Abstract
Trm1 is a tRNA specific m22G methyltransferase shared by nuclei and mitochondria in Saccharomyces cerevisiae. In nuclei Trm1 is peripherally associated with the inner nuclear membrane (INM). We investigated the mechanism delivering/tethering Trm1 to the INM. Analyses of mutations of the Ran pathway and nuclear pore components showed that Trm1 accesses the nucleoplasm via the classical nuclear import pathway. We identified a Trm1 cis-acting sequence sufficient to target passenger proteins to the INM. Detailed mutagenesis of this region uncovered specific amino acids necessary for authentic Trm1 to locate at the INM. The INM information is contained within a sequence of <20 amino acids, defining the first motif for addressing a peripheral protein to this important subnuclear location. The combined studies provide a multi-step process to direct Trm1 to the INM: (1) translation in the cytoplasm; (2) Ran-dependent import into the nucleoplasm; and (3) redistribution from the nucleoplasm to the INM via the INM motif. Furthermore, we demonstrate that the Trm1 mitochondrial targeting and nuclear localization signals are in competition with each other, as Trm1 becomes mitochondrial if prevented from entering the nucleus.
Keywords: inner nuclear membrane, nucleus organization, targeting motif, mitochondrial location
Introduction
The nucleus is a complex organelle containing many subdomains. Distinct subnuclear locations include the nucleolus, nuclear pores, the inner nuclear membrane (INM) and various other nucleoplasmic structures such as cajal bodies (Review: (1)). Because membranes do not separate these areas from one another, their structure, biogenesis, and maintenance likely result from poorly described interactions among resident macromolecules. The INM is of particular interest because chromatin located at this region can be silenced ((2) and references therein) or, if in the vicinity of the nuclear pore complexes (NPC), highly activated (Review: (3)). Moreover, numerous human disorders, laminopathies, are caused by failure to correctly localize INM proteins to the nuclear rim (Reviews: (4); (5)).
Both integral and peripheral membrane proteins are located at the INM. In metazoan cells numerous integral INM proteins have been identified and the mechanism by which they are targeted and maintained at this location have been described (6); Reviews: (7, 8). In brief, integral INM proteins synthesized on and anchored to the endoplasmic reticulum (ER) are directed from the ER/outer nuclear membrane to the INM, presumably through nuclear pore lateral chanels. Subsequent studies in yeast showed that the Heh1/Heh2 integral INM proteins are directed from the ER to nuclear pores via a process requiring the Ran pathway, the nuclear localization signals (NLS) in Heh1/Heh2 proteins, and the karyopherins, importin-α and importin-β, that interact with these NLSs (9). Transport across the pore membrane remains a poorly described process(es) that depends on the size of the protein, the strength of the NLS, the efficiency with which individual cargoes interact with karyopherins, and particular NPC proteins (10); Reviews: (7, 11). Retention of integral proteins at the INM generally requires binding to nuclear components. In metazoan cells lamins play a pivotal role in INM retention, but other proteins as well as chromatin serve as anchors to prevent diffusion to the ER (diffusion–retention; (6)). Yeast lack lamins; so, prevention of retrograde movement to the ER must depend on other nuclear components.
In contrast to integral membrane proteins, delivery of peripheral proteins to the INM has not been investigated in great detail, even though there are a large number of such proteins (7, 12). The best-characterized peripheral INM protein in metazoans is lamin. Although it is formally possible that peripheral INM proteins bind to an integral membrane protein(s) destined to the INM through protein-protein interactions at the ER and in that way are brought to the nucleus via the integral membrane pathway type mechanism, it appears that lamin A, is imported into the nucleus as a soluble protein and then redistributed to the INM (13, 14). Association of lamins A and B3 with the INM requires hydrophobic modification of the motif CAAX at its carboxyl terminus via isoprenylation (15), whereas association of lamin C requires myristoylation at the amino terminus (16). Hydrophobic modifications of lamins, although required for association, are not sufficient for stable association of these proteins with the INM (17); stable association likely results from interactions with integral INM lamin-binding proteins (Reviews: (5, 7)).
Soluble proteins generally achieve their correct subcellular destination via topogenic motifs of defined consensus or structure. No such motifs have been previously described for peripheral INM proteins, although studies of INM association of a mutant version of LAP2 missing its transmembrane domain showed that this mutant protein required a 76 amino acid region that binds lamin in order to stably associate with the INM (18).
The goal of the work described here is to gain an understanding of how proteins peripherally associated with the INM are targeted to and maintained at this location. We employ yeast Trm1, a sorting isozyme found in nuclei and mitochondria, as a reporter to investigate how such peripheral proteins are targeted to the INM. In wild-type cells, two forms of the protein are produced by alternative translation starts. The longer form, Trm1-I, representing about 10% of the Trm1 pool, appears to be exclusively located in mitochondria; the shorter form, Trm1-II, is located in both nuclei and mitochondria, but is predominately targeted to the nucleus (19). We previously identified cis-acting NLS and mitochondrial targeting sequences (MTS) necessary and sufficient for targeting Trm1 to both organelles (Review: (20)). Trm1-II has a distinct subnuclear distribution, as it is evenly distributed at the INM. Several lines of evidence document that it is periperally associated with the INM. First, Trm1-II does not contain a predicted transmembrane domain. Second, Trm1-II is released from the INM by reagents that release peripheral proteins (21). Third, mutant forms of Trm1-II unable to associate with the INM accumulate in the nucleoplasm, not the ER (22).
To discover gene products that function in delivery/tethering Trm1 to the INM, Murthi and Hopper (22) conducted a genome-wide screen to identify mutations of genes that disrupt the INM association of Trm1. These studies uncovered Ice2, an integral membrane protein of the ER; Ice2 may serve as a regulator of a Trm1 tether. The studies also found that deletion of any one of the three genes coding for subunits of the Nat C N-terminal acetyltransferase complex abolishes the association of Trm1 with the INM. While N-terminal acetylation of Trm1-II is necessary for its INM association, its N-acetylation is not sufficient.
In this study we show that the mechanism for the location of Trm1-II to the INM differs significantly from the mechanism described for integral proteins to achieve INM location, but resembles, in part, the pathway proposed to deliver lamins to the INM (13, 14). Trm1-II moves from the cytoplasm to the nucleoplasm via the classical Ran-dependent protein import pathway and then the nucleoplasmic pool is redirected to the INM. We identify the cis-acting motif of Trm1-II that is sufficient and necessary for locating Trm1 to the INM. To identify this motif we generated various fusion of reporter proteins and created mutations in wild-type Trm1. We identified a sequence block that is sufficient for locating three reporter proteins to the INM. We further conducted detailed mutagenesis to define particular amino acids within the region that are necessary for authentic Trm1-II to locate at the INM. These studies identify for the first time a targeting/tethering motif for a protein peripherally associated with the INM. Finally our studies uncover regions of Trm1-II involved in the competition between Trm1-II’s distribution between the mitochondria and the nucleus.
Results
Trm1 is delivered from the cytoplasm to the nucleoplasm via the Ran-dependent nuclear import pathway
A possible path for endogenous Trm1 to peripherally associate with the INM is for it to first enter the nuclear interior via the Ran-dependent nuclear import pathway and then target to the INM. We reasoned that if this was the case, newly synthesized Trm1-II-GFP should locate throughout the cytoplasm if the Ran gradient was disrupted. If instead Trm1-II-GFP achieved its INM location via interaction directly or indirectly with the outer nuclear membrane or the ER, disruption of the Ran pathway should result in the distribution of Trm1-II-GFP to the ER, as occurs for integral INM proteins (9).
