Abstract
Lectins are proteins with specificity of binding to certain monosaccharides or oligosaccharides. They can detect abnormal glycosylation patterns on immunoglobulins in patients with various chronic inflammatory diseases, including rheumatoid arthritis and IgA nephropathy (IgAN). However, lectins exhibit binding heterogeneity, depending on their source and methods of isolation. To characterize potential differences in recognition of terminal N-acetylgalactosamine (GalNAc) on IgA1, we evaluated the binding characteristics of several commercial preparations of GalNAc-specific lectins using a panel of IgA1 and, as controls, IgA2 and IgG myeloma proteins. These lectins were from snails Helix aspersa (HAA) and Helix pomatia (HPA), and the plant Vicia villosa (VV). Only HAA and HPA bound exclusively to IgA1, with its O-linked glycans composed of GalNAc, galactose, and sialic acid. In contrast, VV reacted with sugars of both IgA subclasses and IgG, indicating that it also recognized N-linked glycans without GalNAc. Furthermore, HAA and HPA from several manufacturers differed in their ability to bind various IgA1 myeloma proteins and other GalNAc-containing glycoproteins in ELISA and western blot. For serum samples from IgAN patients, HAA was the optimal lectin to study IgA1 glycosylation in ELISA and western blot assays, including identification of the sites of attachment of the aberrant glycans. The Gal-deficient glycans were site-specific, localized mostly at Thr228 and/or Ser230. Because of the heterogeneity of GalNAc-specific lectins, they should be carefully characterized with appropriate substrates before undertaking any study.
Keywords: IgA nephropathy, IgA, lectins, glycosylation, N-acetylgalactosamine
1. Introduction
Lectins are proteins of plant or animal origin with specificity for a certain monosaccharide or oligosaccharide and have been employed in different areas of cell biology, immunology, and pathology (Carpenter et al., 1997; Mestecky et al., 1993; Mody et al., 1995; Tomana et al., 1999; Rudd et al., 2001; Tabares et al., 2005; Wold et al., 1994). Lectin-based assays can be used to diagnose certain types of cancer and also to evaluate progression and treatment of these malignancies (Brooks, 2000; Mody et al., 1995; Tomana, 1996). In addition, lectins were used to detect markers of various chronic inflammatory diseases, including immunoglobulins (Ig) in diseases of an autoimmune (Mullinax and Mullinax, 1975; Ezaki et al., 2001; Mackiewicz and Mackiewicz, 1995; Malhotra et al., 1995; Novak et al., 2001; Tomana, 1996) or infectious nature (Moore et al., 2005).
Lectin-based assays have been extensively used to study the glycosylation of IgA1 in patients with IgA nephropathy (IgAN), the most common form of primary glomerulonephritis in the world (Julian et al., 1988). In the hinge region of human serum IgA1, 3–5 Ser/Thr residues are occupied by O-glycans of variable composition; the prevailing forms include Gal-GalNAc disaccharide, and its mono- and di-sialylated forms (Mattu et al., 1998; Novak et al., 2000). A variant with terminal GalNAc or sialylated GalNAc in serum IgA1 is more common in patients with IgAN (Allen et al., 2001; Allen et al., 1995; Hiki et al., 1995; Julian and Novak, 2004; Mestecky et al., 1993; Novak et al., 2001; Smith and Feehally, 2003; Tomana et al., 1997; Tomana et al., 1999) than in normal individuals (Field et al., 1989; Mattu et al., 1998). Although many GalNAc-specific lectins are available, those from the snails Helix aspersa (HAA) and Helix pomatia (HPA), and from the plant Vicia villosa (VV), have been most commonly used to evaluate the galactosylation of O-linked glycans on IgA1 of IgAN patients (Allen et al., 2001; Allen et al., 1995; Novak et al., 2001; Tomana et al., 1997; Tomana et al., 1999).
In the past, we have found that lectins are useful and informative for the analyses of glycans; however, the results may be assay-specific. Moreover, the specificity of some lectins (including jacalin, commonly used for the isolation of IgA1 because it binds to the hinge-region O-linked glycans containing a GalNAc-Gal disaccharide) may vary depending on their geographical origin, the part of the plant or animal from which the compound is extracted, and the method of preparation (Bourne et al., 2002; Jeyaprakash et al., 2003; Kabir, 1998; Kobayashi et al., 1988; Raval et al., 2004; Van Damme et al., 2002; Wu et al., 2003). Recently, we encountered such a problem with a new batch of HAA lectin from our usual supplier that did not recognize Gal-deficient IgA1 myeloma proteins, unlike previous preparations from the same company (Tomana et al., 1997; Tomana et al., 1999). This prompted us to evaluate several lectins with specificity for GalNAc to identify the best candidate for the analysis of samples from patients with IgAN.
To the best of our knowledge, a study comparing the specificity and the binding characteristics of HAA, HPA, and VV in different assays has not been published. Because these lectins are used quite extensively by many researchers in immunology, nephrology, and oncology, we undertook this study to determine the binding characteristics of these GalNAc-specific lectins using supplies purchased from different manufacturers. Our study demonstrated that the specificities varied substantially. Therefore, we recommend that investigators not rely on the declared specificity of the lectin, but rather determine the precise reactivity in the particular assay of interest. Furthermore, we demonstrated the utility of the best-performing lectin, HAA, for the analysis of glycosylation of IgA1 from IgAN patients in ELISA and western blot analyses.
