Introduction
The primary goal of rehabilitation is to restore physical, psychological, and social functions and improve the quality of life for individuals with various motor, sensory, and/or cognitive impairments. While treatment options vary from pharmacological agents to physical exercise, this review paper focuses on neural interface technology, also called neuroprosthetics, which functions by directly interacting with the nervous system either electrically or magnetically. Luigi Galvani documented more than two hundred years ago that leg muscle contraction can be elicited by electrical stimulation of the femoral nerve 1,2. However, it is only in the last several decades that neural interface technology started to receive increasing attention from biomedical engineers, neuroscientists, and clinicians, largely due to great advance in electronic medical devices and system neuroscience research. The U.S. Food and Drug Administration (FDA) approved cochlear implants for treating hearing loss in 1984 and deep brain stimulators (DBS) for treating Parkinson's disease in 2000 3. The National Institutes of Health currently holds a bi-annual Neural Interface Conference with the goal of bringing industry partners, clinicians and researchers together to discuss key issues and future directions related to neural interface technology 4.
This review classifies neural interface devices and systems into two categories, neural recording systems and neural stimulation systems, based on the direction of information flow. Neural recording systems retrieve information from the nervous system through electrophysiological recording methods, such as electroencephalography (EEG) and microelectrode recording of single-neuron activities. Neural stimulation systems feed information into the nervous system by electrically or magnetically activating or inhibiting neural activity. This is a simplified classification scheme, as certain neural interface devices are capable of both neural recording and stimulation, such as implantable responsive neuro-stimulators for epilepsy treatment 5,6. As the term neural interface technology covers a broad range of devices and systems, it is a daunting task to provide a detailed and informative discussion on each device and system in one review paper. Furthermore, there exist multiple books, special journal issues, and review papers that are excellent references for neural interface technology 7-15. Hence, this paper aims to review neural interface technology from three unique perspectives. First, it will focus primarily on neural interface systems that are currently under active research but have not yet reached clinical practice, hoping to illustrate not only where we are, but also where we will be in the near future. Second, this paper will exemplify neural interface technology with systems that the authors have directly worked with in their rehabilitation research and clinical practice to provide a first-hand view of neural interface technology. Last and the most important, this paper will review neural interface technology in association with neuroplasticity, a foundation for neurological rehabilitation, and demonstrate that those two concepts work symbiotically to improve the quality of life for individuals with disabilities, as illustrated in Figure 1. Neuroplasticity will help those individuals to make better use of their neural interface devices 16-19, and neural interface technology can also promote neuroplasticity for functional recovery 7,20.
Figure 1.
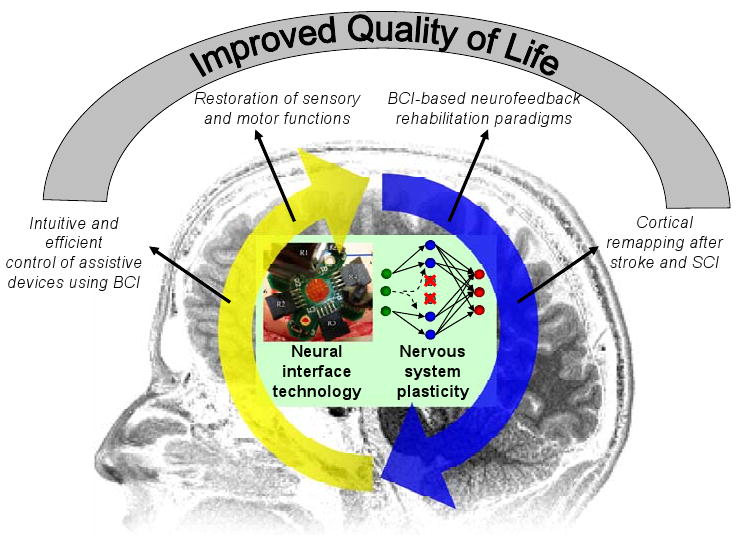
Schematic illustration showing the symbiotic relationship between neural interface technology and nervous system plasticity. Their close interaction leads to increased efficacy of neural interface devices and improved functional recovery of the nervous system, which eventually leads to better quality of life. The neural interface technology is exemplified by a photo of a micro-electrode array for cortical surface recording (courtesy of Dr. Justin Williams at University of Wisconsin-Madison and James A. Hokanson at the University of Pittsburgh). Nervous system plasticity is illustrated with a schematic drawing of a three-layer neural network model. When a group of neurons in the intermediate layer were damaged due to neurological disorders (marked with red “×”), such as stroke, the input layer neurons (green dots) develop or strengthen connections (dashed lines) with spared neurons (blue dots) in the intermediate layer promoting functional recovery.
Neural Interface Technology For Neural Recording
This section focuses the application of neural interface technology to support brain-computer interface (BCI) devices. The primary goal of BCI technology is to establish a direct communication pathway between the brain and external devices enabling faster and more intuitive communication and control for individuals with motor disabilities caused by stroke, spinal cord injury, limb amputation, and degenerative neurological disorders. In this sense, BCI is a type of assistive technology, and what differentiates it from traditional assistive devices is that user commands are extracted directly from brain activity without the need for users to exert any overt movement. Due to the capability to provide real-time feedback of neural activity, BCI systems have also received considerable attention as a rehabilitation tool for stroke and spinal cord injury 7,20. The goal is to induce neuroplasticity through the operation of BCI devices for functional recovery instead of using BCI systems permanently as assistive technology. In a typical BCI setup, multiple channels of neural signals are recorded simultaneously and fed into a decoding module. The decoding module then processes and decodes neural signals in real-time to extract a BCI signal that can be used to control movement of a computer cursor or other external devices, such as a robotic arm or a communication aid.
Three factors are critical for the success of BCI applications. The first is the neural substrate, i.e. neural activities, which encode a certain type of information that can be decoded as a BCI control signal. Many BCI applications were developed based on cortical representation of movement 19,21,22. Motor system neurophysiology studies have shown that activity of motor cortical neurons are modulated by movement and that firing rates of a population of motor cortical neurons can be used to predict hand movement direction, speed, and position 23-30. This provides a neural substrate for extracting movement signals from the brain. The second factor for success is cortical plasticity induced by training through biofeedback. When subjects are trained to operate a BCI system, visual or other sensory feedback of the decoded movement is presented to subjects in real-time. It has been observed that, over time, modulation of recorded neural signals was enhanced as user performance improved 16-19. Neuroplasticity induced through BCI training not only helps BCI operation itself, but also has the potential to promote functional recovery after damage to the nervous system from stroke and spinal cord injury 31. The third factor is an appropriate neural recording method that has sufficient spatial and temporal resolution to take full advantage of aforementioned neural substrates and neuroplasticity. Various neural recording modalities have been used in BCI systems, including microelectrode recording of single-neuron activity 19,21,22,32,33, subdural electrocorticography 34,35, scalp electroencephalography (EEG) 36,37, magnetoencephalography (MEG) 38, and functional magnetic resonance imaging (fMRI) 39. Depending on the targeted application, different BCI systems may have different requirements for the neural recording method. For example, a BCI system that acts as an assistive device needs to be portable, potentially fully-implantable, and has the capability to record highly specific neural activity from a small cortical area. On the contrary, portability may be less critical for a BCI system that serves as a rehabilitation tool if it can offer whole-head coverage with reasonable spatial and temporal resolution non-invasively, such as an MEG-based BCI system.
Brain-computer interface as assistive technology
Great strides have been made using various neural recording methods, such as microelectrode recording of single-unit activities and scalp EEG 19,21,22,33,40. Each recording modality has its own advantages and disadvantages. Single-unit recording requires craniotomy and surgical insertion of microelectrode arrays into the cortex, and it has been suggested that microelectrode recording may lack long-term reliability due to foreign body reaction 15. In addition, single-unit recording typically requires sophisticated hardware and software systems. To capture the occurrence of spikes associated with action potentials, advanced data acquisition hardware with a very high sampling rate (typically 25,000 to 50,000 Hz) is required. However, it offers the highest spatial and temporal resolution and can extract BCI control signals with multiple degrees of freedom with high accuracy. Researchers have demonstrated that non-human primates can achieve high-precision control of robotic arms to perform a self-feeding task 22, and clinical trials in human subjects are currently underway to test the safety and efficacy of BCI systems based on microelectrode recording of neuronal activity 21. On the other side of the spectrum, scalp EEG can be recorded non-invasively with much simpler recording systems (e.g. sampling rate can be less than 1000 Hz), but it has a low spatial resolution (30 mm) 41 and a low signal-to-noise ratio, especially at higher frequencies (>60 Hz) due to signal attenuation caused by the skull. It has been demonstrated that subjects can control the movement of a two-dimensional cursor with EEG by modulating their sensorimotor rhythm (10-30 Hz) 40, but extensive training is often required.