We determined the subcellular location of Trm1-II-GFP encoded by plasmid pGP54a-Trm1-II-GFP in cells with a temperature sensitive RanGAP mutation, rna1-1 (23). When cells are shifted to the nonpermissive temperature immediately after induction of Trm1-II-GFP by addition of galactose, newly synthesized Trm1-II-GFP fails to localize to the nucleus (Fig. 1A). In contrast, if cells are shifted to the nonpermissive temperature 1 hr post induction, Trm1-II-GFP distributes evenly around the INM (Fig. 1B) as does wild-type Trm1 without GFP and regulated by its endogenous promoter (22, 24). Thus, the Ran pathway is not necessary for maintenance of Trm1-II-GFP at the INM, but rather only for its delivery to the nuclear interior. The data support the hypothesis that Trm1-II is delivered to the INM via the classical nuclear import pathway.
Figure 1. Location of Trm1-II-GFP at the INM depends on the Ran pathway.
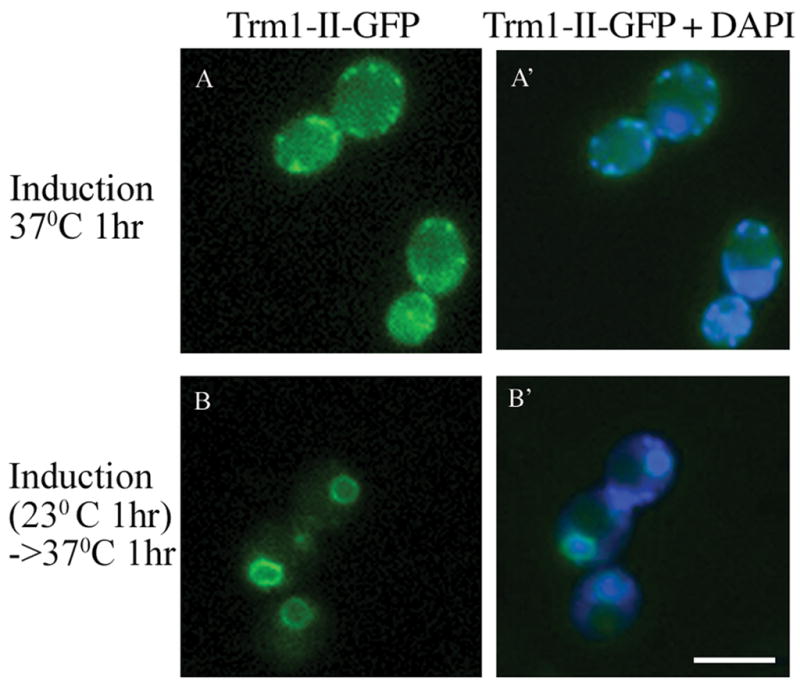
Live cell imaging of the location of Trm1-II-GFP in rna1-1 cells. The cells in panels A were induced by addition of galactose and then immediately shifted to the nonpermissive temperature for 1 hr. Cells in panels B were induced by addition of galactose for 1 hr prior to the shifting the cells to the nonpermissive temperature. Panels A′ and B′ are overlays of panels A and B, respectively, with DAPI staining. Bar =5μm.
When the Ran-GTP gradient is defective there is no detectable nuclear pool of Trm1-II. Interestingly, part of the cytoplasmic Trm1-II pool co-localizes with mitochondria (Fig. 1A, A′). Mitochondrial location for Trm1-II is consistent with our previous studies that showed that Trm1-II contains both nuclear and mitochondrial targeting information (19, 25) and supports the hypothesis that distribution of Trm1-II between mitochondria and the nucleus results from the competing MTS and NLS motifs, located between amino acids 17–48 and 95–102, respectively. Since >90% of Trm1-II normally is located at the INM, its nuclear import predominates mitochondrial import. The data presented here show that the Ran pathway plays an important role in the dominant nuclear location of Trm1-II.
We also determined the consequences of altering the composition of the nuclear pores upon Trm1-II-GFP distribution. Nup133 is essential for growth and nucleus-cytoplasm transport at elevated temperatures, but not at low temperatures (23°C); however, its deletion causes NPC clustering to a subdomain of the nuclear envelope (NE) at permissive temperatures ((26) and references therein). We expressed galactose-inducible Trm1-II-GFP in wild-type and nup133Δ cells. After 1 ½ hr of induction by addition of galactose, Trm1-II-GFP was located at the nuclear rim in wild-type cells; in these cells the NPC protein, Nup49-mCherry was uniformly distributed about the NE (Fig. 2). In contrast, in nup133Δ cells Nup49-mCherry was clustered to a subportion of the NE, as predicted (Fig. 2) ((26) and references therein). For ~75% of the galactose-induced nup133Δ cells viewed (81 of 104 cells counted), Trm1-II-GFP correctly located to the NE; for the remaining ~25% of the cells (23 of 104 cells counted), Trm1-II-GFP clustered on the NE. We also studied the distribution of Trm1-II-GFP in cells with defects in other NPC components. Trm1-II-GFP was normally located in nup2Δ, nup100Δ, nup188Δ, nup53Δ, and pom152Δ cells (data not shown). The data demonstrate that capping of nuclear pores that occurs in nup133Δ cells does not affect all peripheral proteins associated with the INM.
Figure 2. Location of Trm1-II-GFP and Nup49-mCherry in nup133Δ cells.
Left: distributions of Trm1-II-GFP and Nup49-mCherry in wild-type cells. Trm1-II-GFP is located at the nuclear rim (top row), as is Nup49-mCherry (middle row). Bottom row is the overlay of the GFP and mCherry signals. Right: distributions of Trm1-II-GFP and Nup49-mCherry in nup133Δ cells. Trm1-II-GFP distribution is identical to its distribution in wild-type cells in the majority of nup133Δ cells. As expected, Nup49-mCherry is aberrantly distributed to one or two foci on the nuclear envelop (middle row). Bottom row is the overlay of the GFP and mCherry signals. Bar = 5μm.
A region of Trm1 including ADEPT 2 is sufficient to target reporter proteins to the INM
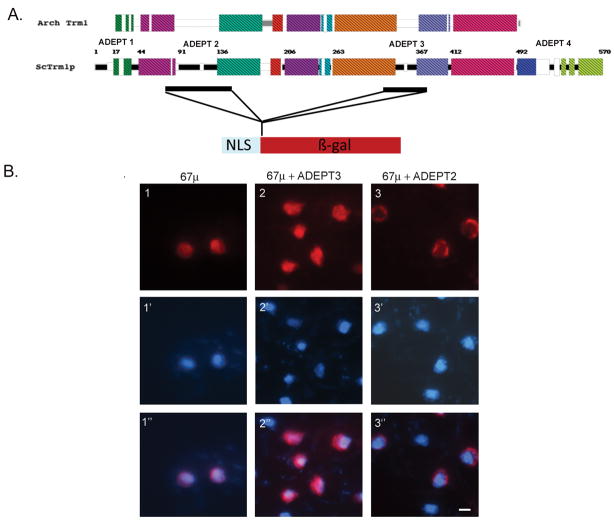
The above data show that Trm1-II is directed to the INM from the nucleoplasm. We sought to identify regions of Trm1 that function in targeting to the INM. We were initially guided in selecting regions of focus by our previous work comparing conserved prokaryotic and eukaryotic protein counterparts. These studies revealed additional domains in eukaryotic members (ADEPTs) that serve to target eukaryotic proteins to particular subcellular locations (27). Trm1 possesses four such additional domains (Fig. 3A).
