Abstract
Autophagy is a well-established mechanism to degrade intracellular components and provide a nutrient source to promote survival of cells in metabolic distress. Such stress can be caused by a lack of available nutrients or by insufficient rates of nutrient uptake. Indeed, growth factor deprivation leads to internalization and degradation of nutrient transporters, leaving cells with limited means to access extracellular nutrients even when plentiful. This loss of growth factor signaling and extracellular nutrients ultimately leads to apoptosis, but also activates autophagy, which may degrade intracellular components and provide fuel for mitochondrial bioenergetics. The precise metabolic role of autophagy and how it intersects with the apoptotic pathways in growth factor withdrawal, however, has been uncertain. Our recent findings in growth factor-deprived hematopoietic cells show that autophagy can simultaneously contribute to cell metabolism and initiate a pathway to sensitize cells to apoptotic death. This pathway may promote tissue homeostasis by ensuring that only cells with high resistance to apoptosis may utilize autophagy as a survival mechanism when growth factors are limiting and nutrient uptake decreases.
Keywords: autophagy, apoptosis, metabolism, Bcl-2, Bim, chop
It is now clear that metabolism, apoptosis and autophagy are inexorably linked. Expression of anti-apoptotic, or loss of proapoptotic, Bcl-2 family proteins can prevent apoptosis of growth factor-deprived cells but does not prevent decreased nutrient uptake. When Bak and Bax are absent, apoptosis cannot occur and cells may persist long-term, eventually using autophagy as a primary metabolic source. Bcl-2 or Bcl-xL expression, in contrast, inhibits but does not preclude apoptosis and inhibits autophagy in acute settings of nutrient deprivation. If Bcl-2 or Bcl-xL prevents autophagy induction when nutrients are limiting, however, we posed the question: Where do growth factor-withdrawn cells obtain nutrients necessary for Bcl-2 or Bcl-xL to prevent death?
To address this issue, we used an interleukin-3 (IL3)-dependent cell model and investigated autophagy after growth factor withdrawal. When removed from IL3, cells rapidly decreased glucose uptake, upregulated pro-apoptotic BH3-only proteins such as Bim and Puma, and died within 24 hours. Bcl-2 or Bcl-xL can delay this apoptotic death in a dose-dependent manner by interfering with the pro-apoptotic actions of Bim and Puma. Unlike in acute nutrient deprivation where Bcl-2 can prevent autophagy, we observed rapid induction of autophagy after growth factor withdrawal regardless of Bcl-2 or Bcl-xL expression (Fig. 1). This autophagy produced long-chain fatty acids and amino acids for mitochondrial oxidation, suggesting that autophagic breakdown of membrane-enclosed bodies did indeed provide a fuel source to replace the reduced glucose uptake.
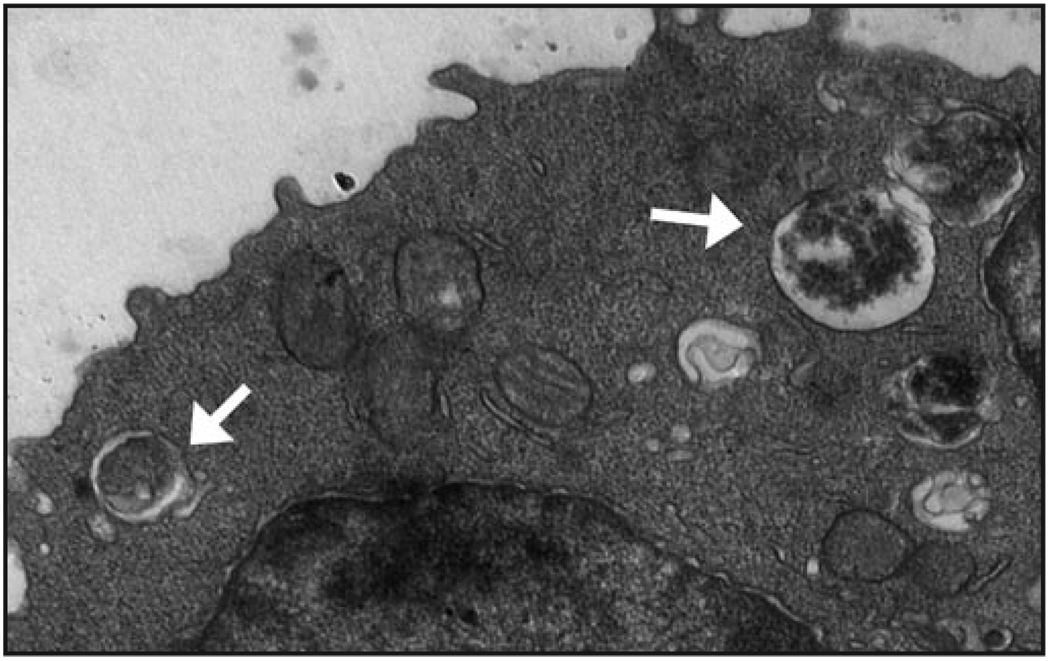
Figure 1.
