Abstract
Neurobiology has entered a new era in which optical methods are challenging electrophysiological techniques for their value in measuring and manipulating neuronal activity. This change is occurring largely because of the development of new photochemical tools, some synthesized by chemists and some provided by nature. This review is focused on the three types of photochemical tools for neuronal control that have emerged in recent years. Caged neurotransmitters, including caged glutamate, are synthetic molecules that enable highly localized activation of neurotransmitter receptors in response to light. Natural photosensitive proteins, including channelrhodopsin-2 and halorhodopsin, can be exogenously expressed in neurons and enable rapid photocontrol of action potential firing. Synthetic small-molecule photoswitches can bestow light-sensitivity on native or exogenously expressed proteins, including K+ channels and glutamate receptors, allowing photocontrol of action potential firing and synaptic events. At a rapid pace, these tools are being improved and new tools are being introduced, thanks to molecular biology and synthetic chemistry. The three families of photochemical tools have different capabilities and uses, but they all share in enabling precise and non-invasive exploration of neural function with light.
Introduction
In the beginning, neurophysiologists invented electrodes to learn about electrical excitability and the functioning of neural circuits. But it soon became clear that the nervous system is much too complex to rely entirely on recordings from one, two, or even several neurons at a time. Even within an individual neuron, membrane potential and ion concentrations may not be homogeneous, limiting the usefulness of electrode-based methods that record from a single point in a cell. At least in theory, optical-based recording methods could provide a much more detailed view of activities, either within the complex architecture of an individual neuron or across populations of neurons. The hunt for optical neurophysiological methods was on.
The first breakthrough was the development of optical methods for monitoring activity. Investigators developed a wealth of fluorescent dyes that report on voltage, synaptic vesicle release, Ca2+, and other ions. These indicators have opened new windows for observing different aspects of neuronal signaling within individual neurons and in neural circuits. Small molecule indicators, most notably for Ca2+, have revolutionized our understanding of synaptic transmission. More recently, genetically-expressed GFP-based indicators have been introduced, and they provide insights into many aspects of signal transduction. The search for new indicators continues at a fast pace, but there is still room for improvement. Perhaps the most pressing need is for a genetically-expressed voltage indicator that can resolve single action potentials in individual neurons embedded in a circuit. At the same time, new developments in microscopy are allowing investigators to peer into neural tissue deeper, faster, and with greater spatial resolution than ever before, allowing us to see various aspects of neural activity in real time, and in vivo.
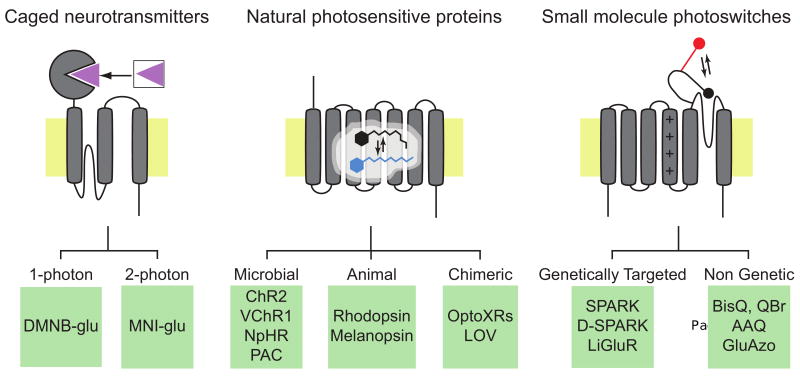
Until recently, optical methods for manipulating neural activity lagged behind methods for measuring activity. But the past few years have seen an explosion of photochemical tools that can be used for controlling neurons, and these tools are the subject of this review. Most of the tools developed to date can be placed in one of three categories: caged neurotransmitters, natural photosensitive proteins, and small molecule photoswitches that bestow light sensitivity on ion channels and receptors (Fig. 1). Each type of tool has its own unique advantages and limitations. When asking a particular neurobiological question, it is important to “choose the right tool for the right job”. We will address the state-of-the-art for each photochemical tool, but it is important to note that this is a rapidly developing field, and we are cataloging the available toolkit at a moment in time, knowing full well that new tools with improved properties and different functionalities are right around the corner.
Figure 1. Photochemical tools for control of neuronal activity.
Three categories of tools are illustrated: Caged molecules (left), natural photosensitive proteins (center) and small molecule photoswitches (right). Specific examples are shown below in the green boxes.
Abbreviations are as follows: DMNB-glu, 4,5-dimethoxy-2-nitrobenzyl-glutamate [4]; MNI-glu, 4-methoxy-7-nitroindolinyl-caged-glutamate [6]; ChR2 (channelrhodopsin-2), a cation channel from Chlamydomonas reinhardtii [22]; VChR1 (channelrodhopsin-1), a cation channel from Volvox carteri [38]; NpHR (halorhodopsin), a chloride transporter from Natronomonas pharaonis [29,30]; PAC, a photoactivatable adenylyl cyclase isolated from Euglena gracilis [39]; OptoXRs, rhodopsin/α1- or rhodopsin/β2-adrenergic receptor chimeras [42]; LOV, the phototropin1 light oxygen voltage domain from Avena sativa [43]; SPARK, a synthetic photoisomerizable azobenzene-regulated K+ channel; D-SPARK [46], a depolarizing SPARK channel [47]; LiGluR, a light-activated glutamate receptor [48]; BisQ, -3,3′-bis[α-(trimethylammonium)methyl]azobenzene dibromide [44]; QBr, 3-(alpha-bromomethyl)-3′-[alpha-(trimethylammonium)methyl]azobenzene bromide [45]; AAQ, acryl-azobenzene-quaternary ammonium [51]; GluAzo, azobenzene-glutamate [53].
