Abstract
Vitamin B12 (cobalamin, Cbl) is indispensable for proper brain development and functioning, suggesting that it has neurotrophic effects beside its well-known importance in metabolism. The molecular basis of these effects remains hypothetical, one of the reasons being that no efficient cell model has been made available for investigating the consequences of B12 cellular deficiency in neuronal cells. Here, we designed an approach by stable transfection of NIE115 neuroblastoma cells to impose the anchorage of a chimeric B12-binding protein, transcobalamin-oleosin (TO) to the intracellular membrane. This model produced an intracellular sequestration of B12 evidenced by decreased methyl-Cbl and S-adenosylmethionine and increased homocysteine and methylmalonic acid concentrations. B12 deficiency affected the proliferation of NIE115 cells through an overall increase in catalytic protein phosphatase 2A (PP2A), despite its demethylation. It promoted cellular differentiation by improving initial outgrowth of neurites and, at the molecular level, by augmenting the levels of proNGF and p75NTR. The up-regulation of PP2A and pro-nerve growth factor (NGF) triggered changes in ERK1/2 and Akt, two signaling pathways that influence the balance between proliferation and neurite outgrowth. Compared with control cells, a 2-fold increase of p75NTR-regulated intramembraneous proteolysis (RIP) was observed in proliferating TO cells (P < 0.0001) that was associated with an increased expression of two tumor necrosis factor (TNF)-α converting enzyme (TACE) secretase enzymes, Adam 10 and Adam 17. In conclusion, our data show that B12 cellular deficiency produces a slower proliferation and a speedier differentiation of neuroblastoma cells through interacting signaling pathways that are related with increased expression of PP2A, proNGF, and TACE.
Keywords: homocysteine, neurotrophin
Vitamin B12 (B12, also named cobalamin, Cbl) deficiency has long been associated with pernicious anaemia (1) and neurological disorders that range from minor behavior changes to severe neurodegenerative disorders (2). Molecular mechanisms are still lacking to explain how the deficiency can bring about all of the symptoms observed. Indeed, only two enzymes are B12-dependent in mammalian cells: the mitochondrial enzyme l-methylmalonyl-CoA mutase (EC 5.4.99.2) and the cytoplasmic homocysteine methyltransferase, also referred as methionine synthase (EC 2.1.1.13). Inferences are thus based on the two direct consequences of B12 deficiency: the accumulation of methylmalonic acid (MMA) and homocysteine (Hcy). Until now, the consequences of B12 deficiency in the brain have been difficult to evidence because of the experimental limitations of the classical cell models. Indeed, the minute amount of vitamin B12 needed by cells can be provided in vitro by B12 from the FCS, which can bind the “autocrine” B12 carrier protein, transcobalamin (TC) (3, 4).
To delineate the role of B12 in neurological disorders, we designed a neuronal cell model made deficient in B12 through the stable expression of a chimeric protein associating TC with oleosin, a plant protein that is targeted to oil bodies via the endoplasmic reticulum (ER) (5). The expression of oleosin in reticulum remains when oleosin is heterologously expressed in mammalian cells (6). TC is the protein that displays the highest affinity for B12; it is constitutively secreted into plasma to bind the circulating vitamin B12, internalized into cells via receptor-mediated endocytosis, and subsequently addressed in cytosol and mitochondria (3, 4, 7). We linked TC to the hydrophobic oleosin to sequester B12 in the cytosolic face of intracellular membranes. We designed this approach to create cellular models deficient in vitamin B12 (8). The consequences were here studied to reveal the molecular mechanisms affecting growth and differentiation of neuronal cells (Fig. 1). Our results indicated that the B12-impaired metabolism induced by the overexpression of TC-oleosin influences interacting signaling pathways related to PP2A (protein phosphatase 2A), pro-nerve growth factor (NGF), and TNF-α converting enzyme (TACE) and consequently produces a slower growth and a speedier differentiation of NIE115 cells.
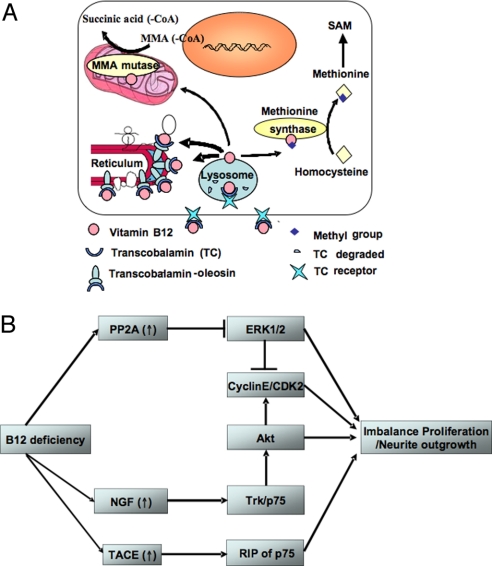
Fig. 1.
