Abstract
Background
This study evaluated the therapeutic efficacy of combining vascular disrupting agents with antiangiogenic agents.
Materials and Methods
Human clear cell renal carcinoma (Caki-1) tumors were established in nude mice. Treatments consisted of Avastin (2 mg/kg) administered twice a week; CA4P (100 mg/kg) or OXi4503 (25 mg/kg) administered 3 times a week for a period of 2 weeks; or a combination of Avastin and CA4P or OXi4503. Tumor response was assessed by growth delay.
Results
The tumor growth delays were 8, 6, and 18 days for Avastin, CA4P, and OXi4503, respectively. When the two therapies were combined, there was a significantly greater tumor response than what was achieved with single-agent treatments. For example, Avastin plus CA4P led to a growth delay of 13 days, and 27 days for Avastin plus OX14503.
Conclusion
Vascular-directed therapies that include both antiangiogenic and vascular disrupting therapeutics can result in significantly enhanced antitumor effects.
Keywords: Antiangiogenic therapy, vascular disrupting agents, combination therapy, renal cell carcinoma
Blood vessels in tumors have long been recognized to differ greatly in structure and function from those found in normal tissues (1). Tumor vasculature is highly irregular, heterogenic and tortuous, and typically lacks smooth muscle and pericyte support. Functionally, these tumor vessels are highly permeable and angiogenic. Despite these abnormalities, a tumor’s growth, spread, and survival are utterly dependent on its ability to maintain a functional blood vessel network (2). Consequently, intense research has pursued and successfully established a variety of new therapeutic approaches and agents directed specifically against this component of solid tumors. Indeed, the therapeutic potential of targeting the tumor vasculature is now widely recognized and accepted (3, 4). Two distinct therapeutic approaches have emerged: antiangiogenic agents (AIs), which act primarily by interfering with new blood vessel formation, and vascular disrupting agents (VDAs), whose principal mode of action is to damage established tumor blood vessels (5). These approaches differ not only in their mode of action and treatment regimen, but also in the type and extent of neoplastic disease that is likely to be susceptible (5). Rather than being redundant, these two approaches are likely to be complementary. Preclinical data support the rationale of combining AIs and VDAs to enhance antitumor efficacy (6, 7).
Avastin (bevacizumab) is a recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGF). Since VEGF is the key angiogenic factor in tumors, blocking VEGF signal transduction can lead to tumor growth arrest and inhibition of metastasis (8). Avastin is the first antiangiogenic agent to demonstrate a survival benefit in patients with any type of cancer. It improved patient survival by 30% in metastatic colorectal cancer when combined with standard chemotherapy (9). Consequently, Avastin is the first antiangiogenic agent to be approved for the first-line treatment of metastatic colorectal cancer in combination with 5-fluorouracil-based chemotherapy. Recently, encouraging results of clinical studies of Avastin as a single therapy or part of combination therapy for breast cancer, non-small cell lung cancer, renal cell carcinoma, and other solid tumors were also reported (10–15).
VDAs take advantage of the unique properties of tumor endothelium to selectively disrupt the tumor vasculature. Combretastatin A4 (CA4P) is a lead small molecule VDA (16) which potently inhibits tubulin polymerization (17, 18). It has been shown to be highly effective in a wide variety of tumor models (3, 19), and is currently undergoing assessment in cancer patients (20, 21). The entrance of CA4P into clinical trials has spurred the development of second generation VDAs such as OXi4503 (the diphosphate prodrug of CA1P (22)). Recent preclinical studies have demonstrated this agent to have even greater antivascular and antitumor efficacy than CA4P (23–25).
Given their disparate vascular targets, that is, interference with new vessel formation versus damaging the established tumor blood vessel network, it should be clear from a therapeutic perspective that targeting the tumor vasculature with AIs and VDAs is a complementary treatment strategy (7). In the current study, we evaluated the antitumor efficacy of combining Avastin with CA4P or OXi4503 in a tumor model of human clear cell renal cell carcinoma, Caki-1. Renal cell carcinoma is highly vascularized; with abundant angiogenesis and abnormal blood vessel development, it is considered a primary candidate for novel therapies targeting the blood vessel network.