Caged neurotransmittters
Caged molecules have a photolabile protecting group that is removed by exposure to light, liberating a bioactive compound. The most widely used caged molecules in neurobiology have been caged agonists for neurotransmitter receptors, although studies have also utilized caged calcium buffers, caged nucleotides, and even caged peptides that influence intracellular signal transduction pathways. The first caged neurotransmitter agonists were o-nitrobenzyl derivatives of carbamoylcholine, an activator of acetylcholine receptors that was released in response to UV light [1,2]. These molecules enabled a rapid jump in agonist concentration in response to light, leading to a better understanding of the kinetics of acetylcholine receptor activation. But it was the development of caged glutamate [3] that had a major impact on neurobiology. Dalva and Katz were the first to use a laser to locally uncage glutamate in an intact brain slice [4]. Laser photostimulation of presynaptic neurons revealed that the pattern of connections to visual cortical neurons changes during development, a finding that would have been difficult to obtain without local glutamate uncaging.
Unfortunately, however, light scattering limits the spatial precision of laser uncaging. This problem motivated the development of caged molecules that could be photolysed by two-photon illumination, which can pinpoint the liberation of neurotransmitter to individual neurons and even individual dendritic spines. MNI-caged glutamate (4-methoxy-7-nitroindolinyl-caged L-glutamate) has a favorable 2-photon cross-section, and because of this, it is now the most popular form of caged glutamate (for review see [5]). Adding to its usefulness, MNI-caged glutamate has a very low rate of spontaneous glutamate liberation in the dark and the free “cage” that is formed as a byproduct of the photolysis reaction has no apparent effect on neuronal function.
Two-photon uncaging of MNI-glutamate can trigger responses that simulate the kinetics and magnitude of individual synaptic events on single dendritic spines [6]. Fortunately, abundant and highly active glutamate transporters rapidly remove the liberated glutamate, minimizing spillover onto neighboring spines. Uncaging at single spines is beneficial for several reasons. It allows direct comparison of spine geometry and post-synaptic responsiveness. It allows precise measurement of spatial summation across neighboring spines. It removes any ambiguity in attributing plastic changes in synaptic function to the pre- vs. the post-synaptic cell.
Highly localized and rapid uncaging of glutamate requires very bright light and a high concentration of caged compound (mM range). These requirements present potential problems of phototoxicity and off-target effects on other types of receptors. The development of new types of caged glutamate with an even more favorable 2-photon cross-section may help alleviate these problems [7,8]. At the same time, investigators are developing forms of caged glutamate that can be uncaged by exposure to visible light [9,10]. These molecules are beneficial because the optical instrumentation required for their use is simpler and less expensive, but two-photon sensitive caged molecules are still the best-suited reagents for ensuring spatial and temporal precision.
Caged versions of many other neurotransmitters have also been synthesized, and these are useful for understanding both intercellular and intracellular signaling systems. Recent developments include new visible light-sensitive [11] and 2-photon-sensitive [12] forms of caged GABA, new types of caged glycine [13] and caged anandamides for local activation or inhibition of endocannabinoid receptors [14]. New tools for studying intracellular signaling include various types of caged Ca2+ [15], a caged IP3 that is two-photon sensitive [16], and caged peptides that interfere with synaptic vesicle exocytosis [17].
A different type of photosensitive compound can irreversibly disrupt the function of certain types of glutamate receptors in response to light [18]. ANQX is an azide containing analog of commonly used AMPA receptor antagonists (e.g. CNQX and DNQX). Exposure of bound ANQX to UV light results in covalent attachment to the AMPA receptor, permanently preventing the binding of glutamate or other agonists. ANQX has been useful for probing the turnover of AMPA receptors in synapses between hippocampal neurons, a process that is thought to play a crucial role in long-term synaptic plasticity and learning and memory [19]. So far, studies utilizing ANQX have been limited to neurons in culture, but compounds with different properties, including perhaps a more favorable 2-photon cross-section, could enable ANQX to reveal receptor trafficking in more intact preparations including brain slices. Azido derivatives of antagonists of other neurotransmitter receptors might be used in a similar manner to explore receptor turnover and its possible activity-dependence.
Natural photosensitive proteins
It is hard to believe that is was only 6 short years ago that Nagel and colleagues discovered ChR2, a directly light-sensitive cation channel from the algae Chlamydomonas. After publication of their paper [20], at least a few astute neurobiologists recognized that ChR2 could potentially make a very useful tool for photostimulation of neurons. However, there were two hurdles that first needed to be overcome: 1) the gene encoding ChR2 would need to be expressed and the protein trafficked to the membrane of neurons in sufficiently high quantities, and 2) enough of the chromophore (all-trans-retinal) would need to be present to convert the channelrhodopsin-2 apoprotein (sometimes called ChOP2) into a light-sensitive holoprotein (ChR2). Electroporation of the ChR2 gene into chick spinal cord neurons was sufficient to allow optical stimulation [21], but an important technical breakthrough came when Karl Deisseroth and colleagues “humanized” the codon usage of the ChR2 gene to optimize its expression in mammalian cells [22]. They also found that ordinary cell culture media had sufficient retinal to render cultured neurons highly sensitive to light, and remarkably, they later found that there is enough chromophore present in the mammalian brain that addition of exogenous retinal is unnecessary. It should be noted, that in invertebrates, (e.g. Drosophila and C. elegans) exogenous chromophore is required before neurons can respond to light.