Figure 3. Indirect immunofluoresence analysis of the subcellular location of H2B 1-67-Trm1-β–galactosidase fusion proteins containing various ADEPTs.
Panel A, Cartoon diagramming the experimental strategy. The top block diagram (after Stanford et al., (27) ) depicts the consensus of conserved sequences among archaea organisms. The lower block diagram is S. cerevisiae Trm1. Color blocks represent uninterrupted sequence similarity of at least 35% between archaea and all eukaryotic counterparts. Black lines represent regions of the proteins that are found only in eukaryotes but are not conserved between eukaryotes and the C-terminal purple and green regions represent eukaryotic additions that are conserved throughout eukaryotes. The Trm1 regions used for these studies and inserted at the BamHI site in vector pFB1-67μ are depicted as black bars below the S. cerevisiae block diagram. Trm1 sequences were inserted between the regions encoding histone H2B amino acids1-67 that include its NLS and β-galactosidase. Panel B. Immunofluorescence images. Panel 1, vector alone; panel 2, amino acids 323-375 (ADEPT 3); and panel 3, 73-151 (ADEPT 2) of Trm1. Rows 1-3: β–galactosidase viewed with Cy3. Rows 1′-3′: DNA viewed by DAPI staining. Rows 1″-3″ are overlays of 1-3 and 1′-3′, respectively. White size bar = 1μm.
The additional domains were tested for the ability to deliver nucleoplasmic reporter proteins to the INM. We generated fusion proteins containing amino acids of the relevant peptides between histone H2B sequences with an NLS to promote nuclear location and β-galactosidase (Fig. 3A). Studies were first conducted on ADEPT 3 (amino acids 323–375) and ADEPT 4 (amino acids 492–570) because prior studies demonstrated that ADEPT 1 and ADEPT 2 contain MTS and NLS information, respectively (24, 25). The locations of the fusion proteins was determined by indirect immunofluoresence using anti-β-galactosidase primary antibody (28). As anticipated, the NLS-β-galactosidase construct lacking any Trm1 information located diffusely throughout the nucleus (Fig. 3B, panel 1). Neither the fusion protein containing ADEPT 3 nor ADEPT 4 localized to the INM (Fig. 3B, panel 2 and data not shown). Although the data provide no support of the ability for these Trm1 regions to deliver a passenger to the INM, they do not eliminate INM targeting roles because the fusion constructs do not encode the Trm1-II amino-terminus (located at AUG codon #17) and our previous data showed that N-acetylation of Trm1-II and the amino-terminal sequence is required for its appropriate INM distribution (22).
As the blocks of information in ADEPTs 1 (data not shown), 3, and 4 appeared not to contain information sufficient to target a reporter protein to the INM, we tested a region containing ADEPT 2 (amino acids 73–151) for a possible role in INM targeting/tethering. The data show that this Trm1 region is sufficient for INM location because the resulting fusion protein, Trm1(73–151)-β-galactosidase, is located at the nuclear rim (Fig. 3B, panel 3). However, the nuclear rim staining is somewhat different than authentic Trm1 as it is not evenly distributed around the entire nuclear rim in all cells.
To confirm that the Trm1(73–151)-β-galactosidase fusion protein is indeed located at the nuclear membrane, we employed indirect immunofluoresence to co-localize the fusion protein and the nuclear pore protein, Nsp1, in the same cells. The fusion protein was detected with anti-β-galactosidase primary antibody. Endogenous Nsp1 was detected with anti-Nsp1 primary antibody (29). In contrast to the β-galactosidase signal that often appeared as a “half-moon” type of location, Nsp1 was located all around the INM (Fig. 4A and B). In all cells that had both β-galactosidase and Nsp1 staining the signals overlap (Fig. 4C), supporting the notion that the Trm1(73–151)-β-galactosidase fusion protein is located at the nuclear rim.
Figure 4. Trm1-β–galactosidase fusion protein locates to a region of the nuclear membrane as judged by co-location with Nsp1.
Amino acids 73-151 of Trm1 between amino acids 1-14 from histone H2B at the amino terminus and β–galactosidase at the carboxyl terminus of the protein. A. Immunofluorescence of the fusion protein; B. immunofluorescence of Nsp1 and C. an overlay of A and B. Bar = 1μm
To further analyze the role of Trm1 amino acids 73-151 in INM targeting, we moved this sequence to two additional reporter proteins: NLS-GFP [generating Trm1(73-151)-GFP] and NLS-Trm7-GFP – a cytoplasmic tRNA modification enzyme supplied with an ectopic NLS [generating NLS-(73-151)-Trm7-GFP], and viewed their distributions. Trm1(73-151)-GFP and NLS-(73-151)-Trm7-GFP located to the nuclear rim. However, their distributions at the nuclear rim differed from authentic Trm1 in that they were restricted to a small region of the nuclear periphery as determined by co-localization with nuclear pore proteins, Nsp1 and Nup49 (Fig. 5A and B; also see below).
Figure 5. Trm1 amino acids 73-151 deliver NLS-GFP and NLS-Trm7 to the INM.
A. Trm1-GFP fusion protein locates to a region of the nuclear membrane as judged by partial co-location with Nsp1. Trm1 amino acids 73-151 between amino acids 1-67 from histone H2B at the amino-terminus and two tandem GFPs at the carboxyl-terminus of the protein. Left panel: Trm1(73-151)-GFP fusion protein; middle panel: GFP signal overlaid with DAPI staining of DNA; right panel: an overlay of GFP and the nucleoporin Nsp1 as detected by anti-Nsp1 monoclonal antibody and Cy-3 goat anti-mouse secondary antibody. Bar = 1μm. B. Delivery of Trm7 with Trm1 amino acids 73-151 to a sub-region of the nuclear envelop is dependent upon the Ran pathway. Wild-type and rna1-1 mutant cells contain vectors encoding constitutively expressed Nup49-mCherry and galactose-inducible NLS-(73-151)-Trm7-GFP. Location of proteins was determined in live cells after 1 hr induction at permissive (23°C) or nonpermissive (37°C) temperatures by addition of galactose to a final concentration of 2%. Bar = 5μm.
The above data show that fusion proteins containing Trm1 amino acids 73-151 locate to the nuclear rim, but provide no information as to whether the fusion proteins are located at the INM as is endogenous Trm1 (22) or, instead, are incorrectly located on the outer surface of the nucleus. To address this, we studied the distribution of Trm1(73-151)-GFP and NLS-(73-151)-Trm7-GFP in rna1-1 cells at the nonpermissive temperature. We reasoned that if these proteins were located at the INM, newly synthesized protein should locate throughout the cytoplasm if the Ran-GTP gradient was disrupted. In contrast, if Trm1(73-151)-GFP and NLS-(73-151)-Trm7-GFP were inappropriately adhered to the outer nuclear membrane or at the ER, their locations should not be dependent upon the Ran gradient. As predicted, newly synthesized Trm1(73-151)-GFP (not shown) and NLS-(73-151)-Trm7-GFP (Fig. 5B) are largely cytoplasmic in rna1-1 cells at the nonpermissive temperature; the data support the hypothesis that fusion proteins containing Trm1-II amino acids 73-151 are delivered to the INM, rather than the outer nuclear membrane.
