Abstract
Light-activated ion channels provide a new opportunity to precisely and remotely control neuronal activity for experimental applications in neurobiology. In the past few years, several strategies have arisen that allow light to control ion channels and therefore neuronal function. Light-based triggers for ion channel control include caged compounds, which release active neurotransmitters when photolyzed with light, and natural photoreceptive proteins, which can be expressed exogenously in neurons. More recently, a third type of light trigger has been introduced: a photoisomerizable tethered ligand that directly controls ion channel activity in a light-dependent manner. Beyond the experimental applications for light-gated ion channels, there may be clinical applications in which these light-sensitive ion channels could prove advantageous over traditional methods. Electrodes for neural stimulation to control disease symptoms are invasive and often difficult to reposition between cells in tissue. Stimulation by chemical agents is difficult to constrain to individual cells and has limited temporal accuracy in tissue due to diffusional limitations. In contrast, ion channels that can be directly activated with light allow control with unparalleled spatial and temporal precision. The goal of this chapter is to describe light-regulated ion channels and how they have been tailored to control different aspects of neural activity, and how to use these channels to manipulate and better understand development, function, and plasticity of neurons and neural circuits.
I. Introduction
Neurons possess ion channels that are opened by changes in membrane voltage, changes in temperature, ligand binding, and mechanical forces, but none are known to be directly sensitive to light. Therefore, when experimentalists want to modulate neuronal activity mediated by ion channels, they typically will apply external electrical or chemical stimuli. This entails either the placement of electrodes or perfusion devices into or onto a neuronal preparation. Light stimulation from afar could in many instances be preferable to stimulation with physically invasive electrodes or inaccurate perfusion devices. Light can be projected onto a tissue preparation with great temporal and spatial precision. It can be focused onto single cells or even subcellular structures and it can also be quickly redirected between different cells in a population. With modern laser scanning technology, patterns of light can be “painted” on large groups of neurons, enabling precise control over neural networks through the light-mediated activation of ion channels allowing for much more advanced experiments than have been previously possible. In addition, three-dimensional two-photon excitation offers the promise of activating structures deep inside tissue; areas that were once inaccessible without physical damage to the tissue.
A. Photorelease of Caged Compounds
The caged neurotransmitter approach utilizes small, drug-like molecules that contain a neurotransmitter (e.g., glutamate (GLU)) that is protected by a photolabile accessory group (the “cage”). Illumination with light of the correct frequency rapidly removes the cage from the molecule, releasing active neurotransmitter. Photo-induced release of caged GLU has been used to accurately mimic the kinetics of synaptic release (Callaway and Yuste, 2002; Katz and Dalva, 1994) with spatial control that has allowed mapping of neuronal connections (Callaway and Katz, 1993; Matsuzaki et al., 2008; Shoham et al., 2005). However, the approach does have drawbacks that could hinder some experiments. Uncaged neurotransmitter can diffuse through the extracellular milieu and linger for some time, potentially activating neighboring, untargeted neurons and limiting the frequency of repetitive stimulation. Moreover, other liberated photoproducts (i.e., the free cage) may result in unintended side effects on exposed cells. While the global concentration of caged neurotransmitters is typically rather high (millimolar range), the local concentration of the caged compound may become depleted with even moderately long illumination (>1 s). This approach may be inappropriate for eliciting long-term responses like those thought to underlie long-term potentiation that result from homeostatic regulation of ion channels and receptors.
B. Photoactivation Using Natural Photoproteins
The natural photoprotein approach involves exogenous expression of an opsin-related protein (e.g., rhodopsin). The protein does not itself sense light, but operates through a covalently attached photoisomerizable chromophore (e.g., retinal). Usually, the light-activated protein signals downstream cellular events through a G-protein-coupled mediated cascade and at least some of these accessory proteins must be coexpressed to support a heterologous light response (Zemelman et al., 2002). Light responses triggered by photoproteins such as rhodopsin (Zemelman et al., 2002) or melanopsin (Melyan et al., 2005) are typically slow and variable in type, probably reflecting differences in the downstream targets activated by the signaling cascade (i.e., the specific type of ion channel). However, a rhodopsin homolog with intrinsic ion channel activity has recently been borrowed from green algae (Nagel et al., 2003). This protein, called channelopsin-2 (CHOP-2), can be expressed in mammalian neurons, where it covalently attaches to naturally-occurring retinal to form light-sensitive channel rhodopsin which when activated with 460 nm light elicits rapid depolarization in response to brief flashes of light (Boyden et al, 2005). Light triggers an “On” response in transfected retinal neurons due to the nonselective cation channel properties of CHOP-2, simulating the normal light response of On-retinal ganglion cells (Bi et al., 2006). CHOP-2 is a promising tool for allowing optical activation of neuronal firing and has been adopted by many biologists as a turn-key solution to light activation of cells. More recently, another opsin-related protein has been studied that hyperpolarizes cells in response to 580 nm light. Halorhodopsin is a selective chloride channel found in the archaebacterium Natronomas pharaonis (Han and Boyden, 2007; Zhang et al., 2007). This protein, in conjunction with CHOP-2 now offers biologists the ability to use light to activate some cells, while inactivating others, allowing much more complicated experiments to be performed.
