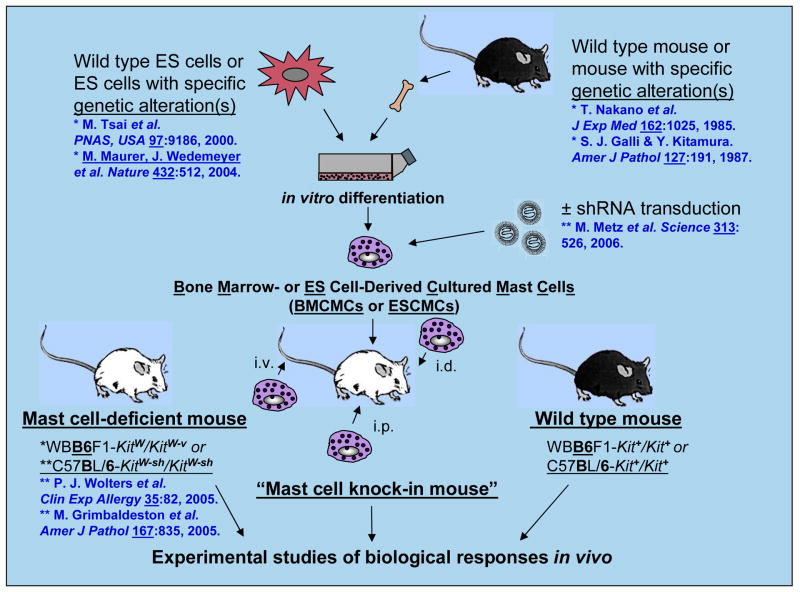
Fig. 1.
How to make mast cell knock-in mice, c-kit mutant genetically mast cell-deficient mice which have been selectively engrafted with genetically-compatible in vitro-derived mast cells. Mast cells are generated from bone marrow cells (or other hematopoietic cells, e.g., those in the fetal liver) from wild-type mice or from mice with specific genetic alterations. Alternatively, embryonic stem (ES) cell-derived cultured mast cells (ESCMCs) can be generated from wild-type or genetically-altered ES cells or mast cells can be transduced in vitro with shRNA to silence specific genes of interest. Those cultured mast cells can then be transplanted intra-venously (i.v.), intra-peritoneally (i.p.) or intra-dermally (i.d.) into mast cell-deficient c-kit mutant mice, such as WBB6F1-KitW/KitW-v or C57BL/6-KitW-sh/KitW-sh mice, to produce “mast cell knock-in mice”. A suitable interval is then allowed for the engraftment and phenotypic maturation of the adoptively transferred mast cells (the length of this interval can vary based on the route of mast cell transfer, the anatomical site of interest, etc.). The importance of mast cell function(s) in biological responses can be analyzed by comparing the responses in wild-type, c-kit mutant mast cell-deficient and selectively mast cell-engrafted c-kit mutant mice (mast cell knock-in mice). The contributions of specific mast cell products (surface structures, signaling molecules, secreted products, etc.) to such biological responses are analyzed by comparison of the features of the responses of interest in mast cell knock-in mice engrafted with wild-type mast cells versus mast cells derived from mice or ES cells that lack or express genetically-altered forms of such products or that have been transduced with shRNA to silence the specific genes that encode these products. Reproduced, with the permission of the publisher, from reference [4].
The mast cell deficiency of C57BL/6-KitW-sh/W-sh mice, like that of WBB6F1-KitW/W-v mice, can be selectively “repaired” by the adoptive transfer of genetically compatible, in vitro-derived mast cells [39, 40] (Fig. 1). Such mast cells can be derived from bone marrow cells (i.e., bone marrow-derived, cultured mast cells) of the congenic wild-type mice or various transgenic or mutant mice [36], from BMCMCs that are transduced with shRNA in order to reduce expression of proteins of interest [26], from other sources of hematopoietic cells (such as fetal liver), or directly from embryonic stem cells (embryonic stem cell-derived cultured mast cells [ESCMCs])[37]. These in vitro-derived mast cells can be administered by intravenous, intraperitoneal, or intradermal injection [14, 36, 39] or by direct injection into the anterior wall of the stomach [41] to create so-called “mast cell knock-in mice” (Fig. 1).
Mast cell knock-in mice can be used to assess the extent to which differences in the expression of biological responses observed in c-kit mutant mast cell-deficient mice and the corresponding wild-type mice reflect the absence of mast cells (or the reduction or absence of specific proteins expressed by mast cells), as opposed to consequences of the c-kit mutations that are independent of their effects on mast cells. However, in conducting studies of this type, it is important to recognize that the phenotypic characteristics, and the anatomical distribution and numbers, of adoptively-transferred mast cells change in the interval after the injection of the mast cell populations into the recipient c-kit mutant mice. Therefore, decisions about how to design and interpret the results of experiments employing such mice should take these facts into consideration, as described in detail elsewhere [5, 28].
