Abstract
Objectives
The purpose of this study was to examine the release of nitric oxide (NO) and cGMP in response to electroacupuncture (EA) stimulation in acupuncture point (acupoint) compared to non-meridian control area.
Methods
Thirty dermal microdialysis data were collected from 24 volunteers at pericardium (PC) 4 and control area. EA was applied to PC 3 by using a 5 voltage pulse with duration of 1.0 msec at 10 Hz for 15 min. Dialysate samples were continuously collected 20 min each before, during and after EA for 2 hr. Total nitrite and nitrate (NOx-) and cGMP in the dialysate were quantified in a blinded fashion.
Results
Dialysate NOx- concentrations were decreased during a 120-min dialysis in all groups but reduced NOx- levels were attenuated predominantly in PC 4 acupoint at 20-40 min after EA PC 3. cGMP concentrations were significantly enhanced in acupoint PC 4 by EA PC 3, but not in non-meridian area.
Conclusion
We suggest that attenuation of NOx- reduction during dialysis reflects an increase in NO release induced by EA stimulation in acupoint and that cGMP mediates the signaling functions of NO to improve local microcirculation, which at least in part contributes to the effects of acupuncture.
Keywords: nitric oxide, nitrate and nitrite, cGMP, electroacupuncture, acupuncture points, dermal microdialysis
I. Introduction
In traditional Chinese medicine, acupuncture points (acupoints) are located on meridian lines (channels and collaterals, jingluo) on the body surface, and Quze (PC 3) is the main acupoint in the pericardium (PC) Hand Jue-Yin Meridian. Electroacupuncture (EA) or acupuncture stimulation of PC 3 has long been used for treatment of cardiac and gastric pain or symptoms of irritability [1]. Numerous studies in both animals and humans have demonstrated that EA causes multiple biological responses [2,7,12,15,31]. These responses can occur locally, i.e., at or close to the site of application, or at a distance [2,7]. The distant effect is thought to be mediated mainly by sensory neurons that project to various structures within the central nervous system and affect various physiological systems in the brain [7,12,15,31]. However, the specificity of acupoint and biochemical process of acupuncture signal transmission still remains largely unknown.
Anatomical studies have shown that most acupoints are located intimately at the distribution of nerve trunks and blood vessels [7,14,35]. A number of studies have demonstrated that acupoints possess characteristics of low impedance, high electric potential and hypersensitivity to pain [6,13,37,42,43]. Skin electrical resistance depends on the activity of the sympathetic nervous system [24,40] and nitric oxide (NO) stimulates norepinephrine (NE) release from the central and peripheral nervous systems [29,32,33,38]. Our recent studies have demonstrated that NO content and expression of neuronal NO synthase (nNOS) are greater in the skin acupoints/meridians in rats [30]. L-arginine-derived NO synthesis increases low resistance characteristics of acupoints [4], and NE turnover rate in acupoints/meridians is facilitated by presence of NO [5]. Human studies have demonstrated that warm needling increases NO level in the blood [27], and enhanced local circulation correlates with an increase in NO concentration in the plasma from the arm receiving acupuncture [41].
NO is a signaling molecular involved in a critical range of processes, including vasodilatation, neurotransmission, endocrine signal transduction, and other activities [3,11,28]. NO activates soluble isoforms of guanylate cyclases, which results in the production of cyclic 3′,5′-guanosine monophosphate (cGMP), and cGMP serves as the second messenger of NO. The NO-cGMP pathway serves as an important signal transduction system in the cells, and the increase of cGMP produces vasodilatation and various biological functions [11,28]. It has been well-demonstrated that the chemical lability of NO in cells and tissues is attributed to a rapid oxidation to both nitrite and nitrate [17,18]. Previous studies show that measurements of these two stable metabolites are very adequate indicators of the changes in NO activity and production in the tissue [17,18,30]. Dermal microdialysis has been developed and used to study releases of NO, cGMP and other substances in human skin [8-10,26].
The purpose of this study is to quantify the release of total nitrate plus nitrite (NOx-) concentrations from skin acupoint compared to their release from corresponding non-meridian area by using dermal microdialysis in humans. Dermal microdialysis, which can simultaneously collect and analyze the chemical composition of dialysate samples, was used to investigate the NO and cGMP release/generation upon EA versus no EA in the areas. Whether the effects of EA induction of NO and cGMP releases are specific to acupoint are determined by quantification of NO and cGMP concentrations from the groups of dialysis at acupoint and non-meridian area following the treatment.
II. Materials and Methods
1. Human Subjects
Twenty-four men and women (18 to 58 years old) recruited at Harbor-UCLA Medical Center volunteered for the following studies. Some subjects participated in more than one protocol (a total of thirty dermal microdialysis experiments) in a randomized, blind, and crossover fashion. Researchers in operation of the chemiluminescence Nitrogen Oxide Analyzer, and performing the Assay of cGMP, and analyzing the data obtained were blinded to acupuncture treatment. All subjects were healthy nonsmokers who did not have major surgery in the past 12 months nor history of cardiovascular disease. The characteristics of the volunteers and their attitudes and experience of acupuncture are summarized in Table 1. Volunteers with dermatological problems, allergic diseases, infectious diseases, and prescribed medication were excluded from the study. The protocol was approved by John F. Wolf, MD Human Subjects Committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. They were given a detailed oral instruction of the study and their informed consent was obtained. Female participants were not in their menstrual period on the study day. The room temperature was maintained at 25-27 °C. Experiments were performed with the subject supine and their arms at heart level for the PC dialysis. All subjects were seated comfortably 30 min before the start of the experiments. Experimental sessions lasted approximately 2 hours.