ChR2 has several favorable properties that make it an effective tool for optically stimulating neuronal firing [23]. The channels can be activated very rapidly (within 30 μs) in response to visible light (λmax=500 nm), and the channel closes quickly at light offset. Brief flashes of light can trigger trains of neuronal spikes to the maximal firing frequency limit of a neuron. This allows precise programming of patterns of spike activity, which may be useful for mimicking and therefore better understanding temporal coding in neural systems, and should also be a benefit for studying synaptic events with strict temporal requirements, including spike-timing dependent plasticity.
The single channel conductance of ChR2 (40 fS) [24] is several orders of magnitude smaller than the endogenous voltage-gated channels that normally control neuronal firing (typically 5-240 pS). So a great abundance of ChR2 protein must be expressed and delivered to the plasma membrane for photostimulation to be fast and effective. However, with an appropriately strong promoter driving ChR2 gene expression (e.g. CMV or EF-1α), the expressed protein level has proven to be sufficient. Other than being light-sensitive, ChR2-expressing neurons have normal electrophysiological properties, and there is no evidence of the high level of ChR2 expression interfering with the trafficking or membrane density of other channels or receptors, although this has not been studied extensively.
A variety of gene delivery methods, including transfection [22], electroporation [25], viral transduction [26], and transgenesis [27], have been used to deliver the ChR2 gene into mammalian neurons. Expression can be limited to a particular neuronal population by including a cell-type selective gene promoter element upstream of the ChR2 coding sequence. There is already a long list of neuron types that have been rendered light-sensitive with ChR2, and we expect that the list will continue to grow rapidly. The combination of optical control and genetic targeting has given rise to a new term for this approach, called “optogenetics” [28]. Detailed information about obtaining and using ChR2 and related tools can be found at the “Optogenetics Resource Center” website (http://www.stanford.edu/group/dlab/optogenetics/).
ChR2 can effectively trigger neuronal firing in response to light, but it would further benefit neurobiology to have a complementary tool for inhibiting activity with light. Sure enough, Deisseroth and colleagues [29], as well as Han and Boyden [30] reported the discovery and neuronal expression of another light-sensitive protein that suppresses action potentials in response to yellow light (λmax=570nm). Halorhodopsin (NpHR), from halobacteria, is a light-driven Cl- pump, and like ChR2, it employs all-trans retinal as its chromophore. Exogenous expression of NpHR enables light to rapidly suppress action potentials, such that brief flashes can “subtract out” individual action potentials from within a train of spikes. Because the excitation wavelength differs for ChR2 and NpHR, they can be co-expressed and selectively activated in the same neuron, allowing different wavelengths to up-or down-regulate firing [29,30].
So far, NpHR has been applied to far fewer systems than ChR2, largely because of difficulties with achieving high levels of exogenous expression needed for effective light-elicited inhibition. However, investigators are working hard to improve NpHR expression and reduce intracellular retention by improving the signal peptide sequence and adding an ER export signal [31,32].
Other modifications have been made to improve the performance of ChR2. The kinetics, spectral sensitivity, and trafficking of ChR2 have all been modified through mutagenesis. ChR2 activates very rapidly with light, but it also partially inactivates over time, limiting its ability to evoke sustained neuronal activity during prolonged light exposure. To alleviate this problem, mutations have been introduced to convert ChR2 into a bi-stable switch that can generate sustained depolarization in response to brief flashes of light [33]. Chimeric combinations have been made between ChR2 and ChR1, a related light-activated proton channel [34], to elucidate determinants of various functional properties [35]. Some chimeras have faster inactivation kinetics, which might enable a faster spike repetition rate in response to brief light flashes [36]. Another chimeric combination between ChR2 and a protein with a myosin-binding domain localizes the protein to somato-dendritic regions and excludes it from axons [37]. Specific targeting of ChR2 to presynaptic terminals is still unattained. Perhaps this would allow optical stimulation of neurotransmitter release with spatial and temporal precision to rival local 2-photon uncaging of glutamate.
An alternate strategy is to search the genomes of microbes in the hope of finding light-regulated proteins with more desirable properties. This approach resulted in the discovery of a light-activated channelrhodopsin homolog from Volvox (VChR1), whose peak activation wavelength is red-shifted by ∼70 nm [38]. This enables photostimulation of neurons with yellow light, which penetrates deeper into neural tissue and avoids spectral overlap with Ca2+ indicator dyes that are often used for monitoring neural activity. Investigators have also found in the flagellate Euglena a photosensitive adenylyl cylase (PAC), which uses as a blue-light sensitive chromophore, flavin adenine nucleotide, a common cofactor found in all cells. PAC can be exogenously expressed in neurons and other cells, and illumination leads to production of cyclic AMP and activation of downstream effects within tens of msec [39].