4. Functions of mast cells in specific biological responses
We will now provide a few examples of how mast cell knock-in mice can be used to identify and to assess the importance of mast cell functions during adaptive or pathological host responses, particularly those involving the skin, and to define the mechanisms by which mast cells express such functions in these settings.
4.1. Mast cells, and mast cell-derived TNF, can promote elongation of cutaneous nerves during contact hypersensitivity in mice
Several groups have investigated the role of mast cells in contact hypersensitivity (CHS) in mice. As reviewed in detail elsewhere [42], studies in genetically mast cell-deficient c-kit mutant mice and the congenic wild type mice have shown that mast cells can enhance tissue swelling, and leukocyte infiltration, in some models of CHS in mice but not in others. Indeed, we found that, in some experiments assessing the magnitude of CHS responses in mice, reactions measured 24 h after challenge in sensitized mice were slightly but significantly greater in mast cell-deficient mice than in the corresponding wild type mice [43].
In a different model of CHS, mast cells, and antigen-independent effects of IgE, were required for optimal sensitization for CHS, presumably reflecting effects of mast cells on the phenotype, function and/or migration of cutaneous dendritic cells (DCs) in this setting [42]. Mast cells also can promote DC migration from the skin to local lymph nodes in other settings: after activation with IgE and antigen (by a mechanism involving histamine) [44] or, in a model of CHS, during the first 24 h after initial epicutaneous application of fluorescein isothiocyanate (by a mechanism involving mast cell-derived TNF) [45].
Thus, the potential roles of mast cells in CHS are complex, and may vary depending on the details of the CHS protocol tested, such as the type and concentrations of hapten used for sensitization and challenge, and the composition of the vehicle used in that model. However, most studies of the roles of mast cells in such reactions have focused on the increases in vascular permeability, the tissue swelling, and/or the leukocyte infiltration elicited at sites of hapten challenge. We were interested in observations that lesions of contact eczema [46] or atopic dermatitis [47] in humans can exhibit increases in epidermal nerves, and attempted to assess in a mouse model whether mast cells might influence cutaneous nerves at sites of CHS.
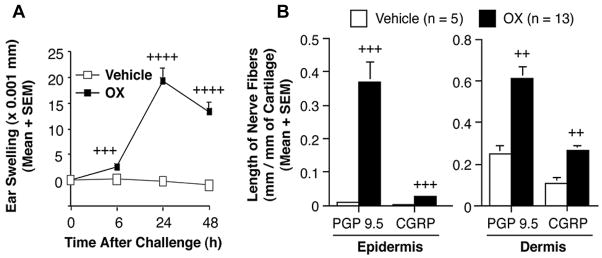
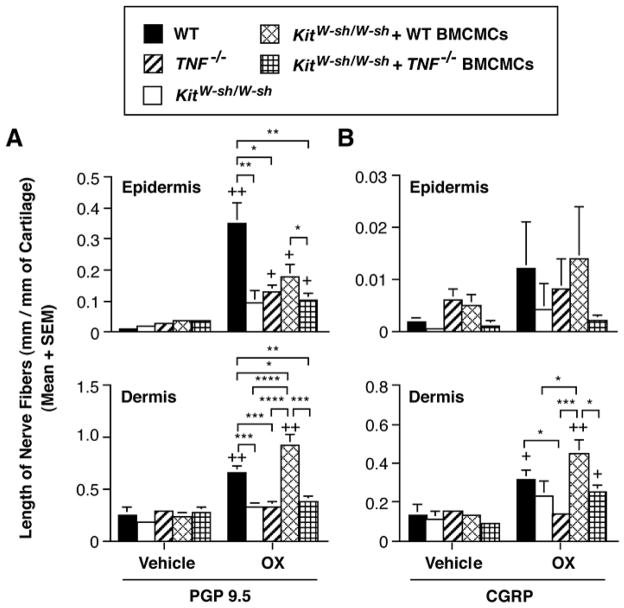
We found that CHS reactions to oxazolone (OX) in mice were associated with significant increases in the length of nerves in the epidermis and dermis [48] (Fig. 2). Using the mast cell knock-in approach, in which genetically mast cell-deficient c-kit mutant mice were engrafted intradermally with either wild type or TNF-deficient mast cells, we found that mast cells, and mast cell-derived TNF, significantly contributed to the elongation of epidermal and dermal PGP 9.5+ nerves and dermal CGRP+ nerves (Fig. 3), as well as to the inflammation, at sites of CHS to OX [48].
Fig. 2.