2. Materials and methods
2.1. Myeloma Proteins
Table 1 lists the IgA1, IgA2, and IgG myeloma proteins with their molecular properties that were isolated from serum or plasma of patients with multiple myeloma, as described previously (Mestecky and Kilian, 1985). Briefly, serum or plasma samples were precipitated with ammonium sulfate (50% saturation), dissolved in phosphate buffer and the dialyzed precipitates were fractionated by ion-exchange chromatography on DEAE-cellulose, affinity chromatography on Jacalin-agarose to capture or remove IgA1 (Sigma Chemical Company, St. Louis, MO) (Tomana et al., 1997). The final purification step included size-exclusion chromatography on columns of Sephadex G-200 or Ultrogel AcA22 (Pharmacia, now Amersham Biosciences, Piscataway, NJ). For the IgA myeloma proteins, the potential contaminant, IgG, was removed by affinity chromatography on staphylococcal protein G immobilized on agarose (Sigma). The purity of the IgA1 preparations was assessed by SDS-PAGE, western blots (using IgA1-specific and IgA2-specific monoclonal antibodies) (Mestecky et al., 1996), and immunoelectrophoresis using polyvalent reagents against IgA, IgG, and human serum (Jackson ImmunoResearch Laboratories, West Grove, PA). The molecular forms of the IgA1 proteins (Table 1) were assessed by size-exclusion chromatography, nonreducing SDS-PAGE, and western blots developed with anti-IgA antibody.
Table 1.
Myeloma proteins used in this study.
| Myeloma protein | Ig Isotype | Molecular form |
|---|---|---|
| Mce | IgA1 | Polymer |
| Ham | IgA1 | Polymer |
| Ste | IgA1 | Polymer |
| Gou | IgA1 | Polymer |
| Lat | IgA1 | Dimer |
| Ber1 | IgA1 | Dimer |
| Sin | IgA1 | Monomer |
| Kni | IgA1 | Monomer |
| Fel | IgA2 | Polymer |
| Ber2 | IgA2 | Polymer |
| Cob | IgA2 | Polymer |
| Hum | IgA2 | Polymer |
| Kur | IgA2 | Polymer |
| Cur | IgA2 | Monomer |
| Mat | IgG |
2.2. Lectin sources
HAA was purchased from Sigma, ICN (Aurora, OH), and EY (San Mateo, CA); HPA, from ICN and EY; and VV, from EY and Vector (Burlingame, CA).
2.3. ELISA
For capture ELISA, Costar 96-well U-bottom plates (Corning Inc., Corning, NY) were coated overnight at 4°C with F(ab′)2 fragment of goat anti-human IgA (BioSource International, Camarillo, CA) at a concentration of 5 μg/ml. Plates were blocked with 1% bovine serum albumin (BSA; Sigma) in PBS containing 0.05% Tween 20 (v/v) for 1 h at 37°C. Ig-containing samples diluted in blocking buffer were added to each well and incubated overnight at 4°C. In some instances, the captured IgA was subsequently desialylated by treatment for 3 h at 37°C with 10 mU/ml neuraminidase from Vibrio cholerae (Roche, Indianapolis, IN) in 100 mM sodium acetate buffer, pH = 5 (Tomana et al., 1997). Samples were then incubated for 2 h at 37°C with biotinylated lectins diluted 1:500 in blocking buffer (Tomana et al., 1997). The bound lectins were measured by the addition of avidin-alkaline phosphatase (Sigma) diluted 1:8,000 and incubated for 1 h at 37°C followed by a chromogenic substrate p-nitrophenyl phosphate (Sigma; one tablet per 5 ml substrate). Absorbance at 405 nm was measured with an EL808 microplate reader (Bio-Tek Instruments, Winooski, VT). Alternatively, avidin-horseradish peroxidase conjugate (ExtrAvidin; Sigma) was used and the reaction developed with the peroxidase chromogenic substrate O-phenylenediamine (OPD)-H2O2 (Sigma). The color reaction was stopped with 1 M sulphuric acid and the absorbance at 490 nm was measured.
For some experiments, ELISA microtiter plates were directly coated with IgA myeloma proteins (2.5 μg/ml) overnight at 4°C. Plates were then blocked, treated with neuraminidase, and developed as described above. The amount of coated IgA was verified by ELISA with anti-IgA antibody.
2.4. Western blot
Lectin binding was further confirmed by SDS-PAGE separation of reduced Ig proteins followed by western blot analysis (Tsuchiya et al., 1993). Briefly, 1-μg aliquots of purified Ig proteins were dissolved in reducing buffer containing 1% (v/v; final concentration) 2-mercaptoethanol, and then electrophoretically separated by SDS-PAGE. These proteins were transferred to PVDF membranes by electroblotting. After blocking with Superblock (Pierce, Rockford, IL) supplemented with 0.05% Tween 20 for 1 h at room temperature, the membranes were incubated for 3 h at 37°C with 10 mU/ml neuraminidase in blocking buffer adjusted to pH 6.0, to remove sialic acid from Ig glycans. The removal of sialic acid was verified by gas-liquid chromatography. After washing, the membranes were incubated overnight at 4°C with biotinylated lectin at a 1:500 dilution. For detection of the IgA heavy chain, a duplicate membrane was incubated overnight at 4°C with biotinylated goat anti-human IgA (BioSource) at a 1:30,000 dilution. After washing, the membranes were incubated with horseradish peroxidase-conjugated NeutrAvidin (Pierce). After addition of SuperSignal West Pico Chemiluminescent Substrate (Pierce) for 10 min at room temperature, the membranes were developed on Biomax MS Film (Eastman Kodak Company, Rochester, NY).
IgA proteins were also digested with IgA proteases from Clostridium ramosum AK183, Streptococcus pneumoniae TIGR 4, and Haemophilus influenzae HK50 (Kilian et al., 1996) before SDS-PAGE separation and blotting on PVDF. The digestions were conducted for 20 h at 37°C in PBS, pH 7.4. Proteins were desialylated on the blot by incubating for 3 h at 37°C with 10 mU/ml neuraminidase in blocking buffer adjusted to pH 6.0. The blots were then probed with HAA and developed following the protocol described above.