Of course, one needn't look to such exotic sources for natural light-sensitive proteins; several are present in the retinal photoreceptors in our own eyes. Rhodopsin and related proteins regulate ion channel activity through a G-protein coupled biochemical cascade. Hence controlling the electrophysiology of ordinary neurons with rhodopsin would seem to require exogenous expression of multiple genes encoding several proteins in this pathway, as demonstrated by Miesenbock and colleagues working in Drosophila [40]. Surprisingly, in vertebrate neurons exogenous rhodopsin can couple to endogenous signaling proteins to regulate native ion channels [21]. Similar results have been obtained with exogenous expression of melanopsin [41], which is normally found only in intrinsically photosensitive retinal ganglion cells. The light response in neurons exogenously expressing rhodopsin or melanopsin tends to be slow and small, and the specific nature of the response is variable, depending on the particular types of native ion channel targets that are present in the host neuron. These factors make the photosensitive proteins from animals less attractive tools than their microbial counterparts.
Rhodopsin is part of the large family of 7-transmembrane receptors that couple to several different G-proteins to exert myriad effects on cells. Recent work has shown that light-sensitivity can be bestowed on two different G-protein signaling systems by generating chimeric combinations between rhodopsin and either the Gq-coupled α1a-adrenergic receptor or the Gs-coupled β2-adrenergic receptor [42]. Exogenous neuronal expression of these chimeric receptors, termed Opto-XRs, resulted in light regulation of the appropriate signaling cascade, leading to opposing effects on spike firing. This exciting study demonstrates that photochemical tools can be applied to metabotropic receptors, potentially regulating diverse and subtle aspects of electrophysiological function.
Very recently, another promising approach has been introduced, utilizing the flavin-binding LOV domain from a plant protein called phototropin1 [43]. The LOV domain was fused onto a constitutively-active mutant of Rac1, a key GTPase that regulates actin cytoskeletal dynamics. Illumination with visible light removed LOV-mediated inhibition of Rac1, resulting in precisely localized changes in cell shape and triggering cell motility. At least in theory, the LOV domain could act as a dominant-negative regulator of many cellular proteins, enabling photoregulation of a broad range of functions.
Small molecule photoswitches
Photosensitive tools for neuronal control can also be rationally designed and manufactured through synthetic chemistry. The general strategy is to couple a photoisomerizable molecule (i.e. a “photoswitch”) onto an ordinary ion channel or receptor to make it sensitive to light. In theory, the photoswitch can be attached in such a way that photoisomerization exerts force on the channel, causing it to open. Alternatively, the photoisomerization could deliver or remove a ligand from a binding site on the channel or receptor, thereby regulating its activity. In practice, the photoswitchable ligand approach has worked nicely with voltage-gated K+ channels and glutamate receptors. However, in theory, the photoswitchable ligand approach could apply to virtually any ion channel or receptor, as long as there are known ligands that regulate activity.
There are several chemical photoswitches available, but the photoisomerizable small molecule azobenzene has emerged as best suited for biological applications. In darkness, azobenzene exists in a linear trans configuration, but 380 nm light promotes transition to the bent cis configuration, which is ∼7Ά shorter. In darkness, the cis form relaxes slowly back to the trans form (over minutes), but this relaxation can be accelerated by exposure to 500 nm light. Azobenzene compounds are relatively easy to synthesize, have well-defined geometries, and show high photochemical stability and little phototoxicity.
Erlanger and colleagues were the first to apply the photoisomerizable ligand approach to control the activity of a receptor [44,45]. They synthesized a soluble photoisomerizable molecule, Bis-Q, and a cysteine-reactive derivative QBr, both of which activate the nicotinic acetylcholine receptor (nAChR) in the trans configuration but not in the cis configuration. QBr covalently attaches to the nAChR, but only after reducing disulfide bonds between native cysteine residues. QBr was particularly useful for rapidly delivering and removing the ligand to minimize desensitization of the nAChR, enabling detailed study of the mechanisms of receptor activation.
The molecular biology revolution has allowed investigators to take the photoswitchable ligand approach one step further. Instead of relying on a native cysteine, a particular channel or receptor can be targeted for photoswitch attachment by genetically engineering a cysteine into the appropriate location on the protein. The first step is to identify a ligand that can be modified so that it can be conjugated to the azobenzene without losing its ability to bind and regulate channel activity. Structural information about ion channels and receptors can guide the engineering of the target protein, in particular the position of the cysteine attachment site.
This approach was first used to generate a Synthetic Photoswitchable Azobenzene-Regulated K+ channel (SPARK) [46]. SPARK channels are generated by coupling a photoswitchable ligand, maleimide-azobenzene-quaternary ammonium (MAQ), onto a genetically engineered Shaker K+ channel. The Maleimide is for cysteine tethering, the Azobenzene is for photoswitching, and the Quaternary ammonium group blocks the pore of the Shaker channel. The channel is only blocked when MAQ is in its extended trans form, and not in the shorter cis form. MAQ enables control of action potential firing only in those neurons that express the cysteine-containing Shaker channel. Visible light blocks SPARK channels, allowing action potential firing. UV light retracts the pore blocker, promoting the flux of K+ through the channel, which hyperpolarizes the neuron and inhibits action potential firing. A mutation that alters the ionic selectivity of the K+ channel changes the polarity of the effects, enabling depolarization and induction of action potentials with UV light [47].