Materials and Methods
Tumor model
The human clear cell renal cell carcinoma (Caki-1) cell line was obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). Female nude mice (NCR, nu/nu), 6 to 8 weeks old, were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The animals were maintained under specific pathogen-free conditions (University of Florida Health Science Center, Gainesville, FL, USA) with food and water supplied ad libitum. Xenografts were initiated by inoculating 1×106 tumor cells intramuscularly into a single hind leg of each animal.
Drug preparations
Avastin (bevacizumab) was obtained from Genentech (South San Francisco, CA, USA). Avastin was diluted in 0.9% saline and injected intravenously at a volume of 0.01 ml/g body weight to give a dose of 2 mg/kg.
CA4P (Combretastatin) and OXi4503 were gifts from OXiGENE (Waltham, MA). Both agents were prepared in 0.9% saline and injected intraperitoneally at volumes of 0.01 ml/g body weight to give a dose of 100 mg/kg (CA4P) and 10 or 25 mg/kg (OXi4503), respectively.
Necrotic area assessment
Twenty-four hours after completing the treatment schedule, tumor-bearing mice were euthanized, tumors were excised, and standard hematoxylin-eosin (H&E) staining was carried out (25). The sections were viewed and captured using a Zeiss Zxiophot 2 microscope (Carl Zeiss Jena GmbH, Jena, Germany) with a Sony DXC970 color camera (Sony Corporation, Tokyo, Japan). Entire tumor sections were reconstructed by tiled field mapping and the total tumor area as well as the area of tumor necrosis was determined using a MCID5.5 image analysis program (Imaging Research Inc., Ontario, Canada) (26).
Tumor size determination
Tumor size was measured every second day by passing the tumor-bearing leg through a series of holes in a plastic plate with increasing diameters. The diameter of the smallest hole a tumor-bearing leg would pass through was recorded and converted to a tumor volume using the formula, tumor volume =[(πd3)/6]-100, where d=the hole’s diameter and 100 represents a volume correction factor determined for a mouse leg without a tumor.