C. Photoactivation Using Bioconjugated Ion Channels
The photoswitch approach utilizes a combination of synthetic chemistry and molecular biology to reengineer ordinary ion channels, so that they are sensitive to light. To date, two disparate types of ion channels have successfully undergone reengineering: a K+-selective ion channel (Banghart et al., 2004) and an ionotropic GLU receptor (Volgraf et al, 2006).
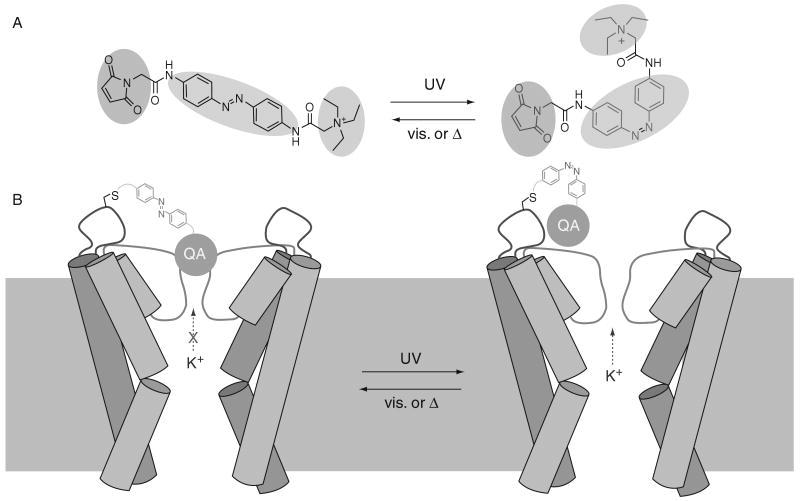
The light-activated K+ channel, called Synthetic Photoisomerizable Azobenzene Regulated K+ (SPARK) channel has two parts: a synthetic photoswitchable small molecule and a modified Shaker K+ channel protein. The photoswitch is a functionalized version of the photoisomerizable molecule azobenzene (AZO) (Fig. 1A). Connected to the AZO on one end is a cysteine-reactive maleimide (MAL) group, which allows attachment to a surface-exposed cysteine residue on the channel, and on the other end a quaternary ammonium (QA) group, which blocks the pore of K+ channels. The system has been designed so that the QA can reach the pore-blocking binding site and arrest ion conduction when the AZO is in its low energy, elongated trans form, but not in its higher energy, bent cis form (Fig. 1B). As UV light photoisomerizes the AZO from trans to cis, it retracts the QA group and unblocks the channel, whereas longer wavelength visible light restores the blocked state by accelerating the reverse cis to trans conversion. The higher energy cis form of AZO is less stable than that of the trans form, so reblocking of the channel also occurs spontaneously, but slowly, if the system is left in the dark.
Fig. 1.
Light regulation of current flow in SPARK channels. (A) The MAL-AZO-QA photoswitch isomerizes to the cis form upon exposure to UV light and returns to the lower energy trans form with visible light or through thermal relaxation. (B) Schematic representation of the K+-selective SPARK channel. The QA group moiety ion conduction when AZO is in its trans configuration (left). UV light induces photoisomerization to the higher energy cis form, retracting the QA group, thus allowing K+ to flow out and hyperpolarize the cell.
The GLU receptor was reengineered to site-specifically attach a tethered analog of GLU that was attached to a photoswitchable AZO to the edge of the ligand-binding domain of the receptor. In one state, the ligand-binding domain could be occupied by the GLU analog allowing activation of the ion channel and in the other, the receptor would be in the unoccupied, native state. Photoisomerization switches between these two states with the low energy, trans state disallowing binding and the high energy, cis state causing agonism of the receptor and thus opening of the channel and depolarization of the cell.
II. Discussion
A. Inhibition of Neuronal Activity with Light-Activated Ion Channels
1. SPARK Channels
There are several methods in current use for photostimulation of action potential firing in neurons, yet methods for optical inhibition remain limited. To impart reversible optical silencing of neural activity, a system has been designed that consists of an engineered Shaker potassium channels that gates in response to light when functionalized with a covalently attached, photoswitchable pore blocker. SPARK channels consist of a synthetic AZO-containing photoswitch and a genetically modified Shaker K+ channel protein. When tested in the heterologous Xenopus oocyte expression system, after microinjection of mRNA encoding the channel, it was found that the SPARK channels open and close rapidly (within seconds) upon exposure to 380 and 500 nm light, respectively (Fig. 2A). Opened channels also close in the dark, but over the much slower time course of several minutes (Fig. 2B).