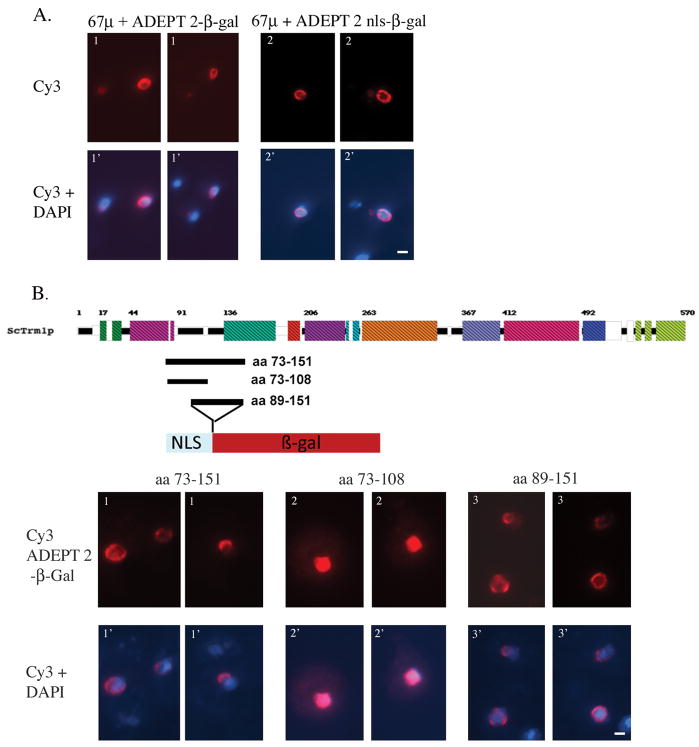
Analysis of the locations of reporter proteins in Figs. 3–5 were carried out with cells that encode endogenous wild-type TRM1. Since affinity capture-MS studies have shown that Trm1 can self-associate (30), Trm1(73-151)-β-galactosidase, Trm1(73-151)-GFP, and NLS-(73-151)-Trm7-GFP could potentially be located at the INM via an interaction between Trm1 amino acids 73-151 with wild-type Trm1 preexisting at the INM, rather than by this block possessing INM targeting information. To learn whether endogenous Trm1 in wild-type cells is required for locating newly synthesized fusion proteins to the INM through protein-protein interactions, we analyzed the location of Trm1(73-151)-β-galactosidase in cells possessing a TRM1 deletion. The location of the fusion protein is identical in TRM1 and trm1Δ cells (Supplemental figure 1), supporting the conclusion that Trm1 amino acids 73-151 alone have INM targeting/tethering information.
To determine whether INM localization requires the Trm1 NLS originally identified by Rose et al (24), we altered this sequence in vector pFB1-67μ that contains the histone H2B ectopic NLS in addition to the wild-type or mutant NLS in Trm1 (Fig. 6A). There was no difference in the subcellular location of NLS-ADEPT 2-β-galactosidase whether it contained the wild-type 95KKSKKKR101 NLS or the mutant 95EESEEER101 sequence previously demonstrated to disrupt nuclear location of Trm1 (24). Thus, the wild-type endogenous Trm1 NLS is not required for location to the INM. To determine whether the information for INM targeting/tethering is located amino-terminal or carboxyl-terminal to the NLS, we created two constructs, one encoding amino acids 73-108 and the other amino acids 89-151. The results obtained with these constructs demonstrate that amino acids 73-108 do not contain information to target β–galactosidase to the INM while amino acids 89-151 do (Fig. 6B).
Figure 6. Mapping targeting information sufficient for INM location.
A. The Trm1 NLS is not necessary for INM localization of fusion proteins. Indirect immunofluorescence location of proteins consisting of H2B 1-67 amino acids (including the NLS), Trm1 amino acids 73-151, and β–galactosidase. Panel 1, wild-type; panel 2, mutant Trm1 NLS. Top row: Cy3 staining forβ–galactosidase. Bottom row: overlay with DAPI staining. B. Information sufficient for INM location locates to amino acids 89-151. Diagram above immunofluroescence data shows the experimental strategy. The block diagram is S. cerevisiae Trm1. The Trm1 regions used and inserted at the BamH1 site in vector pFB1-67μ are depicted as black bars below the S. cerevisiae block diagram. Trm1 sequences were inserted between the regions encoding histone H2B amino acids1-67 that include its NLS andβ-galactosidase. Immunofluorescence data: Top row: panel 1, fusion proteins 1-67 amino acids of H2B, Trm1 amino acids 73-151; panel 2, Trm1 amino acids 73-108; panel 3, Trm1 amino acids 89-151. White size bars = 1μm
Trm1 sequences necessary for INM targeting/tethering
We investigated whether the region in Trm1 sufficient to target reporter proteins to the INM is necessary for authentic Trm1 to achieve its appropriate subnuclear location at the INM using a variety of genetic strategies. First we generated TRM1 internal deletions. The studies described above showed that Trm1 amino acids 89-151 are capable of addressing passenger proteins to the INM and that the NLS located between amino acids 95-101 is not part of this targeting information. To determine which other amino acids within this region are necessary for INM location for Trm1-II, we generated a Trm1-II-GFP construct that deleted all of these amino acids except for the NLS. The resulting Trm1-II-GFP missing amino acids 72–95 and 102-151 failed to appropriately locate to the nucleus despite the presence of the endogenous NLS (data not shown).
To address the possibility that the mutant Trm1-II protein failed to locate to the nucleus because the endogenous NLS was not appropriately exposed, four additional mutants versions were generated. Construct pGP54a-GFP/Trm1Δ72-93 is missing a region amino-terminal to the NLS while pGP54a-GFP/Trm1Δ101-151 is missing information carboxyl-terminal to the NLS. Construct pGP54a-GFP/Trm1Δ 72-151 removes the entire region and pGP54a-GFP/Trm1Δ 72-151 + NLS also removes the entire region but contains an additional NLS at the end of Trm1 before the GFP sequence. Each construct gave the same result. The mutant proteins failed to locate to the nucleus, but instead they located to mitochondria as assessed by co-location with the mitochondrial outer membrane protein, Tom20-mCherry (Fig. 7B–E). The data provide further support for competition between MTS and NLS information in Trm1-II. The results also indicate that for a protein with both nuclear and mitochondrial targeting information, the context of the NLS is important for nuclear import.
Figure 7. Subcellular location of Trm1-II-GFP with various ADEPT 2 deletions.
Top rows: Trm1-II-GFP location in live cells. Middle rows: the locations of mitochondria as assessed by the fluorescence tagged Tom20-mCherry. Bottom rows: overlay of GFP and mCherry. A, wild-type Trm1-II-GFP; B, deletion of amino acids 72-93; C, deletion of amino acids 101-151; D, deletion of amino acids 72-151; and E, deletion of amino acids 72-151 in a construct containing an additional NLS located at the end of the Trm1 coding sequence before the GFP. Bar = 5μm.
We employed in vitro mutagenesis to determine which amino acids within the region sufficient for INM location are necessary to achieve this subnuclear distribution. The DNA region corresponding to codons 89-151 was amplified by mutagenic PCR to generate a library of mutant sequences. The library was then used as a mega primer to introduce the mutations into wild-type TRM1 in plasmid pGP54a-Trm1-II-GFP, generating a library of full length Trm1-II with alterations of amino acids 89-151. The location of encoded mutant Trm1-II-GFP was determined by epifluoresence after induction by addition of galactose. DNA sequencing of randomly chosen candidates demonstrated more than half possessed single or multiple codon changes and that the alterations were distributed throughout the region subjected to PCR mutagenesis (Fig. 8A).