Elongation of cutaneous nerves during CHS responses to oxazolone (OX) in C57BL/6 mice. (A) Tissue swelling responses to vehicle (open squares) or OX (closed squares) in C57BL/6 wild-type mice, which had been sensitized with 100 μl 2.0% OX in ethanol to shaved abdomen and challenged 5 days later with a total of 20 μl of ethanol to right (control) ear (10 μl to each side) and a total of 20 μl 1.0% OX in ethanol to left ear (10 μl to each side). Ear swelling was measured up to 48 h after challenge. (B) The length of PGP 9.5-positive or CGRP-positive nerve fibers in epidermis or dermis, respectively, in vehicle-treated ears (open columns) or OX-challenged ears (closed columns) of OX-sensitized mice 48 h after challenge. Data are the pooled results from three independent experiments, which gave similar results (vehicle: n = 5, OX; n = 13). In A, +++, ++++ = p < 0.001, < 0.0001 vs. corresponding values for vehicle-treated ears; In B, ++, +++ = p < 0.01, < 0.001 vs. corresponding values for vehicle-treated ears (Student’s t test, 2-tailed). Reproduced, with the permission of the publisher, from reference [48].
Fig. 3.
Mast cell-derived TNF contributes to elongation of cutaneous nerves during CHS to OX in C57BL/6 mice. The length of (A) PGP 9.5-positive or (B) CGRP-positive nerve fibers in ears examined 48 h after hapten challenge in OX-sensitized C57BL/6 wild-type (vehicle; n=5, OX; n = 10), TNF−/− mice (vehicle; n=5, OX; n = 10), mast cell-deficient KitW-sh/W-sh mice (vehicle; n=5, OX; n = 10), and KitW-sh/W-sh mice that had been selectively repaired of their mast cell deficiency by the intra-dermal injection of wild type or TNF−/− BMCMCs (vehicle; n=5, OX; n = 9 in each group). Data show the length of nerve fibers at 48 h after OX or vehicle challenge in OX-sensitized mice. +, ++ = p < 0.05, < 0.01 vs. corresponding values for vehicle-treated ears; *, **, ***, **** = p < 0.05, < 0.01, < 0.001, < 0.0001 for the comparisons indicated by brackets (Student’s t test, 2-tailed). Reproduced, with the permission of the publisher, from reference [48].
We do not yet know whether TNF, or other mast cell products, influenced nerve elongation in this reaction directly, indirectly (e.g., via the promotion of local inflammation) or by both direct and indirect mechanisms. However, these observations show that mast cells, and mast cell-derived TNF, can promote the elongation of cutaneous nerve fibers during this model of CHS in the mouse.
4.2. Mast cell-dependent tissue remodeling in a mouse model of chronic asthma
Mast cells also can regulate tissue remodeling in the lungs. For example, we demonstrated that mast cells contribute significantly to increases in both the number of mucus-producing goblet cells in the airways and in lung collagen content in a mouse model of chronic allergic inflammation [49]. Although the mast cell-derived products that are responsible for these “tissue remodeling” effects remain to be defined, we showed that mast cell-dependent enhancement of goblet cell numbers can occur even in mice whose mast cells lack the ability to signal either via the high affinity IgE receptor (FcεRI) or via FcγRIII (which can bind immune complexes of IgG1 and specific antigen). By contrast, other features of this chronic asthma model are markedly diminished in mice in which only mast cells lack the signaling FcR γchain common to FcεRI and FcγRIII. Thus, in this mouse model of chronic asthma, it appears that mast cells markedly enhance the development of multiple features of the response, but they do so in response to factors which activate the cells via either antibody/FcR γ–dependent or alternative mechanisms.
Our studies of the roles of mast cells in various cutaneous or pulmonary responses, and work by others with mast cell knock-in mice showing that mast cells can contribute to the development of pathology in models of EAE [27] or antibody-dependent arthritis [50], strongly suggest that mast cells can contribute to tissue damage and tissue remodeling in various anatomical sites via multiple direct or indirect mechanisms, and can be activated to express such functions by different potential stimuli in different settings.
4.3. Mast cells, and mast cell-derived IL-10, can limit the magnitude and duration of CHS responses to urushiol or DNFB in mice
CHS can develop in response to exposure to plants in the Toxicodendron genus (Anacardiaceae), including poison oak, poison ivy and poison sumac, and the cashew, mango and Japanese lacquer trees [51]. CHS to poison oak (Toxicodendron diversilobum) or poison ivy (T. radicans) is mediated by T lymphocytes specific for catechols (e.g., heptadecylcatechol or pentadecylcatechol) present in urushiol, the allergen-containing sap of such plants [52]. As reviewed in [34], antigen-specific CD8+ T cells are thought to be effectors of urushiol-induced CHS in humans and in mice; CHS to 2,4-dinitrofluorobenzene (DNFB) in mice is also mediated by DNFB-specific CD8+ T cells.
CHS to urushiol in humans can include the development, 8 – 48 h after exposure, of intensely pruritic vesicular skin lesions that can remain for up to 3 weeks [51]. In severe cases, lesions can develop frank necrosis, with ulceration of the epidermis and the development of striking epidermal hyperplasia [51, 53].