Fig. 2.
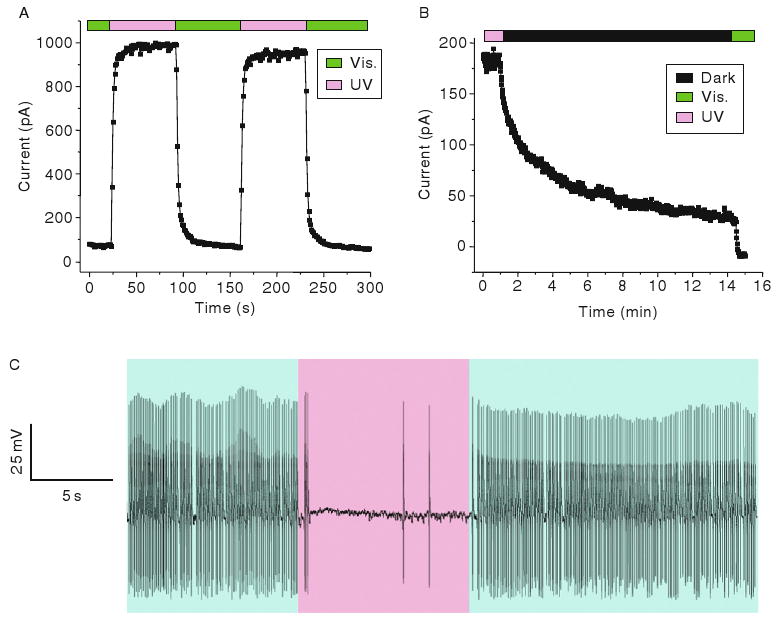
Photoswitching of SPARK channels. (A) UV light opens channels and visible light closes channels in an inside–out patch taken from a MAL-AZO-QA-treated Xenopus oocyte expressing SPARK channels. (B) SPARK channels close slowly in the dark as the photoswitches relaxes back to the trans configuration. (C) Suppression of spontaneous action potentials by UV light (380 nm) in a MAL-AZO-QA-treated cultured hippocampal neuron expressing SPARK channels. Exposure to visible light (500 nm) restores spontaneous firing.
In addition to the inside-out patch clamp Xenopus oocyte experiements, SPARK channels has been used to regulate action potential firing in cultured rat hippocampal neurons (Fig. 2C). Several mutations have been introduced into the Shaker channel to maximize the dynamic range of light-induced voltage changes. N-type inactivation has been minimized by removal of the Shaker “ball” and voltage-dependence of activation shifted to hyperpolarized potentials, presumably making the channel constitutively open at normal neuronal resting potentials. Neurons that have been transfected with the channel gene and treated with the photoswitch become directly sensitive to light, with short wavelengths opening the channels and silencing activity and longer wavelengths closing the channels and allowing spontaneous firing of action potentials to resume. Action potential firing can be switched on and off within seconds and can be controlled repeatedly for tens of minutes or as long as the patched cell-seal remains.
The photoswitch that has been used in most experiments was MAL-AZO-QA and consisted of a cysteine-reactive MAL group, an AZO group, which is photoisomerizable, and a QA group, which is a blocker of the pore of K+ channels. Remarkably, the MAL-AZO-QA photoswitch appears to be selective for the expressed Shaker channel, having no measurable effect on the many other types of K+ channels in these neurons that are known to be blocked by QA ions (Fortin et al., 2008). It is hypothesized that the selectivity exhibited by MAL-AZO-QA is a consequence of the close matching of the molecular length in the trans, or extended, form with the spacing of the engineered cysteine and the QA-binding site on the Shaker channel. The covalent attachment of the MAL group to the introduced cysteine in the channel appears to be accelerated by the binding of the QA group to the pore, which is about 17 Å away. If any MAL-AZO-QA does, in fact, attach to another ion channel family on the neuronal surface, it would likely have little physiological effect because the QA group would not be ideally situated in a position where it could block the pore. The specificity of the photoswitch for the exogenously expressed Shaker channel suggests that SPARK channels can be targeted to different types of neurons simply by targeting gene expression, for example, with cell-type-specific promoters or by using virally-mediated transfection.