In parallel to other groups' work using microelectrode recording and scalp EEG, the authors have been developing BCI systems using electrocorticography (ECoG) and micro-ECoG. ECoG (sometimes also called intracranial EEG) recently received considerable attention as a promising modality for BCI application 34,35. ECoG recordings are often performed for patients with intractable epilepsy for presurgical brain mapping and seizure foci localization. While patients' ECoG signals are recorded continuously for epilepsy monitoring, those signals can be split off and fed into a second neural recording system for BCI research. Since its electrodes are placed inside the skull, ECoG preserves a wide range of high frequency components (40 – 200 Hz) of the electrical field potential generated by neuronal activities. Several groups of researchers, including the authors, found that the power of high frequency bands increases significantly during movement and that the power of high frequency bands varies systematically with movement direction 34,42 (Figure 2). The high frequency band of ECoG signals encodes desired movement direction, similar to the high frequency band of local field potential signals recorded in animal studies 43. Thus, ECoG potentially contains rich information for extracting BCI control signals. Several studies have shown that human subjects can achieve effective control of cursor movement within a very short period of time with ECoG 34,35,42 (Figure 3).
Figure 2.
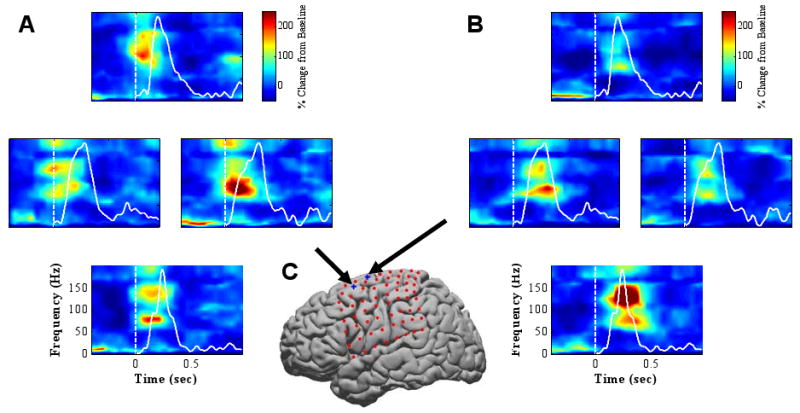
Spectrograms (time-frequency plots) showing movement modulation of ECoG signals recorded from two representative subdural ECoG electrodes above the motor cortical area as the subject performed a center-out movement with a 2-dimensional cursor controlled by a joystick. A & B: Each panel shows four spectrograms arranged corresponding to cursor movement direction (up, down, left, and right). Time 0 represents movement onset (also marked with white vertical lines). Color represents percent change from baseline. Solid white curves are cursor velocity profiles. C: Locations of subdural ECoG electrodes. A grid of 64 electrodes (red dots) covers the left frontal and parietal cortical areas. The two electrodes whose responses are depicted in A and B are marked with blue “+” signs and black arrows. For the high frequency band, there is a significant increase in power during movement. Furthermore, the high frequency band power differs across four different movement directions with a strong activation for rightward movement in A and downward movement in B.
Figure 3.
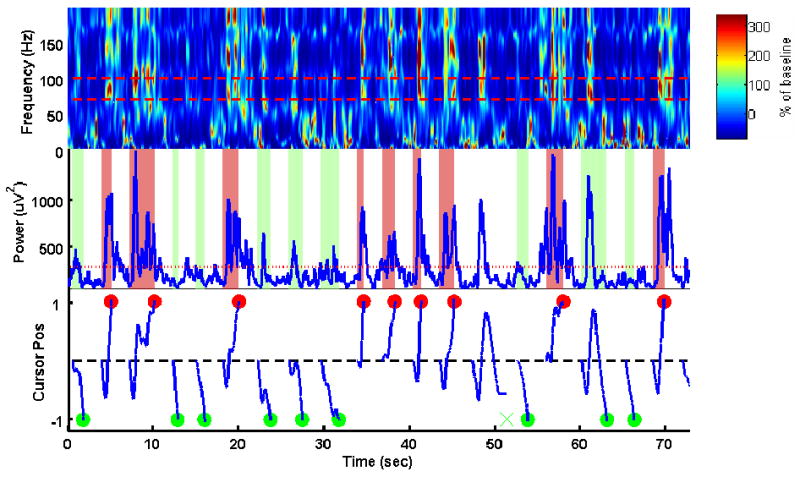
Real-time control of one-dimensional computer cursor movement using an ECoG channel. Top: Spectrogram of the ECoG signal. Color represents percentage change from baseline. The red dashed lines mark the frequency band used to control the cursor (70 – 100 Hz). Middle: Instantaneous power of the 70-100 Hz band (blue curve) and its baseline power (red line). Bottom: Vertical cursor position plotted as a function of time (blue curve). The cursor always started from screen center at the beginning of each trial. The red and green dots indicate the time and cursor vertical position when a top or a bottom target was hit. “×” indicates an unsuccessful trial. For this subject, an accuracy of 73% was achieved within the first 10 minutes of brain-control session while the chance level was 50%.
Implantation of these standard subdural ECoG grids typically requires a craniotomy that exposes a large portion of cortical surface. For BCI applications, such an invasive craniotomy is impractical and can be avoided with a new technique called micro-ECoG. A micro-ECoG electrode grid can be thought of as a miniature version of the regular ECoG grids with smaller electrodes and closer spacing between electrodes. Micro-ECoG grids can record neural activity from a localized cortical volume with high spatial and temporal resolution, which makes it possible to extract even richer and more specific information from micro-ECoG signals for controlling BCI devices. Furthermore, a micro-ECoG grid can be easily implanted through a small burr hole in a minimally invasive surgery, and it can be placed on top of the dura mater. By leaving the dura intact, risks of various complications, such as infection, can be significantly reduced. Previously, the Cleveland Clinic Foundation group reported no serious complications from epilepsy monitoring using epidural electrodes, and there was zero instance of purulent wound infection over 500 epidural electrodes inserted 44. Hence, micro-ECoG recording may provide high quality neural recording with low clinical risks, making it a desirable platform for developing clinical BCI devices.
With approval from the Institutional Review Board at the University of Pittsburgh, the authors recently conducted a micro-ECoG study in a human subject undergoing subdural ECoG recording for epilepsy monitoring purpose. A micro-ECoG grid with 14 recording electrodes was implanted over the motor cortical area, and it was found that the high frequency band of neural signals recorded from this micro-ECoG grid showed significant modulation by hand movement, including individual finger movement 45 and grasping/pinching movement of the hand 46. Modulation of micro-ECoG signals by hand movement offers a neural substrate that is critical to the success of a BCI system aiming to restoring volitional control of finger movement. During the experiment, the subject achieved real-time control of one-dimensional cursor movement within 30 minutes. Offline analysis showed that hand posture (open vs. closed) can be predicted accurately using micro-ECoG signals and that individual finger movement can be decoded with an accuracy of 73% (chance level 20%) 45.
The capability to decode hand movement from micro-ECoG recording has great clinical significance. Loss of hand function leads to difficulties in simple daily tasks, such as grooming, feeding, and dressing, and it significantly affects quality of life. In addition to high-level spinal cord injury patients, many individuals with hand function impairments caused by other neurological disorders, such as stroke 47, may benefit from this technology. A large portion of stroke patients have upper limb paresis when admitted into the hospital (60-70%) 48 and 55-75% of stroke survivors have limited upper-extremity function 49,50. Our study suggests that it is possible to develop a minimally-invasive implantable BCI device based on micro-ECoG recording in order to provide control signals for prosthetic hands or functional electrical stimulators to re-animate paralyzed hands.