2.5. Gas-liquid chromatography
The monosaccharides from purified IgA myeloma proteins were determined as trifluoroacetates of methyl-glycosides by gas-liquid chromatography (Tomana et al., 1976; Tomana et al., 1984). A Hewlett-Packard model 5890 series II gas chromatograph was used with a 25-m fused silica (0.22-mm inner diameter) OV-1701 WCOT column and electron capture detector; GC ChemStation software (Agilent Technologies, Palo Alto, CA) was used for acquisition of chromatographic data and peak integration. About 20 μg of each IgA was used for analyses.
3. Results
3.1. HAA, HPA, and VV from various manufacturers differ in their binding to IgA
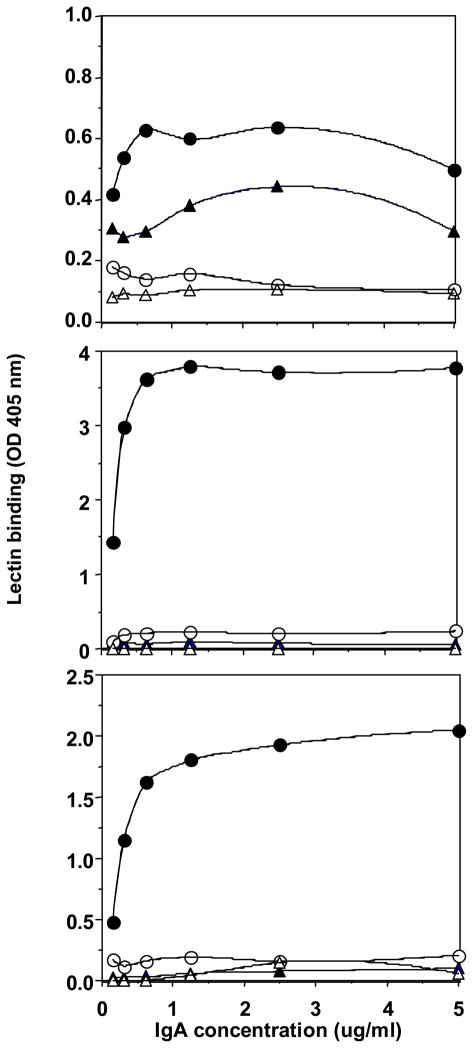
The fact that HAA recently purchased from EY did not reproduce our previous results with an earlier batch of this lectin prompted us to investigate the heterogeneity of lectins purchased from various manufacturers. We screened three lectins purportedly specific for GalNAc (HAA, HPA, VV) for their binding to a panel of IgA1 and IgA2 myeloma proteins (Table 1). The ELISA results for each lectin purchased from two different suppliers are shown in Fig. 1. HAA from Sigma exhibited a broader binding capacity for IgA1 than did HAA from ICN. In addition, HPA and VV from EY bound to the IgA1 myeloma proteins more effectively than did the lectins purchased from ICN and Vector. Based on these results, we selected HAA from Sigma and HPA and VV from EY for further experiments in this study.
Fig. 1.
Capture ELISA of lectin binding to a neuraminidase-treated IgA2 myeloma protein, Fel (triangles), and a neuraminidase-treated IgA1 myeloma protein, Mce (circles). (A) Binding of VV purchased from Vector (open symbols) and EY (closed symbols). (B) Binding of HPA purchased from ICN (open symbols) and EY (closed symbols). (C) Binding of HAA purchased from ICN (open symbols) and Sigma (closed symbols).
3.2. Binding specificity of HAA, HPA, and VV for Gal-deficient IgA1
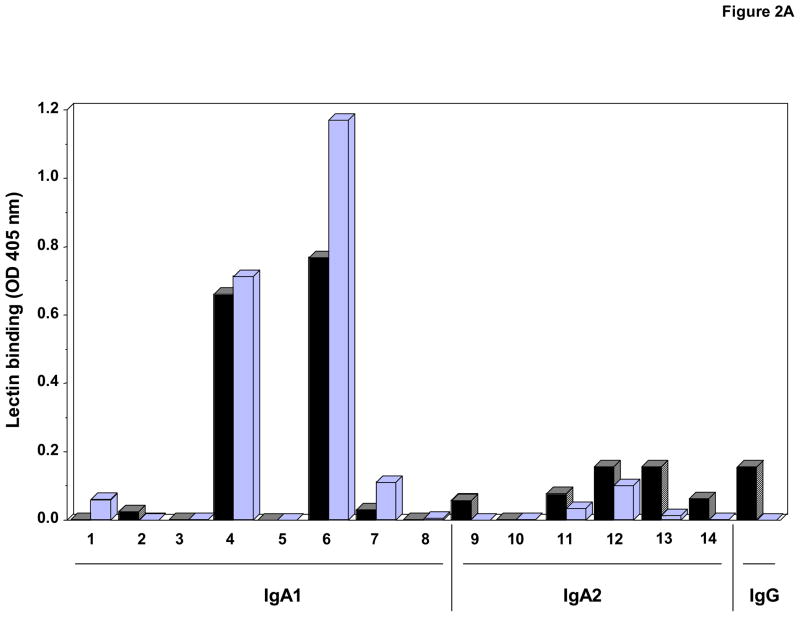
To further examine the binding specificity of these lectins, additional IgA1 and IgA2 myeloma proteins were used. These myeloma proteins were analyzed for molecular form by size-exclusion chromatography and SDS-PAGE and for monosaccharide composition by gas-liquid chromatography; the results confirmed the absence of GalNAc in the IgA2 and IgG preparations. Furthermore, western blot analysis with IgA subclass-specific monoclonal antibodies confirmed the immunochemical purity of the preparations. In addition to IgA1 proteins, VV bound IgA2 in ELISA and western blot (Fig. 2A and B). Surprisingly, VV also bound to an IgG myeloma protein. Thus, this lectin was not specific for GalNAc.
