Abstract
Purpose
The goal of this study was to examine the therapeutic potential of the vascular endothelial growth factor (VEGF) signaling inhibitor cediranib in a human model of renal cell carcinoma (Caki-1).
Methods and Materials
The effects of cediranib treatment on in vitro endothelial cell function (proliferation, migration, and tube formation), as well as in vivo angiogenesis and tumor growth, were determined.
Results
In vitro, cediranib significantly impaired the proliferation and migration of endothelial cells and their ability to form tubes, but had no effect on the proliferation of Caki-1 tumor cells. In vivo, cediranib significantly reduced Caki-1 tumor cell–induced angiogenesis, reduced tumor perfusion, and inhibited the growth of Caki-1 tumor xenografts.
Conclusions
The present results are consistent with the notion that inhibition of VEGF signaling leads to an indirect (i.e., antiangiogenic) antitumor effect, rather than a direct effect on tumor cells. These results further suggest that inhibition of VEGF signaling with cediranib may impair the growth of renal cell carcinoma.
Keywords: Antiangiogenic therapy; Caki-1 renal cell carcinoma; Cediranib (Recentin, AZD2171)
INTRODUCTION
Neovascularization, a rare event in adults confined almost exclusively to the female reproduction system, is an essential feature of solid tumors (1). It is widely accepted that continued tumor growth depends on nutrient supply from a network of microvessels that may originate from angiogenesis, vasculogenesis, vessel intussusception, vascular mimicry, or any combination thereof (2, 3). Angiogenesis involves the migration and proliferation of endothelial cells from existing vessels (4) triggered by the release of such stimulators as the endothelial cell–specific mitogen vascular endothelial growth factor (VEGF), which is secreted as a 45-kDa homo dimer protein (5). The soluble isoform VEGF165 is commonly expressed in a wide variety of human and animal tumors (6) and currently is believed to be the key mediator of tumor angiogenesis (5). In patients, VEGF overexpression has been documented in most types of cancers, and this overexpression often has been associated with poor prognosis (7, 8). This has made the VEGF pathway an attractive target for therapeutic interventions in the treatment of patients with cancer.
Renal cell carcinoma (RCC) is the most common malignancy of the kidney and accounts for about 2% of all adult malignancies (9, 10). Unless discovered at an early stage, RCC has a very unfavorable treatment outcome after conventional therapeutic interventions and is fatal in nearly 80% of its patients (10). The RCC is a highly vascularized neoplasm (11), and increased serum/urine VEGF levels have been associated with malignant progression and poor treatment outcome (12, 13). Consequently, RCC may be an excellent site to investigate VEGF-targeted antiangiogenic therapies.
A number of approaches have been devised to interfere with the VEGF signaling cascade (14, 15); targeting the VEGF protein- or receptor(s) (VEGFR[s])-associated tyrosine kinases has emerged as a key therapeutic strategy. This approach has recently led to clinical validation of the value of antiangiogenic therapy (16, 17) and the first approval of the antiangiogenic therapeutics bevacizumab (Avastin, Genentech, San Francisco, CA) and sunitinib (Sutent, Pfizer Inc., New York, NY; SU11248). Sunitinib and sorafenib (Nexavar, Bayer Pharmaceuticals, West Haven, CT), multi-targeted receptor tyrosine kinase inhibitors, have now been approved by the Food and Drug Administration (FDA), as well as in the European Union, for the treatment of patients with RCC. The goal of the present study was to evaluate the efficacy of cediranib (Recentin; AstraZeneca Pharmaceuticals, Macclesfield, UK), an oral, highly potent, and selective VEGF signaling inhibitor of VEGFR-1, -2, and -3 tyrosine kinases (18) in a human RCC (Caki-1) model.
METHODS AND MATERIALS
Cell culture
The clear-cell RCC cell line Caki-1 was originally received as a gift from Dr. Susan Knox (Stanford University, Palo Alto, CA). Caki-1 cells were grown in Dulbecco’s modified minimum essential medium (D-MEM, Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, NY), 1% penicillin-streptomycin (Invitrogen), and 1% 200-mmol/L l-glutamine (Invitrogen). Human microvascular endothelial cells from the lung (HMVEC-L) were obtained from Clonetics (San Diego, CA). The HMVEC-L cells were grown in EBM-2-MV (Clonetics) supplemented with 5% fetal bovine serum.