Experimental model of vitamin B12 cellular sequestration and proposed effects on the growth and the differentiation of neuronal cultured cells (adapted from ref. 8). (A) The schematic of the TO experimental model is shown. (B) Vitamin B12 deficiency up-regulates PP2Ac, proNGF, and TACE. These reduce proliferation and promote differentiation of neuroblastoma cells by modulating the interacting downstream pathways including ERK1/2, Akt/PKB, and p75NTR.
Results
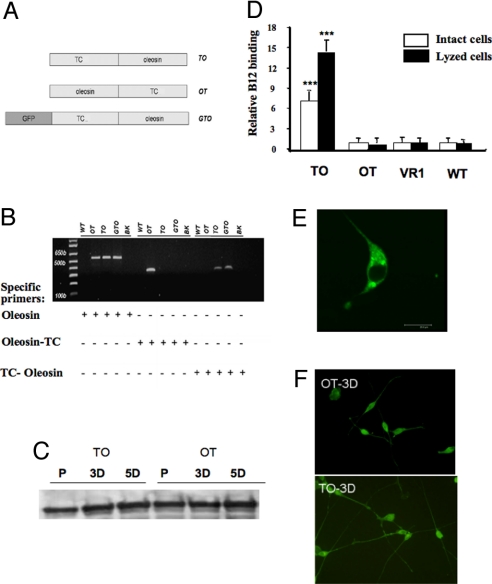
We created several stable NIE115 cell lines that express TC-oleosin (TO), oleosin-TC (OT), and GFP-TC-oleosin (GTO), respectively (Fig. 2). The incorporation of the transgenes was verified by PCR with specific primers (Fig. 2B). As a control for transfection, another stable NIE115 line that expresses an unrelated membrane protein, VR1 (transient receptor potential vanilloid subfamily member 1; TRPV1) was also created. The content of tyrosine hydroxylase remained unmodified in both proliferative and differentiation status of the stably transfected cells (Fig. 2C). Fig. 2D shows the results of the radioactive B12 binding in both intact cells and lysed membrane fraction of the cells after 20 min of incubation. The TO-expressing cells possessed the highest binding capacity to B12, while OT-expressing cells had a binding capacity similar to that of TRPV1-transfected and nontransfected wild-type (WT) cells. The GFP, TO, and OT constructs enabled us to confirm the intracellular membranous localization of the fusion protein in the reticulum (Fig. 2E and Fig. S1).
Fig. 2.
Expression of transcobalamin-fused constructs in NIE115 cells. (A) The schematics of the plasmids TO, OT, and GTO are shown. (B) The incorporation of the three plasmids in NIE115 cells in corresponding stable cell line was evaluated by PCR technique (see Materials and Methods for the specific primers). (C) The relatively constant contents of the tyrosine hydroxylase in TO and OT cells indicate that these transfected cells remain as valid neuronal model (typical result). (D) Radiolabeled B12 (30,000 cpm at 300 μCi/μg) was added to ≈106 cells and then incubated for 20 min at either 37 °C (intact cell) or 4 °C (lysed cell). Compared with OT, VR1, and WT, the expression of TO leads to an increased B12-binding capacity in either intact or lysed cells (P < 0.001). The VR1 expressing cells serve as a control to normalize the B12 binding to the TO, OT, and WT cells. (typical result out of five runs performed in three repeats. Mean and SE are indicated). (E) The confocal image of the NIE-115 cells transfected with GTO shows that the expressed fusion protein is targeted to reticulum (see supplemental figure for TO- and OT-tranfected cells). (F) Three days after initiating the differentiation of the NIE115 cells, more extensive neurite outgrowth was evident in TO cells. Here, cells were immuno-labeled with Adam17 antibody. The secondary antibody was coupled to Alexa Fluor 488 for visualization. P, proliferation; 3D, 3 days after differentiation; 5D, 5 days after differentiation; WT, wild-type NIE115 cells; BK, blank without DNA template; TC, transcobalamin.
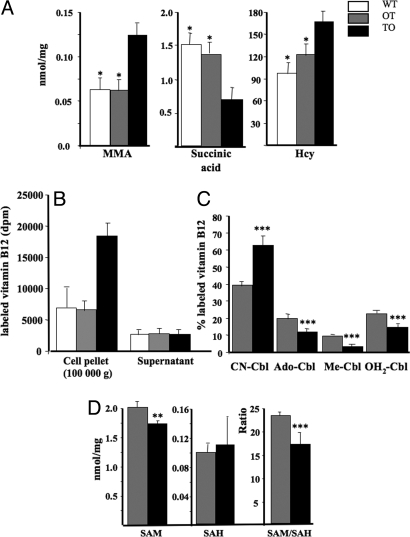
The concentration of Hcy and MMA in culture medium and cellular extract was higher in TO cells compared with OT and WT cells (Fig. 3A). This indicated that the B12 intracellular sequestration impaired the B12-dependent activity of methionine synthase and of MMA mutase, respectively. We chose OT cells as a control here because both TO and OT were transfected cells overexpressing oleosin-containing proteins and cultivated in the same conditions. As shown in Fig. 3, compared with OT cells, TO cells displayed a statistically significant reduction in conversing CN-Cbl into methyl-Cbl, ado-Cbl, and aquo-Cbl and in SAM/SAH ratio.
Fig. 3.