Treatment and tumor growth day assay
Once the Caki-1 xenografts reached a size of ~200 mm3, the animals were randomly assigned to various treatment groups. Avastin was administered at a dose of 2 mg/kg twice a week (Monday and Friday). VDAs were administered three times a week (Monday, Wednesday, and Friday) using doses of 100 mg/kg (CA4P) and 25 or 10 mg/kg (OXi4503), respectively. Treatment periods were for either 2 or 3 weeks. Animals receiving the VDA-AI combinations were given Avastin, CA4P, or OXi4503 on the same schedule as was used for single-agent treatments. On days when both the AI and VDA were given, the VDA was administered 1 hour after the AI. Following treatment, the time taken for the tumors in the various treatment groups to grow from 200 to 1,000 mm3 was recorded and compared using the Wilcoxon rank sum test.
Results
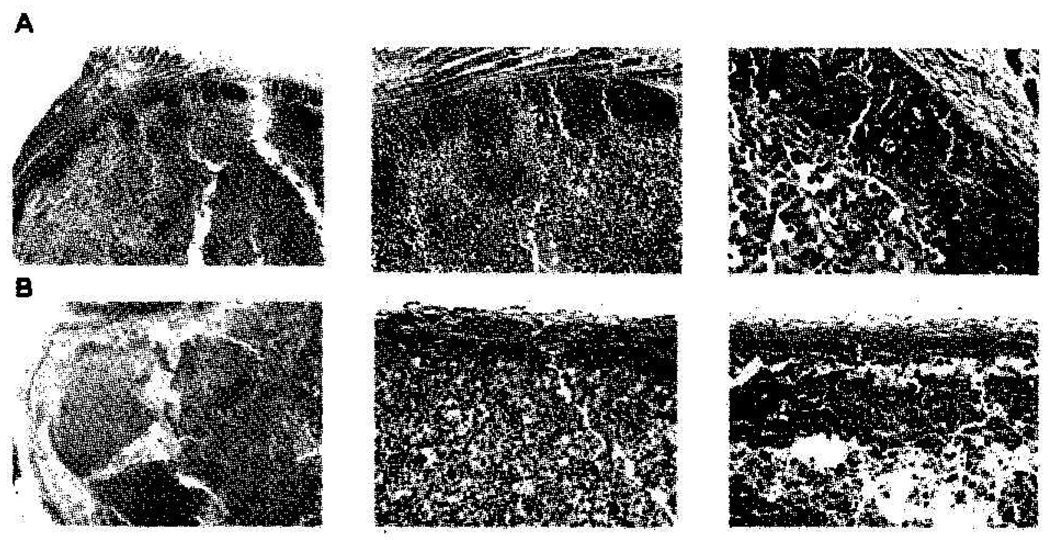
We and others have previously observed that both CA4P and OXi4503 treatments result in a marked reduction of tumor blood flow, loss of patent blood vessels, and secondary tumor cell death due to the induction of ischemia (17, 27); however, OXi4503 has been consistently associated with increased potency (23, 24). Histological assessments carried out using an image analysis system on tumor sections were first used to evaluate CA4P and OXi4503 treatment response. The results showed that while untreated Caki-1 tumors typically demonstrated less than 5% necrosis, treatment with Avastin alone resulted in no significant change of tumor necrotic fraction. VDA treatment resulted in widespread necrosis in these xenografts (Figure 1). Specifically, the viable neoplastic tissue remaining 24 hours after treatment was less than 6% following a 25 mg/kg dose of OXi4503 and 10% to 15% remained in mice treated following