Figure 8. Mutations that do or do not alter Trm1 INM location.
A. Diagram of the mutations analyzed. Green box indicates the area of Trm1 in eukaryotic but not Archael counterparts; magenta box indicates sequences highly conserved in all Trm1 proteins. The region that includes the green and magenta boxes defines the sequence subjected to mutagenesis. Mutations that alter the location of Trm1-II-GFP are shown in colors above the wild-type sequence and those that do not alter the location are shown below. Orange and blue amino acids represent a triple and a double alteration, respectively, which affect Trm1-II-GFP INM location. Magenta and green single amino acid changes above the sequence were generated by site-directed mutagenesis, while the brown alteration was generated by error-prone PCR. Black amino acid changes below the sequence were generated by error-prone PCR; cyan changes below the sequence were generated by site-directed mutagenesis. B. Sample data for error-prone and site-directed mutations: 1, wild-type Trm1-II-GFP; 2, Trm1-II-GFP bearing a triple mutation is mislocated to the nucleoplasm; 3, mutation of A147 to D eliminates Trm1-II-GFP association with the INM; 4, mutation of a highly conserved amino acid (R149 to A) does not affect Trm1-II-GFP subnuclear location; 5, mutation of A151 to D causes mislocation of Trm1-II-GFP to the nucleoplasm. 1, 2, 3, 4, 5: location of GFP signal; 1′, 2′, 3′, 4′, 5′: DAPI staining of DNA; 1″, 2″, 3″, 4″, 5″: overlay of GFP and DAPI signals. Bar = 5μm.
Of the ~250 yeast candidates analyzed most encoded proteins with normal INM location. Sequence analyses showed that alterations of individual and multiple amino acids within the Trm1 region absent from Archael counterparts (Fig. 8A, green colored block) have no effect upon the INM location of Trm1-II (Fig. 8A; see changed amino acids indicated below the Trm1 sequence). We did identify three plasmids that demonstrate altered Trm1-II-GFP subnuclear distribution from INM location to nucleoplasmic (Fig. 8; see brown, orange, and blue amino acid changes above the Trm1 sequence). One contained a single alteration of the last codon (151) of the region subjected to mutagenesis, changing A to D (Fig. 8A, brown amino acid; 8B, panel 5). Another plasmid had multiple changes of amino acids 134, 141, and 142 from I, S, A to K, Q, T, respectively (Fig. 8A, orange amino acids; 8B, panel 2). As we also identified a plasmid that generated normally distributed Trm1 and possessed an individual mutation of I134 to K134 (Fig. 8A), single alteration of position 134 was eliminated as causing nucleoplasmic Trm1 distribution. Subsequent directed mutagenesis of S141 to Q141 or A142 to T142 also resulted in proteins that locate at the INM (data not shown). The data indicate that I134, S141, A142 are not individually necessary for INM location, but that some combination of these amino acids is necessary. The third plasmid encoding a nucleoplasmic Trm1 possessed two codon changes at 133 and 136 from Y and I to K and S (Fig. 8A, blue amino acids above the Trm1 sequence). Site-directed mutagenesis to individually alter these showed that mutation of I136S alone causes Trm1 to become nucleoplasmic and mutation of Y133K had no affect on Trm1 distribution (Fig. 8A, blue amino acid above and cyan amino acid below the Trm1 sequence).
Interestingly, the mutations that caused Trm1 to mislocalize to the nucleoplasm rather than to be appropriately located to the INM all clustered to the extreme carboxyl-terminus of the region subjected to mutagenic PCR (Fig. 8A). As the mutagenic PCR method employed did not result in the alteration of many amino acids in this region, we employed site-directed mutagenesis to obtain additional changes. The results showed that alteration of L145 to S145, I148 to S148, and R149 to A149 do not affect the location of Trm1-II-GFP (Fig. 8 cyan amino acids below Trm1 sequence; 8B, panel 4). In contrast, alteration of R146 to A146 and A147 to D147 each cause Trm1-II-GFP to become nucleoplasmic (Fig. 8, green and magenta colored amino acids above Trm1 sequence; Fig. 8B, panel 3). All single and multiple amino acid changes that alter Trm1-II subnuclear distribution fall into a region of Trm1 that is highly conserved between Archael and eukaryotic counterparts (27) (Fig. 8A, magenta block of amino acids). Not surprisingly, each of the mutations that mapped in the region conserved between Archael and eukaryotic Trm1 proteins lacked tRNA methyltransferase activity (Supplementary Fig. 2). However, it is unlikely that there is a strict correlation between activity and INM location because alterations of completely conserved amino acids in this region that cause loss of enzyme activity (e.g., R149A) have no effect on Trm1 INM location (Figs. 8A and B, panel 4, and S2). In sum, the data show that a block of amino acids shared between Archael and eukaryotic Trm1 proteins is a major determinant in INM targeting/tethering.
The region subjected to PCR mutagenesis included the Trm1 NLS, 95KKSKKKR101. We obtained single amino acid changes of this motif (95IKSKKKR101, 95KKTKKKR101, 95KKSEKKR101 95KKSKMKR101, and 95KKSKKRR101; Fig. 8A) without affecting nuclear import, even though mutation to 95EESEEER101 destroyed NLS activity (24). We also learned that I134, S141, A142 are not individually necessary for INM location, but that some combination of these amino acid is necessary. Thus, our approach to map the region of Trm1-II required for INM location could have missed blocks of the sequence in which no particular single amino acids are required.
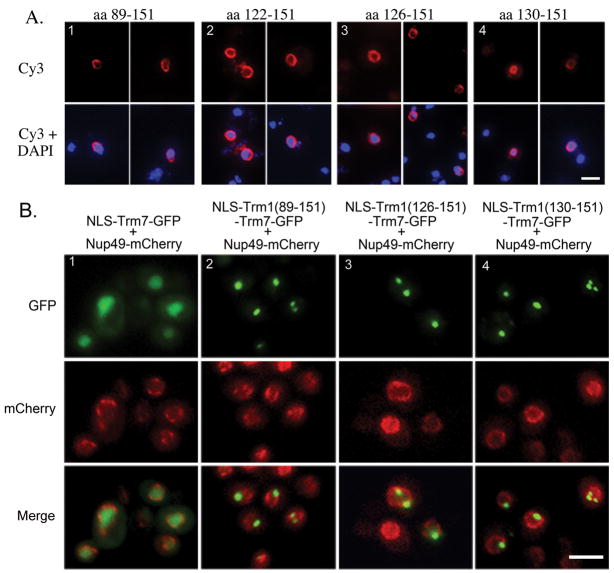
To address the possibility that blocks of amino acids important for INM targeting were missed by alteration of single amino acids we generated a series of overlapping deletions in Trm1-II-GFP throughout the region sufficient to target a reporter to the INM, each missing 5 amino acids. We were unable to assess the consequences of deletions between amino acids 102 and 124 because the altered proteins did not achieve nuclear location but rather accumulated as “spots” in the cytoplasm (Supplementary figure 3). Part of the pools of proteins missing amino acids 122–126, 124–128, or 126–130 also accumulated as spots in the cytoplasm, but for each of these constructs, part of the pool was correctly located at the INM (Fig. S3). Trm1 missing amino acids 128–132 was located at the INM like wild-type (Fig. S3). In contrast, deletion of amino acids 130–134 or 131–135 resulted in small nucleoplasmic pools of Trm1-II-GFP (Fig. S3). The deletion data in combination with the single amino acid mutations place the Trm1 INM information between amino acids 133 and 151. The data eliminate most of ADEPT 2 in targeting Trm1 to the INM and identify a stretch of <20 amino acids, located primarily in a region shared by Archael and eukaryotic Trm1 proteins (Fig. 8A, magenta block), as being necessary for targeting/tethering Trm1 to the INM.