Table 1.
Characteristics of participants
| Characteristics | Participants, n = 24 |
|---|---|
| Women, No. | 11 |
| Men, No. | 13 |
| Age, mean (SEM), y | 26.67 ± 2.60 |
| Body Mass Index, mean (SEM) | 23.96 ± 0.81 |
| Weight, mean (SEM), pounds | 157.25 ± 7.17 |
| Experience with acupuncture | 1 |
| White | 12 |
| Hispanic | 6 |
| Asian | 5 |
| Pacific Islander | 1 |
2. Identification of Acupoints and Electroacupuncture Stimulation
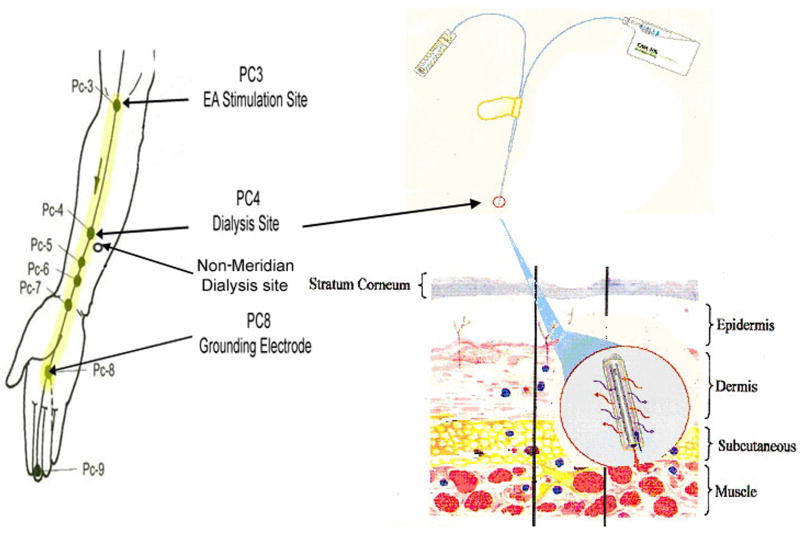
The locations of the PC meridian, and acupoints PC 3, PC 4 were determined by acupoint/meridian map of humans [1]. The PC 3 (Quze) is located on the transverse cubital crease at the ulnar side of the tendon of m. biceps brachii, and the PC 4 (Ximen) is located on the forearm between two tendons; the m. Palmaris longus and the m. flexor carpi radials, as shown in figure 1. The acupoints were further verified by measuring skin electric currents at the points by using an electro-diagnostic device, Dermatron ST (Pitterling Electronic GmbH, Germany) as previously described [4,42].
Figure 1.
Illustrates the acupuncture points of pericardium meridian (PC) of aim (left; reproduced from Beijing College of Traditional Chinese Medicine et al., 1980), the CMA/20 microdialysis probe and a microdialysis pump CMA 102 (right top; reproduced from CMA www.microdialysis.se), and a probe dialysis in subcutaneous tissue (right bottom; reproduced from Zhu and Hao, 1989). PC 4 and the nearby area not on the meridian line represent the targeted sites for microdialysis in acupoint and its non-meridian control. PC 3 denotes the acupoint in which electrical stimulation is applied. The grounding electrode was placed on PC 8. The distance between PC 3 to 8 as meridian line that electrical stimulation was passed through and dialysis was conducted.
The disposable acupuncture needles (0.3 × 25mm) were inserted gently and superficially at acupoint PC 3 in the groups 2 & 3, which is defined in research protocols (below). Figure 1 shows that the stimulating electrode was connected to an acupuncture needle inserted at PC 3 on the arm. Another stainless steel electrode was applied to the skin surface of PC 8 on the hand as a grounding electrode with a distance beyond the paired electrodes for stimulation. EA stimulation was applied to PC 3 on the same arm as PC 4 dialysis, and was conducted initially using a 5 voltage pulse with duration of 1.0 msec at 10 Hz for 15 min. The voltage pulse may have increased depending upon whether the subject experiences a sensation of “de qi” (the feeling of soreness, numbness, distension or pain). During the course of EA stimulation, the subjects were asked whether they still maintained the “de qi” sensation [1,2,7]. If they did not feel the sensation, the voltage pulse was increased until they experienced “de qi” again.
3. Dermal microdialysis
The CMA/20 microdialysis probes of 0.5 mm diameter, 10 mm length, and 20 KDa molecular weight cut-off were sterilized by immersing in a 3% cold renalin solution for 12–24 hours at 4 °C (Minntech Corp., Minneapolis, MN) [8,9,26]. The probes were immersed in a sterile normal saline for 12-24 hours. On the day of the study, the probe was flushed with sterile Perfusion Fluid T1 (Na+ 147 mmol/L, K+ 4 mmol/L, Ca2+ 2,3 mmol/L, Cl- 156 mmol/L, pH 6; CMA Microdialysis Inc., North Chelmsford, MA) followed by a test with Renalin Residual test strips (Minntech Corp., Minneapolis, MN) to be certain that no renalin remained before use. The probes were inserted under topical local anesthetic (EMLA, Wilmington, DE) into the volar surface of the forearm at acupoint PC 4 to lie subcutaneously below the skin surface as described previously [8,9,25]. After a 30 min recovery period, the probes were perfused with Perfusion Fluid T1 at a constant flow rate at 2 μl/min for 2 hour using a 2.5 ml microsyringe driven by using a Microdialysis Pump CMA/102 (CMA Microdialysis Inc., North Chelmsford, MA). The dialysate sample flowed out from the outlet tubing and collected in the sealed ice-cold microcentrifuge tube. All experimental samples were stored at -70 °C for later processing.