Changes in B12-related parameters in TO cells. (A) A higher concentration of Hcy in culture medium, higher content of MMA and lower content of succinic acid in cell extract was observed in TO, compared with OT and WT cells (P < 0.001). (B and C) TO cells had a statistically significant reduction in their SAM/SAH ratio (P < 0.001), with a weaker change in SAM (P = 0.010) and no significant modification in SAH cellular level (P = 0.116). (C) 57Colabeled Cbl (≈300 μCi/μg) at a concentration of 30,000 cpm/μL was incorporated into culture medium (30,000 cpm/mL culture medium) for 3 days. The various forms of vitamin B12 in whole cell extracts were analyzed by HPLC (described in ref. 3). The total amount of radioactivity taken by each cell lines was considered as 100%. Absolute amount of radioactivity in 100 000 × g pellet and supernatant of TO and OT extracts is showed in B. Significantly less Me-Cbl, Ado-Cbl and OH2-Cbl were formed in TO cells, as evidenced in C (P < 0.001). CN-Cbl, cyano-cobalamin; Me-Cbl, methyl-cobalamin; Ado-Cbl, adenosylcobalamin; OH2-Cbl, aquo-cobalamin; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; Hcy, homocysteine. (A–C) Typical result out of five runs performed in three repeats. Mean and SE are indicated.
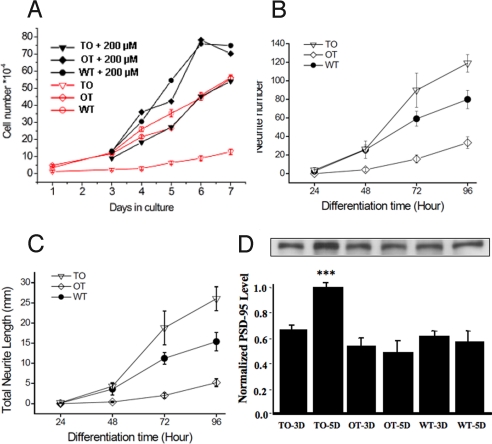
The rate of proliferation of TO cells was 5.5-fold reduced, compared to that of OT-transfected and WT cells (Fig. 4A). The reduction was less dramatic (1.5-fold) when performing these experiments in excess of vitamin B12 in culture medium (200 μM).
Fig. 4.
B12 deficiency leads to slower growth and earlier differentiation. (A) A significant reduced rate of proliferation was associated with the vitamin B12-deficient TO cells, compared with WT and OT cells. When adding 200 μM B12 in culture medium, the growth rate of TO cells became similar to that of WT and OT cells cultivated in normal B12 concentration (number of viable cells was counted by trypan blue exclusion method). (B and C) upon change to differentiation medium, within 48 h TO cells showed a more significant outgrowth of neurite than WT and OT cells; this was evident in total neurite number (B) and total neurite length (C). (D) TO cells had an increased level of PSD 95 at day 5 of differentiation. See Materials and Methods for the analysis of neurite using NeuroJ program. The neurite number and the neurite length were the sum of all cells examined in the optical field (constant density of 130–150 cells). (A) typical result out of three runs performed in three repeats. (B and C) Results from five independent determinations. Mean and SE are indicated.
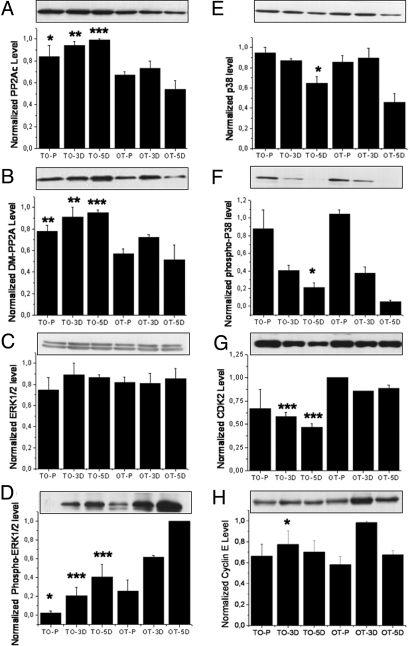
This reduced rate could be related to an increase in PP2A (9). Indeed, both demethylated PP2A and total catalytic PP2AC were increased in TO cells (Fig. 5 A and B). We examined the level of ERK1/2 and p38 because the PP2A-dependent MAPK pathway is known to regulate the cellular proliferation and differentiation processes (10). Phospho-ERK1/2 levels were significantly reduced in the B12-lacking TO cells, while the variation in p38 occurred only at the 5D stage by comparison with OT cells (Fig. 5 D–F). Total CDK2 level in TO cells appeared reduced in cells upon differentiation (Fig. 5G), probably reflecting the inactivation of ERK1/2 in a specific cyclin E-dependent way (Fig. 5H). Cyclin D was not affected.
Fig. 5.