a 100 mg/kg dose of CA4P (Figure 1). In tumors of mice treated with CA4P, this rim is typically several cell layers deep; a finding consistent with published reports (28, 29). In OXi4503-treated tumors, the necrosis extended to the very edge of the tumor, leaving only a thin viable rim of neoplastic tissue, often consisting of only a few cell layers. At times, it was discontinuous, with areas showing no viable tumor at all. This marked difference between the two VDAs has also been reported in other preclinical tumor models (23–25, 30).
Figure 1.
H&E sections of Caki-1 tumors from mice 24 hours after treatment with (A) 100 mg/kg CA4P or (B) 25 mg/kg OXi4503. Arrows indicate the viable rim of tumor tissue surviving VDA treatment.
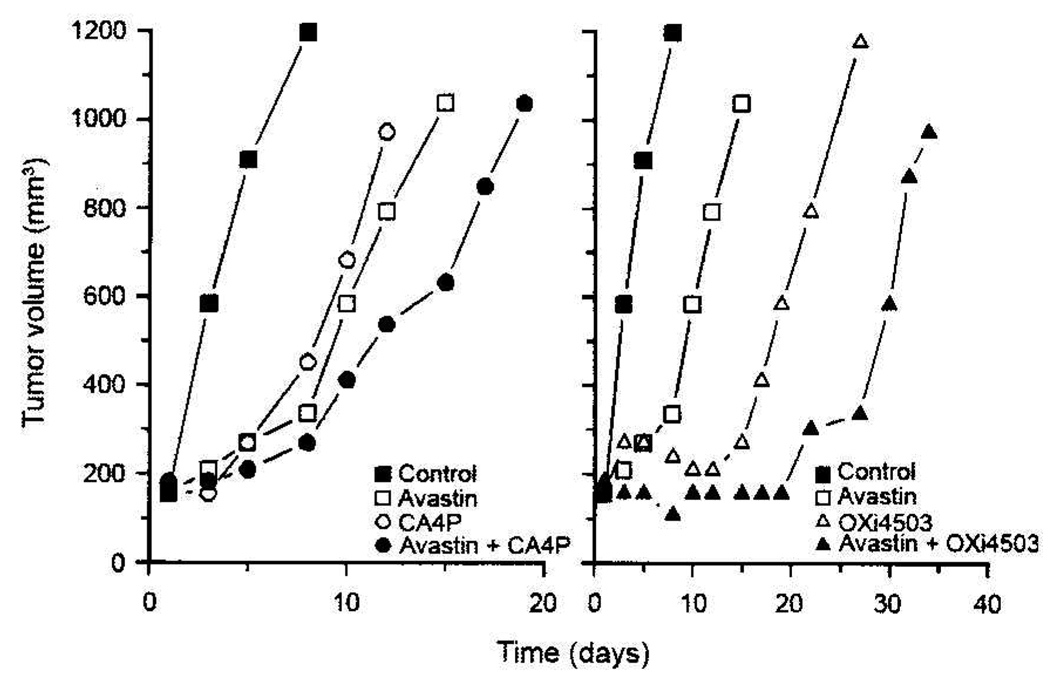
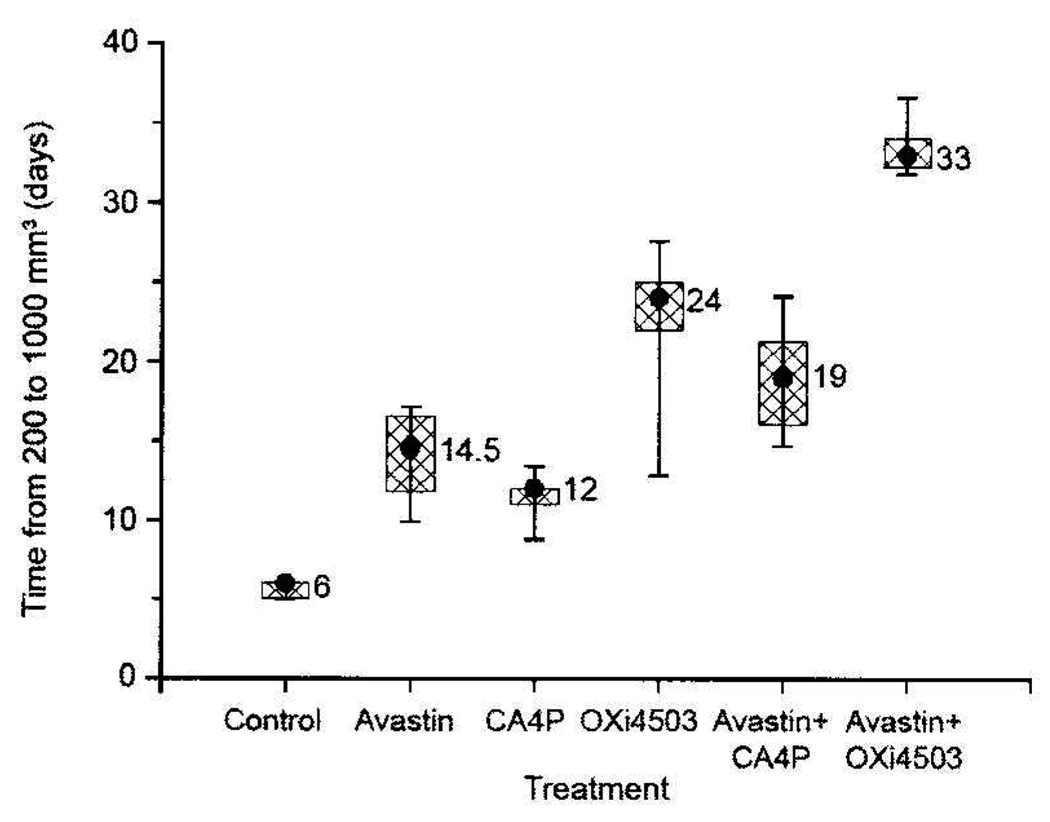
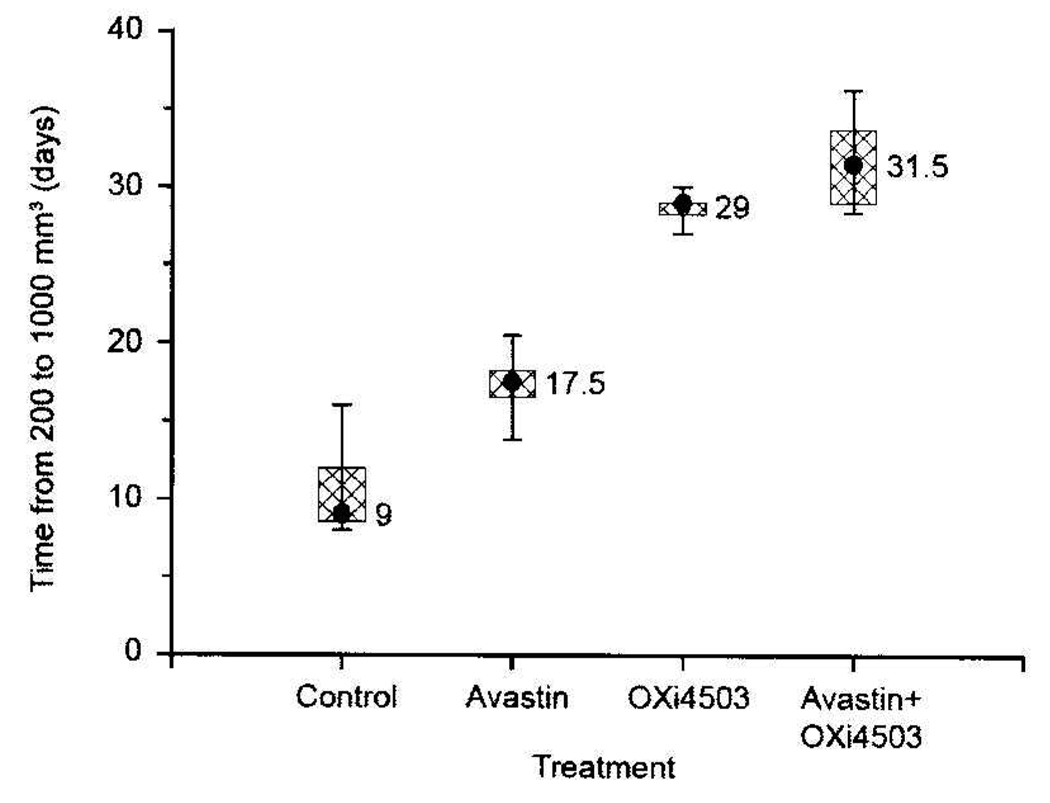
While single-dose CA4P or OXi4503 treatments typically have little impact on tumor growth, repeated drug exposures can result in measurable growth inhibition (16). This was also the case for Caki-1 xenografts, which demonstrated significant growth delays when administered repeatedly (3×/week) over a 2-week period (Figure 2 and Figure 3). A 100 mg/kg dose of CA4P or a 25 mg/kg dose of OXi4503 given Mondays, Wednesdays, and Fridays for 2 weeks resulted in 6 and 18 days of tumor growth delay, respectively. Avastin treatment also impaired Caki-1 tumor growth. When administered daily (Monday through Friday) for 2 weeks, this agent retarded Caki-1 growth by 8.5 days. However, the greatest tumor growth inhibition was achieved when VDA and Avastin treatments were combined (Figure 2 and Figure 3). For example, the combination of Avastin plus CA4P caused a tumor growth delay of 13 days, which was significantly longer than that achieved with either treatment alone (p<0.05). Similarly, in mice treated with a combination of Avastin and OXi4503, a significantly enhanced tumor growth delay (27 days) compared to single-agent therapy (p<0.05) was also observed.