2. PALS—Photoswitchable Affinity Labels
All of the light-activated channels described in this chapter, require exogenous expression of a gene encoding either a foreign or genetically modified ion channel, followed by covalent bioconjugation of a photoswitchable small molecule (e.g., retinal for CHOP-2 or MAL-AZO-QA for SPARK). Exogenous gene expression can be routinely accomplished using a variety of methods, including chemically facilitated gene transfection (e.g., lipofection or calcium phosphate) or viral-mediated gene transfer, but in all cases exogenous gene expression is slow, requiring hours to weeks depending on multiple variables. Due to the time constraints, tissue culture is often employed, either using dissociated neurons in a dish or organotypic cultures (e.g., brain slice cultures). The level of exogenous gene expression varies widely within a population of transfected neurons and successful introduction of foreign genes is often a low-probability event (e.g., in our hands ≪ 10% for calcium phosphate transfection). Foreign ion channel genes can also be introduced into transgenic animals, but this requires months, is rather expensive, and there may be consequences for the proper development of cells and tissues expressing the genes, thereby confounding interpretation of the results. Additionally, using exogenously-expressed genes is sometimes not suitable for multisubunit receptors, since overexpression of monomers of a particular receptor may bias natural receptor subtype composition (Bredt and Nicoll, 2003). For these reasons, a method for conferring light-sensitivity that does not require exogenous gene expression, but rather can be carried out on freshly obtained, native, neural tissue would be a great improvement.
The MAL-AZO-QA photoswitch for SPARK channels contains a MAL group, which presumably reacts with a cysteine residue that has been introduced at the correct position on the channel protein surface. The PAL compound that has been used recently for imparting light sensitivity to K+ channels is identical to MAL-AZO-QA, with one difference: instead of a MAL at one end, the new molecule contains a “promiscuous” acrylamide group, which can react more readily with several nucleophilic sidechains on the protein surface, including lysine, serine, threonine, in addition to cysteine. Presumably, the bioconjugation occurs through the QA first binding to the pore site, slowing its departure from the vicinity of the channel thus, increasing the local effective concentration of the acrylamide moiety. This promotes covalent attachment to the channel protein, if it happens to possess a nucleophilic amino acid sidechain at an appropriate distance from the QA binding site (∼17 Å away). Hence, the labeling of native channels by the highly reactive portion of the molecule is promoted through ligand-binding interaction, as in classical photoaffinity labeling experiments (Chowdhry and Westheimer, 1979). In contrast to SPARK channels, where two components are required to impart light-sensitivity to cells (i.e., the engineered ion channel gene and the MAL-AZO-QA photoswitch), this is a one-component system, where only the PAL molecule is required. This allows PAL to react with and thus impart light-sensitivity on native K+ channels in neurons with no need for exogenous gene expression. Like SPARK channels, PAL-treated channels allow precise and reversible control over membrane potential and action potential firing in neurons, but in a different and complementary way. SPARK can be genetically targeted through ion channel expression and PAL can be globally applied to an experimental preparation.
The possibility of using PAL to activate cells with light was tested first using the Shaker K+ channel as a test system (Fortin et al., 2008). Addition of PAL to a Shaker channel that has a cysteine-substitution at position E422 (E422C) imparts light-sensitivity on the channel in a way very similar to MAL-AZO-QA. However, PAL works equally well on a Shaker channel that does not have the cysteine substitution. Hence, it appears that the PAL molecule can locate an alternative attachment site at an appropriate distance from the pore, such that light-elicited changes in photoswitch geometry can reversibly block and unblock the pore of the channel. Steady-state current–voltage curves from a hippocampal neuron in culture that has been treated with PAL have demonstrated that exposure to 380 nm light increases the voltage-gated outward current at membrane potentials positive to about −20 mV, and 500 nm light reverses the effect. In contrast, MAL-AZO-QA failed to impart light sensitivity on untransfected neurons that express only their native ion channels. PAL treatment also confers light-dependent effects on action potential firing. Under current clamp mode, 380 nm light silences spontaneous firing and 500 nm light allows native firing (Fortin et al., 2008).
B. Excitation of Neuronal Activity with Light-Activated Ion Channels
1. D-SPARK (Depolarizing SPARK) Channels
Wild-type Shaker channels are highly selective for K+ conduction through their pore over other cations (Hille, 1992). To convert the hyperpolarizing and thus silencing SPARK channel into a nonselective cation channel and thus depolarizing (D-SPARK), a single point mutation has been introduced into the pore-lining domain, converting valine-443 into glutamine (V443Q). Mutation of this residue, which is part of the conserved signature sequence of voltage-gated K+ channels, reduces the K+:Na+ permeability ratio of Shaker from <0.02 to about 0.70 (Heginbotham et al., 1994). Other than this single pore mutation, the structure of the D-SPARK channel protein is identical to the SPARK protein. The D-SPARK system also consists of the same MAL-AZO-QA molecule bioconjugated to the protein to impart light-induced gating as the SPARK channel (Chambers et al., 2006).