As noted above, while the importance of mast cells in the elicitation of CHS was once controversial, it is now clear that mast cells can enhance the magnitude of the tissue swelling and leukocyte infiltration associated with some but not all models of CHS in mice. However, the prior studies of the role of mast cells in CHS examined the reactions for only 24, 48 or, in one study, 72 h [43] after a single challenge with hapten. Because we wished to evaluate whether mast cells might influence the long-term consequences of CHS responses, including their resolution, we examined CHS reactions to urushiol or DNFB for up to 15 d after a single epicutaneous challenge with hapten.
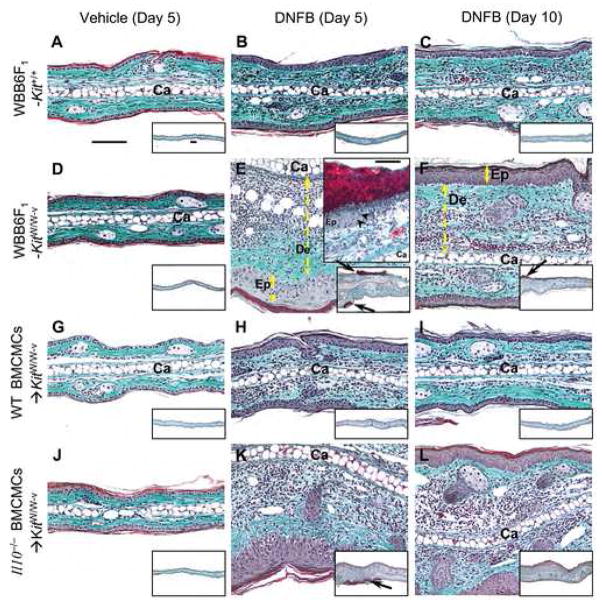
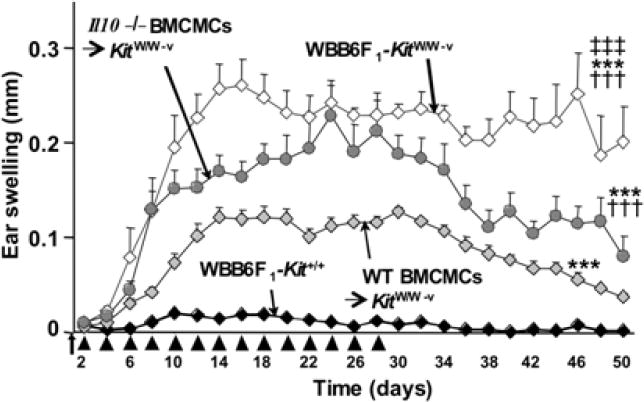
Beginning 3 d after challenge with urushiol or DNFB, mast cell-deficient WBB6F1-KitW/W-v (Fig. 4) or C57BL/6 (B6)-KitW-sh/W-sh mice exhibited significantly stronger CHS responses than did the corresponding wild-type mice [34]. In mast cell-deficient mice, tissue swelling increased progressively and peaked ~ 5 – 7 d after hapten challenge [34]. The tissue swelling and histological evidence of inflammation associated with CHS responses to urushiol or DNFB (Fig. 4) in WT BMCMCs→KitW/W-v mice resembled that in wild-type mice [34]. Comparison of responses in KitW/W-v mice engrafted with WT versus Il10−/− BMCMCs showed that the ability of mast cells to produce IL-10 contributed significantly to their ability to limit and hasten the resolution of the ear swelling associated with CHS responses to either urushiol or DNFB [34]. At 5 d after hapten challenge, reactions in mast cell-deficient KitW/W-v mice or Il10−/− BMCMCs→KitW/W-v mice, compared to those in wild-type mice or in WT BMCMCs→KitW/W-v mice, were associated with substantial dermal leukocyte infiltration, hyperplasia and focal ulceration of the epidermis, and expansion/edema of the dermis.
Fig. 4.
Mast cells and mast cell-derived IL-10 can limit the skin pathology associated with CHS to DNFB. Cross-sections of ears (stained with Masson’s Trichrome) from (A–C) WBB6F1-Kit+/+ (wild-type) mice, (D–F) mast cell-deficient KitW/W-v mice or KitW/W-v mice engrafted with wild-type (WT) BMCMCs (WT BMCMCs→KitW/W-v mice) (G–I) or Il10−/− BMCMCs (Il10−/− BMCMCs→KitW/W-v mice) (J–L) were obtained 5 d after challenge with vehicle (100% acetone) only (A, D, G and J) or 0.2% DNFB (B, E, H and K), or 10 d after challenge with 0.2% DNFB (C, F, I and L). Upper inset in E: arrowheads indicate border between intact epithelium (Ep) and, to the right, complete loss of epithelium at the base of an ulcer covered by an exudate. Ca*: cartilage; double-headed arrows show thickness of dermis (De) or epidermis (Ep); arrows in insets: sites of ulcers with adherent exudate (red). Scale bars in A and in insets in A & E = 100 μm. Photomicrographs are representative of the findings observed in the 6 different experiments we performed, with n = 4–6 mice/group per experiment; each experiment gave similar results. Reproduced, with the permission of the publisher, from reference [34].