Autophagy is induced despite Bcl-2 expression and contributes to both metabolism and apoptosis upon growth factor withdrawal. Bcl-2-expressing FL5.12 cells were deprived IL3 and imaged by electron microscopy. Autophagic vesicles were observed within hours of IL3 withdrawal (indicated by white arrows) showing that unlike in acute nutrient withdrawal, Bcl-2 does not effectively inhibit autophagy when cells are growth factor-deprived. Unexpectedly, autophagy both contributed to cell metabolism as a source of intracellular nutrients and sensitized cells to apoptosis, possibly to ensure only cells with high resistance to apoptosis can survive long-term using nutrients derived from autophagy.
These metabolic data suggest that autophagy may play a positive role in survival of growth factor-deprived cells. Indeed, using RNAi to suppress autophagy led to enhanced death of growth factor-deprived control cells lacking exogenous Bcl-2 expression. Surprisingly, however, RNAi inhibition of autophagy in cells with moderate expression of Bcl-2 or Bcl-xL increased cell survival. This suggested that while autophagy provided nutrients that could benefit metabolism in the absence of growth factor, it could also eventually sensitize to cell death. Consistent with these results, we found that inhibition of autophagy by RNAi against four independent autophagy-essential genes increased the survival of two other IL3-dependent hematopoietic cell lines, and that conditional repression of Atg5 expression increased the survival of mouse embryonic fibroblasts.
These results were contrary to the model that autophagy is essential as a source of intracellular nutrients when extracellular nutrient uptake decreases. Instead, we found that autophagy does not appear to be required to maintain cell metabolism for viability in the early days after growth factor withdrawal. Some amount of autophagy likely remained, however, to provide a minimal source of nutrients due to incomplete RNAi-mediated gene knockdown, and this residual degree of autophagy may have been sufficient to sustain cell viability. Growth factor-withdrawn cells may not require high levels of autophagy since intracellular energy demands decrease. Alternatively, extracellular nutrient uptake does not immediately cease and this may be sufficient to support metabolism in the initial days following growth factor withdrawal. Ultimately, however, nutrient uptake will become negligible and autophagy is likely to provide a critical nutrient source at this point. The metabolic transition after growth factor withdrawal is still not clear, and clarifying this transition will be important to understand the role of autophagy in cell survival.
That autophagy sensitized cells to apoptosis rather than autophagic or necrotic death was also unexpected. This possibility had not been fully explored in previous work because Bak−/−Bax−/− cells or cells with very high levels of Bcl-2 expression used in many studies are apoptosis-incompetent or highly resistant. By using cells with moderate expression of Bcl-2 and Bcl-xL, we identified a pathway in FL5.12 cells in which autophagy appeared to cause a cell stress that augmented expression of the transcription factor CHOP to promote induction of Bim. As Bim levels increased, the pro-apoptotic Bcl-2 family proteins eventually overcame Bcl-2 and caused apoptotic cell death. While this pathway was implicated in apoptosis of FL5.12 cells, it is important to note that despite identical effects of autophagy to promote apoptosis, we were unable to confirm a similar stress signaling pathway in other cell lines. Thus, the central cell stress caused by apoptosis appears shared, but the specific regulation of CHOP and Bim may be cell-type specific.
Anti-apoptotic Bcl-2 family levels are modulated in many systems in the body, notably during development, differentiation of immune cells, and the immune response. In these instances, anti-apoptotic Bcl-2 family protein expression may be adjusted in response to growth factor signaling to maintain tissue homeostasis. If cells are cut off from necessary growth factors or nutrients, autophagy will initially provide benefit, but the mechanism we described will eventually sensitize cells to autophagy-induced death. This death may provide a ‘checkpoint’ to prevent cells with low or moderate Bcl-2 or Bcl-xL expression from long-term survival in the absence of growth factor, only allowing cells with the highest levels of anti-apoptotic protein to ultimately benefit from autophagy-derived nutrients. Autophagy itself, therefore, may promote tissue homeostasis by partially ‘selecting’ which cells may use it as a metabolic and survival strategy.
Acknowledgements
We would like to thank members of the Rathmell lab for comments. This work was supported by R01 CA123350 (to J.C.R.).