For all the BCI systems mentioned above, regardless of the neural signal (single-unit activity, EEG, ECoG, etc.), when a subject operates a BCI system, neuroplasticity plays a critical role. Through closed-loop training with real-time feedback of cursor or robotic arm movement, BCI systems induce neuroplasticity that greatly facilitates BCI control 16-19. Meanwhile, neuroplasticity induced by BCI not only benefit BCI operation itself, but may also potentially facilitate functional recovery. In the next two sections, the goal of using BCI devices changes from simply operating the device to serving as a tool for stroke and spinal cord injury rehabilitation.
Brain-computer interface as a rehabilitation tool
In addition to controlling assistive devices that can replace or augment impaired functions, BCI technology can also facilitate the restoration of function through neuroplasticity 7,20. Traditional therapies for restoring motor function focus on forced use of the impaired limb. Most notably constraint-induced movement therapy and EMG-based biofeedback have proven to be fairly successful strategies for improving muscle strength and performance of activities of daily living 51-55. However, success of these therapies depends on the patient having a moderate degree of residual function to start with. In the case of spinal cord injury (SCI) or severe hemiplegia, alternative therapies for restoration of function need to be explored. In particular, we will focus on research that seeks to engage neuroplasticity using BCI as a neurofeedback tool in order to elicit changes along the corticospinal pathway.
Neurofeedback is a technique in which an individual learns to voluntarily modulate, or change, his or her brain activity 56,57. Current clinical applications of neurofeedback include the treatment of epilepsy, anxiety, and more recently Attention Deficit Hyperactivity Disorder (ADHD) 56-59. The user is provided with real-time feedback of some feature of his neural activity, often the amplitude of oscillatory signals originating within the cortex. Based on this feedback, the individual learns to control the neural signal of interest. Voluntary modulation of sensorimotor rhythm amplitude is often learned through neurofeedback in order to control a BCI device. It is well established that neuroplasticity can be induced and that control of voluntary modulation can be improved with BCI training 16,17,19,21,31,38,60. Besides improving brain control of external devices, such as computer cursors and robotic arms, neuroplasticity induced by BCI training may also directly facilitate motor function recovery. Many previous studies have shown that functional recovery is often associated with cortical activity returning to a state close to that of an unimpaired individual 61-63. BCI training through neurofeedback can directly influence cortical activity and potentially restore normal cortical activation pattern, which could have a positive effect on the downstream neuromuscular systems.
A recent study investigated the feasibility of restoring movement using BCI training in chronic stroke patients 20,31. In that study, subjects learned to modulate their brain activity in order to control an orthosis which opens and closes the hand. Eight participants with chronic hand weakness secondary to stroke underwent training focused on modulating sensorimotor rhythm amplitude as measured by MEG. MEG sensors over the sensorimotor region that were activated during imagery of movement of the paralyzed hand were selected as control channels for the MEG-based BCI. Motor imagery and observation for example of an individual performing the intended task represent powerful inductors of cortical plasticity 64-67. Sensorimotor rhythm amplitude controlled the vertical position of a computer cursor and the subjects tried to hit a top or bottom target. Additionally, the same neural signals were coupled to control of the hand orthosis. The orthosis guided the hand into an open or closed position depending on the sensorimotor rhythm amplitude relative to a predetermined threshold. After 3-8 weeks of training, these eight patients were able to achieve an average success rate of 72% for the two-target task. While this success rate may seem modest, it should be noted that most of these patients were able to achieve control with MEG sensors above the sensorimotor areas ipsilateral to their subcortical stroke lesions. No significant changes in gross hand motor function were measured for any of the patients. Hand function improvement was not expected in those patients, as they started the study with a 0/5 score on the Medical Research Council scale with completely paralyzed hands. To further improve the efficacy of this neurofeedback paradigm, particularly in patients who retain some level of hand motor function, we can combine BCI with functional neuromuscular stimulation to generate brain-controlled muscle contraction. The famous Hebb's rule for neuroplasticity is often summarized as, “the neurons that fire together, wire together” 68,69. The temporally coupled motor cortex activation, muscle contraction, and somatosensory feedback may promote neuroplasticity for motor functional recovery based on similar principles.
A comprehensive rehabilitation approach combining action observation, motor imagery, and BCI neurofeedback
The authors believe that the feasibility and effectiveness of a BCI-based neurofeedback paradigm can be further enhanced by combining BCI-based neurofeedback with two additional training paradigms: action observation and motor imagery. Action observation training is based on the concept of human mirror neuron system (MNS). Neurons in the human MNS fire when an individual acts and when he observes the same action performed by another person 70-73. These neurons “mirror” the behavior, as if the observer is himself acting. The human MNS plays a critical role for motor learning, action imitation, action understanding, speech and social interaction 71,74. In addition to the classic human MNS including inferior parietal lobule, ventral premotor cortex, and the caudal part of inferior frontal gyrus 75,76, multiple human and animal studies have also shown that motor cortex demonstrates congruent activities during both action observation and action execution 75,77-79. Just like action observation, motor imagery, or imagined movement of a body part, can also activate multiple sensory and motor cortical areas. Motor imagery is believed to be a covert stage of action execution that draw upon cortical areas that are typically involved in motor planning and execution, such as the supplemental motor area, premotor cortex, and the primary motor cortex (M1) 80,81.
In essence, both action observation and motor imagery can elicit a certain degree of motor cortical activity in absence of overt movement. They offer clinicians opportunities to directly activate cortical areas that cannot be activated otherwise, e.g. cortical areas representing a paralyzed limb. They may be able to strengthen neural pathways that remain intact and also facilitate activation of motor cortical areas. Multiple studies have demonstrated that action observation and motor imagery can elicit sensorimotor cortical activity and at least temporally improve motor functions of paralyzed limbs after stroke and spinal cord injury 82-84. As discussed in the last section, a BCI-based neurofeedback training paradigm has the potential to promote neuroplasticity and functional recovery. A comprehensive rehabilitation approach that merges BCI neurofeedback, action observation, and motor imagery together will bring even greater benefits to patients than using any of those paradigms alone. First, action observation and motor imagery will facilitate BCI training. They allow a BCI system to optimize its decoding algorithm to extract motor-related information from cortical activity without overt movement from patients. They also offer an intuitive way for patients to learn to voluntarily modulate their cortical activity in order to operate a BCI system. Enhanced performance in BCI operation will make the neurofeedback paradigm more effective, potentially improving motor function recovery. Second, BCI systems can at least partially provide real-time feedback of actions that a patient is imagining, making the motor imagery training more engaging and effective than simple motor imagery without any feedback.
The authors are currently developing such a rehabilitation paradigm to enhance motor cortical modulation in individuals with tetraplegia. In the case of incomplete SCI, it is our goal to maximize the amount of information transferred through remaining corticospinal pathways in order to enhance residual function. For others, maximizing voluntary control of cortical modulation may increase the effectiveness of BCI technology and future spinal cord regeneration therapies. Preliminary studies investigated motor cortical activity in both able-bodied volunteers and individuals with tetraplegia during both action observation and action execution, with a primary focus of hand motor function. The study was approved by the Institutional Review Board at the University of Pittsburgh. Results from two participants are reported here. One participant was a 29-year old male with no history of neuromuscular disease, and the other was a 34-year old male with a C7 level complete SCI that occurred 15 years prior to his participation in this study. A whole-head 306-channel MEG system (Elekta Neuromag®) was used to record cortical activity non-invasively while subjects performed simple hand movements under 4 different conditions:
Observed: Participants watched a video of the movements, while they remained resting.
Imitated: Participants performed the movements along with the video presentation.
Imagined: Participants imagined performing the movements in response to a visual cue.
Overt: Participants overtly performed the specified movement in response to a visual cue.
The time sequence and visual feedback provided during the experimental paradigm are shown in Figure 4. Two different hand movements were tested: 1) grasping an object; 2) tapping an object with the finger tips using wrist flexion and extension. A total of 40 repetitions were collected using a randomized block design for each “condition × movement” combination.