Western blots for proteins relevant to neuronal cell growth. (A) The level of total protein PP2Ac (phosphatase 2A, catalytic subunit) increase in all three cultured states of the TO cells (P = 0.0281, 0.0012, and <0.0001 for P, 3D, and 5D cells, respectively). (B) The level of demethylated PP2Ac also increased in TO cells, with P = 0.0012, 0.0082, and 0.0006 for P, 3D, and 5D cells, respectively. (C and D) No difference of total ERK1/2 (C) could be observed between the TO and the OT cells; phospho-ERK1/2 level (D) was significantly reduced in TO cells (for total ERK1/2, P = 0.4226, 0.3992, and 0.8563 and for phospho-ERK1/2, P = 0.01181, P < 0.0001, and P < 0.0001 for P, 3D, and 5D cells, respectively). (E and F) for both total p38 (E) and phospho-p38 (F), an increase was visible at the 5D stage of the cells (for p38, P = 0.1087, 0.6742, and 0.02038 and for P-p38, P = 0.2513, 0.6881, and 0.0013, respectively for P, 3D, and 5D cells). (G) The total amount of cyclin-dependent protein kinase 2 (CDK2) appeared reduced in TO cells in differentiated cells (for P, 3D, and 5D cells: P = 0.2032, 0.0005, and 0.0004, respectively), reflecting the growth retardation associated with the TO cells. (H) A significant decrease was detected in cyclin E at 3D (for proliferating cells, P = 0.394; for 3D cells, P = 0.0283; for 5D cells, P = 0.709). (A–H) Typical result out of three runs performed in three repeats. Densitometric analysis was normalized to an arbitrary value of 1.0 that represented the maximal value recorded within each experiment series. Mean and SE are indicated.
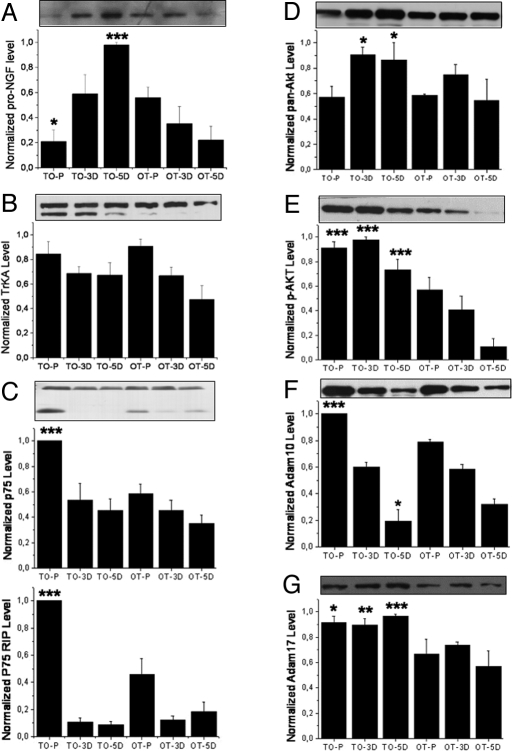
The B12-lacking TO cells started their outgrowth of neurites earlier than OT and WT cells and had an increased level of PSD 95 at day 5 of differentiation (Fig. 4 B–D). This effect was abolished when adding 200 μM B12 in the culture medium. This observation translated into a slightly longer mean neurite length when the outgrowth of neurites was quantified under constant cell density. We found that, once triggered to differentiate, TO cells had an increased level of pro-NGF, compared to OT (Fig. 6A). Tyrosine kinase A (TrkA) receptor, functionally depending on NGF, was unchanged (Fig. 6B), while the p75 neurotrophin receptor (p75NTR), being part of this receptor complex, was up-regulated in proliferating TO cells (Fig. 6C). Up-regulation and activation of the Akt cascade has been linked to the differentiation of human neuroblastoma cells (11, 12). In our model, total Akt and phospho-Akt, detected by an anti-phospho S473 Akt, were both found increased in TO cells (Fig. 6 D and E). Glycogen synthase kinase 3β (GSK-3β) is a key downstream effector of Akt that can be reactivated by PP2A (13). However, no significant difference could be observed between the two TO and OT cell lines for both phospho-GSK-3β and total GSK-3β (phospho-GSK3β: P = 0.477, 0.869, and 0.537 for P, 3D, and 5D cells, respectively, and total GSK-3β: P = 0.934, 0.473, and 0.133 for P, 3D, and 5D cells). This suggests that other pathways may counterbalance the influence of Akt (14).
Fig. 6.