Drug preparation
Cediranib was provided by AstraZeneca Pharmaceuticals. For in vitro investigations, 10 mm of stock solutions prepared in dimethyl sulfoxide were serially diluted in sterile saline such that dimethyl sulfoxide exposure in cell cultures was less than 0.1%. For in vivo studies, stock solutions of drug (10 mm) were prepared by suspending the agent in 10% (vol/vol) Tween-80 (Invitrogen, Grand Island, NY) and N-2-hydroxyethylpiperazine propanesulfonic acid. Working dilutions were made by serial dilution of the stock solution in sterile saline. All drug preparations were kept refrigerated and in the dark and used within 1 week of preparation.
In vitro cell growth
The HMVEC-L or Caki-1 cells (1 × 104) were seeded in triplicate into 60-mm tissue culture dishes and allowed to attach overnight. The next day, drug was added to the medium at the appropriate dose as a solution of 10 µl/ml of medium. At various times later, plates were trypsinized and cells were counted.
Endothelial cell migration
The HMVEC-L cells (1 × 104) were seeded in sextuplet into six-well plates and allowed to reach confluence. A scrape 2-mm wide was then made across the entire length of each well. Cediranib was added to each well at the appropriate dose in a volume of 10 µl/ml. Forty-eight hours later, cells were stained with crystal violet and viewed at original magnification ×5, and the number of endothelial cells that had migrated into the scraped area was determined.
Endothelial cell tube formation
The HMVEC-L cells (6–8 × 104) were plated into 24-well dishes precoated with 200 µl of matrigel. Photos of endothelial tubes at original magnification ×5 were obtained 24 hours after plating the endothelial cells.
Caki-1 xenografts
Tumors were initiated by implanting (1 × 106) Caki-1 tumor cells in the left hind calf muscle of 6–8-week-old female athymic nude mice (NCR nu/nu). All mice were provided sterilized food and water ad libitum and housed in a barrier facility with 12-hour light and dark cycles. All procedures were conducted at the University of Florida, Gainesville, FL, according to guidelines laid out by the Institutional Animal Care and Use Committee. When tumors reached approximately 200 mm3, the animals were randomly assigned to the various treatment groups.
In vivo drug administration
Cediranib (6 mg/kg/d) was administered by means of oral gavage for a 2-week period (Monday to Friday) after tumors reached a volume of 200 mm3.
Intradermal angiogenesis assay
Caki-1 cells (1 × 105) were inoculated intradermally in a volume of 10 µl at four sites on the ventral surface of nude mice. One drop of 0.4% trypan blue was added to the cell suspension, making it lightly colored, simplifying subsequent location of the sites of injection. Three days later, the mice were euthanized, the skin was carefully separated from the underlying muscle, and the number of blood vessels intersecting each inoculate was counted by using a dissecting microscope. The resultant data for each treatment group were pooled for statistical analysis (Wilcoxon’s rank-sum test). Statistical significance at p < 0.05 was used.
Vessel density: CD31 immunohistochemistry
Frozen sections of tumors were cut on a cryostat, air dried, and fixed in acetone/methanol at 4°C for 10 minutes. Tumor microvessels were stained using a mouse monoclonal antibody to the CD31 (PECAM-1) antigen found on endothelial cells (Beckman Coulter, Brea, CA), applied overnight at 4°C at a dilution of 1:50. A secondary antibody conjugated with Cy3 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was applied for 1 hour at room temperature. Staining was followed by standard washing, then slides were allowed to air dry before storage at 4°C.