Figure 4.
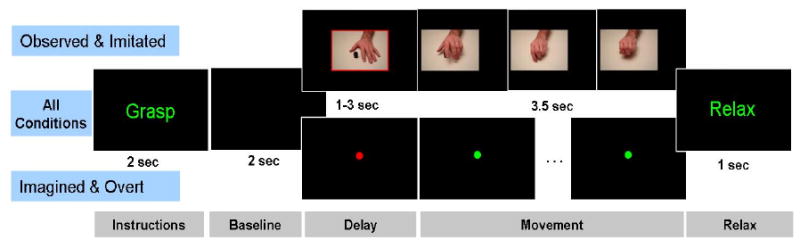
Timeline for the behavioral tasks used to study cortical activity during action observation, imitation, motor imagery, and overt movement. For all conditions an instruction slide specifies a “grasp” or “tapping” trial and is followed by a 2 sec baseline period. For the observed and imitated conditions, a movie of the specified movement is shown following a brief delay period. For the imagined and overt conditions, movement time is indicated by the fixation dot turning from red to green. “Relax” signals the end of the trial.
The power of sensorimotor rhythm (10 – 30 Hz) was used as an indicator of motor cortical activation. Time-frequency plots averaged over all repetitions from a representative MEG channel above the motor cortex is pictured in Figure 5. In general, similar results were obtained from both the able-bodied subjects and the subjects with SCI subjects. For both the overt and the imitated conditions, the power of sensorimotor rhythm decreased preceding movement followed by a “rebound”, i.e. power increase. However, differences in cortical activation between those two subjects were noted, particularly in the imagined condition. The able-bodied subject showed a stronger decrease in sensorimotor rhythm power during the delay period and a clear post-movement rebound. A shortened period of such power decrease was observed in the subject with tetraplegia, while the rebound occurred immediately at movement onset and was earlier than seen in the able-bodied subject. For both subjects, the observed condition led to similar cortical activation as the overt condition, although to a lesser degree. These preliminary results provide strong support for pursuing a rehabilitation paradigm that combines MEG-based BCI and neurofeedback training with action observation and motor imagery. Future research is targeted to determine if this new training paradigm can increase activation of the motor cortex and whether or not this translates to actual functional improvements.
Figure 5.
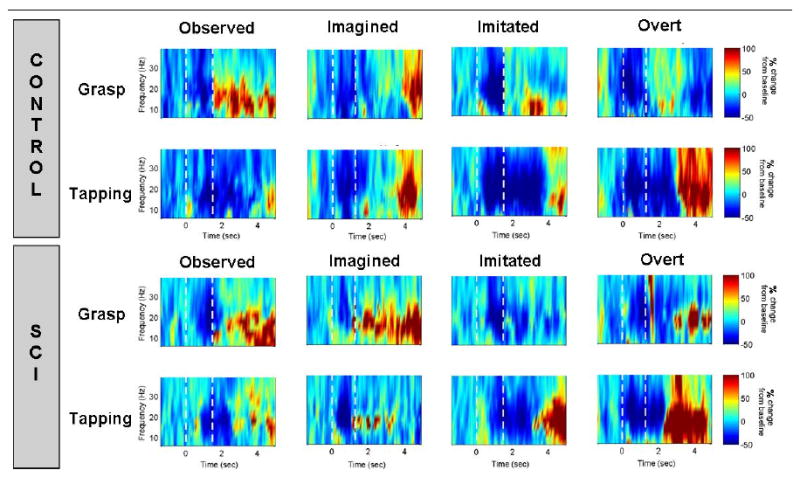
Frequency band power modulation over time for two movements (grasp and tapping) under four conditions (observed, imagined, imitated, and overt) compared between a control subject (top panel) and a subject with tetraplegia (bottom panel). A single representative sensor over the contralateral sensorimotor cortex is pictured. Percent change in power from baseline (resting) is plotted for frequencies ranging from 4-40 Hz. The white dotted line at Time= 0 sec indicates the start of the delay period. The second white dotted line at Time = 1.5 sec indicates the start of the movement period. A characteristic decrease in power prior to movement followed by a “rebound” was observed for both subjects in the imitated and overt conditions. The same activation pattern was observed at a lower intensity for observed and imagined movements.
Neural Interface Technology for Functional Neural Stimulation
Neural stimulation allows us to directly modulate activity of the nervous system. Compared to neural recording devices, to a certain degree, neural stimulation devices have had more success in being translated from basic research into clinical practice with a profitable market to support an active medical device industry in this domain 85. Neural stimulation devices have benefited many patients with sensory, motor, and other neurological disorders 13,14. Just like BCI applications, successful neural stimulation applications also rely on three factors: neural substrate, neuroplasticity, and the appropriate neural stimulation technique. Take cochlear implants (CI) as an example 86. Its neural substrate is the tonotopic organization of the basal membrane and the auditory nerve. Stimulating electrodes are inserted along the auditory nerve to elicit neural activities that represent the different frequency components of sound. Neuroplasticity again plays a critical role, as even the most advanced CI devices currently being marketed only have about 16 electrodes to represent a limited number of frequency components of sound, but neuroplasticity induced by extensive training especially in early childhood allows CI users to learn to discriminate various sounds, understand speech, and even enjoy music 87.
Neural stimulation technology can be used to replace specific sensorimotor functions or treat other neurological and psychiatric disorders. Restoration of motor function is often targeted using functional electrical stimulation (FES) 88. In FES, nerve fibers innervating specific muscles are stimulated to activate its target muscle in a controlled fashion. Substantial work has been done in this area aiming to restore walking or upper limb function. The most recent and exciting advance in this area is the combination of BCI with FES, where motor cortical activity is used to directly control FES devices to re-animate paralyzed limbs bypassing the diseased neural pathway (e.g. the spinal cord) 89,90. Another application of neural stimulation is the restoration of sensory function. As mentioned earlier, the cochlear implant is one of the most successful neural interface technologies, and it has significantly improved the quality of life for many individuals with deafness. Neural interface devices to restore vision are also being investigated in research laboratories and start-up medical device companies 91-93. Finally, neural stimulation targeted to modulate cortical and subcortical activities is being used to treat neurological and psychiatric disorders, such as epilepsy, Parkinson's disease, and depression 13,14.
Just like BCI devices that can serve as assistive technology or as rehabilitation tools, neural stimulation technologies are being investigated in their potential to promote long-term neuroplasticity for rehabilitation applications. Given the capability of both neural recording and stimulation, neural interface technology has the potential to promote neuroplasticity based on Hebbian learning by artificially associating activities of two separate sites within the nervous system. For example, Fetz's group demonstrated that motor cortical reorganization can be induced by coupling action potentials of one motor cortical neuron (neural recording site) with electrical stimulation of another motor cortical neuron (neural stimulation site) 94. In the following section, we provide a sparse, but hopefully informative, sample of neural stimulation technology focusing on neural stimulation for restoring somatosensation and cortical stimulation for stroke rehabilitation.
Neural stimulation as assistive technology for restoring somatosensation
Somatosensory feedback is needed for motor planning, movement control and activity modification. A prosthetic limb that is able to provide sensory information back to its user may allow for greater functional performance. Sensory feedback can be provided as either a substitution or replacement of normal sensation 15. Sensory substitution involves delivering sensory information through a pathway or modality (i.e. eye, ear, or skin) different from the pathway that this type of sensory information is normally delivered or experienced 95. Sensory substitution typically takes a non-invasive approach. An example of sensory substitution would be a prosthetic hand vibrating at its contact point to the residual limb in order to indicate grip force being applied. A number of review articles examined electrotactile and vibration methods of sensory feedback 96,97. Users have indicated that it may be challenging to respond to more than one source of sensory input using this feedback design. For example, a prosthetic hand may provide force feedback of hand opening or closing through vibration, but usually not both 98. A drawback to electrotactile stimulation is pain induced at high levels of stimulation 99, which is a particular concern due to the common presence of phantom limb pain in persons with amputation.