Western blots for proteins relevant to neurite outgrowth. (A) The level of proNGF increased very rapidly in TO cells upon differentiation instead of decrease, as in the case of OTcells (6A: P = 0.03, 0.302, <0.001 for P, 3D, and 5D cells, respectively). (B) For TrkA neurotrophin receptor, only the 140-kDa band was quantified because it represents the mature form of the functional NGF receptor. No difference could be detected between TO and OT cells (P = 0.612, 0.841, and 0.222 for the P, 3D, and 5D cells, respectively). However, in TO cells the 110-kDa form of the TrkA (underglycosylated immature precursor) was up-regulated. (C) The level of neurotrophin receptor p75NTR and its RIP appeared to increase dramatically in proliferating TO cells (upper band: P < 0.001, 0.443, and 0.203; lower band RIP: P < 0.001, 0.734, and 0.245, respectively for P, 3D, and 5D cells). (D and E) The level of total Akt (D) and S473 phospho-Akt (E) were in general increased in TO cells, especially the S473 phosphorylated form of Akt (for pan-Akt: P = 0.789, 0.033, and 0.042 for P, 3D, and 5D cells respectively; for p-Akt: P < 0.001, <0.0001, and <0.0001 for P, 3D, and 5D cells, respectively). (F) The level of Adam 17 increase in TO cells in all states examined (P = 0.0111, 0.002, and <0.001, for P, 3D, and 5D cells, respectively). (G) The level of Adam 10 increased in proliferating but not differentiated TO cells (P < 0.001, 0.5931, and 0.0576 for P, 3D, and 5D cells, respectively). (A–C) Typical result out of three runs performed in three repeats. Densitometric analysis as in Fig. 5. Mean and SE are indicated.
Western blot analysis of p75 neurotrophin receptor (p75NTR) additionally revealed the presence of regulated intramembraneous proteolysis (RIP) in proliferating cells (15), with an ≈2-fold increase, compared with the control OT cells (Fig. 6C). Such RIP of the p75NTR involves the family of TACE. Consequently, two TACE enzymes most implicated in brain were examined: Adam 10 and Adam 17. Fig. 6 F and G show an increase of at least 20% in both Adam 10 and Adam 17 in proliferating TO cells. For 5D TO cells, a nearly 40% higher expression than control in Adam 17 was found.
Discussion
Our experimental cell model was designed to identify the molecular mechanisms of B12 impaired cellular metabolism related to proliferation and early differentiation. It appeared to be not adapted for evaluating the effects of B12 in fully differentiated NIE115 neuroblastoma cells because they became detached and died once reaching fully differentiation status. We compared TO with OT cells rather than with WT cells because (i) they only differed by the B12 binding capacity of the expressed chimera and subsequent B12-impaired metabolism, (ii) both cell lines were cultivated in the presence of G418 aminoglucoside, a condition that cannot be used for WT cells. The lack of B12 trapping effect in OT cells suggests that the integration of TC in the chimera modified the conformation of its N-terminal domain (7, 8).
Recently, we showed that the stable transfection with TO produces a dramatic decrease of methyl B12 and of methionine synthase activity in the cytosol fraction of Caco2 and NIE 115 cells (8). Here, the B12 impaired metabolism in the whole cell lysates of TO cells was confirmed by the decreased conversion of B12 into methyl-Cbl, ado-Cbl, and aquo-Cbl, the accumulation of both Hcy and MMA, and the reduction of succinic acid and SAM/SAH ratio in the whole cell lysate. OT and WT cells proliferated at a much higher speed than TO cells, while neurite outgrowth and PSD 95 expression were significantly higher in TO cells than in WT and OT cells. Only OT cells could be considered as a valid control of TO cells for the mechanistic study of B12 deficiency, because they were cultivated also with G418. Taken together, these lines of evidence suggest that reduced proliferation and earlier differentiation of the transfected TO NIE 115 cells are linked, at least in part, to B12-impaired metabolism.
The molecular effects of B12-impaired metabolism are summarized in Fig. 1B. Total catalytic PP2Ac actually increased in TO cells despite the relative demethylation of its C-terminal leucine residue, which was reported to have key functional consequences (16). This relative demethylation could result either from a deficient transmethylation due to the decreased SAM/SAH ratio, or from the activation of a methylesterase by homocysteine accumulation (17). Our data suggested that the reduction in growth of the TO cells was mediated by an inhibition of the MAPK/ERK1/2 pathway through PP2Ac up-regulation, as reported in vitro and by knock out experiments in Drosophila Schneider 2 cells (18, 19). The increased homocysteine produced in our model had similar effects on MAPKs than those obtained with exogenously added homocysteine, with inactivation of ERK1/2 and inhibition of Erk1/2-dependent expression of cyclin E (20).
The effects of TO transfection on neurite outgrowth and the increased level of PSD 95 are consistent with an influence of B12 impaired metabolism on neuroplasticity. PSD 95 is a major component of postsynaptic densities (PSDs) that promotes dendrite spine formations and multiple axon connections (21). It is expressed in the NIE-115 cell line only after differentiation (22). We examined the level of proNGF in TO and OT cells because neurotrophins trigger differentiation of NIE-115 cells. We found that once triggered to differentiate, TO cells augmented rapidly their proNGF level, in contrast to OT cells (Fig. 6A). ProNGF has been demonstrated to induce neurite outgrowth, albeit to a lower extent than NGF (23, 24). After nerve stimulation, ProNGF is released along with proteases that independently cleave proNGF to NGF (25). For neurite outgrowth, in addition to NGF, evidence points to the participation of the p75NTR neurotrophin and Akt pathways. Earlier works suggest that NGF, TNF-α and their receptors may all be involved in regulating the proliferation and differentiation of B12-lacking cells (26, 27). NGF can induce the synthesis of TNF-α through NF-κB in neurons, and TNF-α can induce the expression of NGF in glia. The receptors for these factors include the heteromeric and monomeric NGF receptors containing TrkA and p75NTR, the latter being up-regulated in TO cells.