Tumor perfusion, patent blood vessels, Hoechst-33342
The fluorescent dye Hoechst-33342 (bisBenzimide; Sigma, Saint Louis, MO) was prepared in 0.9% sterile saline immediately before intravenous administration (40 mg/kg) (18). One minute after Hoechst-33342 injection, the mice were euthanized and the tumors were resected and immediately immersed in liquid nitrogen for subsequent frozen sectioning. The sections were studied under UV illumination by using a fluorescent microscope. Blood vessel outlines were identified by the surrounding halo of fluorescent Hoechst-33342–labeled cells (19).
Vessel counts
For each tumor sample, 10-µm cryostat sections were cut at three different levels between one pole and the equatorial plane. Vessel counts were performed by using a Chalkley point array for random-sample analysis (20). Briefly, three sections were cut per tumor and 10 areas were viewed (at objective magnification × 10) per section. A 25-point Chalkley grid was positioned over the field of view, and any points within the fluorescent areas were scored as positive. Data from three controls and six tumors from cediranib-treated mice were pooled and presented.
Tumor response
Efficacy of treatment was determined by using a tumor growth delay assay. Established tumors were measured every 2 days by using calipers, and volumes were approximated by using the formula: volume = (1/6) πab2 (where a and b represent two perpendicular tumor diameters). Times for tumors in the various treatment groups to grow from 200 to 1,000 mm3 were compared by using Wilcoxon’s rank-sum test. Statistical significance at p < 0.05 was used.
RESULTS
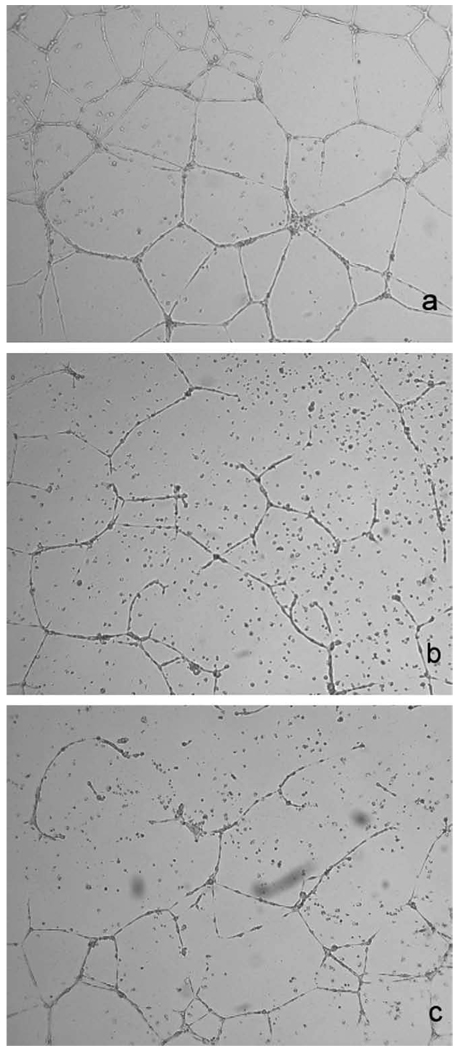
In vitro, cediranib (10–100 nm) inhibited the growth of microvascular endothelial (HMVEC-L) cells; however, doses of 5 µm or less had little impact on renal carcinoma (Caki-1) cell growth (Fig. 1). Cediranib also significantly impaired the migration of endothelial cells in a dose-dependent manner (Fig. 2). For example, a dose of 5 nm of cediranib decreased (by a factor of nearly 2) the number of cells that migrated into a denuded area during a 48-hour period. A 24-hour pretreatment of endothelial cells with low doses of cediranib (5–10 nm) also affected their ability to form tubes (Fig. 3). For example, a 5-nm dose of cedirinib decreased the number of completed structures in a field of view from 14.6 ± 1.6 (SE) to 10.1 ± 1.6 (mean, nine fields of view from three treated and three control groups).
Fig. 1.
Effect of cediranib treatment on growth of Caki-1 cells and human microvascular endothelial cells from the lung (HMVEC-Ls). Cells were treated with a range of doses of cediranib for 9 days. At various times during the course of treatment, the number of cells was determined. Each point represents the mean of two experiments. Growth curves of Caki-1 cells treated with cediranib doses of 10 nm or less were indistinguishable from those of untreated cells and are not shown for clarity.