Very similar findings were obtained when the experiments were repeated in mast cell-deficient KitW-sh/W-sh mice, the corresponding wild-type B6 mice, and B6 (WT) or Il10−/− BMCMCs→KitW-sh/W-sh mice, using either urushiol or DNFB [34].
Thus, work with two different types of genetically mast cell-deficient mice indicates that mast cells, and mast cell-derived IL-10, can markedly limit the magnitude of CHS responses to urushiol or DNFB, including the numbers of activated granulocytes and macrophages, and CD8+ and CD4+ T cells in the reactions sites, and can markedly reduce focal necrosis (Table 2) and ulceration of the epidermis at such sites [34]. Our in vitro and in vivo data indicate that, in the models of CHS tested, the mast cells’ ability to limit the responses reflects, at least in part, the cells’ activation via IgG1 antibodies and FcRIII [34]. Taken together, our findings suggest that, in mice which have been sensitized epicutaneously to certain haptens, the development of an antigen-specific IgG1 response can result in enhanced FcRγ-dependent mast cell IL-10 production, and that this represents one mast cell-dependent mechanism for limiting the extent and duration of such CHS reactions. However, our finding that several aspects of the pathology associated with DNFB-induced CHS in Fcer1g−/− BMCMC-engrafted mast cell-deficient mice (in which mast cell signaling via FcγRIII/FcRγ could not occur) were intermediate in magnitude between those of WT BMCMC-engrafted mast cell-deficient mice and mast cell-deficient mice indicates that mast cells may be activated to express anti-inflammatory and/or immunosuppressive functions in this setting by at least one additional mechanism, that is not mediated via FcRγ.
Table 2.
Mast cell-derived IL-10 limits epidermal necrosis associated with CHS to DNFB.
| Mice with full thickness epidermal necrosis |
||
|---|---|---|
| Time after DNFB challenge | 5 days | 10 days |
| WBB6F1-Kit+/+ | 0/19 | 0/5 |
| WBB6F1-KitW/W-v | 8/10* | 2/3* |
| WT BMCMCs→KitW/W-v | 0/16 | 0/3 |
| Il10−/− BMCMCs→KitW/W-v | 11/15* | 2/3* |
Values indicate numbers of mice whose ears exhibited full thickness epidermal necrosis, and, in some cases, ulcers as assessed by histology at 5 or 10 days after challenge with DNFB (mice with necrosis/total mice).
P < 0.05, wild type (Kit+/+) mice or WT (wild type) BMCMCs→KitW/W-v mice versus the corresponding mast cell-deficient KitW/W-v mice or Il10−/− BMCMCs→KitW/W-v mice. Data are from three experiments with similar results on day 5 (3–6 mice per group per experiment) or one experiment on day 10 (3–5 mice per group).
Reproduced, with the permission of the publisher, from reference [34].
4.4. Mast cells can limit responses to chronic UVB irradiation
To assess whether mast cells and mast cell-derived IL-10 might have anti-inflammatory functions in a setting other than CHS, we investigated an example of an innate tissue response to a ubiquitous environmental stimulus: UVB irradiation. As reviewed in [34], excessive exposure to solar UV radiation (particularly UVB and UVA radiation with wavelengths between 280–320 nm and 320–400 nm, respectively) causes a variety of acute and chronic photocutaneous reactions with several deleterious effects, including sunburn and photoaging (accelerated aging of the skin caused by environmental UV exposure.
To test whether mast cells contribute to the pathology observed in chronically UVB-irradiated skin, we exposed the ears of wild-type mice, mast cell-deficient KitW/W-v mice and WT or Il10−/− BMCMCs→KitW/W-v mice to 1 minimal erythema dose (MED) of UVB every 2 d for a total of 15 exposures (total cumulative dose = 30 kJ/m2). KitW/W-v mice and Il10−/− BMCMCs→KitW/W-v mice developed more striking ear erythema and tissue damage, and significantly greater ear thickness, after such UVB irradiation than did the corresponding wild-type mice or WT BMCMCs→KitW/W-v mice (Fig. 5), even though both groups of BMCMC-engrafted KitW/W-v mice had similar numbers of mast cells in the ear pinnas [34]. As expected, the smallest responses to UVB irradiation occurred in wild-type mice, the only group of mice with extensive skin pigmentation (they are black, unlike any of the other groups, each of which virtually lacks skin melanocytes because of their c-kit mutation [39].
Fig. 5.
Mast cells are required for optimal resolution of tissue swelling after chronic low-dose UVB irradiation. Ear swelling examined for up to 22 d after the last of 15 exposures of 2 kJ/m2 UVB irradiation in WBB6F1-Kit+/+ (wild-type [WT]) mice, mast cell-deficient KitW/W-v mice, or KitW/W-v mice engrafted with WT BMCMCs (WT BMCMCs→KitW/W-v mice) or Il10−/− BMCMCs (Il10−/−BMCMCs→KitW/W-v mice); n = 4–6 mice/group. The first exposure (arrow) was on day 0, immediately after we measured baseline ear thickness. Subsequent exposures are shown as arrowheads. ***P < 0.0001 versus corresponding UVB-irradiated wild-type mice; †††P < 0.0001 versus corresponding UVB-irradiated WT BMCMCs→KitW/W-v mice; ‡‡‡P < 0.0001 versus corresponding UVB-irradiated Il10−/− BMCMCs→KitW/W-v mice. Reproduced, with the permission of the publisher, from reference [34].