Adam-17 and Adam-10 are TACE secretases that activate pro-TNF-α. The expression of TACE has been documented in neurons (28). The increases of Adam-10 and Adam-17 reported in TO cells have to be considered with earlier studies in both B12-deficient human and animals that had shown the presence of higher level of TNF-α in the cerebrospinal fluid (29–31). We did not find any change in TNF-α transcription level in TO. However, our data suggest that TNF-α may be augmented in the cerebrospinal fluid because of the increased TACE activity. TACE can be regulated by tissue inhibitors of metalloproteinases (TIMPs). TIMPs are regulated by methylation and have been associated with either brain trauma or neurodegenerative diseases (32–35). However, the role of TIMP in TACE expression remains an open question, as we have not been successful in detecting either TIMP1 or TIMP3 at the protein level.
The very effects of B12 deficiency, in particular the up-regulation of p75NTR, TACE, increased RIP of the p75NTR by TACE as well as the production of NGF are consistent with the earlier outgrowth of neurites upon differentiation of the TO cells. This is in agreement with the studies by Ahmed et al. (36) and Logan et al. (37), who showed that in dorsal root ganglion culture and optic nerve, TACE induced the RIPs of Nogo-66 receptor and of p75NTR that disinhibit the outgrowth of neurites and promote their branching. The RIP of Nogo-66 receptor was not present in our culture system, indicating that the RIP of p75NTR alone is sufficient for disinhibiting neurite outgrowth, at least in NIE115 cells.
In conclusion, our results showed that impaired cellular metabolism of B12 influences cell cycle progression and differentiation of neuroblastoma cells through interacting signaling pathways related with the increased expression of PP2A, proNGF, and TACE.
Materials and Methods
PCDNA3 Plasmids for Mammalian Cells.
The oleosin sequence was determined as described in ref. 38. The schematics of the recombinant plasmids are given in Fig. 2A. The preparation of plasmids, the transfection, the RT-PCR and primers for PCR verification of the transgene in the stably transfected NIE115 cells, and the characteriztion of the fusion proteins have been described in ref. 8.
Cell Culture.
The NIE115 cells are neuroblastoma cells chosen here as a neuronal model (39). The number of cell passages was limited to five after the establishment of the stable cell lines. B12 binding was found constant within the five passages used for the experiments. This assay was performed before all cell experiments. The cell number was evaluated using the classical trypan blue method using a hemocytometer. For proliferation studies, cells were cultured in a medium containing 90% DMEM with 4.5 g/L glucose (Gibco, supplemented with sodium pyruvate) and 10% FCS at 37 °C in an atmosphere of air (95%) and carbon dioxide (5%). For differentiation studies, 1 day before the initiation of differentiation, cells were trypsinized gently and replated in cultured dishes at a density of 4–8 × 104 per 60-mm Petri dish. The differentiation medium contained 2.5% FCS and 1.25% DMSO (Sigma) in DMEM with 4.5 g/L glucose. We have quantified the neurite amount and length using the NeuroJ plugin developed for the ImageJ program (Erik Meijering). Briefly, cells were grown and differentiated for up to 96 h in 65-mm culture dishes to a constant density of 130–150 cells per optical field (4× plane, Olympus BX51WI). The living cells were fixed and stained using the Neurite Outgrowth Assay Kit (Millipore) and then photographed by Olympus digital camera for quantitative analysis by NeuroJ. The neurite number and the neurite length were the sum of all cells examined in the optical filed. The data reported were obtained from five independent experiments. Penicillin and streptomycin were added to all media; G418 at 1 mg/mL was included in the culture medium for the cells stably transfected with all pCDNA3-based vectors. The VR1 construct used as a transfection control expresses a nonspecific cationic channel (40) and was a gift from Dr. Dejian Ren of the University of Pennsylvania.
Radiolabeled B12 Binding, SAM, SAH, Hcy, Succinic Acid, and MMA Determination.
The binding of radiolabeled B12 in membrane extracts from cultured cells was determined in NIE 115 cells as described for Caco 2 cells (8). The method for determination of SAM and SAH was adapted from Miller et al. (41) as described in ref. 42. Hcy, succinic acid and MMA were determined by a single UPLC-MS/MS procedure adapted from previously published articles, with an Acquity UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm, Waters Corporation) (8, 43, 44).
Protein Analysis by Western Blot.