Figure 2.
Response of Caki-1 tumors to Avastin (2 mg/kg, twice a week for 2 weeks), CA4P or Oxi4503 (100 mg/kg or 25 mg/kg, respectively, 3 times a week for 2 weeks), or the combination of AI and VDA. Data shown represent median tumor responses of groups of 8 to 10 mice.
Figure 3.
Tumor growth delay in Caki-1 tumors treated with Avastin and/or either CA4P or OXi4503, administered as described in Figure 2. Data shown are the median, 75th and 90th percentiles of responses of tumors treated at a starting size of 200 mm3. Results in mice treated with combination therapy were significantly greater than those obtained in mice treated only with a single therapy (p<0.05, Wilcoxon rank sum test).
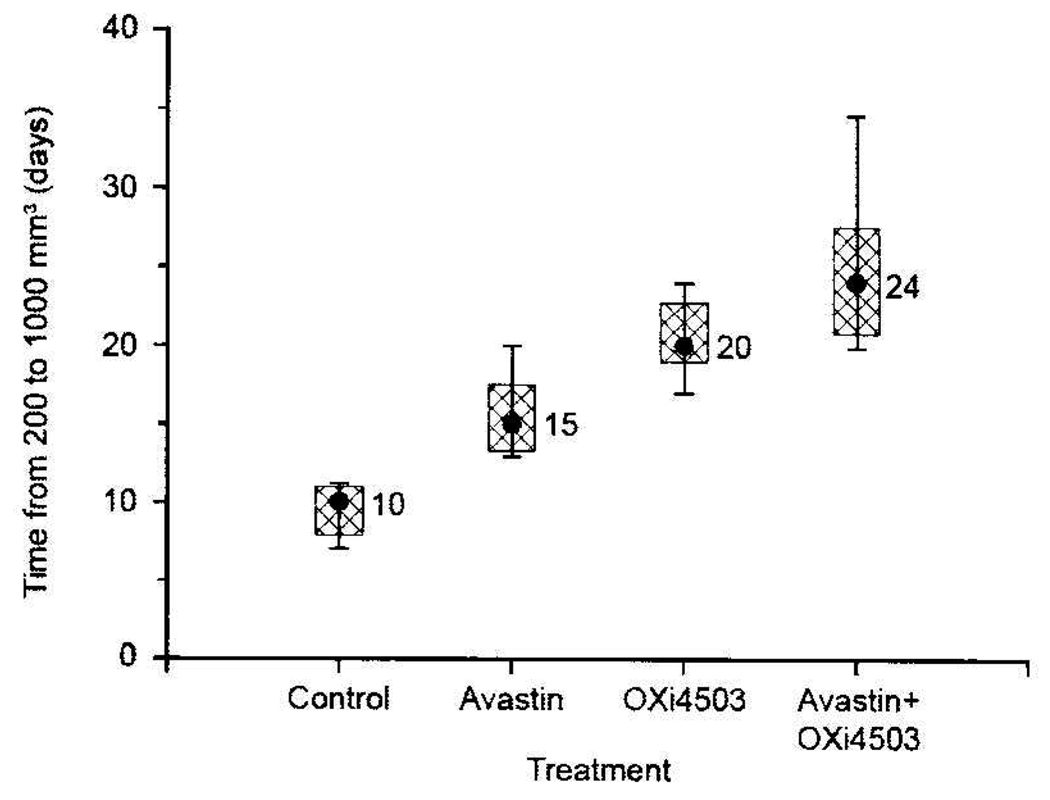
A reduction in OXi4503 dose from 25 to 10 mg/kg in the 2-week schedule resulted in a less effective treatment regimen for the VDA (a reduction in growth delay from 18 to 10 days; Figure 3 and Figure 4). But even this reduced dose of OXi4503, when applied in combination with Avastin, resulted in a Caki-1 tumor response significantly greater than that achieved with either therapy alone (p<0.05, Figure 4).
Figure 4.
Tumor growth delay of Caki-1 tumors treated with Avastin and/or OXi4503. The treatment schedule is described in Figure 2; however, the dose of OX14503 was 10 mg/kg. The data shown are the median, 75th and 90th percentiles of responses of tumors treated at a starting size of 200 mm3.
When the treatment period of these two agents (Avastin, 2 mg/kg; OXi4503, 10 mg/kg; Monday, Wednesday, Friday) was extended from 2 to 3 weeks, the prolonged treatment period resulted in effective growth inhibition by both Avastin and OXi4503 monotherapy and a significant increase in tumor growth delay in mice treated with the OXi4503-Avastin combination (p<0.05; Figure 5).
Figure 5.