Glutamate receptors are another class of ion channels where photswitchable tethered ligands have been successfully applied. In 2006, a light-gated ionotropic glutamate receptor (LiGluR) was introduced [48]. This system is based on a glutamate derivative covalently attached to a genetically engineered kainate receptor (iGluR6) via an azobenzene tether. In its original embodiment, the tethered neurotransmitter was presented to the clamshell-like binding site in the cis configuration (380 nm light) and retracted in the trans configuration (500 nm light). Changing the attachment site reversed the polarity, with 500 nm turning the receptor on and 380 nm light turning it off [49]. LiGluR and its modifications can be employed to control neural activity in vitro and in vivo [50]. Very recently, LiGluR has proven to be a valuable tool for the dissection of neural circuits that control behavior in zebrafish [60].
The first-generation light-activated K+ channel (SPARK) and glutamate receptor (LiGluR) were designed specifically for light-induced neuronal inhibition and excitation. Each of these photoswitch-ready channels was derived from a particular generic channel, chosen because of prior structure-function information and favorable properties. However, there is no reason why many other K+ channels and glutamate receptors would not become photoswitchable, if a cysteine attachment site were included in the correct position on the channel. For example, we have now generated several light-regulated K+ channels, including Kv3.1 and SK2 (unpublished results). This gives neurobiologists optical tools for selectively regulating functions carried out by different K+ channels. Indeed, given sufficient motivation by chemists and neurobiologists, the photoswitchable tethered ligand approach should be applicable to many other types of voltage-and ligand-gated channels.
SPARK channels and LiGluR, like ChR2 and NpHR, are genetically-encoded tools and therefore can be targeted to particular types of neurons by selective gene expression. The neuronal specificity that comes from genetic targeting is often a big advantage, but in some cases exogenous gene expression is not practical and may not even be desirable (e.g., in humans). This has motivated the development of small molecule photoswitches that act on native channels or receptors without requiring exogenous gene expression. We have developed a family of azobenzene-containing molecules that photosensitize a wide variety of native voltage-gated K+ channels [51]. We have shown that one of these molecules, AAQ, imparts light-sensitivity on neurons in cell culture, in brain slices, and in intact retina. Light-elicited blockade of K+ channels can cause membrane depolarization, leading to action potential firing. Unlike the genetically-encoded tools that enable light to over-ride the normal activity of the neuron, AAQ enables light to alter the intrinsic excitability of the neuron. Hence the properties that are regulated by K+ channel activity, including action potential threshold, propensity for repetitive firing, and spike afterhyperpolarization, can also be regulated by light in AAQ-treated neurons.
AAQ and most analogs block K+ channels when the molecule is in the trans form (500 nm light) and unblock in the cis form (380 nm light). However, a related molecule named PrAQ, blocks in the cis and unblocks in the trans form. Hence, this molecule should allow light to regulate neuronal firing in the opposite manner to AAQ [52]. AAQ-mediated photocontrol of neurons persists for up to 24 hours after treatment, and it was initially proposed that AAQ covalently attaches to K+ channels. However, related photoswitch molecules lack a reactive group and yet still impart light-sensitivity on native K+ channels, suggesting that they linger in or near the channels without covalent attachment. Whatever the mechanism of photosensitization, these tools share in their ability to impart light-sensitivity without involving the introduction of exogenous genes.
An azobenzene-containing photoswitch has also been developed that enables photoregulation of native glutamate receptors [53]. This “reversibly caged” glutamate (Glu-Azo) was shown to act on kainate receptors and reversibly trigger action potential firing in dissociated hippocampal neurons. Although its reversibility might be considered an advantage over classical caged glutamate, its usefulness in brain slices and live animals remains to be demonstrated.
The right tool for the right job
It has been suggested that neurobiologists need a “Consumers Guide” to provide an unbiased comparison of the various photochemical tools currently available for controlling neuronal activity. The reality is that all of the tools covered in this review have merits---choosing the right tool depends on the specific question and experimental system that is being explored. We expect that new photochemical tools will continue to emerge, filling up the toolbox of methods for optically manipulating many aspects of neuronal function.
At present, the tool that is best suited for studying the kinetics and localization of synaptic events is caged glutamate. This is largely a consequence of two-photon uncaging, which has enabled investigators to locally liberate glutamate with great temporal and spatial precision, mimicking neurotransmitter release elicited by a single action potential as it invades a single presynaptic bouton. Abundant glutamate transporters on glial cells ensure that the liberated glutamate is rapidly cleared without spilling over to neighboring synapses. However, repeated or prolonged photolysis can lead to local depletion of caged glutamate, so this method is less appropriate for simulating glutamate release occurring during high frequency or prolonged spike trains.
The natural photosensitive proteins, particularly ChR2, have several characteristics that are very well-suited for controlling activity and mapping circuit connections in specific neural pathways. They can be genetically-targeted, but photosensitizing a particular cell type depends on the availability of a promoter that is specific for that cell type. An alternative is to use transgenic mice that express ChR2 under the control of the Thy-1 promoter [27]. This results in mouse lines that express the channel in different neuronal populations, hopefully including one that the experimenter finds interesting. ChR2-expressing neurons can be activated by visible light, but there is uncertainty about 2-photon activation with infrared light, which would penetrate deeper into tissue. The most important advantage of ChR2 is the bioavailability of its chromophore (retinal) in the mammalian brain, which greatly facilitates experiments, particularly those performed in vivo.