Cells were lysed directly with a solution containing 0.05 M NaH2PO4 (pH 8), 0.15 M NaCl, 0.1 M imidazole, 0.5% Chaps, and Complete Protease Inhibitors (Roche). Lysates were then centrifuged twice at 5,000 × g for 10 min. The protein concentration of the supernatant was determined using Advanced Protein Assay Reagent (Cytoskeleton) and BSA as standard protein. In general, 20 μg total protein was loaded per lane for SDS/PAGE. Depending on the molecular mass of the protein, the stacking and the separating gel contained 4 and 8–12% of acrylamide, respectively. Proteins were electrotransferred onto PVDF membranes (Millipore) in 25 mM Tris buffer containing 192 mM glycine and 20% (vol/vol) methanol. The membranes were then blocked with 5% nonfat milk for 1 h at room temperature. The PVDF membrane was then probed overnight with various primary antibodies (all antibodies of the PI3K and ERK pathways, PP2Ac, and demethylated PP2A were obtained from Cell Signaling Technology; proNGF, p75NTR, TrkA, Adam10, Adam17, and tyrosine hydroxylase were obtained from Millipore; CDK2, TIMP1, and 3 were obtained from Santa Cruz; anti-postsynaptic density-95 kDa (PSD-95) from Cell Signalling. Appropriate secondary antibodies conjugated to HRP were used for detection with ECL or ECL PLUS reagent (Amersham). Densitometric analysis was normalized to an arbitrary value of 1.0 that represented the maximal value recorded within each experiment series.
Statistics.
All results were expressed as means ± standard error (SE). The significance between the effects of culture conditions was determined by analysis of variance (ANOVA). Results of paired Student's t tests are denoted by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Supplementary Material
Acknowledgments.
Institutional grants were received from Institut national de la santé et de la recherche médicale, Region Lorraine, and Agence Nationale de la Recherche Nutrivigene.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811794106/DCSupplemental.
References
- 1.Russell JSR, Batten FE, Collier J. Subacute combined degeneration of the spinal cord. Brain. 1900;23:39–110. [Google Scholar]
- 2.Gillette Guyonnet S, et al. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–152. [PubMed] [Google Scholar]
- 3.Pons L, Guy M, Lambert D, Hatier R, Guéant J. Transcytosis and coenzymatic conversion of [(57)Co]cobalamin bound to either endogenous transcobalamin or exogenous intrinsic factor in caco-2 cells. Cell Physiol Biochem. 2000;10:135–148. doi: 10.1159/000016344. [DOI] [PubMed] [Google Scholar]
- 4.McLean GR, et al. Antibodies to transcobalamin II block in vitro proliferation of leukemic cells. Blood. 1997;89:235–242. [PubMed] [Google Scholar]
- 5.Hsieh K, Huang AH. Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J. 2005;43:889–899. doi: 10.1111/j.1365-313X.2005.02502.x. [DOI] [PubMed] [Google Scholar]
- 6.Hope RG, Murphy DJ, McLauchlan J. The domains required to direct core proteins of hepatitis C virus and GB virus-B to lipid droplets share common features with plant oleosin proteins. J Biol Chem. 2002;277:4261–4270. doi: 10.1074/jbc.M108798200. [DOI] [PubMed] [Google Scholar]
- 7.Wuerges J, et al. Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci USA. 2006;103:4386–4391. doi: 10.1073/pnas.0509099103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pons L, et al. Anchoring secreted proteins in endoplasmic reticulum by plant oleosin: The example of vitamin B12 cellular sequestration by transcobalamin. PLoS One. 2009;4:e6325. doi: 10.1371/journal.pone.0006325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y, Mumby MC. Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2a regulates the activities of G(1) cyclin-dependent kinases. J Biol Chem. 1999;274:31917–31924. doi: 10.1074/jbc.274.45.31917. [DOI] [PubMed] [Google Scholar]
- 10.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 11.López-Carballo G, Moreno L, Masiá S, Pérez P, Barettino D. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–25304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 12.Evangelopoulos ME, Weis J, Krüttgen A. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene. 2005;24:3309–3318. doi: 10.1038/sj.onc.1208494. [DOI] [PubMed] [Google Scholar]
- 13.Lee YI, et al. Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3-beta, and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells. J Biol Chem. 2005;280:22044–22052. doi: 10.1074/jbc.M413987200. [DOI] [PubMed] [Google Scholar]
- 14.Hernández F, Avila J. The role of glycogen synthase kinase 3 in the early stages of Alzheimer's disease. FEBS Lett. 2008;582:3848–3854. doi: 10.1016/j.febslet.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Domeniconi M, et al. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Favre B, Zolnierowicz S, Turowski P, Hemmings BA. The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J Biol Chem. 1994;269:16311–16317. [PubMed] [Google Scholar]
- 17.Zhang CE, et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging. 2008;29:1654–1665. doi: 10.1016/j.neurobiolaging.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Zhou B, Wang ZX, Zhao Y, Brautigan DL, Zhang ZY. The specificity of extracellular signal-regulated kinase 2 dephosphorylation by protein phosphatases. J Biol Chem. 2002;277:31818–31825. doi: 10.1074/jbc.M203969200. [DOI] [PubMed] [Google Scholar]