Fig. 2. VV binding to IgA1 and IgA2 myeloma proteins using ELISA (A) and western blot analysis (B). The numbers identify the same proteins in both figures.
A) VV binding to myeloma proteins (0.625 μg/ml) determined by capture ELISA. Protein were native (black bars) or treated with neuraminidase to remove sialic acid (light bars).
B) One μg IgA1 and IgA2 protein were loaded per well. Selected western blot membranes were treated with neuraminidase (NA), then developed with VV. IgA myeloma proteins used: (1) Mce, (2) Ham, (3) Ste, (4) Gou, (5) Lat, (6) Ber1, (7) Sin, (8) Kni, (9) Fel, (10) Ber2, (11) Hum, (12) Kur, (13) Cur, and (14) Cob. An IgG myeloma protein (Mat) was used as a negative control. Anti-IgA staining was performed as a loading control.
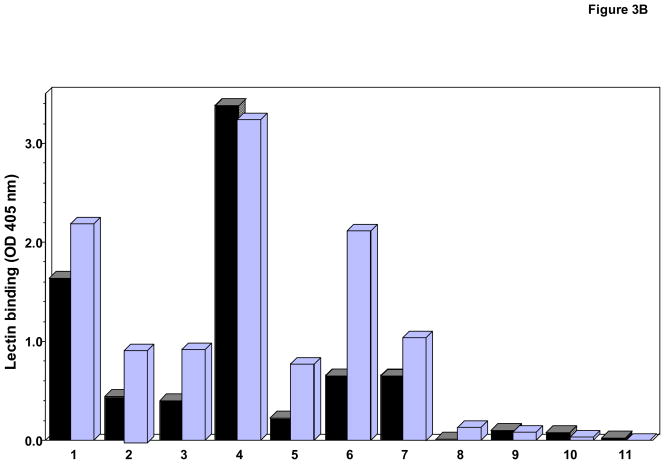
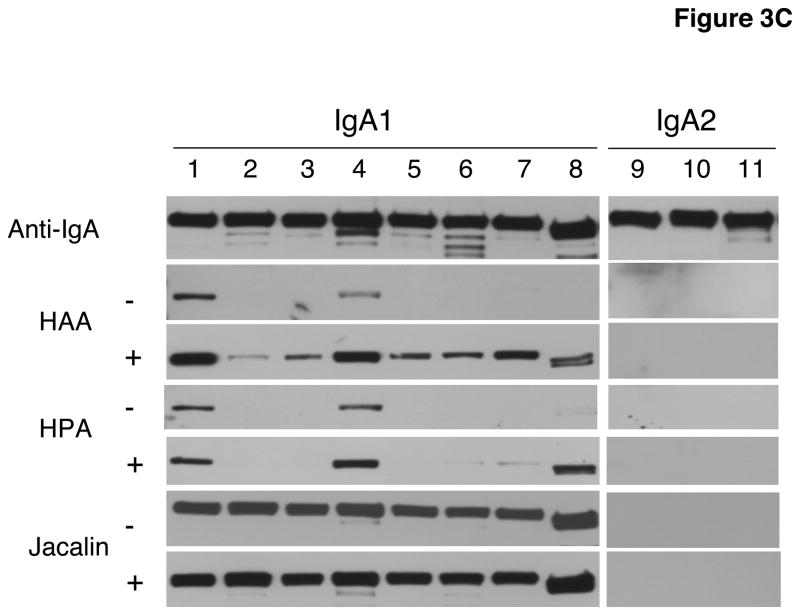
We compared the binding efficiency of HAA and HPA by ELISA and western blot analysis (Fig. 3A, B, and C). Although these two lectins purportedly have similar binding specificities (Dahr et al., 1975; Ishiyama and Uhlenbruck, 1972), we found disparate characteristics (Figs. 1 and 3). Results from ELISA showed that although both lectins bound all IgA1 myeloma proteins except Kni, HPA bound some IgA1 proteins more effectively than did HAA. In contrast, in the neuraminidase-treated western blots, HPA bound only four of the eight tested proteins whereas HAA bound to all. Using biotinylated jacalin, we confirmed the presence of O-linked GalNAc-Gal in all IgA1 myeloma proteins by western blot analysis (Fig. 3C).
Fig. 3. Comparative binding of HPA and HAA binding in ELISA (A and B) and western blot analysis (C).
A) and B). Binding of HAA and HPA, respectively, to myeloma proteins (0.625 μg/ml) determined by capture ELISA. Proteins were native (black bars) or treated with neuraminidase to remove sialic acid (light bars).
C) One μg myeloma IgA1 and IgA2 proteins loaded per well. Membranes were not treated (−) or treated (+) with neuraminidase. Anti-IgA and jacalin were used as controls for loading and presence of GalNAc-Gal disaccharides, respectively. The same numbers identify the same proteins in A through C. IgA myeloma proteins used: (1) Mce, (2) Ham, (3) Ste, (4) Gou, (5) Lat, (6) Ber1, (7) Sin, (8) Kni, (9) Fel, (10) Ber2, and (11) Cob.
The difference in binding HAA to NA-treated and untreated IgA1 proteins in the western blot analysis demonstrated heterogeneity of sialylation of their GalNAc moieties (Fig. 3C). After treatment with neuraminidase to remove sialic acid and thereby increase the number of exposed GalNAc moieties, the number of IgA1 myeloma proteins binding to HAA increased from two to eight, irrespective of the molecular form (see Table 1). For instance, after neuraminidase treatment, HPA bound IgA1 polymer (Mce) and IgA1 monomer (Kni) to the same degree (Fig. 3). In summary, the removal of sialic acid improved the accuracy of the measurement of total GalNAc content in the hinge-region IgA1 O-linked glycans and reduced the assay-dependent variability.