4. Quantification of NO Metabolites
The total NOx- concentration (NO2- and NO3-) was measured in the dialysate using an ozone phase chemiluminescence method (NOA280i, GE Analytical Instruments, Boulder, CO) as described previously [17,30]. Briefly, five microliter samples of fresh or previous frozen dialysate were reduced using a Vanadium (III)/HCl solution. The nitrate calibration curve was established using known concentrations of NaNO3 dissolved in the sterile nitrogen-free water. The total amount of NO3- in each dialysate sample was calculated by integration of the signal peaks using the nitrate calibration curve. All samples were measured in duplicate. The presence of (NO2-) in our dialysate samples was close to the water basal level. Therefore, the final NOx- concentration in the dialysate was expressed as μM with no allowance made for NO2-. The minimum sensitivity level of NO amount is 1.0 picomole. The measurements were done in a blinded manner.
5. Measurement of cyclic GMP
The concentration of cGMP in the dialysate was assayed using a competitive Enzyme-Linked Immunosorbent Assay (Parameter™ Cyclic GMP Assay, R&D Systems, Minneapolis, MN). The minimal detection level of cGMP is 1.14 pmol/ml. Briefly, forty microliter dialysate was diluted in 160 μl Calibrator Diluent RD5P, and the diluted sample was then added to the 96 well microplate coated with a goat anti-rabbit polyclonal antibody (100 μl/well). Control (non-specific binding) and standard were included in each assay. Cyclic GMP conjugate to horseradish peroxidase (HRP) and rabbit polyclonal antibody to cGMP were added to the diluted sample in the wells. The optical densities of yellow color development in the wells were read using a microplate reader set to 450 nm with a wavelength correction to 570 nm (Molecular Devices Emax, Sunnyvale, CA). The cGMP concentration in the dialysate was calculated based on a standard curve established by cGMP standards (2.1, 6.2, 18.5, 56 pmol/ml) in each assay.
6. Research Protocols
Volunteers were randomly asked to participate in one or more of the following 3 experimental groups: 1) PC 4 dialysis control with no EA (n=8); 2) Non-meridian dialysis with EA PC 3 (n=8); and 3) PC 4 dialysis with EA PC 3 (n=14). Six subjects participated in both groups 1 and 3, and served as self controls in a randomized, blind fashion. The microdialysis probe was embedded at PC 4 acupoint or non-meridian region on the front side of the forearm, as shown in figure 1. The perfusion was continuously performed for 2 hours in all groups. After 40-min continuous perfusion, the acupuncture needle was inserted into PC 3 acupoint and EA stimulation was conducted for 15 min with the same procedure in groups 2 & 3 in a blind fashion for acupoint or non-meridian dialysis. Volunteers in the PC 4 dialysis control (group 1) did not receive EA during the 2-hr perfusion time. A total of six dialysate samples (2 samples for before EA, one for during EA, and 3 for after EA) for each participant were collected at 20-min intervals during 2-hr perfusion.
7. Statistical Analysis
All numerical data were expressed as mean ± standard error of the mean (SEM). Analysis of variance (one-way ANOVA and Tukey HSD Post Hoc Test), and Student's t-test (unpaired) were used to analyze significant difference using software SSPS 11.5 (SSPS Inc., Chicago, IL). P-value < 0.05 is considered significant.
III. Results
Baseline NOx- and cGMP concentrations in non-meridian area compared to acupoint
The baseline NOx- concentration was examined in the acupoint, PC 4, and its non-meridian area following dermal microdialysis as illustrated in figure 1 in twenty four healthy volunteers. The characteristics of the participants are detailed in table 1. The mean value of dialysate NOx- concentration in PC 4 was 7.6±1.2 μM (mean±SE) measured in 19 probes for the first 20 min collection. The baseline levels of NOx- concentrations in the PC 4 dialysis compared to non-meridian dialysis suggested an elevation, although the increase fell short of statistical significance due to large variability (Figure 2). The next 20-min PC 4 dialysis was less variable (5.6±0.8 μM) after the first collection. This NOx- concentration measured in the 20-40 min dialysate sample was used as a baseline value in the following experiment.
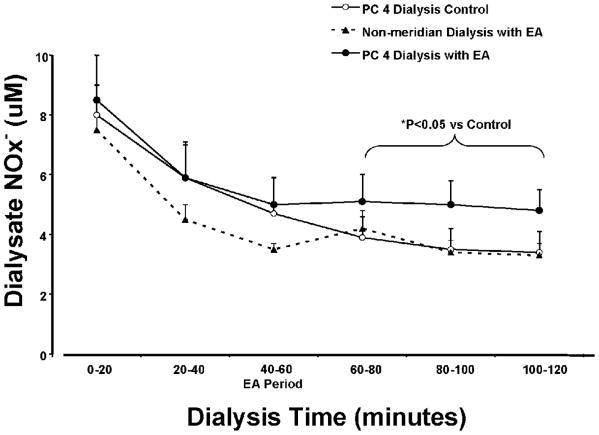
Figure 2.