Fig. 2.
The effect of cediranib treatment on migration of human microvascular endothelial cells from the lung (HMVEC-Ls) in vitro. Cells were plated in sextuplet into six-well plates and allowed to reach confluence. A 2-mm scrape was made in each well, and drug was added. Forty-eight hours later, cells were stained with crystal violet and the number of endothelial cells that migrated into the denuded area was counted. Data are mean ± SE of three experiments. *p < 0.05 compared with control, determined by using unpaired Student’s t-test.
Fig. 3.
Impact of cediranib pretreatment on the ability of human microvascular endothelial cells from the lung (HMVEC-Ls) to form tubes. Endothelial cells were exposed to 5 or 10 nm of cediranib for 24 hours before plating them in dishes coated with 200 µl of matrigel. Tube formation was visualized 24 hours later at original magnification ×5. (a) Control, (b) 5 nm of cediranib, and (c) 10 nm of cediranib. Cells were not exposed to the drug during the 24-hour tube formation period.
An intradermal assay was used to study the effect of cediranib treatment on Caki-1 tumor cell–induced angiogenesis in vivo. Nude mice received cediranib (3, 6, or 9 mg/kg/d) or vehicle by means of oral gavage starting on the day before tumor cell inoculation. Caki-1 cells (1 × 105) were injected intradermally at four sites on the ventral surface of each of four mice. Three days after inoculation, the mice were killed, the skin flap containing the inoculation site was excised, and the number of blood vessels induced was counted. Results (Fig. 4) showed that compared with controls, skin flaps from mice of all cediranib-treated groups showed significant reductions in the number of blood vessels induced by the Caki-1 tumor cells. In addition, the difference between the 3- and 6-mg/kg/d groups was significant, but neither was significantly different from the 9-mg/kg/d group.
Fig. 4.
Effect of cediranib treatment on the number of blood vessels induced by inoculii of 1 × 105 Caki-1 tumor cells. Nude mice received cediranib (3, 6, or 9 mg/kg/d) or vehicle by means of oral gavage starting on the day before tumor cell inoculation. Caki-1 cells were injected intradermally at four sites on the ventral surface of each of four mice. Three days after inoculation, the mice were humanely killed, the skin flap containing the inoculation site was excised, and the number of blood vessels induced was counted. Data shown are median responses. Bars and hatched areas = 90% and 75% confidence intervals, respectively; stars = statistical significance of p < 0.05 (Wilcoxon’s rank-sum test) compared with controls.
The antitumor efficacy of cediranib was assessed in mice bearing Caki-1 xenografts. When tumor volume reached approximately 200 mm3, mice were randomly assigned to receive either no treatment or cediranib administered as 6 mg/kg/d orally for 2 weeks (Monday to Friday). The antitumor efficacy of cediranib treatment was assessed by using a tumor growth delay assay. Results of three experiments showed that in animals receiving cediranib, tumors took twice as long to reach five times the starting size compared with untreated control mice (Fig. 5).
Fig. 5.
Response of Caki-1 xenografts to cediranib treatment. Tumor-bearing mice were treated for a 2-week period (Monday to Friday) with daily 6-mg/kg doses of cediranib administered by means of gavage. Data are results of three experiments, points are the medians of groups of nine mice, and bars and hatched areas are 90% and 75% confidence intervals, respectively. Treated groups were significantly different from untreated controls compared by using Wilcoxon’s rank-sum test (p < 0.05).
To evaluate the impact of cediranib treatment on tumor vasculature, tumor sections were prepared at the end of the treatment period and analyzed for tumor vascularity (CD31 immunohistochemistry) and patent blood vessel number (Hoechst 33342 fluorescence). No significant differences in CD31 staining were noted between treated and control tumors (data not shown). However, the number of patent blood vessels was reduced in tumors of mice undergoing cediranib therapy compared with that in tumors of mice in the control group (Fig. 6).
Fig. 6.
