Electron capture dissociation (ECD)[1] results from the mutual storage of thermal electrons with multiply protonated peptide cations – an experiment generally performed within the high magnetic field of a Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR-MS). The technique is particularly useful as it generates random backbone cleavage with little regard to the presence of post-translational modifications (PTMs), amino acid composition, or peptide length. Electron transfer dissociation (ETD),[2] the ion-ion analogue of ECD, is conducted in radio frequency (RF) quadrupole ion trap devices where radical anions serve as electron donors. Because it can be implemented on virtually any mass spectrometer with an RF ion transfer or storage device, ETD has become an increasingly widespread dissociation method.
The capture of an electron can trigger a free radical-driven rearrangement resulting in N–Cα backbone cleavage and c- and z•-type fragment ion production. Sometimes, however, the precursor cation captures the electron and forms a long-lived, charge-reduced species that does not separate (ECnoD/ETnoD).[3] This phenomenon becomes more probable with increasing precursor mass-to-charge (m/z) ratio. As the charge density decreases, the magnitude of intramolecular non-covalent interactions increases so that following electron capture and cleavage the newly formed c- and z•-type fragment ions often remain bound – an obstacle of higher consequence for ETD as it is conducted under elevated pressure conditions.[4] McLafferty and others reported that photon bombardment of the precursor cation prior to ECD (activated ion-ECD, AI-ECD) decreased non-dissociative electron capture,[5] presumably by destroying the peptide cation secondary structure prior to electron capture.
ETD is conducted at pressures ~ 106 higher than in ECD (~ 1mTorr) so that precursor cations are considerably cooler and pre-activation with either photons or collisions is expected to produce only short-lived (< 1 ms) unfolding. Recently we examined the use of collisions to coerce the ETnoD products to dissociate via a technique coined ETcaD.[3] The method increased the number and intensity of N–Cα backbone cleavages; however, the majority of the newly formed fragment ions displayed evidence of H atom rearrangement to produce even electron z-type fragments and odd electron c•-type products. ECD practicioners propose that such rearrangements result from the c- and z•-type fragment ions being held in close proximity so that an H atom can be abstracted from the c-type and directed to the z•-type product (note this happens prior to the separation of the two).[6,7] For large-scale sequencing applications these rearrangements are problematic as the mass window needed to define a possible fragment becomes too large.
We considered collisionally activating the precursor cations via resonant excitation during the entire ion-ion reaction period, which would inhibit collisional cooling and refolding prior to electron transfer. A side effect of resonant excitation, however, is that the precursor cations undergo an increase in velocity. Ion-ion reaction rates are governed by the velocity of the participants and such increases inhibit ion-ion reactions.[8] McLuckey and colleagues reported the use of elevated bath gas temperatures to increase the degree of ETD fragmentation, but did not discuss the impact of the method on H atom abstraction.[9] We reasoned that infrared photons (10.6 µm) could be used, in conjunction with ion-ion reactions, to continuously increase precursor ion internal energy, destroy non-covalent interactions, and limit H atom migration. Note recent results of Kaplan et al.[10] show that photo-activation of peptide cations undergoing ETD can increase direct fragmentation; however, no evaluation of H atom migration was performed. To test our hypothesis we modified a dual cell linear ion trap system such that an IR beam irradiated the axial length of both linear ion traps. Peptide cations and reagent anions (azobenzene) were generated at atmospheric pressure (AP) and introduced through a single inlet.