Response of Caki-1 tumors to Avastin (2 mg/kg, twice a week for 3 weeks), OXi4503 (10 mg/kg, 3 times a week for 3 weeks), and a combination of both. The data shown are the median, 75th and 90th percentiles of responses of tumors treated at a starting size of 200 mm3.
Discussion
We previously showed that tumors in mice treated with CA4P (100 mg/kg) or OXi4503 (25 mg/kg) demonstrate rapid and extensive shutdown in vascular function when vascular changes are assessed by fluorescence microscopy using Hoechst 33342 dye or magnetic resonance imaging (MRI) with the contrast agent gadolinium-diethylene-triaminepentaacetic acid (GdDTPA) (25, 30). The tumors undergo frank necrosis as intratumoral blood vessels are irreversibly compromised. Following treatment with OXi4503, vascular shutdown lasts longer and the induction of necrosis is more severe (20, 25, 30). Still, even after OXi4503 treatment, some surviving tumor cells persist in a viable rim typically only a few cell layers thick at the tumor periphery (Figure 1) (20, 25). The absence of damage to vessels within the adjacent peritumoral tissue suggests that these vessels aid the survival of tumor cells at the tumor’s edge and support the initiation of subsequent tumor regrowth (3). Because this regrowth requires the re-establishment of a vascular network, active angiogenic response is expected as tumors recover from VDA treatment. Recent investigations examining the pathophysiological impact of CA4P treatment have indicated that vascularity in the rim appears to increase after treatment, suggesting increased angiogenic activity in those parts of the tumor that survive treatment (36). Moreover, evidence that VEGF increases following OXi4503 treatment has been reported in the MHEC5-T tumor model (31). We therefore hypothesized and subsequently provided experimental evidence supporting the notion that the combination of AI and VDA treatments should prove particularly efficient at compromising the tumor blood vessel network (7, 32).