Vessel counts in Caki-1 xenografts at the end of a 2-week course of treatment with 6 mg/kg of cediranib. For comparison, equal-sized untreated control tumors were assessed. Mice were injected with a 40-mg/kg dose of Hoechst 33342 to identify patent blood vessels, and the tumors were removed 1 minute later. Counting was performed by using a Chalkley point array for random sample analysis. Data are mean ± SE of three (control) and six (cediranib-treated) tumors. *Significant difference (p < 0.05) from untreated controls (Wilcoxon’s rank-sum test).
DISCUSSION
The VEGF acts as a survival factor for newly formed vasculature, enhances the permeability of blood vessels, and has been implicated as the key factor in the angiogenic process of solid tumors (1, 4, 7, 8). Thus, interfering with VEGF signaling has become a major strategy to inhibit tumor growth and spread (7, 8, 14). In addition to targeting the VEGF ligand (bevacizumab), another approach that has received considerable attention in the clinic is the targeting of VEGFR-associated tyrosine kinase signaling (reviewed in [21]).
Histopathologic characteristics of RCC (highly vascularized neoplasm, abundant angiogenesis) coupled with its poor response to traditional anticancer therapies has led to the investigation of novel blood vessel–directed molecular targeting strategies in this disease setting. Bevacizumab was approved by the FDA for use in combination with fluorouracil-based chemotherapy for the first-line treatment of patients with metastatic colorectal carcinoma after results showed that the addition of this VEGF-specific antibody led to improved overall survival in previously treated and untreated patients with colorectal cancer (16). In patients with RCC, bevacizumab has been studied as a single agent (22) in combination with thalidomide (23) or interferon-α (24). In general, response rates were poor, although some improvement in time to progression was noted for patients receiving bevacizumab (22). The strategy of using multitargeted tyrosine kinase inhibitors, including those that inhibit VEGF signaling, also has been vigorously pursued in patients with RCC. Lead agents that have emerged included sorafenib and sunitinib. The former, although also showing a lack of objective responses, provided clear evidence of disease stabilization and prolonged time to disease progression (25). Conversely, sunitinib, in two separate Phase II studies, showed consistent response rates in approximately 40% of patients in whom cytokine therapy had already failed, and in a Phase III study, showed superior efficacy compared with interferon-α, as well as a doubling of progression-free survival (17). As a result, the FDA has approved sunitinib for first-line use and sorafenib for treatment of patients with advanced RCC. Taken together, these data strongly support a role for this class of drug in the clinical management of patients with RCC.
Cediranib is an oral highly potent and selective VEGF signaling inhibitor of VEGFR-1, -2, and -3 tyrosine kinases (18) that currently is undergoing Phase II–III evaluation (26–28). Preliminary investigations of cediranib in patients with advanced RCC in two Phase I studies have shown encouraging antitumor activity (overall response rate, 37%) and a manageable toxicity profile across a wide range of doses (29). In addition, an ongoing Phase II study of cediranib, 45 mg/d, showed preliminary antitumor activity (overall response rate, 31%) in the first-line treatment of patients with metastatic RCC (30).
Preclinical investigations have shown it to have antitumor efficacy when used alone or in combination with other targeted molecules or conventional anticancer therapy in a variety of xenograft models, including colon, head-and-neck, and lung cancer (31–33). The present study reports on the efficacy of cediranib in human RCC (Caki-1) cells in tissue culture and solid tumor xenografts.
VEGF is a key stimulant of endothelial cell migration, and targeting VEGF signaling by means of cediranib treatment significantly impaired this endothelial cell function in a dose-dependent manner (Fig. 2). This inhibitory effect occurred at cediranib doses (≥10 nm) that were significantly lower than those required to affect endothelial cell proliferation (Fig. 1). Pretreatment of endothelial cells (24 hours) with cediranib also impaired the ability of endothelial cells to form tubes (Fig. 3), but did not induce clonogenic or apoptotic endothelial cell death (data not shown). In vivo, cediranib exposure significantly inhibited the ability of Caki-1 tumor cells to initiate new blood vessel formation (Fig. 4). Even a relatively short tumor treatment period of 3 days reduced the number of vessels reaching Caki-1 tumor cells by a factor of approximately 2 (Fig. 4). In light of the in vitro observations, this in situ result likely reflects an effect of cediranib on endothelial cell function (proliferation, migration, and tube formation) and not a direct cytotoxic action of this agent.