Time-response curves of dialysate nitrite plus nitrate (NOx-) from non-meridian area and PC 4 dialysis in three groups of healthy volunteers with and without EA PC 3. Dialysate NOx- was collected at 20-minute intervals over 2 hours as follows: 0-20 min, and 20-40 min collections before EA, 40-60 min during EA stimulation, 60-80 min, 80-100 min, and 100-120 min. Each point represents mean ± S.E. (n = 8-13). Dialysate NOx- concentration was increased in PC 4 dialysis with EA stimulation compared to the PC 4 control and non-meridian dialysis groups (ANOVA, *: P<0.05).
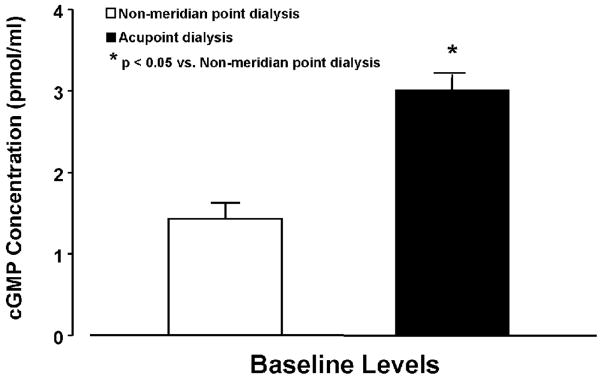
The baseline level of dialysate cGMP concentrations (pmol/ml) were examined in the PC 4 dialysis (n = 14) and non-meridian group (n = 8). As shown in figure 3, the cGMP concentration of the 0-20 min dialysis in the PC 4 dialysate was significantly higher than those of the non-meridian dialysate (P<0.05).
Figure 3.
Baseline levels of cGMP concentrations in acupoint (PC 4) dialysis and dialysate from its non-meridian. cGMP concentrations (pmol/ml) in PC 4 dialysis (n = 14) were significantly higher than the dialysate in non-meridian area (n = 8). Each bar represents the mean values and vertical bars represent S.E.M. *: p<0.05, compared with non-meridian area dialysis.
Time responses of dialysate NO metabolites in non-meridian area and PC 4 with and without EA stimulation
Thirty dermal microdialysis experiments were performed with three experimental groups: PC 4 dialysis control (n = 8), Non-meridian dialysis with EA PC 3 (n = 8), and PC 4 dialysis with EA PC 3 (n = 14). Six subjects participated in both PC 4 dialysis with and without EA PC 3, and served as self controls. Figure 2 shows the time intervals of NO releases in three dialysis groups during the 2-hr dialysis time. The NOx- concentrations were gradually decreased during a 120-min dialysis with a large fall during 0-40 min dialysis in all groups, which are consistent with the time curves of dialysate NOx- contents from other studies using dermal microdialysis in humans [8,9,23].
After a 15-min EA stimulation of PC 3, the gradually decrease in NOx- concentrations was attenuated as seen in the dialysis response curve of the PC 4 dialysis with EA group compared to those responses in the control and non-meridian dialysis groups (Figure 2). The level of NOx- reductions in PC 4 dialysis at the 40–60 min dialysate (during EA treatment) compared to non-meridian dialysis group suggested a moderate attenuation, but did not produce statistically significant prevention. As shown in figure 2, NOx- concentrations kept falling in the dialysate samples of the PC 4 dialysis control group without EA stimulation. In contrast, the reduction of dialysate NOx- concentrations in the PC 4 dialysis was significantly attenuated by EA PC 3 at the 60–120 min dialysate (0-60 min after EA). In non-meridian area dialysis group, EA stimulation of PC 3 with the same procedure did not prevent the reduction of NOx- concentrations at the periods of 60–120 min dialysis, as shown in figure 2. In the 6 volunteers acting as their own controls, the NOx- concentration at the 80–100 min dialysate was 3.5±0.7 as control without EA and 4.5±0.9 μM following EA stimulation. There is no detectable difference of dialysate NOx- concentrations between male and females in either PC 4 or non-meridian area dialysis.
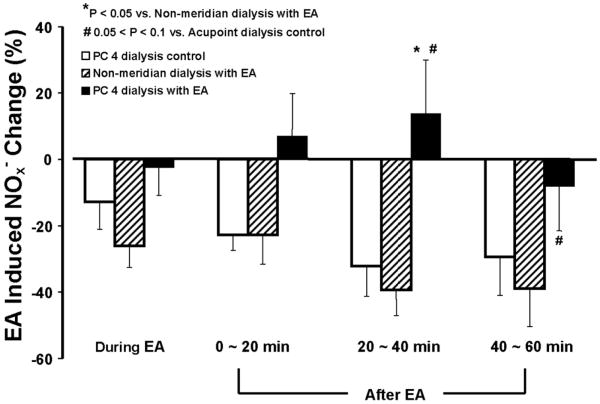
In order to examine EA-induced NO release compared to baseline levels, the percentage changes of NOx- concentrations were calculated based on the differences of the values during the EA, 20 min, 40 min, and 60 min after EA and the basal value of the second collection at 20 minutes after the dialysis. Figure 4 shows percentage changes of NOx- concentrations during EA PC 3 and a period of 60 min after EA stimulation at each 20-min interval in the PC 4 dialysis control, PC 4 and non-meridian area dialysis following EA PC 3. As shown in figure 4, attenuation of NOx- reduction started 0-20 min after the initiation of EA in the PC 4 dialysis group. Prevention of NOx- concentration reduction at 20-40 min after EA was statistically significant in the PC 4 dialysis with EA group compared to both non-meridian dialysis with EA PC 3 and PC 4 dialysis control without EA groups (P<0.05). Dialysate NOx- concentrations in PC 4 at 40-60 min after EA showed marginally significant differences compared to the PC 4 dialysis control group (#: P = 0.077) but were not significantly altered when compared to the non-meridian dialysis with EA PC 3. However, the percentage change of NOx- concentrations became negative number in the dialysate samples of the PC 4 dialysis control and non-meridian area dialysis following EA PC 3 (figure 4). The effect of the EA PC 3 on attenuation of NOx- reduction in PC 4 tended to return in 40-60 min after the treatment.