- 19.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci USA. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabaneda LG, et al. Homocysteine inhibits proliferation of neuronal precursors in the mouse adult brain by impairing the basic fibroblast growth factor signaling cascade and reducing extracellular regulated kinase 1/2-dependent cyclin E expression. FASEB J. 2008;22:3823–3835. doi: 10.1096/fj.08-109306. [DOI] [PubMed] [Google Scholar]
- 21.Nikonenko I, et al. PSD-95 promotes synaptogenesis and multi-innervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183:1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh JE, et al. Cytoskeleton changes following differentiation of N1E-115 neuroblastoma cell line. Amino Acids. 2006;31:289–298. doi: 10.1007/s00726-005-0256-z. [DOI] [PubMed] [Google Scholar]
- 23.Fahnestock M, et al. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shawi R, et al. ProNGF, sortilin, and age-related neurodegeneration. Ann N Y Acad Sci. 2007;1119:208–215. doi: 10.1196/annals.1404.024. [DOI] [PubMed] [Google Scholar]
- 25.Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci USA. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takei Y, Laskey R. Tumor necrosis factor alpha regulates responses to nerve growth factor, promoting neural cell survival but suppressing differentiation of neuroblastoma cells. Mol Biol Cell. 2008;19:855–864. doi: 10.1091/mbc.E07-06-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog Neurobiol. 2009;88:203–220. doi: 10.1016/j.pneurobio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Skovronsky DM, Fath S, Lee VM, Milla ME. Neuronal localization of the TNFalpha converting enzyme (TACE) in brain tissue and its correlation to amyloid plaques. J Neurobiol. 2001;49:40–46. doi: 10.1002/neu.1064. [DOI] [PubMed] [Google Scholar]
- 29.Buccellato FR, et al. Myelinolytic lesions in spinal cord of cobalamin-deficient rats are TNF-α-mediated. FASEB J. 1999;13:297–304. doi: 10.1096/fasebj.13.2.297. [DOI] [PubMed] [Google Scholar]
- 30.Peracchi M, Bamonti Catena F, Pomati M, De Franceschi M, Scalabrino G. Human cobalamin deficiency: Alterations in serum tumour necrosis factor-alpha and epidermal growth factor. Eur J Haematol. 2001;67:123–127. doi: 10.1034/j.1600-0609.2001.t01-1-00507.x. [DOI] [PubMed] [Google Scholar]
- 31.Scalabrino G, et al. High tumor necrosis factor-alpha levels in cerebrospinal fluid of cobalamin-deficient patients. Ann Neurol. 2004;56:886–890. doi: 10.1002/ana.20325. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson's disease. Exp Neurol. 2002;178:13–20. doi: 10.1006/exnr.2002.8019. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzl S, Albers DS, Chirichigno JW, Augood SJ, Beal MF. Elevated levels of matrix metalloproteinases-9 and -1 and of tissue inhibitors of MMPs, TIMP-1 and TIMP-2 in postmortem brain tissue of progressive supranuclear palsy. J Neurol Sci. 2004;218:39–45. doi: 10.1016/j.jns.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Hoe HS, et al. The metalloprotease inhibitor TIMP-3 regulates amyloid precursor protein and apolipoprotein E receptor proteolysis. J Neurosci. 2007;27:10895–10905. doi: 10.1523/JNEUROSCI.3135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JK, et al. Tissue inhibitor of metalloproteinases-3 (TIMP-3) expression is increased during serum deprivation-induced neuronal apoptosis in vitro and in the G93A mouse model of amyotrophic lateral sclerosis: A potential modulator of Fas-mediated apoptosis. Neurobiol Dis. 2008;30(2):174–185. doi: 10.1016/j.nbd.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed Z, et al. TACE-induced cleavage of NgR and p75NTR in dorsal root ganglion cultures disinhibits outgrowth and promotes branching of neurites in the presence of inhibitory CNS myelin. FASEB J. 2006;20:1939–1941. doi: 10.1096/fj.05-5339fje. [DOI] [PubMed] [Google Scholar]
- 37.Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006;129:490–502. doi: 10.1093/brain/awh706. [DOI] [PubMed] [Google Scholar]
- 38.Pons L, et al. The 18 kDa peanut oleosin is a candidate allergen for IgE-mediated reactions to peanuts. Allergy. 2002;57:88–93. doi: 10.1034/j.1398-9995.57.s72.16.x. [DOI] [PubMed] [Google Scholar]
- 39.Marler KJ, et al. Outgrowth of neurites from NIE-115 neuroblastoma cells is prevented on repulsive substrates through the action of PAK. Mol Cell Biol. 2005;25:5226–5241. doi: 10.1128/MCB.25.12.5226-5241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 41.Miller JW, Nadeau MR, Smith J, Smith D, Selhub J. Folate-deficiency-induced homocysteinaemia in rats: Disruption of S-adenosylmethionine's co-ordinate regulation of homocysteine metabolism. Biochem. 1994;298:415–419. doi: 10.1042/bj2980415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaise SA, et al. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am J Pathol. 2007;170:667–679. doi: 10.2353/ajpath.2007.060339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ducros V, Belva-Besnet H, Casetta B, Favier A. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin Chem Lab Med. 2006;44:987–990. doi: 10.1515/CCLM.2006.178. [DOI] [PubMed] [Google Scholar]
- 44.Hempen C, Wanschers H, van der Sluijs Veer G. A fast liquid chromatographic tandem mass spectrometric method for the simultaneous determination of total homocysteine and methylmalonic acid. Anal Bioanal Chem. 2008;391(1):263–270. doi: 10.1007/s00216-008-1953-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.