The small molecule photoswitch approach brings to the table tremendous flexibility in the design of tools for neuronal control. The photoswitch molecules are synthesized from scratch, so at least in theory, the awesome power of chemistry can be used to generate the exact molecule that can activate or inhibit the function of a given ion channel or receptor. It should be relatively easy, for instance, to turn a tethered agonist into a tethered antagonist, to red-shift the action spectrum of the photoswitch so that UV light is no longer necessary, or to tinker with the relaxation kinetics of the photoswitch. The polarity of photoswitching can be reversed by changing the site of covalent attachment or the nature of the anchoring group, as demonstrated for both light-regulated K+ channels and light-regulated glutamate receptors. Photoswitches with a favorable 2-photon cross-section could be developed to allow precise spatial control of transmembrane potentials, for instance at individual dendritic spines. Finally, with the right photoswitchable ligands, it might be possible to regulate not only voltage-gated and ligand gated-ion channels, but also G-protein coupled receptors, growth factor receptors and other receptor tyrosine kinases, and transporters.
Like the natural photosensitive protein approach, small molecule photoswitches can be used to “over-ride” the normal activity of neurons to provide insights into neural circuits. However, the photoswitch approach also provides an opportunity for learning about the roles of channels and receptors that normally control neuronal signaling. This feature applies to photoswitches acting on wild-type channels, such as AAQ, but the implications are particularly important for photoswitches that couple to cysteine-containing mutant channels. A photoswitch-ready version of a specific type of K+ channel or glutamate receptor can be genetically targeted to a particular cell type, and after adding the photoswitch, only that specific target will be photosensitive. Instead of searching for a drug that is specific for a given channel, the combination of genetic engineering and chemical engineering results in an entirely new way to manipulate specific target proteins, a step beyond conventional pharmacology. The ultimate application of this approach lies in gene knock-in technology, which will enable the substitution of a wild-type channel with the cysteine-containing mutant channel in a living animal. Addition of the photoswitch will result in the ability to specifically photoregulate one specific type of channel to the exclusion of others. In addition to precise temporal and spatial control, this approach provides the new dimension of precise biochemical control of a specific ion channel or receptor.
A common challenge: delivering light to the nervous system
All of the photochemical tools described in this review require the effective delivery of light to the part of the nervous system being controlled. Projecting light onto neurons in culture or in brain slices is relatively easy, but delivering light onto neurons in vivo presents a major challenge. The brain is encased in an opaque cranium which presents a formidable barrier. Even after removal of cranial bone and the overlying dura, brain tissue tends to scatter light which limits spatial precision and makes it more difficult to affect structures far from the illuminated surface.
The retina is the one part of the nervous system that is normally exposed to light, making it a useful platform for testing photochemical tools. Of course, the retina is an interesting and important part of the central nervous system in its own right, and there is great clinical interest in developing tools that can impart light-sensitivity on retinal neurons that are not normally photoresponsive. Retinitis pigmentosa and macular degeneration are degenerative blinding diseases in which the normal rod and cone photoreceptors are destroyed, leaving the retina with no effective way to signal the visual cortex about light. Expression of ChR2 in either retinal ganglion cells [54] or bipolar cells [55] can restore visual sensitivity to retinas of animals with mutations that cause rods and cone degeneration. Expression of melanopsin [41] or halorhodopsin [56] is also effective, as is intra-ocular injection of AAQ (unpublished results). Photoregulation of all of these tools require high intensity light, and azobenzene-based photoswitches require short wavelength illumination, which can be damaging over a prolonged time. For these reasons, there is a need for red-shifted photochemical tools that also have enhanced light-sensitivity. Nevertheless, these studies provide hope that some neurological disorders might be treatable in a relatively non-invasive manner, using light to regulate activity in the parts of a neuron circuit that lie downstream from sites of damage or degeneration. The photochemical tools allowing this to occur may be genetically-expressed, but small molecule photoswitches provide an alternate means for imparting light-sensitivity while avoiding gene therapy.
Despite the obvious difficulties, bioengineers have succeeded in delivering light into the brain with implanted fiber optics. Fiber coupled systems have been used for optical measurement [57] or manipulation [58] of neural activity. Recent studies [58] raise the possibility of substituting light for electrodes in “deep brain stimulation”, a procedure that is being used increasingly for treatment of Parkinson's disease and other neuropsychiatric disorders.
Finally, the delivery of light for neural control involves an important, but rarely discussed trade-off between effectiveness and precision. On one hand, a highly localized optical stimulus that illuminates part of a single neuron could ensure exclusive stimulation of only that cell. On the other hand, the light-regulated proteins are usually distributed over much of the cell surface, and more widespread illumination will activate more of these proteins resulting in a faster and more powerful effect. There has been considerable interest in developing photosensitive molecules that are highly sensitive to 2-photon illumination, because this would permit deeper and more precise photocontrol in neural tissue. However, the benefits of pinpoint accuracy will be offset by the asynchronous recruitment of photoactivated proteins as the 2-photon laser scans through a given focal plane within the tissue. New optical methods involving holographic illumination may help solve this problem by allowing simultaneous activation of distributed photosensitive molecules, with spatial and temporal precision that rivals two-photon liberation of caged glutamate [59].