A 2-week course of cediranib administered to mice bearing Caki-1 xenografts did not lead to a change in tumor vessel density, determined by means of CD31 staining, but decreased tumor perfusion, determined by the uptake and distribution of the fluorescent dye Hoechst 33342 (Fig. 5), implying that some tumor blood vessels lost patency as a consequence of cediranib therapy. These results differ from the observed decrease in tumor microvessel density reported when Calu-6 lung tumor xenograft-bearing mice were treated with cediranib (32). However, it should be noted that a decrease in tumor blood vessel patency without change in vessel density as seen in the Caki-1 xenografts is not unprecedented, having been reported in other studies of anti-VEGF therapy (34), although the mechanistic basis for this observation has yet to be established. As reported in head-and-neck (CAL33), lung (Calu-6), and colon (LoVo) tumor xenografts (31, 33), cediranib treatment also led to significant antitumor effects in the present study; i.e., an approximately two-fold delay in the time for Caki-1 tumor xenografts to reach five times the starting size (Fig. 6). These results suggest that inhibition of VEGF signaling with cediranib may impair the growth of RCC.
Based on these results, studies investigating the efficacy of this agent in combination with chemotherapy and radiotherapy in several preclinical tumor models including RCC are ongoing. Preliminary observations of enhanced antitumor activity combined with radiation have been made in the Caki-1 model. Specifically, administering the 2-week cediranib treatment protocol after a single 10-Gy dose of radiation resulted in significantly greater tumor growth delay (p < 0.05; Wilcoxon’s rank-sum test) than either radiation or cediranib treatment alone. Although encouraging and consistent with results of previous studies assessing the cediranib-radiation combination in other tumor models (31, 33), it is recognized that it will be most prudent to assess the efficacy of cediranib in combination with other RCC therapies given that RCC is a disease not typically treated with radiation therapy.
In summary, results of this study further support the therapeutic value of targeting VEGF-associated angiogenic signaling in RCC. Treatment with the small-molecule VEGF signaling inhibitor cediranib was effective at impairing endothelial cell function in vitro and renal cancer cell induction of blood vessel formation in vivo. Most importantly, systemic administration of cediranib to mice bearing macroscopic tumors resulted in significant inhibition of the growth of the Caki-1 RCC model. Taken together, these findings suggest that cediranib therapy may have utility in the management of RCC.
Acknowledgments
The authors thank S. Lepler and C. Pampo for excellent technical support.
Supported in part by a grant from AstraZeneca and US Public Health Service Grant CA089655 from the U.S. National Cancer Institute.
Footnotes
Conflict of interest: These studies were supported in part by a research grant to Dr. Siemann from AstraZeneca. Dr. Juliane Jürgensmeier is an employee of AstraZeneca.