3.3 HAA and HPA binding to Gal-deficient IgA1: differences between ELISA and western blot results
In addition to lectin-dependent differences in binding to various IgA1 myeloma proteins, we also found marked assay-dependent differences. For example, HPA bound only to myeloma proteins Mce, Gou, and neuraminidase-treated Kni on the western blot. In contrast, in ELISA, HPA did not significantly bind IgA1 (Kni) protein although it bound other IgA1 myeloma proteins, such as Sin and Ber1, at high levels. We speculated that the discrepancies in the results of the ELISA and the western blot may have been due to a difference in sample preparation (reduction of disulphide bonds in samples for western blot analysis) and/or different three-dimensional positioning and exposure of the binding sites (GalNAc). To test whether reduction of IgA1 accounts for the observed differences, we used these samples in ELISA. We observed lower levels of lectin binding to the reduced IgA1 proteins, particularly Gou, compared to the unreduced samples (Table 2). Although in ELISA the pattern of binding was similar, there was no correlation with the pattern on western blots. We propose that the discrepancy was caused by differences in the exposure of hinge-region glycans in ELISA and western blot rather than by the reduction of disulphide bridges.
Table 2.
HPA binding to reduced and native IgA myeloma proteins in ELISA.
| Myeloma protein | Reduced (Capture) | Reduced (Direct) | Native (Capture) | Native (Direct) |
|---|---|---|---|---|
| NA-treated | ||||
| Mce | 1.055a | 1.175 | 2.407 | 2.719 |
| Ham | 0.196 | 0.375 | 1.017 | 1.434 |
| Ste | 0.101 | 0.398 | 1.050 | 1.972 |
| Gou | 0.689 | 0.602 | 3.326 | 3.771 |
| Lat | 0.182 | 0.294 | 0.754 | 1.604 |
| Ber1 | 0.984 | 0.629 | 2.065 | 2.462 |
| Sin | 0.404 | 0.622 | 1.026 | 2.108 |
| Kni | 0.034 | 0.013 | 0.074 | 0.064 |
| Fel | 0.000 | 0.003 | 0.000 | 0.000 |
| Ber2 | 0.000 | 0.000 | 0.000 | 0.000 |
| Cob | 0.000 | 0.001 | 0.000 | 0.000 |
| Without NA | ||||
| Mce | 0.523 | 0.380 | 1.888 | 2.001 |
| Ham | 0.092 | 0.059 | 0.447 | 0.679 |
| Ste | 0.079 | 0.043 | 0.395 | 0.723 |
| Gou | 0.867 | 0.343 | 3.388 | 3.667 |
| Lat | 0.105 | 0.091 | 0.301 | 0.984 |
| Ber1 | 0.341 | 0.089 | 0.803 | 0.720 |
| Sin | 0.383 | 0.251 | 0.740 | 1.643 |
| Kni | 0.080 | 0.020 | 0.000 | 0.029 |
| Fel | 0.000 | 0.004 | 0.003 | 0.003 |
| Ber2 | 0.000 | 0.007 | 0.000 | 0.000 |
| Cob | 0.009 | 0.002 | 0.000 | 0.000 |
Absorbance at 405 nm.
NA, neuraminidase
To better understand the possible effect of three-dimensional conformation on the lectin-IgA1 interactions, we compared the binding of HAA and HPA to several IgA1 myeloma proteins, either directly bound to ELISA plates or captured by anti-IgA antibody (Table 2). For most of the IgA1 proteins, direct coating resulted in better overall binding; similar results were observed with native and reduced samples. The pattern of HAA and HPA binding was generally the same as that obtained by capture ELISA (Fig. 1, Table 2) and did not correlate with the results of western blots. We postulate that the three-dimensional conformation of hinge-region GalNAc plays an important role in the lectin binding and that the accessibility of the glycans differs between assays (ELISA vs. western blot).