In contrast to our findings in the CHS models, we detected no significant requirement for FcRγ–dependent mast cell activation in the mast cell’s ability to limit the magnitude or duration of cutaneous responses to chronic irradiation with UVB [34]. Moreover, even though the ear swelling responses in Il10−/−BMCMCs→KitW/W-v mice were very similar to those of KitW/W-v mice during the 30 d period of UVB treatment, the reactions in the Il10−/− BMCMCs→KitW/W-v mice resolved more rapidly than those in KitW/W-v mice after UVB treatment was stopped (Fig. 5). This finding indicates that mast cells can contribute to the resolution of the UVB-induced responses at least in part by mechanisms that are independent of the ability of mast cells to produce IL-10.
4.5. Mast cells can limit the pathology associated with snake or honeybee venoms
We found that mast cells can limit the toxicity of the endogenous peptide ET-1 in vivo, and that this represents one of the mechanisms by which mast cells can contribute to survival in the cecal ligation and puncture model of sepsis [25]. The amino acid sequence of ET-1 exhibits striking similarity to that of sarafotoxin 6b [54], the most toxic component of the venom of the Israeli mole viper (Atractaspis engaddensis). This raised the possibility that mast cells might be able to enhance resistance in mice to envenomation with Atractaspis engaddensis venom.
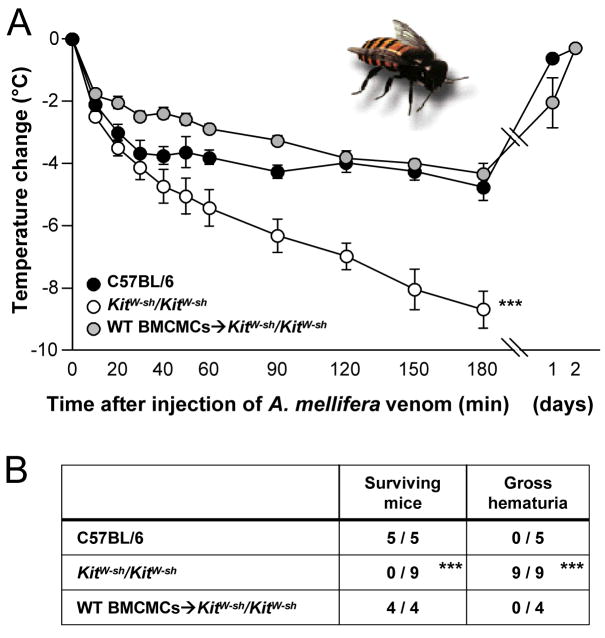
Indeed, we found that mast cell deficient mice were ~ 10 times more susceptible than wild-type mice to the deadly effects of A. engaddensis venom, and both pharmacological and shRNA knock down experiments implicated mouse mast cell carboxypeptidase A (mMC-CPA) as an important contributor the mast cell’s ability to “detoxify” either sarafotoxin 6b or the whole venom [26]. Mast cells also enhanced resistance in mice to the venom of two species of pit vipers (Crotalinae) and the honeybee (Apis mellifera) (Fig. 6); in these settings, the ability of mast cells to enhance resistance appeared to reflect, at least in part, the ability of mast cells to release proteases [26].
Fig. 6.
Mast cells can limit the toxicity of honeybee venom. (A) Changes in rectal temperature and (B) 24-hour survival and occurrence of gross hematuria after subcutaneous injection of A. mellifera venom (A.m.v.) at five different sites (three injections distributed over the length of the back skin and two into the belly skin, each containing 100 μg A.m.v. in 50 μl PBS) into WT mice, mast cell–deficient KitW-sh/KitW-sh mice, or KitW-sh/KitW-sh mice that had been engrafted intra-dermally, 6 weeks earlier, with 1.5 × 106 BMCMCs into each of the five injection areas (WT BMCMCs→KitW-sh/KitW-sh). The amount of venom per injection (100 μg) roughly reflects the amount that can be delivered by one bee sting. All of the WT or WT BMCMCs→KitW-sh/KitW-sh mice appeared healthy, and their body temperatures returned to baseline within 2 days. ***P < 0.005 versus either C57BL/6 or WT BMCMCs→KitW-sh/KitW-sh mice. Reproduced, with the permission of the publisher, from reference [26].