Acknowledgments
We thank the entire Kramer Lab for helpful discussions. RH Kramer is supported by grants from the National Eye Institute (EY018957), the National Institute of Mental Health (MH088484), and the Nanomedicine Development Center for the Optical Control of Biological Function (PN2 EY018241).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Walker JW, McCray JA, Hess GP. Photolabile protecting groups for an acetylcholine receptor ligand. Synthesis and photochemistry of a new class of o-nitrobenzyl derivatives and their effects on receptor function. Biochemistry. 1986;25:1799–1805. doi: 10.1021/bi00355a052. [DOI] [PubMed] [Google Scholar]
- 2.Milburn T, Matsubara N, Billington AP, Udgaonkar JB, Walker JW, Carpenter BK, Webb WW, Marque J, Denk W, McCray JA, et al. Synthesis, photochemistry, and biological activity of a caged photolabile acetylcholine receptor ligand. Biochemistry. 1989;28:49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- 3.Wieboldt R, Gee KR, Niu L, Ramesh D, Carpenter BK, Hess GP. Photolabile precursors of glutamate: synthesis, photochemical properties, and activation of glutamate receptors on a microsecond time scale. Proc Natl Acad Sci U S A. 1994;91:8752–8756. doi: 10.1073/pnas.91.19.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalva MB, Katz LC. Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science. 1994;265:255–258. doi: 10.1126/science.7912852. [DOI] [PubMed] [Google Scholar]
- 5.Ellis-Davies GC. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This is an excellent recent review of the design principles and practical usage of a variety of caged compounds, including new molecules that are particularly sensitive to 2-photon uncaging.
- 6.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]; **First reported use of MNI-glutamate, a caged neurotransmitter with a favorable two-photon cross-section. Two-photon uncaging of MNI-glutamate results in highly localized responses in single dendritic spines, allowing direct comparison of receptor function and spine geometry.
- 7.Momotake A, Lindegger N, Niggli E, Barsotti RJ, Ellis-Davies GC. The nitrodibenzofuran chromophore: a new caging group for ultra-efficient photolysis in living cells. Nat Methods. 2006;3:35–40. doi: 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- 8.Ellis-Davies GC, Matsuzaki M, Paukert M, Kasai H, Bergles DE. 4-Carboxymethoxy-5,7-dinitroindolinyl-Glu: an improved caged glutamate for expeditious ultraviolet and two-photon photolysis in brain slices. J Neurosci. 2007;27:6601–6604. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shembekar VR, Chen Y, Carpenter BK, Hess GP. A protecting group for carboxylic acids that can be photolyzed by visible light. Biochemistry. 2005;44:7107–7114. doi: 10.1021/bi047665o. [DOI] [PubMed] [Google Scholar]
- 10.Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: Two-Photon and Visible-Light Photoactivation of Neurons and Dendritic spines. Front Neural Circuits. 2009;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Front Neural Circuits. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Visual light photorelease of GABA with a novel caged-GABA compound that uses a ruthenium complex as the photosensor.
- 12.Curten B, Kullmann PH, Bier ME, Kandler K, Schmidt BF. Synthesis, photophysical, photochemical and biological properties of caged GABA, 4-[[(2H-1-benzopyran-2-one-7-amino-4-methoxy) carbonyl] amino] butanoic acid. Photochem Photobiol. 2005;81:641–648. doi: 10.1562/2004-07-08-RA-226. [DOI] [PubMed] [Google Scholar]
- 13.Shembekar VR, Chen Y, Carpenter BK, Hess GP. Coumarin-caged glycine that can be photolyzed within 3 microseconds by visible light. Biochemistry. 2007;46:5479–5484. doi: 10.1021/bi700280e. [DOI] [PubMed] [Google Scholar]
- 14.Heinbockel T, Brager DH, Reich CG, Zhao J, Muralidharan S, Alger BE, Kao JP. Endocannabinoid signaling dynamics probed with optical tools. J Neurosci. 2005;25:9449–9459. doi: 10.1523/JNEUROSCI.2078-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis-Davies GC. Neurobiology with caged calcium. Chem Rev. 2008;108:1603–1613. doi: 10.1021/cr078210i. [DOI] [PubMed] [Google Scholar]
- 16.Kantevari S, Hoang CJ, Ogrodnik J, Egger M, Niggli E, Ellis-Davies GC. Synthesis and two-photon photolysis of 6-(ortho-nitroveratryl)-caged IP3 in living cells. Chembiochem. 2006;7:174–180. doi: 10.1002/cbic.200500345. [DOI] [PubMed] [Google Scholar]
- 17.Kuner T, Li Y, Gee KR, Bonewald LF, Augustine GJ. Photolysis of a caged peptide reveals rapid action of N-ethylmaleimide sensitive factor before neurotransmitter release. Proc Natl Acad Sci U S A. 2008;105:347–352. doi: 10.1073/pnas.0707197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers JJ, Gouda H, Young DM, Kuntz ID, England PM. Photochemically knocking out glutamate receptors in vivo. J Am Chem Soc. 2004;126:13886–13887. doi: 10.1021/ja048331p. [DOI] [PubMed] [Google Scholar]; *A photoaffinity label that binds to AMPA receptors and permanently eliminates function in response to UV light.
- 19.Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]; *One of the first papers demonstrating heterologous functional expression of rhodopsin and ChR2 in neurons. Genes encoding these proteins were electroporated into chick spinal cord and the two photosensitive proteins were used to alter neuronal activity in an antagonistic manner with high temporal precision.