3.4. HAA binding to IgA1 from serum samples of IgAN patients and healthy controls
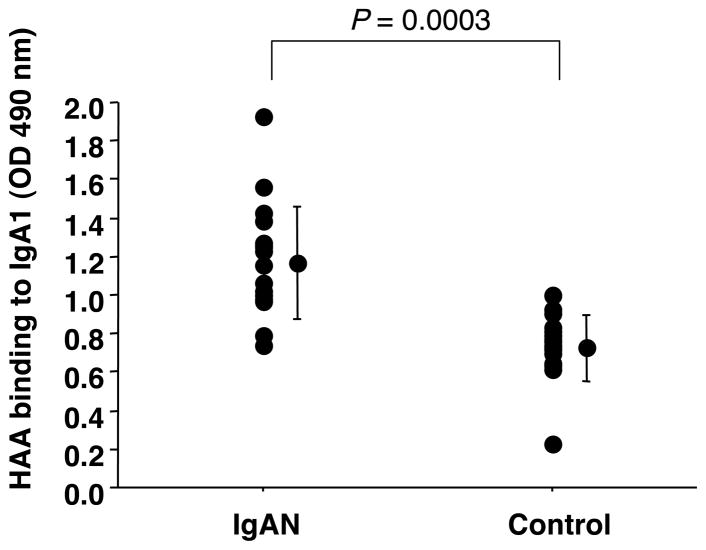
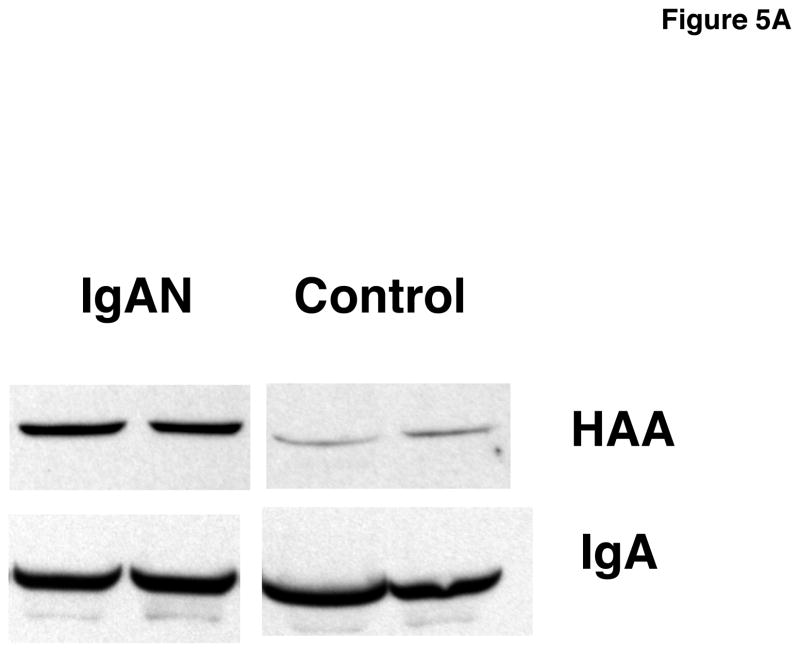
Due to its ability to bind seven of eight IgA1 myeloma proteins in both assays, we concluded that HAA from Sigma was the best lectin to detect Gal-deficient IgA1. We tested the HAA binding to IgA1 from serum samples of IgAN patients and healthy controls using ELISA with neuraminidase treatment of captured IgA1 (see Methods 2.3) (Fig. 4). HAA bound more to IgA1 from IgAN patients than to IgA1 from healthy controls (p = 0.0003). Western blot analysis confirmed the ELISA results (Fig. 5A).
Fig. 4.
HAA (Sigma) binding to serum IgA1 from IgAN patients (n = 17) and healthy controls (n = 16) measured by capture ELISA with neuraminidase treatment of IgA1. Concentration of IgA1 was normalized to 1 μg per well. Samples were treated with neuraminidase after capture.
Fig. 5.
A) Western blot of serum samples from patients with IgAN and healthy controls developed with HAA after neuraminidase treatment on the membranes. Load of serum samples was normalized to IgA concentration of 0.25 μg/well for separation by SDS-PAGE under reducing conditions.
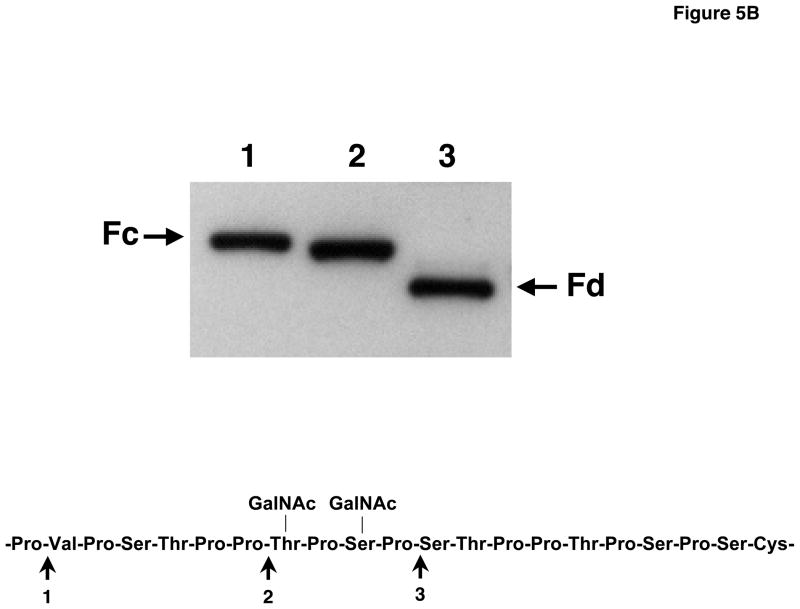
B) Western blot of purified IgA1 isolated from serum of an IgAN patient and digested with 3 different IgA proteases (1, Clostridium ramosum AK183; 2, Streptococcus pneumoniae TIGR 4; 3, Haemophilus influenzae HK50), desialylated and probed with HAA. The amino acid(s) with a Gal-deficient O-glycans was localized to a site between the cleavage sites 2 and 3.
3.5. HAA reveals Gal-deficient sites in IgA1 from IgAN patients
We isolated serum IgA1 from 3 patients with IgAN and digested the proteins with three IgA proteases. These enzymes cleaved after Pro 221, 227, and 231 (Kilian et al., 1996). The fragments generated, Fc and Fd, were separated by SDS-PAGE, blotted and after desialylation probed with HAA. The lectin reacted with the Fc fragment after digestion with proteases from AK183 or TIGR 4 (see Methods) but with Fd fragment after digestion with protease from HK50; this finding localized the Gal-deficient sites to Thr228 and/or Ser230 (Fig. 5B). One IgA1 protein also had additional Gal-deficient sites at or after Ser 232.