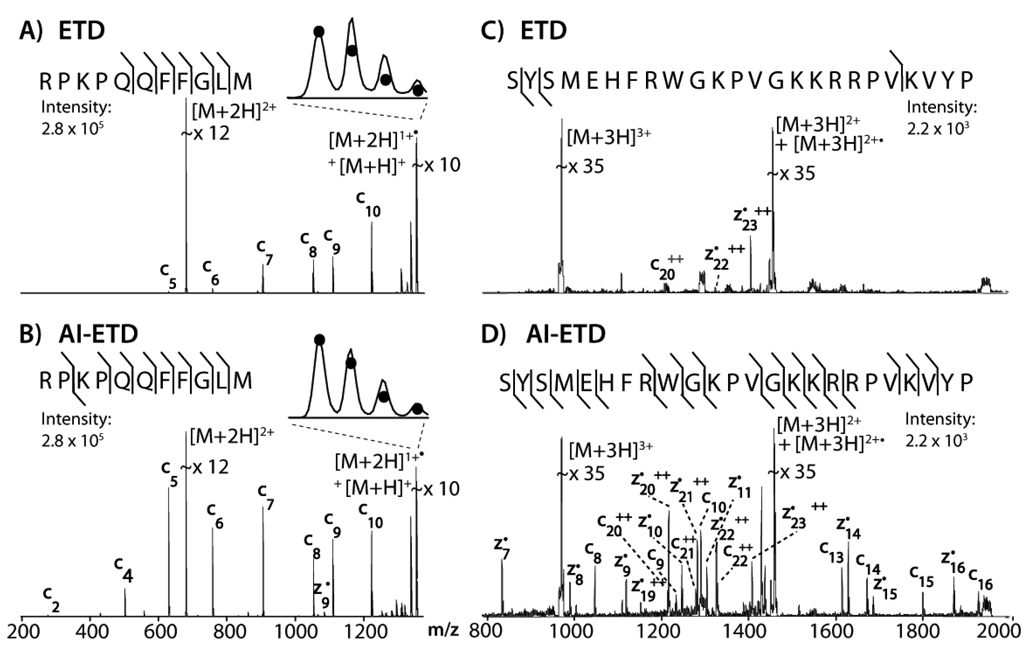
Panels A and B of Figure 1 present single scan tandem mass spectra acquired following a 100 ms reaction of doubly protonated Substance P cations (RPKPQQFFGLM, m/z =674) with radical anions of azobenzene either without (ETD, panel A) or with concomitant photon bombardment (AI-ETD, panel B). ETD results in the production of six c-type product ions. Irradiation of the reactants during the ETD process results in the formation of nine c- and z•-type fragments, with virtually all of them showing significant increases in intensity. The inset of panel A displays the isotopic envelope of the charge-reduced precursor, which comprises products of both ETnoD and proton abstraction. Proton abstraction is a competing side-reaction to electron transfer and the partitioning is largely dependent upon the reagent anion[11] – azobenzene engages in more proton abstraction than our preferred reagent fluoranthene, but is conveniently produced under AP conditions. Shown on the inset are the theoretical isotopic envelopes of the proton transfer charge-reduction product (i.e., [M+H]+). The heightened distribution of the 13C isotopic cluster provides an estimate of the ETnoD population. From Panel B we conclude that AI-ETD significantly lessens the degree of ETnoD, presumably by disrupting gas-phase secondary structure and increasing the probability of direct dissociation. Panels C and D of Figure 1 display the ETD and AI-ETD mass spectra resulting from ETD activation of the triply protonated precursor of the ACTH peptide (m/z = 978). Similar results were obtained, except in this case ETD produced only three fragment ions whilst AI-ETD resulted in twenty three c- or z•-type products. For an expanded dataset and the effect of laser power, see Supporting Information (SI) Figure 1. As power is increased from 50 to 99% (50W max) so does the intensity of the z7•-type product ion. A simultaneous reduction in the H-atom transfer product (i.e., z-type) is likewise observed, consistent with previous AI-ECD studies.[7] Due to the relatively high pressure of the system (~ 6 mTorr) IR activation did not induce detectable b-, w-, or y-type fragment formation, even at the highest power settings.
Figure 1.
The ETD and AI-ETD spectra of RPKPQQFFGLM (A/B) and ACTH (C/D) generated via 100 ms reaction with radical azobenzene anions. For AI-ETD, laser power was 99%.