To address whether the newly defined region necessary for Trm1 INM location is also sufficient for this subnuclear location, we generated additional β-galactosidase, GFP, and Trm7 fusion proteins containing short peptides from Trm1. Significantly, fusion proteins containing Trm1 amino acids 122–151, 126–151 and 130–151 cause the redistribution of β–galactosidase from nucleoplasmic to location at the INM (Fig. 9A). Unlike, other fusion proteins containing large regions of ADEPT 2 (Fig. 9A, panel 1) in addition to the region necessary for INM location, the fusion proteins that contain these smaller regions (Fig. 9A, panels, 2–4) have a rather even distribution around the INM, as if the extra ADEPT 2 sequences interfered with β-galactosidase being evenly distributed at the INM. These same short Trm1 regions also deliver NLS-GFP (data not shown) and NLS-Trm7 (Fig. 9B) to the INM. Thus, a short peptide within Trm1 is both necessary and sufficient for the peripheral association of proteins to the inner nuclear membrane.
Figure 9. Fine mapping of the Trm1 region sufficient to target β-galactosidase and NLS-Trm7-GFP to the INM.
A. β-galactosidase studies: Trm1 amino acids 89-151 (1), 122-151 (2), 126-151 (3), and 130-151 (4) were inserted in between H2B acids 1-67 and β–galactosidase and the location was determined by indirect immunofluorescence. Top: Cy3 β–galactosidase staining; bottom: overlay of Cy3 and DAPI staining. Bar = 5μm. B. NLS-Trm7-GFP location in live cells. Cells were transformed with a vector encoding the mCherry-tagged nuclear pore protein, Nup49 and various versions of NLS-Trm7-GFP. NLS-Trm7-GFP is nucleoplasmic in the absence of Trm1 sequences. Insertions of Trm1 amino acids 89-151 (2), 126-151 (3), and 130-151 (4) between H2B acids 1-67 and Trm7-GFP redistribute Trm7 from the nucleoplasm to a sub-region of the nuclear envelope. Top row: Trm7-GFP, middle row: Nup49-mCherry; bottom row, merged images of GFP and mCherry signals. Bar = 5μm.
Discussion
We previously demonstrated that Trm1 possesses amino-terminal mitochondrial targeting information as well as a canonical basic NLS (24, 25). Two of the four ADEPTs identified by comparing eukaryotic and Archael m22G dimethyltransferases contain these sequences. In the work reported here, we tested the hypothesis that the other ADEPTs might play a role in INM localization and found that neither of the two remaining additions possesses information sufficient to target passenger proteins to the INM. Rather, we mapped INM information to a region shared among all Trm1 proteins. Thus, although the ADEPT hypothesis which tenets that conserved proteins will have cell biological information encoded in domains found in eukaryotic proteins and missing in the prokaryotic counterparts, has proven useful for locating sorting information for several proteins (27), it failed to correctly predict the domain that targets/tethers Trm1-II to its correct subnuclear location at the INM.
Competition between nuclear and mitochondrial targeting information
Although our goal was to learn how a soluble protein can be targeted to the INM, unexpectedly, data obtained in this study also provide information concerning the distribution of Trm1 between nuclei and mitochondria. The relative distribution of the Trm1 long form, Trm1-I, and short form, Trm1-II, between the nucleus and mitochondria is different because Trm1-I is more efficiently imported into mitochondria (25). In fact, it appears as if Trm1-I is exclusively mitochondrial (19) even though it contains an NLS. We observed here that we could also turn Trm1-II into a mitochondrial protein either by altering the Ran pathway or by generating mutations in the ADEPT 2 region that contains the NLS. This was true even if the NLS itself is not changed or if an ectopic NLS is provided at the carboxyl-terminus of the protein. It is difficult to see how such mutations could increase the efficiency of mitochondrial import per se, but they could change the conformation of the protein and in that way prevent nuclear import.
Targeting of peripheral proteins to the INM
Our studies support the model that Trm1 achieves its INM location in at least three steps: (1) translation on free polysomes, (2) Ran-dependent nuclear import through nuclear pore complexes to the nucleoplasm via the NLS; (3) redistribution from the nucleoplasm to the INM via the INM targeting/tethering motif we describe here. Some reporter proteins, like β-galactosidase, harboring the Trm1 INM targeting/tethering motif distribute throughout the INM, whereas others distribute to one or a few spots on the INM. A possible explanation is that the INM motif directs proteins to a particular subdomain of the INM and that distribution throughout the INM requires a 4th step of unknown mechanism. If the transition between step 1 and 2 is inhibited, Trm1-II is directed to mitochondria. All three steps to locate Trm1-II to the INM differ from the steps which deliver/tether integral membrane proteins to the INM (Review: (7)), but resembles the proposed steps for the association of lamins to the INM in metazoans. However, C-terminal isoprenylation or N-terminal myristolyation are necessary for lamin’s distribution to the INM, but not for Trm1-II’s INM location. Rather, Trm1 possesses a topogenic short peptide that specifies INM targeting/tethering. We believe our studies characterizing the short sequence that is necessary and sufficient to target/tether Trm1-II to the INM provides the first description of such a motif. Moreover, we learned that the Ran pathway is required for nuclear import of Trm1-II and that retention of Trm1-II at the INM is Ran-independent. Finally, our studies show that the mechanism(s) that assures wide distribution of nuclear pores around the NE is different than the mechanism by which Trm1-II associates with the INM as “capping” of nuclear pores in the nup133Δ mutant is not accompanied by “capping” of Trm1-II in the majority of cells.
In all eukaryotes the nuclear envelope possesses numerous resident peripheral proteins, including those at specific domains like the nuclear pore proteins and those evenly distributed around the envelope, like lamins in higher eukaryotes and Esc1 in yeast; yet other proteins only transiently associate with the INM (Reviews: (7, 8, 31)). We attempted to identify other yeast proteins distributed evenly about the INM (12) that are predicted not to contain transmembrane regions (and hence likely to be peripherally associated with the INM as is Trm1 (21)) to learn whether these proteins contain cis-acting sequences that match the INM targeting/tethering motif described here. BLAST or Patch-Match searches did not uncover such proteins. However, further amino acid substitutions in the region of interest will be required before the Trm1 INM motif can be uniquely delineated. In any case, like for NLSs (simple basic, bipartite basic, basic and hydrophobic, PY-NLS, etc. (Review: (32–34)) and NESs (leucine rich, glycine rich, phosphorylated region; (35–38); (Review: (38)), it is probable that there is more than one motif that targets peripheral nuclear proteins to the INM and that these motifs may vary depending on which interactions, with which partners, serve to locate them to this subnuclear domain.
Why does Trm1-II require both amino-terminal acetylation (22) and the INM targeting/tethering motif described here to achieve its INM location? Perhaps, the INM domain is sufficient for targeting passenger proteins to the INM, but in the absence of N-acetylation, some portion of this domain is not exposed and unable to interact with its partner at the INM. Indeed, results of homology modeling based upon the crystal structure of Pyrococcus horikoshii Trm1 using SWISS-MODEL suggest that while the yeast Trm1 NLS is predicted to be on the surface (Supplementary figure 4, red amino acids), the INM motif is not (Fig. S4, magenta amino acids). Thus, it is possible that N-acetylation alters the conformation of Trm1-II to expose the INM targeting/tethering domain. This would be consistent with documented roles for N-acetylation in protein structure and protein-protein interactions (Review: (39)).