4. Discussion
Lectins are important tools to isolate and characterize glycan moieties on glycoproteins (Baenziger and Kornfeld, 1974; Frangione and Wolfenstein-Todel, 1972; Mattu et al., 1998; Rudd et al., 2001; Tabares et al., 2005). Because some glycoproteins or glycolipids are available in only small quantities, they may not be amenable to direct carbohydrate analyses. Therefore, we had previously developed a lectin-based ELISA to determine glycosylation abnormalities on IgA1 from IgAN patients (Tomana et al., 1997; Tomana et al., 1999). In this assay, IgA1 from some patients with IgAN reacted more effectively with GalNAc-specific lectins than did IgA1 from healthy controls (Tomana et al., 1997; Tomana et al., 1999). This finding indicated structural differences in GalNAc-containing glycans in the hinge region of IgA1 (Baenziger and Kornfeld, 1974; Frangione and Wolfenstein-Todel, 1972; Mattu et al., 1998). Specifically, our data showed that IgA1 from IgAN patients contained fewer terminal Gal residues, thus exposing the underlying GalNAc. Animal and plant lectins are usually available from several sources and each manufacturer uses its own proprietary purification process. Recently, we observed unexpected discrepancies in the binding of different batches of HAA from the same manufacturer to Gal-deficient IgA1 and asialo ovine submaxillary mucin. Upon inquiry, we discovered that the manufacturer had changed the supplier of the snails.
We compared the binding capabilities of three lectins purportedly specific for GalNAc, using well-defined standard IgA1 and control IgA2 and IgG myeloma proteins. HAA, HPA, and VV from different suppliers displayed variable specificities in the binding. Furthermore, HAA and HPA recognized terminal GalNAc in IgA1 while VV, with its previously claimed specificity for GalNAc (Tollefsen and Kornfeld, 1983), recognized other glycans in addition to GalNAc. The type of assay used in lectin binding analyses, in our case ELISA and western blot, profoundly influenced the outcome of measurements. When we compared the binding of HAA and HPA to IgA1 in these two assays, the reactivity of HAA and HPA varied substantially. In western blot analysis, HAA reacted with all IgA1 myeloma proteins tested whereas HPA reacted with only a few. However, in ELISA, HPA bound the IgA1 proteins more effectively than did HAA. The differential binding by HAA and HPA may have been a result of a variation in content or exposure of GalNAc on the IgA1 myeloma proteins.
Using HAA, we demonstrated by western blotting that a well characterized lectin can be useful not only in generally confirming the ELISA data, but also in providing site-specific information about the Gal-deficiency of IgA1 in patients with IgAN (Fig. 5B). If verified, this information would imply that the Gal deficiency in IgAN is not random, but rather is site-specific.
In summary, these results demonstrated a variability in the binding characteristics of several, purportedly GalNAc-specific, lectins based on their source and the assay used. Therefore, when lectins are used to analyze disease-associated glycans, we recommend that their binding properties be evaluated by using standard substrates in the assay of interest.
Acknowledgments
This work was supported by grants DK57750, DK61525, and DE13694 from the National Institutes of Health and by the General Clinical Research Center of the University of Alabama at Birmingham M01 RR00032. The authors express their appreciation to Catherine Barker and Sue Woodford (University of Alabama at Birmingham) for help with collecting clinical samples, and Stacy Hall for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AC, Bailey EM, Brenchley PEC, Buck KS, Barrat J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- Bourne Y, Astoul CH, Zamboni V, Peumans WJ, Menu-Bouaouiche L, Van Damme EJ, Barre A, Rouge P. Structural basis for the unusual carbohydrate-binding specificity of jacalin towards galactose and mannose. Biochem J. 2002;364:173–180. doi: 10.1042/bj3640173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SA. The involvement of Helix pomatia lectin (HPA) binding N-acetylgalactosamine glycans in cancer progression. Histol Histopathol. 2000;15:143–158. doi: 10.14670/HH-15.143. [DOI] [PubMed] [Google Scholar]
- Carpenter GH, Proctor GB, Shori DK. O-glycosylation of salivary IgA as determined by lectin analysis. Biochem Soc Trans. 1997;25:S659. doi: 10.1042/bst025s659. [DOI] [PubMed] [Google Scholar]
- Dahr W, Uhlenbruck G, Bird GW. Further characterization of some heterophile agglutinins reacting with alkali-labile carbohydrate chains of human erythrocyte glycoproteins. Vox Sang. 1975;28:133–148. doi: 10.1111/j.1423-0410.1975.tb02751.x. [DOI] [PubMed] [Google Scholar]
- Ezaki T, Baluk P, Thurston G, La Barbara A, Woo C, McDonald DM. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Dwek RA, Edge CJ, Rademacher TW. O-linked oligosaccharides from human serum immunoglobulin A1. Biochem Soc Trans. 1989;17:1034–1035. doi: 10.1042/bst0171034. [DOI] [PubMed] [Google Scholar]
- Frangione B, Wolfenstein-Todel C. Partial duplication in the “hinge” region of IgA1 myeloma proteins. Proc Natl Acad Sci USA. 1972;69:3673–3676. doi: 10.1073/pnas.69.12.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiki Y, Horii A, Iwase H, Tanaka A, Toda Y, Hotta K, Kobayashi Y. O-linked oligosaccharide on IgA1 hinge region in IgA nephropathy. Fundamental study for precise structure and possible role. Contrib Nephrol. 1995;111:73–84. [PubMed] [Google Scholar]