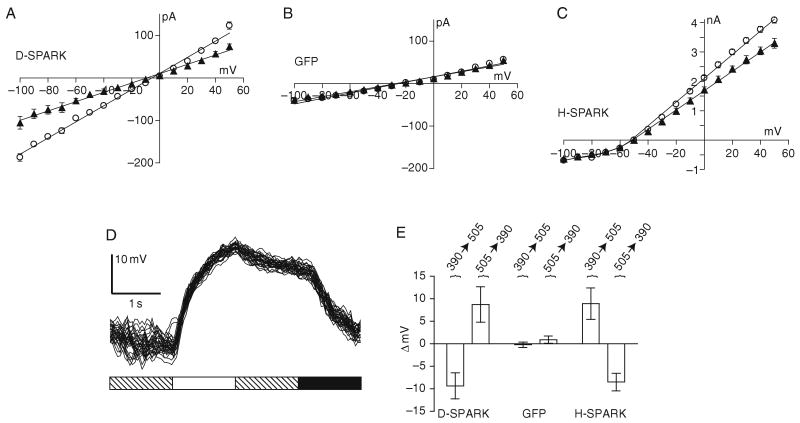
To characterize the biophysical properties of D-SPARK channels steady-state current–voltage curves were recorded and show that exposure to 380 and 500 nm light, increased and decreased the conductance, respectively, and that the reversal potential of the light-regulated conductance was near 0 mV (Fig. 3A), which is indicative of a nonselective ion channel. The light-activation effect was not observed when control cells were tested (Fig. 3B). Light had similar effects on cells expressing hyperpolarizing SPARK, but the reversal potential was closer to −70 mV, as expected for a K+-selective channel (Fig. 3C). A point mutation (L366A) has been introduced into the voltage-sensing domain of both SPARK and D-SPARK to shift the activation potential to about −70 mV as compared to −35 mV for wild-type channels (Lopez et al., 1991). This mutation along with deletion of fast N-type inactivation renders SPARK constitutively active at physiological membrane potentials (>−65 mV), but the channel can be closed at potentials <−65 mV. A surprising difference between the voltage-dependency of gating for SPARK and D-SPARK channels was observed. D-SPARK remained open at membrane potentials down to −100 mV. It appears that the combination of the pore mutation and a voltage-sensor mutation disrupts the normal voltage-control over the permeation pathway, a finding that is not without precedent (Molina et al., 1998).
Fig. 3.
Properties of SPARK and D-SPARK channels expressed in CHO cells. Steady-state I–V curves showing the effect of photoswitching. (A) Cells transfected with GFP-tagged D-SPARK channels and treated with MAL-AZO-QA elicit more current when stepped to any given voltage when UV light is used for illumination (open circles) when compared to visible light (closed triangles). (B) Control cells transfected with GFP plasmid and similarly treated with MAL-AZO-QA do not respond to light. (C) Cells expressing SPARK channels and treated with MAL-AZO-QA also conduct more current upon exposure to UV light. (D) Current clamp recording from a CHO cell showing repeatable depolarization by exposure to UV light (white bar) and hyperpolarization in visible light (black bar). D-SPARK channels closed slowly in the dark (hashed bar) through thermal relaxation of the photoswitch. (E) Average membrane potential changes elicited by light (n = 10 CHO cells for each treatment).
In experiments, where current-clamp configuration is employed, D-SPARK cells depolarize when illuminated with 380 nm light, and hyperpolarize when illuminated with 500 nm light, reaching steady-state within several seconds (Fig. 3D). Channels that are opened with 380 nm light are observed to close spontaneously in the dark, but the rate of closure is slow (tens of seconds). When cells were current clamped to a membrane potential similar to the resting potential of many cells (−40 mV), opening and closing the channels had opposite effects on cells expressing D-SPARK and SPARK channels (Fig. 3E). Cells expressing D-SPARK depolarized by about 9 mV upon exposure to 380 nm light, whereas cells expressing SPARK hyperpolarized by about 8 mV. Switching to 500 nm light reversed the effect in both cell-types.
D-SPARK channels could also be expressed and photoswitched in mammalian neurons. The gene encoding the D-SPARK channel protein was transfected into hippocampal neurons in culture using a calcium phosphate transfection method (Dudek et al., 2003). It was found that D-SPARK-transfected cells showed an increase in membrane conductance upon exposure to 380 nm light and a decrease upon exposure to 500 nm light under voltage clamp conditions. Under current clamp configuration, 380 nm light depolarized transfected neurons and triggered bursts of action potentials, and 500 nm light reversed the depolarization, halting action potential bursting (Fig. 4).
Fig. 4.