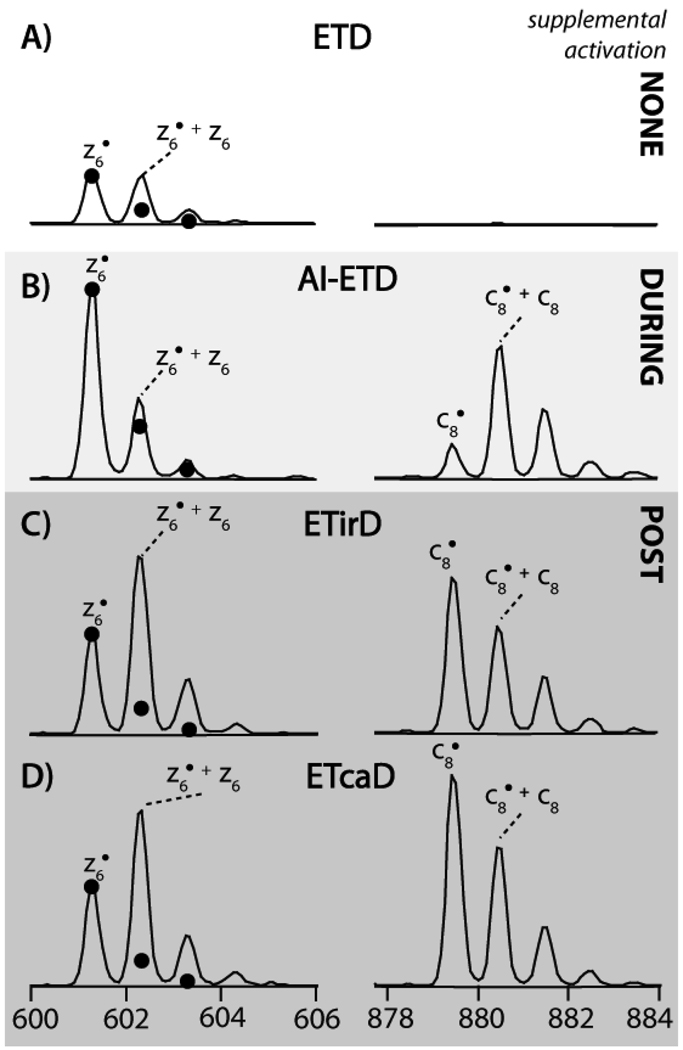
We next explored the degree of H-atom abstraction as this was a significant limitation of our previous ETcaD approach. Figure 2 displays the product ion distributions of the z6• and c8 product ions formed following dissociation of doubly protonated peptide cations having the sequence FSWGAEGQR by several methods. These methods are tripartite: (1) ETD with no other activation (A), (2) ETD with concurrent photo-activation (AI-ETD, B), and (3) ETD with post-activation via either photons (ETirD, C) or collisions (ETcaD, D). Note the intensity scale is identical for all panels, closed circles denote the theoretical isotopic distribution of the z•-type ion. Two principal conclusions can be drawn from these data: (1) AI-ETD results in a marked improvement in intensity and a diminished proclivity for H-atom abstraction as compared to ETD and (2) both post-activation methods (photons and collisions) produce remarkably identical results – increased product formation, but this improvement is primarily accompanied by H atom abstraction. These results are consistent across all peptides examined
Figure 2.
The isotopic distributions of the z6• and c8 product ions for ETD (A), AI-ETD (B), ETirD (C), and ETcaD (D) generated from a 100 ms reaction with radical azobenzene. For AI-ETD and ETirD laser power was set at 99%. Closed circles denote the theoretical distribution of typical ETD product ions.
To further confirm our guiding supposition – that concurrent photo-activation (to the ion/ion reactions) of precursor peptide/protein cations can induce gas-phase unfolding that is maintained in the high pressure environment of the ion trap – we examined ubiquitin cations with charges ranging from +7 to +10. Clemmer et al. have studied ubiquitin conformations under similar pressures.[12] Their work revealed the +7 charge state to be in a compact conformation (cross section ~ 1000 Å2) during the time-scale of our experiments (i.e., 10 ms). As charge state increases, so does the cross-section so that the +10 precursor is described as elongated (~ 1500 Å2). Direct ETD of + 7 precursors produced no detectable fragmentation, low level dissociation was detected for the +8, with the +9 and +10 generating significant fragmentation (data not shown). SI Figure 3 displays selected fragments following ETD for precursors from +7 to +10 and those produced from AI-ETD of the +7. We note the AI-ETD spectrum possesses numerous fragments found only in the +9 or +10 ETD spectra. These data provide further evidence that concomitant photo-activation reduces precursor cation secondary structure during ETD reactions to yield increased fragmentation and reduced H atom rearrangement.