We do not have good candidates for partners of Trm1 that are also at the INM and, so far, no candidates have been identified in proteome-wide partner screens; nor have our multiple attempts via two–hybrid procedures or co-purification led to the identification of a protein tether (unpublished data). Our inability to identify the tether by these methods lead us to propose that the partner may be an integral protein, difficult to identify by the techniques employed, or it may be a different macromolecule, such as a lipid. The partner(s) is unlikely to be in limiting quantities because over-expressed Trm1-II locates to the INM as effectively as endogenously expressed Trm1 ((22); Stauffer and Hopper, data not shown). Continued analyses of Trm1 INM mutants will help define key determinants of at least one INM targeting/tethering domain and will serve as a tool to identify macromolecules that interact with such elements.
Materials and Methods
Yeast strains and methods
BY4741 (MATa his3Δ leu2Δ met15Δ ura3Δ; Open Biosystems, Huntsville, AL; (40)) was used for most experiments. The trm1Δ strain with a Kanr replacement for TRM1 was derived from BY4741 and obtained from the yeast deletion collection (Open Biosystems, Huntsville, AL; (40)). The yeast strain with the rna1-1 allele was derived from BY4741 by gene replacement and was a gift from Dr. Charles Boone. Transformation was carried out as described in Chen et al. (41). Cultures were incubated at 42°C or 45°C for 30 min, plated on appropriate media and grown for 2–3 days.
Plasmid constructions and DNA manipulations
All constructs described in these studies were confirmed by DNA sequencing. Plasmid constructions were propagated using Eschericia coli strain DH5α. Pfu Ultra High Fidelity DNA polymerase (Stratagene) was used to generate TRM1 PCR fragments for cloning. Blunt end PCR products were ligated into either the TOPO Blunt vector (Invitrogen) or pGEM-T vector (Promega) following the manufacturer’s instructions. Plasmid pGP54a-GFP was derived from pGP54a, a pRS416-based plasmid containing the GAL1-GAL10 regulatory region (22), by insertion of a PCR amplified GFP sequence into the HindIII XhoI polylinker sites. This plasmid encodes galactose-inducible Trm1-II-GFP.
Trm1-β-galactosidase fusion proteins – To identify Trm1 regions sufficient for INM location, we fused portions of Trm1 toβ–galactosidase. ADEPT 1 constructs contain Trm1 amino acids 17–48, ADEPT 2 constructs contain amino acids 73-151, ADEPT 3 construct contain amino acids 323–375, and ADEPT4 constructs contain amino acids 492–570. Each amplified sequence was cloned into multi-copy vectors in-frame at the BamHI site behind the histone H2B promoter and either amino acids 1–14 (pFB1–7μ; (42)) or amino acids 1-67 (pFB1-67μ; (42)). After learning that sequences in the ADEPT 2 carboxyl-terminal region and continuing into the Trm1 conserved region were important for INM location, we generated additional constructs that contain Trm1 amino acids 122-151, 126-151, and 130-151 inserted in vector pFB1-67μ.
Fluorescent tagged protein fusions - Trm1(73-151)-GFP was derived from vector pIGoutA (43). pIGoutA/Trm1(73-151)-GFP contains a galactose-inducible gene fusion of histone H2B amino acids 1-67 with an NLS, Trm1 amino acids 73-151, and an in-frame fusion of two tandem GFP proteins. To generate an NLS-Trm7-GFP tagged with the same region of Trm1 (73-151), the Trm7 ORF amplified with terminal EcoR1 sites was cloned into the EcoR1 site of Trm1(73-151)-GFP. This generated vector NLS-(73-151)-Trm7-GFP which encodes a version of Trm7 with amino-terminal NLS and the Trm1 NLS INM targeting domain preceding the TRM7 ORF and a C-terminal tandem GFP following the TRM7 ORF. The protein encoded by this construct maintains catalytic activity (Murthi et al., in prep.). Additional constructs that contain Trm1 amino acids 89-151, 126-151, and 130-151 amino-terminal to Trm7-GFP were generated in a similar fashion. To generate Nup49-mCherry and Tom20-mCherry we employed a systematic collection of the Saccharomyces cerevisiae genome in a high-copy vector (44) to amplify the open reading frames of TOM20, and NUP49. The PCR products were ligated into pGEM-T vector to form pGEM-T/Tom20 and pGEM-T/Nup49. pGEM-T/Tom20 and pGEM-T/Nup49 were digested with NheI and AatII and the linear TOM20 and NUP49 DNAs were cloned into a plasmid which contains the constitutive ADH2 promoter and the mCherry reporter, generating pTPL2 and pTPL3, respectively.
Trm1-GFP deletions – The constructs were cloned into pGP54a-GFP that expresses Trm1-II-GFP under the control of the GAL1-10 promotor. PCR products were purified and phosphoylated using polynucleotide kinase, followed by ligation and transfomation into E. coli. The plasmids were digested with EcoRI and HindIII and the linear mutant TRM1 DNAs were ligated into pGP54a-GFP vector to form pGP54a-GFP/Trm1Δ72-93, pGP54a-GFP/Trm1Δ101-151, and pGP54a-GFP/Trm1Δ72-151 plasmids. To generate plasmid pGEM-T/Trm1Δ72-151+NLS, we used with pGEM-T/Trm1Δ72-151 as a template and a 3′ primer containing sequences complementary to the Trm1 NLS. PCR products were ligated into pGEM-T vector to generate pGEM-T/Trm1Δ72-151+NLS and the Trm1Δ72-151+NLS sequence was ligated into vector pGP54a-GFP between EcoRI and HindIII sites creating pGP54a-GFP/Trm1Δ72-151+NLS.
For fine mapping of Trm1 sequences necessary for INM targeting/tethering, we employed a series of constructs, each missing 5 amino acids of Trm1 sequences from 102-151. Each construct was generated by ligation of two fragments generated by PCR amplification using pGEM-T/Trm1-II as the template and Pfu Ultra High Fidelity DNA polymerase (Stratagene). The first fragment for each construct was generated by using a T7 primer and the appropriate TRM1 primer. The second fragments were generated using a SP6 primer and appropriate downstream primers for each construct. The first and second fragments were digested with EcoRI and HindIII to remove the sequences from pGEM-T vector and the resulting fragments were treated with polynucleotide kinase and then ligated into pGP54a-GFP.
Mutagenic PCR and site-directed mutations – We employed error prone mutagenesis using a GeneMorph® II EZClone Domain Mutagenesis Kit (Stratagene) to generate random mutations of Trm1 amino acids 89-151. Error-prone PCR was performed under following conditions: 1 cycle, 3 min at 94•; 30 cycles, 30 sec at 94•, 30 sec at 61•, 1min at 72•; 1 cycle, 5 min at 72• using pGP54a-Trm1-II-GFP as the template. This created a library that contained 1–5 nucleotide mutations in the region encoding amino acids 89-151. The library was then used as a mega primer to introduce the mutations into wild-type TRM1 in plasmid pGP54a-Trm1-II-GFP following the manufacturer’s instructions except adding an extra 1 μl Pfu Ultra High Fidelity DNA polymerase (Stratagene) into 50 μl EZClone reaction mix. After treatment with Dpn1 to destroy plasmids not subjected to PCR, the plasmid library was transformed into E. coli. To generate site-directed mutations we used the same procedures as were used to generate the constructs with 5 amino acid deletions.