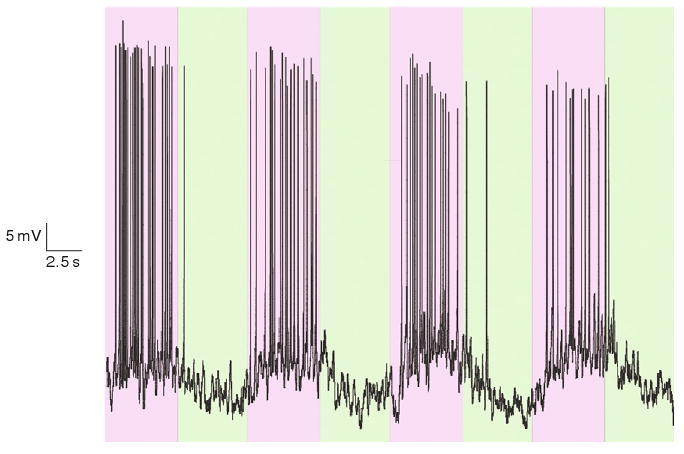
Opening of D-SPARK channels causes action potential firing in mammalian neurons. Bursts of action potentials are elicited when a D-SPARK transfected and MAL-AZO-QA treated neuron is illuminated with UV light purple background. The action potential bursts are halted when the cell is illuminated with visible light green background.
D-SPARK channels are designed to be constitutively open in neurons before the addition of the bioconjugated photoswitch. To investigate concerns about toxicity of D-SPARK tonic activity prior to photoswitch application, cell viability was measured. A constitutively open cation channel could lead to tonic depolarization, lowered input resistance, continual Ca2+ influx, and possibly, cell death. Remarkably, expression of the D-SPARK protein without MAL-AZO-QA application did not reduce the viability of hippocampal neurons in culture when compared to control groups. The percentage of neurons successfully transfected with GFP-tagged D-SPARK or GFP alone were the same (about 0.5% in both cases), and there was no significant difference in the fraction of each that tested positive for DEAD Red nuclear staining. In fact, culturing transfected neurons in the presence of other Shaker channel blockers including 4-aminopyridine, dendrotoxin-K, or charybdotoxin, did not change viability, and therefore was not necessary for future experiments. D-SPARK expression had no significant effect on input resistance or on resting membrane potential. Interestingly, addition of MAL-AZO-QA to D-SPARK-expressing neurons also had no significant effect on these measures. There are several possible explanations for these surprising observations. First, hippocampal neurons may compensate for D-SPARK expression by homeostatically altering expression levels of other channels in order to maintain the cell-programmed “set point” (Turrigiano and Nelson, 2004). This could help explain the similar values of resting membrane potential and cell viability between control and D-SPARK expressing cells, but would not explain why input resistance is unaffected by D-SPARK expression. Second, D-SPARK channels may be preferentially expressed in electrotonically distant portions of the neurons, such that blocking and unblocking them with the photoswitch has no measurable effect on input resistance or resting potential measured in the cell body, but it has a very strong influence on action potential initiation. Compartments of the neuron may have a high local input resistance, such that opening or closing a small number of channels may have a large impact on action potential firing frequency. Finally, a likely scenario is that D-SPARK channel subunits may coassemble with native K+ channel subunits in neurons to produce heteromeric channels whose properties differ from those of homomeric D-SPARK channels expressed in CHO cells including their persistent activity. A heteromeric ion channel consisting of some D-SPARK subunits may display hybrid electrophyiscal properties.
2. LiGluR (Ligand-Gated Ionotropic GLU Receptor)
Recently, a light-gated, agonist-mediated design has been developed for use on the ionotropic GLU receptor. A molecule similar to MAL-AZO-QA but instead MAL-AZO-GLU was covalently attached to the ligand-binding domain of an ionotropic glutamate receptor (iGluR6). This compound consists of a cysteine-reactive MAL, a photoswitchable AZO moiety, and an agonist analog of GLU. In the extended trans form of the AZO, the ligand-binding domain cannot bind the tethered agonist and close. Only after photoisomerization to the cis form can the photoswitchable tether present the agonist to the binding site, thus causing closure of the domain and opening of the ion channel (Fig. 5). Overall, the reversible switching of an AZO allosterically triggers the opening and closing of the entire ion channel, mediated by the clamshell-like movement of the ligand-binding domain. LiGluR has also been used to trigger action potentials in neuronal culture using 1–5 ms pulses of light to achieve responses very similar to those observed during normal neuronal functioning (Szobota et al., 2007).
Fig. 5.
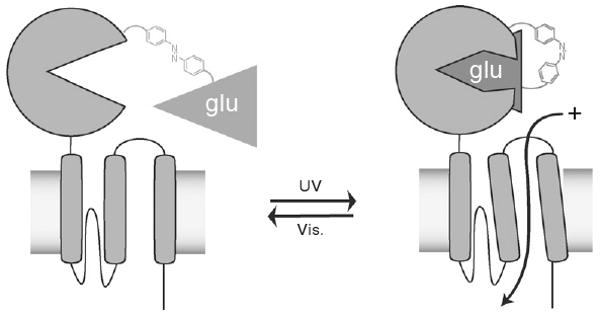
Illumination of LiGluR channels with UV light allows the tethered glutamate (GLU) analog to bind to the ligand-binding domain. Then a conformational change occurs that allows the ion channel to open and cations to flow through the channel, thus depolarizing the cell.