Figure 4.
Changes in dialysate NOx- concentrations in PC 4 control, non-meridian area and PC 4 dialysis following EA PC3. The percentage change of NO releases in PC 4 and non-meridian area was calculated based on the differences of the NOx- concentrations during the EA, 20 min, 40 min, and 60 min after EA and the baseline value of the second 20-min dialysate sample. Each bar represents the mean values and vertical bars represent S.E.M. (n = 8-12). The NOx- concentration of the PC 4 dialysis group was statistically increased at 20-40 min after the EA stimulation compared to the non-meridian dialysis group and the PC 4 dialysis control group (ANOVA, *: P<0.05). NOx- concentrations in PC 4 at 40-60 min after EA was marginally significant differences compared to the PC 4 dialysis control group (#: P = 0.077).
For comparisons of the percentage change of maximum NO releases in the PC 4 and non-meridian area following EA PC 3, the highest NOx- amount was obtained in one of the last three 20-min dialysate samples after the EA stimulation. The maximum change in EA-induced NOx- concentration for each subject was calculated by subtracting the baseline NOx- concentration from the highest NOx- concentration, then dividing by the baseline NOx- concentration. In the PC 4 dialysis (group 3), 76% (10/14) of the subjects displayed NO increases after the EA stimulation as compared to their individual baseline NOx- levels before EA treatment. In contrast, only 25% (2/8) of the subjects in the PC 4 dialysis control and 12.5% (1/8) of the non-meridian dialysis with EA (group 2) exhibited positive NO increases. The maximum NO release was significantly higher in the PC 4 dialysis following EA PC 3 as compared to those in the PC 4 control and non-meridian dialysis groups (P<0.05).
Effects of EA-induced cGMP releases in PC 4 compared to non-meridian area
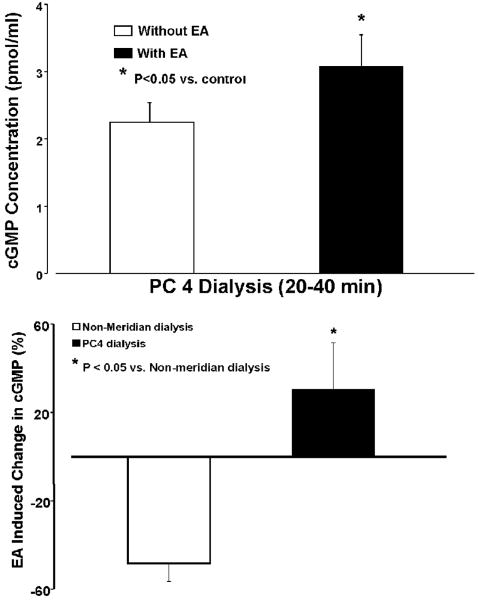
Figure 5, top panel shows dialysate cGMP concentrations in PC 4 dialysis with and without EA PC3 during the same period of collection at 20 min after EA stimulation. Without EA stimulation, the dialysate cGMP concentration in the PC 4 dialysis was 3.0±0.2 (pmol/ml Mean±SE) during the first 20 min dialysis (n=13) and 2.3±0.1 pmol/ml during the subsequent collection. The cGMP concentration in PC 4 was reduced during the subsequent collection compared to the first collection, which is consistent with the time curves of dialysate NOx- contents. With EA PC 3, cGMP concentration in PC 4 dialysis was 3.1±0.5 during the second collection, which was significantly higher than the PC 4 dialysate in the same period without EA (n=6, p<0.05). However, cGMP concentration was 1.53±0.2 pmol/ml in non-meridian dialysis before EA and 1.24±0.2 pmol/ml after EA stimulation.
Figure 5.
Effect of EA PC3 on dialysate PC 4 cGMP concentration (top) and percentage change in cGMP at PC 4 dialysis and dialysate from its non-meridian area following EA PC 3. Top panels show that PC 4 dialysate cGMP concentrations (pmol/ml) were significantly increased 20 min after EA PC 3 compared to the same period of dialysis without EA stimulation. Bottom panels show percentage change in cGMP 20 min after EA PC 3 at PC 4 and non-meridian area dialysis compared to PC 4 dialysis without EA. The mean basal level of cGMP in the second collection of PC 4 dialysis (n=8; 2.3±0.5 pmol/ml) without EA stimulation was used to calculate the percentage change in cGMP. Each bar represents the mean values and vertical bars represent S.E.M. Percentage change in cGMP levels from PC 4 dialysis was significantly higher than those of the non-meridian dialysate following EA PC 3 (unpaired t test; *: P<0.05).