REFERENCES
- 1.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix MJ, Seftor EA, Hess AR, et al. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 3.Streubel B, Chott A, Huber D, et al. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med. 2004;351:250–259. doi: 10.1056/NEJMoa033153. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9 Suppl. 1:2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 8.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin JK, Lipworth L. Epidemiologic aspects of renal cell cancer. Semin Oncol. 2000;27:115–123. [PubMed] [Google Scholar]
- 10.Tsui KH, Shvarts O, Smith RB, et al. Prognostic indicators for renal cell carcinoma: A multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090–1095. doi: 10.1016/s0022-5347(05)67699-9. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino S, Kato M, Okada K. Clinical significance of angiogenesis, proliferation and apoptosis in renal cell carcinoma. Anticancer Res. 2000;20:591–594. [PubMed] [Google Scholar]
- 12.Jacobsen J, Rasmuson T, Grankvist K, et al. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J Urol. 2000;163:343–347. [PubMed] [Google Scholar]
- 13.Tomisawa M, Tokunaga T, Oshika Y, et al. Expression pattern of vascular endothelial growth factor isoform is closely correlated with tumour stage and vascularisation in renal cell carcinoma. Eur J Cancer. 1999;35:133–137. doi: 10.1016/s0959-8049(98)00278-0. [DOI] [PubMed] [Google Scholar]
- 14.Harris AL. Antiangiogenesis for cancer therapy. Lancet. 1997;349 Suppl. 2:SII13–SII15. doi: 10.1016/s0140-6736(97)90014-3. [DOI] [PubMed] [Google Scholar]
- 15.Kerbel RS. Tumor angiogenesis: Past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizurnab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. doi: 10.1200/JCO.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 18.Wedge SR, Kendrew J, Hennequin LF, et al. AZD2171: A highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 19.Smith KA, Hill SA, Begg AC, et al. Validation of the fluorescent dye Hoechst 33342 as a vascular space marker in tumours. Br J Cancer. 1988;57:247–253. doi: 10.1038/bjc.1988.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis ACS. Area and volume measurements by random sampling methods. Med Biol Illustra. 1960;10:261–266. [PubMed] [Google Scholar]
- 21.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 22.Yang JC, Haworth L, Sherry RM, et al. Arandomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elaraj DM, White DE, Steinberg SM, et al. A pilot study of antiangiogenic therapy with bevacizumab and thalidomide in patients with metastatic renal cell carcinoma. J Immunother. 2004;27:259–264. doi: 10.1097/00002371-200407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rini BI, Halabi S, Taylor J, et al. Cancer and Leukemia Group B 90206: A randomized phase III trial of interferon-alpha or interferon-alpha plus anti-vascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma. Clin Cancer Res. 2004;10:2584–2586. doi: 10.1158/1078-0432.ccr-03-0605. [DOI] [PubMed] [Google Scholar]
- 25.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 26.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 27.Hanrahan EO, Heymach JV. Vascular endothelial growth factor receptor tyrosine kinase inhibitors vandetanib (ZD6474) and AZD2171 in lung cancer. Clin Cancer Res. 2007;13 Suppl.:S4617–S4622. doi: 10.1158/1078-0432.CCR-07-0539. [DOI] [PubMed] [Google Scholar]
- 28.Ryan CJ, Stadler WM, Roth B, et al. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25:445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 29.van Herpen C, Drevs J, van Cruijsen H, et al. Evaluation of AZD2171, an oral, highly potent and selective VEGFR signaling inhibitor, in renal cell carcinoma (RCC): Combined results from two phase I studies [Abstract] Proc Am Soc Clin Oncol. 2007;25:3560. [Google Scholar]
- 30.Sridhar SS, Hotte SJ, Mackenzie MJ, et al. Phase II study of the angiogenesis inhibitor AZD2171 in first line, progressive, unresectable, advanced metastatic renal cell carcinoma (RCC): A trial of the PMH Phase II Consortium [Abstract] Proc Am Soc Clin Oncol. 2007;5:5093. [Google Scholar]
- 31.Bozec A, Formento P, Lassalle S, et al. Dual inhibition of EGFR and VEGFR pathways in combination with irradiation: Antitumour supra-additive effects on human head and neck cancer xenografts. Br J Cancer. 2007;97:65–72. doi: 10.1038/sj.bjc.6603791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith NR, James NH, Oakley I, et al. Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther. 2007;6:2198–2208. doi: 10.1158/1535-7163.MCT-07-0142. [DOI] [PubMed] [Google Scholar]
- 33.Williams KJ, Telfer BA, Shannon AM, et al. Combining radiotherapy with AZD2171, a potent inhibitor of vascular endothelial growth factor signaling: Pathophysiologic effects and therapeutic benefit. Mol Cancer Ther. 2007;6:599–606. doi: 10.1158/1535-7163.MCT-06-0508. [DOI] [PubMed] [Google Scholar]
- 34.Horsman MR, Siemann DW. Pathophysiological effects of vascular targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]