C. Possible Future Directions for Light-Gated Ion Channels
1. Thermostable Photoswitches
Since the trans configuration of AZO is the low energy state of the MAL-AZO-QA photoswitch, for instance, in the dark and in visible light, covalent attachment of MAL-AZO-QA to ion channels in neurons will result in tonic K+ channel blockade and neuronal depolarization. This can be overcome by maintained illumination with 380 nm light. However, perhaps a better way to turn off MAL-AZO-QA-treated channels is with a thermostable photoswitch, in which the cis form can persist for many hours. With a molecule such as this, a brief flash of UV light would be all that is required to maintain native K+ channels in their normal, unblocked state, even though a photoswitch was still attached. Then, at any moment, a flash of 500 nm light could be used to block the channels and stimulate the cells. Open SPARK channels revert to their closed state within minutes because the cis configuration of MAL-AZO-QA spontaneously relaxes to the trans configuration. As long as SPARK channels are exposed to 380 nm light they will remain open, but prolonged illumination may not always be desirable.
2. MultiPhoton-Sensitive Photoswitches
With the further development of optical techniques, advances could be used to the advantage of light-gated ion channels. Instead of using AZO as the photoswitchable unit, other multiphoton sensitive chromophores will surely be explored that will allow more robust and deeper tissue activation.
3. Applications of Light-Activated Ion Channels
One potential application for light-activated ion channels of particular interest is the retina. Light-activated ion channels provide an alternative approach to building a retinal prosthetic. In the case of light-activated ion channels, the prosthetic device would be downstream from the neuron itself (e.g., a retinal ganglion cell), which is induced to become directly sensitive to light. Expression of silencing channels in retinal ganglion cells could produce “virtual Off-cells,” which are inhibited by illumination. Expression of stimulating channels should produce “virtual On-cells” that are stimulated with light. Since the SPARK and D-SPARK channels use the same photoswitch, the spectral sensitivity of channel opening and closing is the same. This greatly simplifies the task of regulating the activity of retinal ganglion cells, assuming the appropriate channel type could be expressed selectively in the appropriate cell type. The identification of proteins that are expressed differentially in On-versus Off-retinal ganglion cells (Yamagata et al., 2002) could lead to identification of gene regulatory elements for allowing selective expression of light-activated channels in their appropriate neuronal populations. This would greatly simplify the task of regulating the activity of retinal ganglion cells, assuming the appropriate channel type could be expressed selectively in the appropriate cell type.
Light-activated ion channels may be useful for allowing selective and noninvasive activation with light of individual neurons in a neural circuit. With a high enough photon flux to fully open or close all the channels instantaneously, the temporal resolution of activation with D-SPARK may be similar to that achieved with either GLU uncaging or channelrhodposin-2 stimulation (Boyden et al., 2005; Ishizuka et al., 2006; Nagel et al., 2003). However, unlike neurotransmitter uncaging, SPARK activation is specific to targeted cells and does not produce diffusible products that can have unintended effects on neighboring cells. Unlike the use of natural photoproteins, the photoswitch approach is versatile because it is easy to modify either the ion channel protein or the photoswitch.
III. Methods
CHO cells were cultured on glass coverslips in 10% FBS/F12 media to ∼70% confluency. Primary dissociated hippocampal cultures were prepared from embryonic day E18–19 Sprague-Dawley rat embryos and were cultured on polylysine-coated glass coverslips in serum-containing medium. Both mammalian cell types were grown in 7% CO2 in air at 37 °C. All animal care and experimental protocols were approved by the Animal Care and Use Committee at UC, Berkeley.