Figure 5, bottom panel, shows the percentage change of cGMP production in the PC 4 dialysis and non-meridian area dialysis 20 min after EA PC 3 compared to PC 4 dialysis without EA. The percentage change of cGMP concentrations became a negative number in the non-meridian area dialysis following EA PC 3. However, dialysate cGMP concentration in the PC 4 was significantly increased after the EA stimulation compared to the control group without EA (Figure 5, bottom). Compared to the baseline values before and after EA in the same group, the percentage change of cGMP concentrations was slightly elevated (2.2±6.1%) in the PC 4 dialysis at 20 min after EA PC 3 but reduced (-15.5±6.1%) in the non-meridian area dialysis with EA PC 3 in the same period of collection. There is significant difference between cGMP concentrations in acupoint dialysis and its non-meridian dialysate following EA PC 3 (P < 0.05).
IV. Discussion
We examined the releases of NO and cGMP in acupoint and non-meridian area along the PC meridian using dermal microdialysis in humans. The influences of EA stimulation on NO-cGMP releases were studied by chemical analysis of the dialysate samples collected from continuous dialysis of PC 4 and non-meridian area following EA PC 3. The major new findings of this study are that: 1) Baseline dialysate cGMP content was higher and NOx- concentration tended to be higher in PC 4 acupoint than in non-meridian area; 2) Dialysate NOx- concentrations were gradually reduced during a 120-min dialysis in all groups; 3) Reduced NOx- levels were attenuated predominantly in PC 4 acupoint at 20-40 min after EA PC 3 but not in the non-meridian area; and 4) EA-induced cGMP generation was parallel to an increased NOx- concentration. This is the first evidence showing that EA stimulation of PC 3 prevents reduction of dialysate NOx- release in the PC 4 acupoint but does not alter NO release in non-meridian area. The attenuation of NOx- reduction during dialysis reflects an increase in NO release in acupoint induced by EA stimulation. The time response to elevation of NO release in acupoints began at 0-20 min, reached a maximum at 20-40 min, and returned at 60-80 min after EA stimulation. The increased release of NO in PC 4 acupoint is parallel to the elevated amount of cGMP followed by EA stimulation. The elevation of both molecules induced by EA suggest that enhanced NO production, which stimulate guanylate cyclases to generate cGMP, which results in the subsequent biological effects through NO-cGMP signal system. These findings suggest that EA stimulation induced NO and cGMP release is specific to acupoints in humans. Enhanced chemicals following EA stimulation suggests a release/generation of NO-cGMP, which may mediate signaling pathway involved in the therapeutic effects of EA processes.
Dermal microdialysis has been successfully used to measure different substances in the extracellular space, such as NO, cGMP, histamine, glucose, and catecholamine in human skin [8-10,24,28]. Present studies show that there is a fall in NOx- concentrations during a 120-min dialysis especially in the initial dialysis period. The apparent fall in NO level during continued dermal dialysis has been reported previously [8,9,23]. Possible explanations for this fall include: 1) Probe insertion and perfusion may cause changes in microvascular blood flow and/or interstitial environment resulting in an attenuation of NO production and release; and 2) Dialysis itself depletes the interstitial space of NO/nitrite [8]. Our results are consistent with the previous studies using dermal microdialysis in humans [8,23] and further suggest there are necessary to compare the treatment and control groups under the same dialysis period. In addition, our findings cannot exclude other factors against a falling baseline NOx- concentrations involved in the interpretation of the EA effects. Although there is a large fall in NOx- concentrations during 0-40 min dialysis resulting in large variability in dialysate recovery, the present results from the chemical analysis of the dialysate samples of acupoints suggest that dermal microdialysis is an effective approach to monitor chemical releases in acupoints along their meridian areas in vivo and examine acupoint specificity in humans.
The mechanism responsible for an increase in NO and cGMP release in acupoints by EA stimulation is unclear. Anatomical studies have shown that most acupoints are located at a nerve trunk and situated at or adjacent to an artery and/or vein [7,14,35]. Previous studies have also demonstrated that acupuncture induces the local releases of sensory neuropeptides [19,20] and modified sensory function [36] which cause peripheral vasodilation and increase in blood flow. Our results support these studies and further suggest that enhanced baseline and EA stimulation–evoked NO-cGMP concentrations in the acupoint may result from the rich distribution of blood vessels and neural fibers in the area. A number of international studies have demonstrated that, in both humans and animals, the majority of acupoints correspond to high electrical conductance and low skin resistance [6,13,37,42,43]. Recent studies from our lab showed that NO content and nNOS expressions are consistently higher in the skin acupoints/meridians associated with low electric resistance in rats [30]. L-arginine-derived NO synthesis modifies noradrenergic function, which contributes to low resistance characteristics of acupoints [4]. Consistently, enhanced 3H-NE synthesis/release in acupoints/meridians is facilitated by the presence of an exogenous NO donor and inhibited by an inhibitor of NO synthesis [5]. Recent studies have demonstrated that EA stimulation of ST 36 increases the production of NO in arterioles [22]. It is well-demonstrated that three distinct forms of NO synthase (NOS)-nNOS, endothelial NOS (eNOS), and inducible NOS that catalyze the transformation of arginine to NO [3,11], and the activities of L-arginine-derived NO synthesis, both neuronal and endothelial sources, are demonstrated in the skin tissues [4,5,9,10,30]. The present results support the previous results reporting that activation of L-arginine-derived NO synthesis is high at acupoints and the local NO production is induced by EA stimulation. It is highly possible that elevation of NO-cGMP in the acupoint could be achieved through the activation of endothelial and/or neuronal NO synthesis/release system.