In this study, we examined the utility of combining Avastin treatment with the second-generation VDA OXi4503 currently in phase I clinical trials (20, 33). OXi4503 appears to be a more potent VDA that achieves not only extensive tumor necrosis but also leaves a smaller surviving viable rim of tumor tissue than do other VDAs (23–25, 31). Folkes and colleagues have suggested that the further reduction of the viable rim by OXi4503 may relate to the fact that this agent is an ortho-quinone prodrug (34). Thus, unlike other VDAs, this feature may provide OXi4503 with a novel cytotoxic effect in addition to its vascular targeting capabilities mediated by its action on the tubulin cytoskeleton (34).
We previously have shown that combining agents that target the angiogenic process with those that damage the existing tumor blood vessel can be highly complementary and not redundant (7, 32, 35). Our studies combining Avastin with the VDAs CA4P and OXi4503 confirm those findings, indicating marked enhancements of the antitumor effect in the combination treatments (Figure 2 and Figure 3). We subsequently extended those studies to lower doses of OXi4503 (Figure 4) and longer treatment times (Figure 5). The results showed that enhanced antitumor effects could still be achieved even when the dose of OXi4503 was decreased from 25 to 10 mg/kg (Figure 4). In addition, an equivalent degree of tumor response enhancement was attained by the higher dose of OXi4503 and could be achieved by using the lower dose of the agent for an extended time (3 weeks) in combination with Avastin. This suggests that the effectiveness of such a combination can be retained even with sub-optimal doses of the VDA.
Conclusion
The current study demonstrates marked enhancement of the tumor response of mice treated with Avastin and either CA4P or OXi4503 in a preclinical setting and provide an experimental basis for the consideration of such a treatment strategy in the clinic. The results provide additional evidence to support the use of agents targeting angiogenesis (AIs) when used in combination with therapies that damage the existing tumor blood vessel network (VDAs) as a novel anticancer strategy that targets the tumor vasculature.
Acknowledgements
This work was supported by a grant from the US National Cancer Institute (USPHS grant CA-84408). The authors gratefully acknowledge the Optical Microscopy Facility, Evelyn F. & William L. McKnight Brain Institute of the University Of Florida, Gainesville, Florida in the performance of these studies. The authors also wish to acknowledge Dr. Amyn Rojiani of the University of South Florida for assistance with the assessments of tumor histology.
References
- 1.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 2.Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes Memorial Award lecture. Cancer Res. 1986;46:467–473. [PubMed] [Google Scholar]
- 3.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- 4.O’Reilly MS. Antiangiogenesis and vascular endothelial growth factor/vascular endothelial growth factor receptor targeting as part of a combined-modality approach to the treatment of cancer. Int J Radiat Oncol Biol Phys. 2007;69:S64–S66. doi: 10.1016/j.ijrobp.2007.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siemann DW, Bibby MC, Dark GG, et al. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- 6.Siemann DW, Horsman MR. Targeting the tumor vasculature: A strategy to improve radiation therapy. Expert Rev Anticancer Ther. 2004;4:321–327. doi: 10.1586/14737140.4.2.321. [DOI] [PubMed] [Google Scholar]
- 7.Siemann DW, Shi W. Efficacy of combined antiangiogenic and vascular disrupting agents in treatment of solid tumors. Int J Radiat Oncol Biol Phys. 2004;60:1233–1240. doi: 10.1016/j.ijrobp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 9.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.D’Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol. 2005;23:7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 11.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Arquette M, Shin DM, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5578–5587. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 15.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaplin D, Dougherty G, Siemann DW. Vascular disrupting agents. In: Abbruzzese J, Davis D, Herbst R, editors. Anti-angiogenic cancer therapy. CRC Press, Talylor and Francis Group; 2007. pp. 329–364. [Google Scholar]