In contrast to sensory substitution, sensory replacement engages, as much as possible, the neural pathways normally involved in sensory reception and processing via somatosensory neural interfaces (SSNIs). SSNIs involve stimulation of an individual's nervous system to deliver information directly to a user's neural networks supporting perception and feedback control. A prosthetic hand utilizing a SSNI may directly stimulate the nervous system of the user to provide sensation of touch and position of the prosthetic hand. Direct stimulation of the nervous system more closely approximates sensation that is experienced by an unimpaired individual and also offers the potential to relay a greater “bandwidth” of information. Stimulation can occur anywhere in the nervous system, from the most peripheral elements to central structures including the somatosensory cortex via penetrating or surface electrodes of various types.
Electrical stimulation of peripheral nerves to provide sensory feedback to a prosthetic user has been investigated for several decades 100,101. In the last decade, we have seen the development of implantable electrodes capable of delivering stimulation to nerves through multiple channels, thus providing more specific and richer sensory feedback 102. Among the electrode systems reviewed by Riso (1999), longitudinally inserted intrafascicular electrodes have since been refined and can be used for individual finger sensory input 103. This system has been utilized to provide feedback of grip force and joint position to individuals with upper limb amputation at or below elbow level while controlling a robotic arm 104. This system allowed the user to adjust the position of the robot arm with no visual feedback from the unit. More investigation is needed to see if chronic systems based on this technology are feasible, as concerns regarding the stiffness of the implanted metal filaments relative to the surrounding nerve tissue have been raised 102.
More central stimulation of neural elements is also a possibility to provide sensory feedback for prosthetic applications. Work by Weber and colleagues in cats indicate that penetrating electrodes inserted into the dorsal root ganglion (DRG) can be used for both sensory recording and sensory stimulation 105. Cortical responses seen from DRG stimulation are similar to those seen with physiologic motion. Given the access to neural architecture and large action potentials visualized when recording from it, the DRG is an excellent structure to consider for neural prosthetic applications. Spinal cord stimulation, although commonly performed for pain management 106, may be another potential approach for providing neuroprosthetic sensory feedback. A similar scenario presents itself in the case of the thalamus, which, like the spinal cord, has also been investigated for stimulation for pain control 107. Cortical stimulation for sensory feedback has been investigated through a variety of methods including intracortical microstimulation 108, transcranial magnetic stimulation 109,110 and transcranial direct current stimulation 111 and are likely routes of continued research.
Neural stimulation as a rehabilitation tool for promoting neuroplasticity
In addition to directly replacing or supplementing lost motor and sensory functions, it has been suggested that neural stimulation may be able to promote neuroplasticity for rehabilitation by directly modulating activity of the nervous system 112,113. Earlier studies have shown that motor cortical representation of limbs can be altered through intracortical microelectrical stimulation in animals 114, as well as in humans through direct noninvasive cortical stimulation 115. Recent studies using animal stroke models have shown that upper limb function can be improved after cortical surface electrical stimulation 116. These studies led to clinical trials of the first fully-implantable cortical surface electrical stimulation device aiming to improve upper extremity function 113,117. Stimulating electrodes were implanted epidurally above M1, and the electrodes were tunneled underneath the skin to the chest and connected to a subclavicular pulse generator 113. Focal high-frequency stimulation (e.g. 50 or 100 Hz) was applied to the motor cortex. Several small scale studies examined the changes in upper limb function measured as the Fugel-Meyer score following invasive cortical stimulation 113. Subjects received cortical stimulation along with motor training showed higher Fugel-Meyer score than those only received motor training at 12 week post-implant 113. However, a larger scale Phase III clinical trial recently reported contradictory results showing little difference in motor function improvement between motor training alone and combined motor training and cortical stimulation 118. In their 2009 paper 118, Plow and colleagues discussed various factors that need to be further investigated and controlled for in future studies using invasive cortical stimulation, such as localization of cortical stimulation sites, descending pathway viability, and differences between animal and human studies. It was also suggested that non-invasive cortical stimulation will provide important insights into the efficacy of invasive cortical stimulation.
Non-invasive cortical stimulation can be performed using repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) 112. At least two approaches have been explored using those two non-invasive stimulation techniques 112. One is to directly enhance motor cortical excitability of the stroke-affected hemisphere using ipsilesional cortical stimulation, which is also the approach taken by the implantable electrical cortical stimulators 112. The other is to reduce inter-hemispheric inhibition from the intact to the affected hemisphere by stimulating the hemisphere contralateral to stroke lesion. Both approaches have been shown to improve motor performance for stroke survivors, and motor function improvement was greater when cortical stimulation was coupled with motor training than using cortical stimulation alone 113,119. Previously, it was reported that improvement in motor performance typically only lasts for a rather limited period of time after cortical stimulation, e.g. less than an hour 113. However, a recent study demonstrated that anodal tDCS led to prolonged motor skill enhancement over multiple days by potentially acting on motor skill consolidation mechanisms 120. While clinical protocols of cortical stimulation, either invasive or non-invasive, still need to be refined and their efficacy needs to be further investigated, non-invasive cortical stimulation using rTMS has been applied to modulate excitability of targeted cortical area in order to study various sensorimotor and cognitive functions. The rest of this section aims to review in detail the effects of rTMS on cortical excitability and the potential mechanisms underlying these effects, which will eventually guide clinical application of cortical stimulation.
rTMS is a novel and painless way to stimulate the human brain non-invasively with the purpose of modulating functions of stimulated cortical regions or interconnected areas. Several studies have examined the effects of rTMS on the excitability of the hand 121 and leg 122,123 motor representations in M1. The duration of after-effects of rTMS over M1 are often between 15-60 min, and it depends on stimulation parameters such as number of pulses applied, rate of application and intensity of each stimulus. In some cases, the stimulus intensity used is below the threshold for evoking a muscle twitch in relaxed muscles, so that any effects observed could not be attributed to sensory input produced by movement. In this regard, stimulation of M1 at a subthreshold intensity and at a frequency of 1 Hz for about 25 min (1,500 total stimuli) reduced the size of motor evoked potentials (MEPs) evoked in finger muscles for the next 30 min 124. Stimulation at frequencies higher than 1 Hz tends to increase rather than decrease cortical excitability. The after-effects of rTMS also depend on the pattern of the individual TMS pulses. For example, Huang and collaborators 125 used theta-burst stimulation (TBS), a protocol in which three 50 Hz pulses are applied regularly 5 times per second for 20-40 sec. In this protocol, low intensities of stimulation produce suppression of MEP size and if each TBS burst is applied for only 2 sec followed by a pause of 8 sec and repeated the after-effect becomes facilitatory.
Several studies have documented that rTMS induces adaptations in cortical neuronal circuitries 121,126-128. In this regard, high-frequency subthreshold rTMS can reduce short-interval intracortical inhibition in the stimulated M1 121,129. Although the cellular mechanisms of after-effects of rTMS are not yet understood, some hypotheses have been postulated. It has been proposed that operating mechanisms include changes in the effectiveness of synapses between cortical neurons (long-term depression and potentiation (LTD and LTP) of synaptic connections). Like LTP/LTD, there is evidence from pharmacological interventions that the after-effects of rTMS depend on the glutamatergic NMDA (N-methyl-D-aspartate) receptor, as they are blocked by a single dose of the NMDA-receptor antagonist dextromethorphan 130. Another example is the use of an NMDA-receptor antagonist, which can block the suppressive and facilitatory effect of some rTMS protocols 131-133. Importantly, another study demonstrated that after-effects of rTMS on M1 excitability disappear when MEPs are tested in actively contracting muscle rather than at rest 134. In this study the authors suggested that rTMS was to some extent changing the level of excitability of the resting corticospinal system, rather than changing the effectiveness of transmission at synapses within the cortex. It is possible that both effects occur in different degrees depending on the parameters of rTMS.