With regard to the potential role of EA-induced NO-cGMP release in acupoint, one view holds that acupoints are discrete sites on the body where needling or electrical stimulation can activate production via appropriate neural pathways to produce central effects [7,12,15,31]. Dermal microdialysis studies show that NO release is essential in sustaining cutaneous dilation during heating [21,39], and endogenous NO and noradrenaline contribute to the temperature threshold of the axon reflex response to heating on the skin [16]. Endothelium-derived NO plays a role in reactive hyperemia [34] and local anesthesia inhibits reactive hyperemia which suggests that postocclusion reactive hyperemia on forearm skin is mediated by a local reflex involved sensory nerve [25]. In addition, acupuncture also works locally, and stimulation of the acupoint located on the ground of the pressure pain site (Ashi-point) has been often used for treatment of pain-related syndromes and soft-tissue damage in acupuncture clinical practices [1,2,41]. Previous human studies have demonstrated that NO level is increased in the blood after warm needling [27], and local circulation is enhanced by acupuncture stimulation correlated with NO increase in treated regions [41]. It has been well-documented that NO-cGMP causes direct vasodilation and increase in blood flow [11,18]. The present assay shows that NO and cGMP levels are consistently increased by EA stimulation in the PC acupoint but not in the non-meridian area. These results support the possibility that acupuncture induces local release of NO and cGMP [27,41]. Elevated NO-cGMP results in peripheral vasodilation and improved local microcirculation, which contributes to the therapeutic effects of acupuncture.
In summary, these results show that EA stimulation induces a significant release of NO and cGMP in the acupoint, but not in non-meridian area. Time intervals of NO collections show that EA-induced increase in NO release in PC 4 are significantly higher at the 20-40 min interval than those collected from the non-meridian area. The results suggest that the elevation of both molecules induced by EA is specific to the acupoint, and acupoints with enriched blood vessels and neuronal components may contribute to NO generation, which stimulate guanylate cyclases to generate cGMP. Elevated NO-cGMP release evoked by EA stimulation mediates the therapeutic effects of acupuncture through local vasodilation and other possible mechanisms.
Acknowledgments
We are grateful to Xi-Yan Li who provided excellent technical support and Dr. Peter Christenson, a Biostatistician from NIH-supported General Clinical Study Center, for statistical advice. These studies were conducted at the biomedical research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
This project was made possible by NIH Grants (AT002478 and AT004620) from the National Center for Complementary and Alternative Medicine, and a Research Award (ADA 7-07-RA-100) from the American Diabetes Association to Dr. Ma.
References
- 1.Beijing College of Traditional Chinese Medicine, Shanghai College of Traditional Chinese Medicine, Nanjing College of Traditional Chinese Medicine, Institute of Acupuncture and Moxibustion, China Academy of Traditional Chinese Medicine. Essentials of Chinese Acupuncture. Beijing: Foreign Languages Press; 1980. pp. 301–310. [Google Scholar]
- 2.Berman B. A 60-year-old woman considering acupuncture for knee pain. JAMA. 2007;297:1697–1707. doi: 10.1001/jama.297.15.1697. [DOI] [PubMed] [Google Scholar]
- 3.Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 4.Chen JX, Ma SX. Effects of nitric oxide and noradrenergic function on skin electric resistance of acupoints and meridians. J Altern Complement Med. 2005;11:423–431. doi: 10.1089/acm.2005.11.423. [DOI] [PubMed] [Google Scholar]
- 5.Chen JX, Ma SX. Nitric oxide on modulation of norepinephrine production in acupuncture points. Life Sci. 2006;79:2157–2164. doi: 10.1016/j.lfs.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Chiou SY, Chao CK, Yang YW. Topography of low skin resistance points (LSRP) in rats. Am J Chin Med. 1998;26:19–27. doi: 10.1142/S0192415X9800004X. [DOI] [PubMed] [Google Scholar]
- 7.Chan SHH. What is being stimulated in acupuncture: evaluation of the existence of a specific substrate? Neurosci Biobehav Rev. 1984;8:25–33. doi: 10.1016/0149-7634(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 8.Clough GF. Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo. J Physiol. 1997;516:549–557. doi: 10.1111/j.1469-7793.1999.0549v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clough GF, Bennett AR, Church MK. Measurement of nitric oxide concentration in human skin in vivo using dermal microdialysis. Exp Physiol. 1998;83:431–434. doi: 10.1113/expphysiol.1998.sp004126. [DOI] [PubMed] [Google Scholar]
- 10.Clough G, Bennett AR, Church MK. Relationship between nitric oxide, cyclic GMP and vasodilation in human skin in vivo. J Physiol. 1998;513 [Google Scholar]
- 11.Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 12.Foster JM, Sweeney BP. The mechanisms of acupuncture analgesia. Br J Hosp Med. 1987;38:308–312. [PubMed] [Google Scholar]
- 13.Fraden J. Active acupuncture point impedance and potential measurements. Am J Acupunct. 1979;7:137–144. [Google Scholar]