Animal venoms can contain toxins which act by a variety of mechanisms, and most venoms represent complex mixtures of toxins (reviexed in [26]). Accordingly, we think it very unlikely that mast cells can enhance resistance to all venoms. On the other hand, we think that it will be of great interest to investigate the extent to which mast cells might be able to modulate host responses to the diverse toxins found in nature, including those from microbes and plants, as well as animals. Indeed, enhancing innate host defense against environmental toxins may represent one of the most ancient functions of mast cells.
6. Conclusions
Mast cells have been proposed to have important roles in host defense against pathogens, as well as in many diseases characterized by inflammation and tissue remodeling, including those associated with IgE antibodies (such as asthma, atopic dermatitis and other allergic disorders) and those in which IgE antibodies are not thought to have a significant role. Many of these conditions are associated with changes in the numbers or the phenotype of mast cell populations at the affected organ or anatomical site, and, in many of these settings, there is clear morphological evidence of various forms of “mast cell activation”. Moreover, many mast cell-derived products have effects that can significantly influence the survival, proliferation, phenotype or function of many other cell types that participate in inflammation, immunity and tissue remodeling.
We have proposed that genetic approaches that permit the roles of mast cells to be investigated in wild type mice, c-kit mutant mast cell-deficient mice, and mast cell-engrafted c-kit mutant mice (i.e., mast cell knock-in mice) can provide useful direct evidence for or against significant roles of mast cells, and some of their specific products, in experimental models of adaptive host responses or diseases.
In humans, it is much more difficult to be sure of the roles of mast cells in pathological or adaptive biological responses. Not only do mouse and human mast cells differ in some aspects of phenotype and function, but, so far, no “experiment of nature” that has resulted in a selective defect in human mast cells has been reported.
Therefore, in attempting to decide on the potential importance of mast cells in biological responses in humans, one must use a combination of several lines of indirect evidence, including: inference from mouse and other animal models of disease; observational studies in humans (e.g., employing biopsies of affected sites, measurements of mast cell-associated mediators in sites of disease or in the blood or urine); and the clinical results of interventions that “target” particular mast cell-associated mediators (many of which can be produced by other cell types as well) or that attempt to influence mast cell activation.
Eventually, it may be possible to evaluate the results of clinical trials of agents that have been designed to interfere specifically with mast cell activation, or that perhaps can ablate this cell selectively. If these agents actually are specific in suppressing mast cell function or eliminating mast cells in humans, then it may be possible to know in which disorders this cell is a friend or a foe, or, in more complex situations, at what stages of the particular biological response mast cells work for us or against us.
Acknowledgments
We thank each of the many colleagues who contributed to the studies reviewed in this article, and apologize to the many authors whose work could not be specifically cited because of restrictions on the numbers of references for reviews of this type. This work is supported by United States Public Health Service grants (to S.J.G.) AI23990, AI070813, CA72074 and HL67674, project 1.
Biography
 Stephen J. Galli, M.D.
Stephen J. Galli, M.D.
Stephen Galli received his BA and MD from Harvard, in 1968 and 1973, respectively, and completed a residency in Anatomic Pathology at Massachusetts General Hospital in 1977. He served on the faculty at Harvard until 1999, when he moved to Stanford as chair of the Department of Pathology and the Mary Hewitt Loveless, MD, Professor. Dr. Galli’s research focuses on the development and function of mast cells and basophils, and on developing new animal models to study the diverse roles of mast cells in health and disease. Dr. Galli received a MERIT Award from the National Institutes of Health and the 1997 Scientific Achievement Award from the International Association of Allergy & Clinical Immunology. He was President of the American Society for Investigative Pathology (2005–2006) and has been elected to the Pluto Club (Association of University Pathologists), the Collegium Internationale Allergologicum (in which he is the Vice President), the American Society for Clinical Investigation, the Association of American Physicians, and the Accademia Nazionale dei Lincei in Rome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Recklinghausen F. Ueber Eiter- und Bindegewebskörperchen. Virchows Arch Path Anat. 1863;28:157–97. [Google Scholar]
- 2.Crivellato E, Beltrami C, Mallardi F, Ribatti D. Paul Ehrlich’s doctoral thesis: a milestone in the study of mast cells. Br J Haematol. 2003;123:19–21. doi: 10.1046/j.1365-2141.2003.04573.x. [DOI] [PubMed] [Google Scholar]
- 3.Marone G, Triggiani M, Genovese A, Paulis AD. Role of human mast cells and basophils in bronchial asthma. Adv Immunol. 2005;88:97–160. doi: 10.1016/S0065-2776(05)88004-6. [DOI] [PubMed] [Google Scholar]
- 4.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak AM. Ultrastructural analysis is necessary and sufficient for identification of basophils and mast cells. Chem Immunol Allergy. 2005;85:10–67. doi: 10.1159/000086505. [DOI] [PubMed] [Google Scholar]
- 7.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–99. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 10.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001;194:F1–5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003;111:24–32. doi: 10.1067/mai.2003.60. [DOI] [PubMed] [Google Scholar]