In humans and non-human primates, corticospinal cells exert modulation over a large group of spinal interneurons 135. Therefore, it is possible that activating corticospinal neurons by rTMS may also induce changes in spinal neuronal circuitries. Perez and collaborators 122 applied 15 trains of 20 pulses at 5 Hz at intensities between 75 to 120% of resting motor threshold of the tibialis anterior muscle and reported a decrease in the size of the soleus H-reflex at stimulus intensities ranging from 92% to 120% of the resting motor threshold. In this study the authors demonstrated that rTMS increased the level of presynaptic inhibition at the terminals of Ia afferent fibers but did not change the level of disynaptic reciprocal Ia inhibition. At rTMS frequencies of 1 Hz changes also have been reported in spinal cord reflexes. Valero-Cabré et al. 136 applied 600 pulses of 1-Hz rTMS at 90% of resting motor threshold of the flexor carpi radialis (FCR) muscle and reported a lasting decrease in threshold and an increase in size of the FCR H-reflex. It is possible that different stimulation frequency will have a different effect on spinal cord excitability.
Overall, rTMS studies in both able-bodied volunteers and stroke survivors have demonstrated that it can non-invasively modulate motor cortical excitability and influence motor functions in a controlled fashion. Furthermore, the other form of non-invasive cortical stimulation, tDCS, can also modulate motor and cognitive functions in a polarity specific manner in health and disease and facilitate the design of sham interventions 137-139. However, better understanding of the effects and underlying mechanism of cortical stimulation techniques is needed in order to provide scientific guidance for future clinical trials aiming to promote neuroplasticity and functional recovery using invasive or non-invasive cortical stimulation.
Summary
The current paper reviewed neural interface technology and its relationship with neuroplasticity. Two types of neural interface technology are reviewed highlighting specific technologies that the authors directly work with: 1) neural interface technology for neural recording, such as the micro-ECoG BCI system for hand prosthesis control and the comprehensive rehabilitation paradigm combining MEG-BCI, action observation and motor imagery training; 2) neural interface technology for functional neural stimulation, such as somatosensory neural stimulation for restoring somatosensation and non-invasive cortical stimulation using rTMS and tDCS for modulating cortical excitability and stroke rehabilitation. The close interaction between neural interface devices and neuroplasticity leads to increased efficacy of neural interface devices and improved functional recovery of the nervous system. This symbiotic relationship between neural interface technology and the nervous system is expected to maximize functional gain for individuals with various sensory, motor, and cognitive impairments, eventually leading to better quality of life.
Acknowledgments
This work was supported by the National Science Foundation under Cooperative Agreement EEC-0540865, Telemedicine and Advanced Technology Research Center (TATRC) of U.S. Army Medical Research and Material Command Agreement W81XWH-07-1-0716, a special grant from the Office of the Senior Vice Chancellor for the Health Sciences at University of Pittsburgh, National Institutes of Health (NIH) grants from the NIBIB (1R01EB007749) and NINDS (1R21NS056136) and Grant Number 5 UL1 RR024153 from the National Center for Research Resources (NCRR), a component of NIH and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Galvani L. De viribus electricitatis in motu musculari. Commentarius (Commentary on the effects of electricity on muscular motion) De Bononiesi Scientarium et Ertium Instituto atque Academia Commentarii. 1791;7:363. [Google Scholar]
- 2.Malmivuo J, Plonsey R. Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields. Oxford University Press; USA: 1995. [Google Scholar]
- 3.Pena C, Bowsher K, Costello A, et al. An overview of FDA medical device regulation as it relates to deep brain stimulation devices. IEEE Trans Neural Syst Rehabil Eng. 2007;15:421. doi: 10.1109/TNSRE.2007.903973. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Fertig SJ, Kleitman N, et al. Advances in neural interfaces: report from the 2006 NIH Neural Interfaces Workshop. J Neural Eng. 2007;4:S137. doi: 10.1088/1741-2560/4/3/S01. [DOI] [PubMed] [Google Scholar]
- 5.Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 6.Skarpaas TL, Morrell MJ. Intracranial stimulation therapy for epilepsy. Neurotherapeutics. 2009;6:238. doi: 10.1016/j.nurt.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- 8.Donoghue JP. Bridging the brain to the world: a perspective on neural interface systems. Neuron. 2008;60:511. doi: 10.1016/j.neuron.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Donoghue JP, Nurmikko A, Black M, et al. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J Physiol. 2007;579:603. doi: 10.1113/jphysiol.2006.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finn WE, LoPresti PG. Handbook of neuroprosthetic methods. CRC; 2002. [Google Scholar]
- 11.Horch KW, Dhillon G. Neuroprosthetics: theory and practice. World Scientific Publishing Company; 2004. [Google Scholar]
- 12.Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29:536. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Sakas DE, Panourias IG, Simpson BA. An introduction to neural networks surgery, a field of neuromodulation which is based on advances in neural networks science and digitised brain imaging. Acta Neurochir Suppl. 2007;97:3. doi: 10.1007/978-3-211-33081-4_1. [DOI] [PubMed] [Google Scholar]
- 14.Sakas DE, Panourias IG, Simpson BA, et al. An introduction to operative neuromodulation and functional neuroprosthetics, the new frontiers of clinical neuroscience and biotechnology. Acta Neurochir Suppl. 2007;97:3. doi: 10.1007/978-3-211-33079-1_1. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AB, Cui XT, Weber DJ, et al. Brain-controlled interfaces: movement restoration with neural prosthetics. Neuron. 2006;52:205. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Gage GJ, Ludwig KA, Otto KJ, et al. Naive coadaptive cortical control. J Neural Eng. 2005;2:52. doi: 10.1088/1741-2560/2/2/006. [DOI] [PubMed] [Google Scholar]
- 17.Helms Tillery SI, Taylor DM, Schwartz AB. Training in cortical control of neuroprosthetic devices improves signal extraction from small neuronal ensembles. Rev Neurosci. 2003;14:107. doi: 10.1515/revneuro.2003.14.1-2.107. [DOI] [PubMed] [Google Scholar]
- 18.Jarosiewicz B, Chase SM, Fraser GW, et al. Functional network reorganization during learning in a brain-computer interface paradigm. Proc Natl Acad Sci U S A. 2008;105:19486. doi: 10.1073/pnas.0808113105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 20.Birbaumer N, Cohen LG. Brain-computer interfaces: communication and restoration of movement in paralysis. J Physiol. 2007;579:621. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 22.Velliste M, Perel S, Spalding MC, et al. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 23.Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex. 1994;4:590. doi: 10.1093/cercor/4.6.590. [DOI] [PubMed] [Google Scholar]
- 24.Georgopoulos AP, Kettner RE, Schwartz AB. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci. 1988;8:2928. doi: 10.1523/JNEUROSCI.08-08-02928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- 26.Kettner RE, Schwartz AB, Georgopoulos AP. Primate motor cortex and free arm movements to visual targets in three-dimensional space. III. Positional gradients and population coding of movement direction from various movement origins. J Neurosci. 1988;8:2938. doi: 10.1523/JNEUROSCI.08-08-02938.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol. 1999;82:2676. doi: 10.1152/jn.1999.82.5.2676. [DOI] [PubMed] [Google Scholar]
- 28.Paninski L, Fellows MR, Hatsopoulos NG, et al. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. J Neurophysiol. 2004;91:515. doi: 10.1152/jn.00587.2002. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz AB, Kettner RE, Georgopoulos AP. Primate motor cortex and free arm movements to visual targets in three-dimensional space. I. Relations between single cell discharge and direction of movement. J Neurosci. 1988;8:2913. doi: 10.1523/JNEUROSCI.08-08-02913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Chan SS, Heldman DA, et al. Motor cortical representation of position and velocity during reaching. J Neurophysiol. 2007;97:4258. doi: 10.1152/jn.01180.2006. [DOI] [PubMed] [Google Scholar]