- 17.Li L, Rojiani AM, Siemann DW. Preclinical evaluations of therapies combining the vascular targeting agent combretastatin A4 disodium phosphate and conventional anticancer therapies in the treatment of Kaposi’s sarcoma. Acta Oncol. 2002;41:91–97. doi: 10.1080/028418602317314127. [DOI] [PubMed] [Google Scholar]
- 18.Pettit GR, Singh SB, Niven ML, et al. Isolation, structure, and synthesis of combretastatins A-1 and B-1, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- 19.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 20.Siemann DW, Horsman M. Small molecule vascular disrupting agents in cancer therapy. In: Ellis L, Teicher B, editors. Antiangiogenesis agents. Humana Press; 2007. [Google Scholar]
- 21.Siemann DW, Chaplin D. An update of he clinical development of drugs to disable tumor vasculature. Expert Opin Drug Disco. 2007;2:1357–1367. doi: 10.1517/17460441.2.10.1357. [DOI] [PubMed] [Google Scholar]
- 22.Pettit GR, Singh SB, Hamel E, et al. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 23.Hill SA, Toze GM, Pettit GR, et al. Preclinical evaluation of the antitumour activity of the novel vascular targeting agent Oxi 4503. Anticancer Res. 2002;22:1453–1458. [PubMed] [Google Scholar]
- 24.Hua J, Sheng Y, Pinney KG, et al. Oxi4503, a novel vascular targeting agent: effects on blood flow and antitumor activity in comparison to combretastatin A-4 phosphate. Anticancer Res. 2003;23:1433–1440. [PubMed] [Google Scholar]
- 25.Rojiani M, Rojiani AM. Morphological manifestations of vascular-disrupting agents in preclinical models. In: Siemann DW, editor. Vascular Targeted Therapies in Oncolog. Wiley & Sons; 2006. pp. 81–94. [Google Scholar]
- 26.Shi W, Teschendorf C, Muzyczka N, et al. Gene therapy delivery of endostatin enhances the treatment efficacy of radiation. Radiother Oncol. 2003;66:1–9. doi: 10.1016/s0167-8140(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 27.Dark GG, Hill SA, Prise VE, et al. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57:1829–1834. [PubMed] [Google Scholar]
- 28.Siemann DW, Mercer E, Lepler S, et al. Vascular targeting agents enhance chemotherapeutic agent activities in solid tumor therapy. Int J Cancer. 2002;99:1–6. doi: 10.1002/ijc.10316. [DOI] [PubMed] [Google Scholar]
- 29.Rojiani AM, Li L, Rise L, et al. Activity of the vascular targeting agent combretastatin A4 disodium phosphate in a xenograft model of AIDS-associated Kaposi’s sarcoma. Acta Oncol. 2002;41:98–105. doi: 10.1080/028418602317314136. [DOI] [PubMed] [Google Scholar]
- 30.Salmon HW, Siemann DW. Effect of the second-generation vascular disrupting agent OXi4503 on tumor vascularity. Clin Cancer Res. 2006;12:4090–4094. doi: 10.1158/1078-0432.CCR-06-0163. [DOI] [PubMed] [Google Scholar]
- 31.Sheng Y, Hua J, Pinney KG, et al. Combretastatin family member OXI4503 induces tumor vascular collapse through the induction of endothelial apoptosis. Int J Cancer. 2004;111:604–610. doi: 10.1002/ijc.20297. [DOI] [PubMed] [Google Scholar]
- 32.Shi W, Siemann DW. Targeting the tumor vasculature: enhancing antitumor efficacy through combination treatment with ZD6126 and ZD6474. In Vivo. 2005;19:1045–1050. [PubMed] [Google Scholar]
- 33.Siemann DW, Horsman M. Small molecule vascular disrupting agents in cancer therapy. In: Ellis L, Teicher B, editors. Antiangiogenesis Agents. Humana Press; 2008. pp. 297–310. [Google Scholar]
- 34.Folkes LK, Christlieb M, Madej E, et al. Oxidative metabolism of combretastatin A-1 produces quinone intermediates with the potential to bind to nucleophiles and to enhance oxidative stress via free radicals. Chem Res Toxicol. 2007;20:1885–1894. doi: 10.1021/tx7002195. [DOI] [PubMed] [Google Scholar]
- 35.Siemann DW, Warrington KH, Horsman MR. Targeting tumor blood vessels: An adjuvant strategy for radiation therapy. [Review] Radiother Oncol. 2000;57:5–12. doi: 10.1016/s0167-8140(00)00243-7. [DOI] [PubMed] [Google Scholar]
- 36.Salmon BA, Siemann DW. Characterizing the tumor response to CA4P treatment. Int J Radiat Oncol Phys. 2007;68:211–217. doi: 10.1016/j.ijrobp.2006.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]