- 13.Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18:751–60. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 15.Bienenstock J, Befus AD, Denburg J, Goodacre R, Pearce F, Shanahan F. Mast cell heterogeneity. Monogr Allergy. 1983;18:124–8. [PubMed] [Google Scholar]
- 16.Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- 17.Enerbäck L. The differentiation and maturation of inflammatory cells involved in the allergic response: mast cells and basophils. Allergy. 1997;52:4–10. doi: 10.1111/j.1398-9995.1997.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 18.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–25. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 20.Daëron M, Prouvost-Danon A, Voisin GA. Mast cell membrane antigens and Fc receptors in anaphylaxis. II. Functionally distinct receptors for IgG and for IgE on mouse mast cells. Cell Immunol. 1980;49:178–89. doi: 10.1016/0008-8749(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 21.Tkaczyk C, Okayama Y, Metcalfe DD, Gilfillan AM. Fcgamma receptors on mast cells: activatory and inhibitory regulation of mediator release. Int Arch Allergy Immunol. 2004;133:305–15. doi: 10.1159/000077213. [DOI] [PubMed] [Google Scholar]
- 22.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 23.Galli SJ, Maurer M, Lantz CS. Mast cells as sentinels of innate immunity. Curr Opin Immunol. 1999;11:53–9. doi: 10.1016/s0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 24.Malaviya R, Abraham SN. Mast cell modulation of immune responses to bacteria. Immunol Rev. 2001;179:16–24. doi: 10.1034/j.1600-065x.2001.790102.x. [DOI] [PubMed] [Google Scholar]
- 25.Maurer M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–6. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 26.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 27.Christy AL, Brown MA. The multitasking mast cell: positive and negative roles in the progression of autoimmunity. J Immunol. 2007;179:2673–9. doi: 10.4049/jimmunol.179.5.2673. [DOI] [PubMed] [Google Scholar]
- 28.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 29.Miller HR, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 2002;105:375–90. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–6. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hallgren J, Pejler G. Biology of mast cell tryptase. An inflammatory mediator FEBS J. 2006;273:1871–95. doi: 10.1111/j.1742-4658.2006.05211.x. [DOI] [PubMed] [Google Scholar]
- 32.Gordon JR, Galli SJ. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the FcεRI. Role for mast cell-derived transforming growth factor β and tumor necrosis factor α. J Exp Med. 1994;180:2027–37. doi: 10.1084/jem.180.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall JC, Li XH, Galli SJ, Gordon JR. Promotion of mouse fibroblast proliferation by IgE-dependent activation of mouse mast cells: role for mast cell tumor necrosis factor-α and transforming growth factor-β 1. J Allergy Clin Immunol. 1997;99:113–23. doi: 10.1016/s0091-6749(97)70308-7. [DOI] [PubMed] [Google Scholar]
- 34.Grimbaldeston MA, Nakae S, Kalesnikoff K, Tsai M, Galli SJ. Mast cell–derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet. B Nat Immunol. doi: 10.1038/ni503. Published online: 2 September 2007. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–52. [PubMed] [Google Scholar]
- 36.Nakano T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–43. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai M, Tam SY, Wedemeyer J, Galli SJ. Mast cells derived from embryonic stem cells: a model system for studying the effects of genetic manipulations on mast cell development, phenotype, and function in vitro and in vivo. Int J Hematol. 2002;75:345–9. doi: 10.1007/BF02982122. [DOI] [PubMed] [Google Scholar]
- 38.Lyon MF, Glenister PH. A new allele sash (Wsh) at the W-locus and a spontaneous recessive lethal in mice. Genet Res. 1982;39:315–22. doi: 10.1017/s001667230002098x. [DOI] [PubMed] [Google Scholar]
- 39.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin Exp Allergy. 2005;35:82–8. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wershil BK, Furuta GT, Wang ZS, Galli SJ. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996;110:1482–90. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- 42.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–92. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 43.Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984;226:710–3. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- 44.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J Immunol. 2004;173:5275–82. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 45.Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–12. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 46.Kinkelin I, Motzing S, Koltenzenburg M, Brocker EB. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–7. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- 47.Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90:613–22. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 48.Kakurai M, Monteforte R, Suto H, Tsai M, Nakae S, Galli SJ. Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am J Pathol. 2006;169:1713–21. doi: 10.2353/ajpath.2006.060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116:1633–41. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 51.Walker SL, Lear JT, Beck MH. Toxicodendron dermatitis in the UK. Int J Dermatol. 2006;45:810–3. doi: 10.1111/j.1365-4632.2006.02825.x. [DOI] [PubMed] [Google Scholar]
- 52.Kalish RS, Johnson KL. Enrichment and function of urushiol (poison ivy)-specific T lymphocytes in lesions of allergic contact dermatitis to urushiol. J Immunol. 1990;145:3706–13. [PubMed] [Google Scholar]
- 53.Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–4. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- 54.Kloog Y, Ambar I, Sokolovsky M, Kochva E, Wollberg Z, Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988;242:268–70. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]