For experiments, involving expression of exogenous ion channels, both cell line and primary cell culture were typically transfected with 0.8 mg DNA per 12 mm coverslip encoding either eGFP or eGFP-tagged Shaker H4 channels, with the following mutations: Δ6–46, L366A, E422C, V443Q (for D-SPARK only), and T449 V. The eGPF was tagged to the N-terminal of the Shaker gene using standard PCR and molecular cloning techniques. Transfections with CaPO4 were carried out for 10–13 days in vitro for the primary culture; and electrophysiological recording was performed ∼48 h later (Dudek et al., 2003). Coverslips containing cells were typically treated for 15 min with 300 mM of photoswitch at 37 °C in an extracellular recording solution containing 138 mM NaCl, 1.5 mM KCl, 1.2 mM MgCl2, 5 mM HEPES, 2.5 mM CaCl2, and 10 mM glucose at pH 7.4. The concentration of DMSO in the treatment bath did not exceed 0.1%. Patch pipettes (4–8 MΩ) were filled with 10 mM NaCl, 135 mM K-gluconate, 10 mM HEPES, 2 mM MgCl2, 2 mM Mg-ATP, and 1 mM EGTA at pH 7.4. After washout of unbound photoswitch with extracellular solution, whole-cell patch was established. Voltage-clamp configuration was used to generate I–V data and then the configuration was changed to current-clamp and the membrane potential was recorded. Initial recordings were made at the resting potential to evaluate the effects of light on spontaneous activity in neurons. Pulse protocols and measurements were carried out with pCLAMP 8.0 software, a DigiData 1200 series interface, and an Axo-Patch 200A amplifier (Axon Instruments). Samples were taken at 10 kHz, and the data were filtered at 1 kHz. Seals with a leak current of >200 pA were not included in analyses. Cells were irradiated using a Lambda-LS illuminator containing a 125-W xenon arc lamp (Sutter Instruments Company) equipped with narrow-bandpass (±10 nm) filters through a Fluor 20×, 0.5 n.a. objective lens (Nikon). Cell viability assays were performed according to the protocol provided by the vendor of the Live/Dead Kit (Invitrogen). Variability among data is expressed as Mean ± SD.
IV. Conclusion
The photoswitch approach, the caged neurotransmitter approach, and the natural photoprotein approach each provide new opportunities to precisely and remotely control neuronal activity for experimental and potentially medical applications. Each approach has strengths and weaknesses and is best used in specific contexts. The probable outcome of the current state of research in this field is that all of these methods will continue to find important uses in cell biology in general, and neurobiology in particular. These techniques may eventually be used as medical tools for the noninvasive and precise input of information into the nervous system, downstream of sites of degeneration or injury. It is worth noting that the remarkable advances in this field are driven not only by new discoveries in chemical and molecular biology, but also by new and supremely accurate and powerful optical technology. Together, these tools constitute a new optical interface for controlling the function of cells, tissues, and perhaps entire organisms.
Acknowledgments
We thank S. Ahituv for technical support, M. Banghart, K. Borges, D. Fortin, I. Hafez, E. Isacoff, D. Trauner for critical experiments, discussion, and advice. This work was supported by grants to RHK from the NIH (EY016249) and Fight-for-Sight. JJC was supported by a NIH NRSA fellowship.
References
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50(1):23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40(2):361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci USA. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Yuste R. Stimulating neurons with light. Curr Opin Neurobiol. 2002;12:587–592. doi: 10.1016/s0959-4388(02)00364-1. [DOI] [PubMed] [Google Scholar]
- Chambers JJ, Banghart MR, Trauner D, Kramer RH. J Neurophysiol. Vol. 96. 2006. Light-induced depolarization of neurons using a modified shaker K+ channel and a molecular photoswitch; pp. 2792–2796. [DOI] [PubMed] [Google Scholar]
- Chowdhry V, Westheimer FH. Photoaffinity labeling of biological systems. Annu Rev Biochem. 1979;48:293–325. doi: 10.1146/annurev.bi.48.070179.001453. [DOI] [PubMed] [Google Scholar]
- Dudek H, Ghosh A, Greenberg ME. Calcium phosphate transfection of DNA into neurons in primary culture. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray SJ, editors. Current Protocols in Neuroscience. Wiley; Chichester: 2003. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MV, Otis TS, Kristan WB, Trauner D, Kramer RH. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2(3):e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sinauer Associates; Sunderland, MA: 1992. [Google Scholar]
- Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54:85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Katz LC, Dalva MB. Scanning laser photostimulation: A new approach for analyzing brain circuits. J Neurosci Methods. 1994;54:205–218. doi: 10.1016/0165-0270(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Lopez GA, Jan YN, Jan LY. Hydrophobic substitution mutations in the S4 sequence alter voltage-dependent gating in Shaker K + channels. Neuron. 1991;7:327–336. doi: 10.1016/0896-6273(91)90271-z. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Kasai H. Three-dimensional mapping of unitary synaptic connections by two-photon macro photolysis of caged glutamate. J Neurophysiol. 2008;99(3):1535–1544. doi: 10.1152/jn.01127.2007. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Molina A, Ortega-Saenz P, Lopez-Barneo J. J Physiol. Pt 2. Vol. 509. 1998. Pore mutations alter closing and opening kinetics in Shaker K + channels; pp. 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, O'Connor DH, Sarkisov DV, Wang SS. Rapid neurotransmitter uncaging in spatially defined patterns. Nat Methods. 2005;2(11):837–843. doi: 10.1038/nmeth793. [DOI] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54(4):535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: Synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110(5):649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]