- 22.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]; **This landmark paper showed that exogenous expression of channelrhodopsin-2 can be used to rapidly control neuronal firing with light. Brief light flashes can trigger realistic and precise patterns of spike firing.
- 23.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 24.Feldbauer K, Zimmermann D, Pintschovius V, Spitz J, Bamann C, Bamberg E. Channelrhodopsin-2 is a leaky proton pump. Proc Natl Acad Sci U S A. 2009;106:12317–12322. doi: 10.1073/pnas.0905852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 26.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]; **Introduction of the light-activated Cl- transporter halorhodopsin and use of channelrhodopsin and halorhodopsin to up- and down-regulate neuronal firing with different wavelengths of light.
- 30.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, Deisseroth K, Augustine GJ, Feng G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008;36:141–154. doi: 10.1007/s11068-008-9034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 34.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Sugiyama Y, Hikima T, Sugano E, Tomita H, Takahashi T, Ishizuka T, Yawo H. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from chlamydomonas. J Biol Chem. 2009;284:5685–5696. doi: 10.1074/jbc.M807632200. [DOI] [PubMed] [Google Scholar]
- 36.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis TL, Jr, Mao T, Svoboda K, Arnold DB. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci. 2009;12:568–576. doi: 10.1038/nn.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Prigge M, Beyriere F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroder-Lang S, Schwarzel M, Seifert R, Strunker T, Kateriya S, Looser J, Watanabe M, Kaupp UB, Hegemann P, Nagel G. Fast manipulation of cellular cAMP level by light in vivo. Nat Methods. 2007;4:39–42. doi: 10.1038/nmeth975. [DOI] [PubMed] [Google Scholar]
- 40.Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 41.Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]; * This paper shows that chimeric combinations between rhodopsin and G-protein-coupled receptors yield light-sensitive proteins that initiate G-protein signaling cascades (opto-XRs). Two different optoXRs selectively recruit distinct signaling pathways in response to light, resulting in opposite effects on spike firing in brain neurons.
- 43.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009 doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This paper shows that photosensitivity can be bestowed on an intracellular signaling protein by coupling it with part of the phototropin-1 protein from plants. Specifically, the LOV domain, which binds a flavin chromophore, enables activation of the actin cytoskeletal regulatory protein Rac in response to visible light.
- 44.Bartels E, Wassermann NH, Erlanger BF. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971;68:1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lester HA, Krouse ME, Nass MM, Wassermann NH, Erlanger BF. A covalently bound photoisomerizable agonist: comparison with reversibly bound agonists at Electrophorus electroplaques. J Gen Physiol. 1980;75:207–232. doi: 10.1085/jgp.75.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]; **The first use of a light-activated ion channel to regulate neuronal firing A genetically modified Shaker K+ channel protein was linked to an azobenzene-containing photoswitch, enabling photocontrol of K+ current and action potential firing in cultured hippocampal neurons.
- 47.Chambers JJ, Banghart MR, Trauner D, Kramer RH. Light-induced depolarization of neurons using a modified Shaker K(+) channel and a molecular photoswitch. J Neurophysiol. 2006;96:2792–2796. doi: 10.1152/jn.00318.2006. [DOI] [PubMed] [Google Scholar]
- 48.Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Introduction of a light-activated glutamate receptor (LiGluR), consisting of a glutamate tethered to a kainate receptor protein through an photoisomerizable azobenzene. Photoactivation of LiGluR in cultured neurons results in rapid depolarization and triggering of action potential firing.
- 49.Numano R, Szobota S, Lau AY, Gorostiza P, Volgraf M, Roux B, Trauner D, Isacoff EY. Nanosculpting reversed wavelength sensitivity into a photoswitchable iGluR. Proc Natl Acad Sci U S A. 2009;106:6814–6819. doi: 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D, et al. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Photoswitch molecules that impart light-sensitivity on native K+ channels in neurons, enabling optical control of excitability without exogenous gene expression.
- 52.Banghart MR, Mourot A, Fortin DL, Yao J, Kramer RH, Trauner D. Photochromic blockers of K+ channels. Angewandte Chemie. 2009 doi: 10.1002/anie.200904504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volgraf M, Gorostiza P, Szobota S, Helix MR, Isacoff EY, Trauner D. Reversibly caged glutamate: a photochromic agonist of ionotropic glutamate receptors. J Am Chem Soc. 2007;129:260–261. doi: 10.1021/ja067269o. [DOI] [PubMed] [Google Scholar]
- 54.Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Exogenous expression of channelrhodopsin-2 in retinal ganglion cells imparts light-sensitivity on retinas from mutant mice lacking rods and cones.
- 55.Lagali PS, Balya D, Awatramani GB, Munch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Ivanova E, Bi A, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29:9186–9196. doi: 10.1523/JNEUROSCI.0184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung EL, Schnitzer MJ. Fiberoptic fluorescence imaging. Nat Methods. 2005;2:941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 59.Lutz C, Otis TS, DeSars V, Charpak S, DiGregorio DA, Emiliani V. Holographic photolysis of caged neurotransmitters. Nat Methods. 2008;5:821–827. doi: 10.1038/nmeth.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyart C, Bene FD, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioral module in the vertebrate spinal cord. Nature. 2009 doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]