Here we describe AI-ETD and demonstrate the method can substantially improve the utility of ETD for peptide sequence analysis. The method shows particular promise to enable the use of ETD for low charge density peptide precursors (e.g., precursor m/z ~ > 800) where gas-phase secondary structure prevents direct formation of c- and z•-type fragment ions, permitting extensive H atom migration. By limiting intramolecular interactions, AI-ETD generates lower complexity isotopic cluster peaks that more closely resemble theoretically predicted c- and z•-type product distributions as compared to either ETD or post-activation strategies (i.e., ETcaD and ETirD). Another advantage of AI-ETD over post-activation is that no additional time, above that needed for the ion-ion reaction, is required.
Experimental Section
A dual cell linear ion trap mass spectrometer was fitted with two AP ionization sources, ESI for the generation of cations and APCI for the generation of reagent anions. 10.6 µm radiation was generated by use of a 48-5 SYNRAD laser and was introduced on axis with the dual cell trap through a ZnSe window. Instrument firmware and hardware was modified to externally trigger the laser concurrent with ETD reactions, which were conducted in the higher pressure cell (~ 3 mTorr). For a more detailed description, seethe supporting information.
Supplementary Material
Footnotes
We thank Jae Schwartz, George Stafford, and John Syka for helpful discussions and technical assistance. This work was supported by NIH RO1 GM080148 (JJC) and NSF (CHE-0747990 to JJC and CHE-0718320 to JSB);
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Aaron R. Ledvina, Department of Chemistry, University of Wisconsin-Madison Madison, WI, 53706-1322 (U.S.A.)
Graeme C. McAlister, Department of Chemistry, University of Wisconsin-Madison Madison, WI, 53706-1322 (U.S.A.)
Myles W. Gardener, Department of Chemistry, University of Texas-Austin Austin, Texas, 78712-1167 (U.S.A.)
Suncerae I. Smith, Department of Chemistry, University of Texas-Austin Austin, Texas, 78712-1167 (U.S.A.)
James A. Madsen, Department of Chemistry, University of Texas-Austin Austin, Texas, 78712-1167 (U.S.A.)
Dr. Jennifer S. Brodbelt, Department of Chemistry, University of Texas-Austin Austin, Texas, 78712-1167 (U.S.A.)
Dr. Joshua J. Coon, Department of Chemistry, University of Wisconsin-Madison Madison, WI, 53706-1322 (U.S.A.), Fax: (+1) 608-262-0453, jcoon@chem.wisc.edu
References
- 1.Zubarev RA, Kelleher NL, McLafferty FW. J. Am. Chem. Soc. 1998;120:3265. [Google Scholar]
- 2.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9528. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaney DL, McAlister GC, Wirtala M, Schwartz J, Syka JEP, Coon JJ. Anal. Chem. 2007;79:477. doi: 10.1021/ac061457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamidane H, Chiappe D, Hartmer R, Vorobyev A, Moniatte M, Tsybin YO. J. Am. Soc. Mass Spectrom. 2009;20:567. doi: 10.1016/j.jasms.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Horn DM, Breuker K, Frank AJ, McLafferty FW. J. Am. Chem. Soc. 2001;123:9792. doi: 10.1021/ja003143u. [DOI] [PubMed] [Google Scholar]
- 6.Savitski M, Kjeldson F, Nielsen M, Zubarev RA. J. Am. Soc. Mass Spectrom. 2006;18:113. doi: 10.1016/j.jasms.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Lin C, Cournoyer JJ, O’Connor PB. J. Am. Soc. Mass Spectrom. 2008;19:780. doi: 10.1016/j.jasms.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid GE, Shang H, Hogan JM, Lee GU, McLuckey SA. J. Am. Chem. Soc. 2002;124:7353. doi: 10.1021/ja025966k. [DOI] [PubMed] [Google Scholar]
- 9.Pitteri S, Chrisman P, McLuckey SA. Anal. Chem. 2005;77:5662. doi: 10.1021/ac050666h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan D. Recent Advances in ETD experiments for high resolution mass spectrometry; 6th annual Uppsala conference on electron capture and transfer dissociation.2008. [Google Scholar]
- 11.Gunawardena H, He M, Chrisman P, Pitteri S, Hogan J, Hodges B, McLuckey SA. J. Am. Chem. Soc. 2005;127:12627. doi: 10.1021/ja0526057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myung S, Badman ER, Lee YJ, Clemmer DE. J. Phys. Chem. A. 2002;106:9976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.