- Ishiyama I, Uhlenbruck G. Further studies on the specificity of the anti-A agglutinin from Helix pomatia. Comp Biochem Physiol. 1972;42A:269–276. doi: 10.1016/0300-9629(72)90108-9. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Katiyar S, Swaminathan CP, Sekar K, Surolia A, Vijayan M. Structural basis of the carbohydrate specificities of jacalin: an X-ray and modeling study. J Mol Biol. 2003;332:217–228. doi: 10.1016/s0022-2836(03)00901-x. [DOI] [PubMed] [Google Scholar]
- Julian BA, Novak J. IgA nephropathy: an update. Current Opin Nephrol Hypertens. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med. 1988;84:129–132. doi: 10.1016/0002-9343(88)90019-8. [DOI] [PubMed] [Google Scholar]
- Kabir S. Jacalin: a jackfruit (Artocarpus heterophyllus) seed-derived lectin of versatile applications in immunobiological research. J Immunol Methods. 1998;212:193–211. doi: 10.1016/s0022-1759(98)00021-0. [DOI] [PubMed] [Google Scholar]
- Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen EVG. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kondoh H, Hagiwara K, Vaerman JP. Jacalin: chaos in its immunolglobulin-binding specificity. Mol Immunol. 1988;25:1037–1038. doi: 10.1016/0161-5890(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A, Mackiewicz K. Glycoforms of serum alpha 1-acid glycoprotein as markers of inflammation and cancer. Glycoconjugate J. 1995;12:241–247. doi: 10.1007/BF00731326. [DOI] [PubMed] [Google Scholar]
- Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1:237–243. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Hamilton RG, Magnusson C, Jeffris R, Vaerman JP, Goodall M, de Lange GG, Moro I, Aucouturier P, Radl J, Cambiaso C, Silvain C, Preud’homme JL, Kusama K, Carlone GM, Biewenga J, Kobayashi K, Skvaril F, Reimer CB. Evaluation of monoclonal antibodies with specificity for human IgA, IgA subclasses and secretory component: Results of an IUIS/WHO collaborative study. J Immunol Methods. 1996;193:103–148. doi: 10.1016/0022-1759(95)00289-8. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Tomana M, Crowley-Nowick PA, Moldoveanu Z, Julian BA, Jackson S. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- Mody R, Joshi S, Chaney W. Use of lectins as diagnostic and therapeutic tools for cancer. J Pharmacol Toxicol. 1995;33:1–10. doi: 10.1016/1056-8719(94)00052-6. [DOI] [PubMed] [Google Scholar]
- Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 2005;19:381–389. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- Mullinax F, Mullinax GL. Abnormality of IgG structure in rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 1975;18:417–418. [Google Scholar]
- Novak J, Julian BA, Tomana M, Mestecky J. Progress in molecular and genetic studies of IgA nephropathy. J Clin Immunol. 2001;21:310–327. doi: 10.1023/a:1012284402054. [DOI] [PubMed] [Google Scholar]
- Novak J, Tomana M, Kilian M, Coward L, Kulhavy R, Barnes S, Mestecky J. Heterogeneity of O-glycosylation in the hinge region of human IgA1. Mol Immunol. 2000;37:1047–1056. doi: 10.1016/s0161-5890(01)00019-0. [DOI] [PubMed] [Google Scholar]
- Raval S, Gowda SB, Singh DD, Chandra NR. A database analysis of jacalin-like lectins: sequence-structure-function relationships. Glycobiology. 2004;14:1247–1263. doi: 10.1093/glycob/cwh140. [DOI] [PubMed] [Google Scholar]
- Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- Smith AC, Feehally J. New insights into the pathogenesis of IgA nephropathy. Pathogenesis of IgA nephropathy Springer Semin. Immunopathol. 2003;24:477–493. doi: 10.1007/s00281-002-0115-x. [DOI] [PubMed] [Google Scholar]
- Tabares G, Radcliffe CM, Barrabes S, Ramirez M, Aleixandre RN, Hoesel W, Dwek RA, Rudd PM, Peracaula R, Llorens RD. Different glycan structures in prostate-specific antigen (PSA) from prostate cancer sera in relation to seminal plasma PSA. Glycobiology. 2005;16:132–145. doi: 10.1093/glycob/cwj042. [DOI] [PubMed] [Google Scholar]
- Tollefsen SE, Kornfeld R. The B4 lectin from Vicia villosa seeds interacts with N-acetylgalactosamine residues alpha-linked to serine or threonine residues in cell surface glycoproteins. J Biol Chem. 1983;258:5172–5176. [PubMed] [Google Scholar]
- Tomana M. Glycoproteins in inflammatory bowel disease. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins and Disease. Elsevier; New York, NY: 1996. pp. 291–298. [Google Scholar]
- Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–516. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- Tomana M, Niedermeier W, Mestecky J, Skvaril F. The differences in carbohydrate composition between the subclasses of IgA immunoglobulins. Immunochemistry. 1976;13:325–328. doi: 10.1016/0019-2791(76)90342-6. [DOI] [PubMed] [Google Scholar]
- Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomana M, Prchal JT, Garner LC, Skalka HW, Barker SA. Gas chromatographic analysis of lens monosaccharides. J Lab Clin Med. 1984;103:137–142. [PubMed] [Google Scholar]
- Tsuchiya N, Endo T, Matsuta K, Yoshinoya S, Takeuchi F, Nagano Y, Shiota M, Furukawa K, Kochibe N, Ito K. Detection of glycosylation abnormality in rheumatoid IgG using N- acetylglucosamine-specific Psathyrella velutina lectin. J Immunol. 1993;151:1137–1146. [PubMed] [Google Scholar]
- van Damme EJ, Hause B, Hu J, Barre A, Rouge P, Proost P, Peumans WJ. Two distinct jacalin-related lectins with a different specificity and subcellular location are major vegetative storage proteins in the bark of the black mulberry tree. Plant Physiol. 2002;130:757–769. doi: 10.1104/pp.005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold AE, Motas C, Svanborg C, Mestecky J. Lectin receptors on IgA isotypes. Scand J Immunol. 1994;39:195–201. doi: 10.1111/j.1365-3083.1994.tb03360.x. [DOI] [PubMed] [Google Scholar]
- Wu AM, Wu JH, Lin LH, Lin SH, Liu JH. Binding profile of Artocarpus integrifolia agglutinin (Jacalin) Life Sci. 2003;72:2285–2302. doi: 10.1016/s0024-3205(03)00116-4. [DOI] [PubMed] [Google Scholar]