- 14.Gunn CC, Ditchburn FG, King MH, Renwick GJ. Acupuncture loci: a proposal for their classification according to their relationship to known neural structures. Am J Chin Med. 1976;4:183–195. doi: 10.1142/s0192415x76000238. [DOI] [PubMed] [Google Scholar]
- 15.Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 16.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: Comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1999;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 19.Jansen G, Lundeberg T, Kjartansson J, Samuelson UE. Acupuncture and sensory neuropeptides increase cutaneous blood flow in rats. Neurosci Lett. 1989;97:305–309. doi: 10.1016/0304-3940(89)90615-0. [DOI] [PubMed] [Google Scholar]
- 20.Kashiba H, Ueda Y. Acupuncture to the skin induces release of substance P and calcitonin gene-related peptide from peripheral terminals of primary sensory neurons in the rat. Am J Chin Med. 1991;19:189–197. doi: 10.1142/S0192415X91000260. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg DL, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 22.Kim DD, Pica AM, Duran RG, Duran WN. Acupuncture reduces experimental renovascular hypertension through mechanisms involving nitric oxide synthase. Microcirculation. 2006;13:577–585. doi: 10.1080/10739680600885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-L-arginine-methyl ester on neuropeptide-induced vasodilation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- 24.Korr IM, Thomas PE, Wright HM. Patterns of electrical skin resistance in man. Acta Neuroveg (Wien) 1958;17:77–98. doi: 10.1007/BF01234166. [DOI] [PubMed] [Google Scholar]
- 25.Larkin SW, Williams TJ. Evidence for sensory nerve involvement in cutaneous reactive hyperemia in humans. Circ Res. 1993;73:147–154. doi: 10.1161/01.res.73.1.147. [DOI] [PubMed] [Google Scholar]
- 26.Leis S, Drenkhahn S, Schick C, Arnolt C, Schmelz M, Birklein F, Bickel A. Catecholamine release in human skin--a microdialysis study. Exp Neurol. 2004;188:86–93. doi: 10.1016/j.expneurol.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Chen K, Wu Y, Jiao J, Tao L. Effects of warm needling at Zusanli (ST 36) on NO and IL-2 levels in the middle-aged and old people. J Tradit Chin Med. 2003;23:127–128. [PubMed] [Google Scholar]
- 28.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 29.Lonart G, Wang J, Johnson KM. Nitric oxide induces neurotransmitter release from hippocampal slices. Eur J Pharmacol. 1992;220:271–272. doi: 10.1016/0014-2999(92)90759-w. [DOI] [PubMed] [Google Scholar]
- 30.Ma SX. Enhanced nitric oxide concentrations and expression of nitric oxide synthase in acupuncture points/meridian. J Altern Complement Med. 2003;9:207–215. doi: 10.1089/10755530360623329. [DOI] [PubMed] [Google Scholar]
- 31.Ma SX. Neurobiology of Acupuncture: Toward CAM. eCAM. 2004;1:41–7. doi: 10.1093/ecam/neh017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma SX, Long JP. Effects of nitroglycerin on release, synthesis and metabolism of norepinephrine and activation of tyrosine hydroxylase in guinea-pigs. Eur J Pharmacol. 1991a;199:27–33. doi: 10.1016/0014-2999(91)90633-2. [DOI] [PubMed] [Google Scholar]
- 33.Ma SX, Long JP. Positive chronotropic and inotropic responses to release of norepinephrine from sympathetic nerve terminals produced by nitroglycerin in atria. Arch Int Pharmacodyn Ther. 1991b;309:125–136. [PubMed] [Google Scholar]
- 34.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro-Riviere NA, Hwang YC, Stromberg MW. Light microscopic morphology of low resistance skin points in the guinea pig. Am J Chin Med. 1981;9:155–163. doi: 10.1142/s0192415x81000196. [DOI] [PubMed] [Google Scholar]
- 36.Prado WA, Schiavon VF, Cunha FQ. Dual effect of local application of nitric oxide donors in a model of incision pain in rats. Eur J Pharmacol. 2002;441:57–65. doi: 10.1016/s0014-2999(02)01413-9. [DOI] [PubMed] [Google Scholar]
- 37.Reichmanis M, Marino AA, Becker RO. D.C. skin conductance variation at acupuncture loci. Am J Chin Med. 1976;4:69–72. doi: 10.1142/s0192415x7600010x. [DOI] [PubMed] [Google Scholar]
- 38.Satoh S, Kimura T, Toda M, Miyazaki H, Ono S, Narita H, Murayama T, Nomura Y. NO donors stimulate noradrenaline release from rat hippocampus in a calmodulin-dependent manner in the presence of L-cysteine. J Cell Physiol. 1996;169:87–96. doi: 10.1002/(SICI)1097-4652(199610)169:1<87::AID-JCP9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and L-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- 40.Smith GB, Wilson GR, Curry CH, May SN, Arthurson GM, Robinson DA, Cross GD. Predicting successful brachial plexus block using changes in skin electrical resistance. Br J Anaesth. 1988;60:703–708. doi: 10.1093/bja/60.6.703. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchiya M, Sato EF, Inoue M, Asada A. Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth Analg. 2007;104:301–307. doi: 10.1213/01.ane.0000230622.16367.fb. [DOI] [PubMed] [Google Scholar]
- 42.Voll R. Twenty years of electroacupuncture diagnosis in Germany: a progress report. Am J Acupunct. 1975;3:7–17. [Google Scholar]
- 43.Zhu ZX, Hao JK. Acupuncture Meridian Biophysics-scientific Verification of the First Great Invention of China. Beijing Press; 1989. Electric Characteristics of the Skin Along Meridian Lines. [Google Scholar]