- 31.Buch E, Weber C, Cohen LG, et al. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serruya MD, Hatsopoulos NG, Paninski L, et al. Instant neural control of a movement signal. Nature. 2002;416:141. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 33.Wessberg J, Stambaugh CR, Kralik JD, et al. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- 34.Leuthardt EC, Schalk G, Wolpaw JR, et al. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1:63. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 35.Schalk G, Miller KJ, Anderson NR, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5:75. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabiani GE, McFarland DJ, Wolpaw JR, et al. Conversion of EEG activity into cursor movement by a brain-computer interface (BCI) IEEE Trans Neural Syst Rehabil Eng. 2004;12:331. doi: 10.1109/TNSRE.2004.834627. [DOI] [PubMed] [Google Scholar]
- 37.Sellers EW, Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clin Neurophysiol. 2006;117:538. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 38.Mellinger J, Schalk G, Braun C, et al. An MEG-based brain-computer interface (BCI) Neuroimage. 2007;36:581. doi: 10.1016/j.neuroimage.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Ryu J, Jolesz FA, et al. Brain-machine interface via real-time fMRI: preliminary study on thought-controlled robotic arm. Neurosci Lett. 2009;450:1. doi: 10.1016/j.neulet.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004;101:17849. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman WJ, Holmes MD, Burke BC, et al. Spatial spectra of scalp EEG and EMG from awake humans. Clin Neurophysiol. 2003;114:1053. doi: 10.1016/s1388-2457(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 42.Degenhart AD, Sudre G, Collinger J, et al. Comparison of ECoG signal modulation between hand and brain-controlled cursor movement tasks. Society for Neuroscience; Washington, DC: [Google Scholar]
- 43.Heldman DA, Wang W, Chan SS, et al. Local field potential spectral tuning in motor cortex during reaching. IEEE Trans Neural Syst Rehabil Eng. 2006;14:180. doi: 10.1109/TNSRE.2006.875549. [DOI] [PubMed] [Google Scholar]
- 44.Niedermeyer E, Silva FLd. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5th. Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 45.Wang W, Degenhart AD, Collinger JL, et al. Human motor cortical activity recorded with micro-ECoG electrodes during invividual finger movements. IEEE EMBS; Minneapolis, MN: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinjamuri R, Weber DJ, Degenhart AD, et al. A fuzzy logic model for hand posture control using human cortical activity recorded by micro-ECoG electrodes. IEEE EMBS; Minneapolis, MN: [DOI] [PubMed] [Google Scholar]
- 47.Wisneski KJ, Anderson N, Schalk G, et al. Unique cortical physiology associated with ipsilateral hand movements and neuroprosthetic implications. Stroke. 2008;39:3351. doi: 10.1161/STROKEAHA.108.518175. [DOI] [PubMed] [Google Scholar]
- 48.Hunter S, Crome P. Hand function and stroke. Reviews in Clinical Gerontology. 2002;12:68. [Google Scholar]
- 49.Nakayama H, Jorgensen HS, Pedersen PM, et al. Prevalence and risk factors of incontinence after stroke. The Copenhagen Stroke Study Stroke. 1997;28:58. doi: 10.1161/01.str.28.1.58. [DOI] [PubMed] [Google Scholar]
- 50.Olsen TS. Arm and leg paresis as outcome predictors in stroke rehabilitation. Stroke. 1990;21:247. doi: 10.1161/01.str.21.2.247. [DOI] [PubMed] [Google Scholar]
- 51.Brucker BS, Bulaeva NV. Biofeedback effect on electromyography responses in patients with spinal cord injury. Arch Phys Med Rehabil. 1996;77:133. doi: 10.1016/s0003-9993(96)90157-4. [DOI] [PubMed] [Google Scholar]
- 52.Kohlmeyer KM, Hill JP, Yarkony GM, et al. Electrical stimulation and biofeedback effect on recovery of tenodesis grasp: a controlled study. Arch Phys Med Rehabil. 1996;77:702. doi: 10.1016/s0003-9993(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 53.Lin KC, Wu CY, Liu JS, et al. Constraint-induced therapy versus dose-matched control intervention to improve motor ability, basic/extended daily functions, and quality of life in stroke. Neurorehabil Neural Repair. 2009;23:160. doi: 10.1177/1545968308320642. [DOI] [PubMed] [Google Scholar]
- 54.Petrofsky JS. The use of electromyogram biofeedback to reduce Trendelenburg gait. Eur J Appl Physiol. 2001;85:491. doi: 10.1007/s004210100466. [DOI] [PubMed] [Google Scholar]
- 55.Wolf SL, Winstein CJ, Miller JP, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angelakis E, Stathopoulou S, Frymiare JL, et al. EEG neurofeedback: a brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin Neuropsychol. 2007;21:110. doi: 10.1080/13854040600744839. [DOI] [PubMed] [Google Scholar]
- 57.Heinrich H, Gevensleben H, Strehl U. Annotation: neurofeedback - train your brain to train behaviour. J Child Psychol Psychiatry. 2007;48:3. doi: 10.1111/j.1469-7610.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 58.Monderer RS, Harrison DM, Haut SR. Neurofeedback and epilepsy. Epilepsy Behav. 2002;3:214. doi: 10.1016/s1525-5050(02)00001-x. [DOI] [PubMed] [Google Scholar]
- 59.Sterman MB, Egner T. Foundation and practice of neurofeedback for the treatment of epilepsy. Appl Psychophysiol Biofeedback. 2006;31:21. doi: 10.1007/s10484-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 60.Nijboer F, Furdea A, Gunst I, et al. An auditory brain-computer interface (BCI) J Neurosci Methods. 2008;167:43. doi: 10.1016/j.jneumeth.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaechter JD. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol. 2004;73:61. doi: 10.1016/j.pneurobio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Tecchio F, Zappasodi F, Tombini M, et al. Brain plasticity in recovery from stroke: an MEG assessment. Neuroimage. 2006;32:1326. doi: 10.1016/j.neuroimage.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Jurkiewicz MT, Mikulis DJ, McIlroy WE, et al. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair. 2007;21:527. doi: 10.1177/1545968307301872. [DOI] [PubMed] [Google Scholar]
- 64.Celnik P, Stefan K, Hummel F, et al. Encoding a motor memory in the older adult by action observation. Neuroimage. 2006;29:677. doi: 10.1016/j.neuroimage.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 65.Celnik P, Webster B, Glasser DM, et al. Effects of action observation on physical training after stroke. Stroke. 2008;39:1814. doi: 10.1161/STROKEAHA.107.508184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lotze M, Cohen LG. Volition and imagery in neurorehabilitation. Cogn Behav Neurol. 2006;19:135. doi: 10.1097/01.wnn.0000209875.56060.06. [DOI] [PubMed] [Google Scholar]
- 67.Stefan K, Classen J, Celnik P, et al. Concurrent action observation modulates practice-induced motor memory formation. Eur J Neurosci. 2008;27:730. doi: 10.1111/j.1460-9568.2008.06035.x. [DOI] [PubMed] [Google Scholar]
- 68.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 69.Hebb DO. Organization of behavior. New York: Wiley; 1949. [Google Scholar]
- 70.Buccino G, Vogt S, Ritzl A, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 71.Fabbri-Destro M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda) 2008;23:171. doi: 10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- 72.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 73.Rizzolatti G, Sinigaglia C. Mirror neurons and motor intentionality. Funct Neurol. 2007;22:205. [PubMed] [Google Scholar]
- 74.Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15:632. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Hari R, Forss N, Avikainen S, et al. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci U S A. 1998;95:15061. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iacoboni M, Woods RP, Brass M, et al. Cortical mechanisms of human imitation. Science. 1999;286:2526. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 77.Caetano G, Jousmaki V, Hari R. Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proc Natl Acad Sci U S A. 2007;104:9058. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. J Neurosci. 2007;27:13241. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tkach D, Reimer J, Hatsopoulos NG. Observation-based learning for brain-machine interfaces. Curr Opin Neurobiol. 2008;18:589. doi: 10.1016/j.conb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14:S103. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- 81.Mast FW, Jancke L. Spatial processing in navigation, imagery and perception. Springer; 2007. [Google Scholar]
- 82.Dunsky A, Dickstein R, Marcovitz E, et al. Home-based motor imagery training for gait rehabilitation of people with chronic poststroke hemiparesis. Arch Phys Med Rehabil. 2008;89:1580. doi: 10.1016/j.apmr.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 83.Ertelt D, Small S, Solodkin A, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36 2:T164. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 84.Page SJ, Szaflarski JP, Eliassen JC, et al. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabil Neural Repair. 2009;23:382. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.PRWeb: World Neurostimulation Market to Reach $5.2 Billion by 2012, According to New Report by Global Industry Analysts. 2008 Bio-Medicine.org.
- 86.Middlebrooks JC, Bierer JA, Snyder RL. Cochlear implants: the view from the brain. Curr Opin Neurobiol. 2005;15:488. doi: 10.1016/j.conb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009 doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morita I, Keith MW, Kanno T. Reconstruction of upper limb motor function using functional electrical stimulation (FES) Acta Neurochir Suppl. 2007;97:403. doi: 10.1007/978-3-211-33079-1_53. [DOI] [PubMed] [Google Scholar]
- 89.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morrow MM, Pohlmeyer EA, Miller LE. Control of muscle synergies by cortical ensembles. Adv Exp Med Biol. 2009;629:179. doi: 10.1007/978-0-387-77064-2_9. [DOI] [PubMed] [Google Scholar]
- 91.Caspi A, Dorn JD, McClure KH, et al. Feasibility study of a retinal prosthesis: spatial vision with a 16-electrode implant. Arch Ophthalmol. 2009;127:398. doi: 10.1001/archophthalmol.2009.20. [DOI] [PubMed] [Google Scholar]
- 92.Cohen ED. Prosthetic interfaces with the visual system: biological issues. J Neural Eng. 2007;4:R14. doi: 10.1088/1741-2560/4/2/R02. [DOI] [PubMed] [Google Scholar]
- 93.Wong YT, Chen SC, Kerdraon YA, et al. Efficacy of supra-choroidal, bipolar, electrical stimulation in a vision prosthesis. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1789. doi: 10.1109/IEMBS.2008.4649525. [DOI] [PubMed] [Google Scholar]
- 94.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 95.Kaczmarek KA, Webster JG, Bach-y-Rita P, et al. Electrotactile and vibrotactile displays for sensory substitution systems. IEEE Trans Biomed Eng. 1991;38:1. doi: 10.1109/10.68204. [DOI] [PubMed] [Google Scholar]
- 96.Childress DS. Closed-loop control in prosthetic systems: historical perspective. Ann Biomed Eng. 1980;8:293. doi: 10.1007/BF02363433. [DOI] [PubMed] [Google Scholar]
- 97.Scott RN. Feedback in myoelectric prostheses. Clin Orthop Relat Res. 1990;58 [PubMed] [Google Scholar]
- 98.Prior RE, Lyman J, Case PA, et al. Supplemental sensory feedback for the VA/NU myoelectric hand Background and preliminary designs. Bull Prosthet Res. 1976;170 [PubMed] [Google Scholar]
- 99.Shannon GF. A comparison of alternative means of providing sensory feedback on upper limb prostheses. Med Biol Eng. 1976;14:289. doi: 10.1007/BF02478123. [DOI] [PubMed] [Google Scholar]
- 100.Anani A, Korner L. Discrimination of phantom hand sensations elicited by afferent electrical nerve stimulation in below-elbow amputees. Med Prog Technol. 1979;6:131. [PubMed] [Google Scholar]
- 101.Clippinger FW. A system to provide senstation from an upper extremity amputation prosthesis. In: Fields WL, editor. Neural Organization and Its Relevance to Prosthetics. London: International Medical Book Corporation; 1973. [Google Scholar]
- 102.Riso RR. Strategies for providing upper extremity amputees with tactile and hand position feedback--moving closer to the bionic arm. Technol Health Care. 1999;7:401. [PubMed] [Google Scholar]
- 103.Micera S, Navarro X, Carpaneto J, et al. On the use of longitudinal intrafascicular peripheral interfaces for the control of cybernetic hand prostheses in amputees. IEEE Trans Neural Syst Rehabil Eng. 2008;16:453. doi: 10.1109/TNSRE.2008.2006207. [DOI] [PubMed] [Google Scholar]
- 104.Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans Neural Syst Rehabil Eng. 2005;13:468. doi: 10.1109/TNSRE.2005.856072. [DOI] [PubMed] [Google Scholar]
- 105.Weber DJ, Stein RB, Everaert DG, et al. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng. 2007;4:S168. doi: 10.1088/1741-2560/4/3/S04. [DOI] [PubMed] [Google Scholar]
- 106.Waltz JM. Spinal cord stimulation: a quarter century of development and investigation. A review of its development and effectiveness in 1,336 cases. Stereotact Funct Neurosurg. 1997;69:288. doi: 10.1159/000099890. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto T, Katayama Y, Obuchi T, et al. Thalamic sensory relay nucleus stimulation for the treatment of peripheral deafferentation pain. Stereotact Funct Neurosurg. 2006;84:180. doi: 10.1159/000094958. [DOI] [PubMed] [Google Scholar]
- 108.Romo R, Hernandez A, Zainos A, et al. Sensing without touching: psychophysical performance based on cortical microstimulation. Neuron. 2000;26:273. doi: 10.1016/s0896-6273(00)81156-3. [DOI] [PubMed] [Google Scholar]
- 109.Chapman A. Seeing with your fingers: a transcranial magnetic stimulation investigation of multimodal sensory perception. J Neurosci. 2007;27:7081. doi: 10.1523/JNEUROSCI.2220-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blankenburg F, Ruff CC, Bestmann S, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Antal A, Paulus W. Transcranial direct current stimulation and visual perception. Perception. 2008;37:367. doi: 10.1068/p5872. [DOI] [PubMed] [Google Scholar]
- 112.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 113.Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Top Stroke Rehabil. 2007;14:54. doi: 10.1310/tsr1406-54. [DOI] [PubMed] [Google Scholar]
- 114.Nudo RJ, Jenkins WM, Merzenich MM. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens Mot Res. 1990;7:463. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- 115.Ziemann U, Wittenberg GF, Cohen LG. Stimulation-induced within-representation and across-representation plasticity in human motor cortex. J Neurosci. 2002;22:5563. doi: 10.1523/JNEUROSCI.22-13-05563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 117.NorthstarNeuroscience: Safety and effectiveness of cortical stimulation in the treatment of stroke patients with upper extremity hemiparesis (EVEREST) 2007 http://clinicaltrials.gov/ct2/show/NCT00170716.
- 118.Plow EB, Carey JR, Nudo RJ, et al. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40:1926. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 120.Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pascual-Leone A, Tormos JM, Keenan J, et al. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 122.Perez MA, Lungholt BK, Nielsen JB. Short-term adaptations in spinal cord circuits evoked by repetitive transcranial magnetic stimulation: possible underlying mechanisms. Exp Brain Res. 2005;162:202. doi: 10.1007/s00221-004-2144-2. [DOI] [PubMed] [Google Scholar]
- 123.Zuur AT, Christensen MS, Sinkjaer T, et al. Tibialis anterior stretch reflex in early stance is suppressed by repetitive transcranial magnetic stimulation. J Physiol. 2009;587:1669. doi: 10.1113/jphysiol.2009.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 125.Huang YZ, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 126.Di Lazzaro V, Oliviero A, Mazzone P, et al. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res. 2002;147:108. doi: 10.1007/s00221-002-1223-5. [DOI] [PubMed] [Google Scholar]
- 127.Gilio F, Rizzo V, Siebner HR, et al. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kobayashi M, Hutchinson S, Theoret H, et al. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62:91. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- 129.Peinemann A, Lehner C, Mentschel C, et al. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett. 2000;296:21. doi: 10.1016/s0304-3940(00)01616-5. [DOI] [PubMed] [Google Scholar]
- 130.Stefan K, Kunesch E, Benecke R, et al. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boroojerdi B, Battaglia F, Muellbacher W, et al. Mechanisms underlying rapid experience-dependent plasticity in the human visual cortex. Proc Natl Acad Sci U S A. 2001;98:14698. doi: 10.1073/pnas.251357198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Butefisch CM, Davis BC, Wise SP, et al. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang YZ, Chen RS, Rothwell JC, et al. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 134.Touge T, Gerschlager W, Brown P, et al. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- 135.Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 136.Valero-Cabre A, Oliveri M, Gangitano M, et al. Modulation of spinal cord excitability by subthreshold repetitive transcranial magnetic stimulation of the primary motor cortex in humans. Neuroreport. 2001;12:3845. doi: 10.1097/00001756-200112040-00048. [DOI] [PubMed] [Google Scholar]
- 137.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Hummel FC, Voller B, Celnik P, et al. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ragert P, Vandermeeren Y, Camus M, et al. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin Neurophysiol. 2008;119:805. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