Live cell imaging and microscopy
In vivo localization of various forms of Trm1-II-GFP was assessed using the FITC channel of either a Nikon Microphot-FX microscope and captured using a Sensys charge-coupled device camera (Photometrics, Tucson AZ) and QED software (QED Imaging, Pittsburgh) or a Nikon 90i microscope using a CoolSNAP HQ2 camera and MetaMorph software. Nucleic acid containing organelles were located by staining cells with DAPI. Image processing was performed Adobe photoshop.
Indirect immunofluoresence
Indirect immunofluoresence was carried out as described by Pringle et al. (45) with the modification previously described by Hopper et al. (28). Primary antibodies were affinity-purified rabbit antiβ-galactosidase (28), used at 1:400 or 1:1000 dilutions, mouse monoclonal anti-GFP (Roche), used at 1:250 or 1:500 dilutions, and mouse monoclonal anti-Nsp1p (29), used at 1:10,000 dilution. Secondary antibodies were Cy3-conjugated goat anti-rabbit IgG, Cy3-conjugated goat anti-mouse IgG, FITC-conjugated goat anti-rabbit IgG, and FITC-conjugated goat anti-mouse IgG, each diluted 1:400.
m22G methyltransferase activity assay
The assay followed the procedures described by Ellis et al. (46) with the following changes: trm1Δ cells were transformed with the relevant pGP54a-Trm1-II-GFP plasmids encoding wild-type Trm1-II or mutant Trm1-II proteins with single substitutions of amino acids 131-151. The cells were grown to early log phase in complete minus uracil media with raffinose as the carbon source. Trm1-II synthesis was induced for two hr by the addition of galactose. Crude extracts from 15 ml of each culture were obtained after cell disruption employing vortexing (5 times, 15 sec at 4°C) with an equal volume of glass beads. The reaction, containing 3H, S-adenosylmethionine to track nucleoside methylation, was started by the addition of small RNA containing m22G26-deficient tRNA isolated from trm1Δ cells; the reaction products were precipitated using 10% TCA, collected on glass fiber filters, and the incorporation of 3H, S-adenosylmethionine into tRNA determined.
Structure modeling of S. cerevisiae Trm1-II
We used SWISS-MODEL in the alignment mode after ClustalW alignment of Trm1 and 2Dul (http://www.pdb.org/pdb/explore.do?structureId=2YTZ; Ihsanawati et al., to be published) and viewed the results with PyMol (47–51) to create Supplementary figure 4.
Supplementary Material
Endogenous Trm1 is not required for INM location of Trm1(73-151)-β–galactosidase. Top row: indirect immunofluorescence using wild-type (A) or trm1Δ (B) cells transformed with a gene encoding a fusion protein consisting of 1-14 amino acids of histone H2B, Trm1 amino acids 73-151, andβ–galactosidase. Top row: location of Trm1(73-151)-β–galactosidase Bottom row: overlay of DNA with DAPI staining with Cy3 detection of β–galactosidase. Bar = 1μm.
m22G metyltransferase activity of wild-type Trm1 and mutant versions with single amino acid substitutions. A trm1Δ strain contained plasmids encoding either wild-type (pGP54a-TRM1-II-GFP) or mutant versions generated by error-prone or site-directed mutagenesis. Extracts were generated and the assay conducted as described in the methods. Activities were measured at 0 (blue), 45 (magenta), and 90 (beige) min and the assays were repeated at least twice. Note that all mutations of the Trm1 region 133-151 destroy activity whether the mutations have no affect on INM location (R149A) or whether they cause Trm1 to fail to locate to the INM (I136S, R146A, A147D, A151D).
Intranuclear location of galactose-inducible Trm1-II-GFP mutants containing an overlapping series of 5 amino acid deletions. Data from each of seven mutant constructs missing Trm1-II-GFP amino acids as indicated. Top row: location of Trm1-II-GFP mutant proteins; middle row: the same cells showing DAPI staining of DNA; bottom row; overlay of cells in the top and middle rows. Bar = 5μm.
Predicted structure of S. cerevisiae Trm1 and location of the INM targeting/tethering motif. The same color scheme was used as for Fig. 8A. Magenta: sequence that is sufficient and necessary for Trm1 targeting/tethering to the INM; Green: ADEPT 2: Red: NLS motif within Trm1 ADEPT 2. All other Trm1 regions are colored blue. Note that the proposed structure for the green and red regions is not based on crystallography data because these regions are missing from Pyrococcus horikoshii Trm1. Also, the amino terminus of yeast Trm1-II is not modeled as the homology starts at amino acid 44 of S. cerevisiae Trm1.
Acknowledgments
This work was supported by an NIH grant (GM27930) to AKH and by NSF collaborative grants to NCM and AKH. We thank Charles Boone for the rna1-1 strain and Greetchen Diaz for stimulating conversations. We also thank Nripesh Dhungel and Juan Alfonzo for comments on the manuscript.
References
- 1.Lamond AI, Sleeman JE. Nuclear substructure and dynamics. Curr Biol. 2003;13(21):R825–828. doi: 10.1016/j.cub.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4(3):e1000039. doi: 10.1371/journal.pgen.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CR, Silver PA. Transcriptional regulation at the nuclear pore complex. Curr Opin Genet Dev. 2007;17(2):100–106. doi: 10.1016/j.gde.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313(10):2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parnaik VK. Role of nuclear lamins in nuclear organization, cellular signaling, and inherited diseases. Int Rev Cell Mol Biol. 2008;266:157–206. doi: 10.1016/S1937-6448(07)66004-3. [DOI] [PubMed] [Google Scholar]
- 6.Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995;130(1):15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lusk CP, Blobel G, King MC. Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol. 2007;8(5):414–420. doi: 10.1038/nrm2165. [DOI] [PubMed] [Google Scholar]
- 8.Schirmer EC, Gerace L. The nuclear membrane proteome: extending the envelope. Trends Biochem Sci. 2005;30(10):551–558. doi: 10.1016/j.tibs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.King MC, Lusk CP, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 2006;442(7106):1003–1007. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 10.Ohba T, Schirmer EC, Nishimoto T, Gerace L. Energy- and temperature-dependent transport of integral proteins to the inner nuclear membrane via the nuclear pore. J Cell Biol. 2004;167(6):1051–1062. doi: 10.1083/jcb.200409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuleger N, Korfali N, Schirmer EC. Inner nuclear membrane protein transport is mediated by multiple mechanisms. Biochem Soc Trans. 2008;36(Pt 6):1373–1377. doi: 10.1042/BST0361373. [DOI] [PubMed] [Google Scholar]
- 12.Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425(6959):686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 13.Goldman AE, Moir RD, Montag-Lowy M, Stewart M, Goldman RD. Pathway of incorporation of microinjected lamin A into the nuclear envelope. J Cell Biol. 1992;119(4):725–735. doi: 10.1083/jcb.119.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewinger L, McKeon F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. Embo J. 1988;7(8):2301–2309. doi: 10.1002/j.1460-2075.1988.tb03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtz D, Tanaka RA, Hartwig J, McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989;59(6):969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- 16.Alsheimer M, von Glasenapp E, Schnolzer M, Heid H, Benavente R. Meiotic lamin C2: the unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association. Proc Natl Acad Sci U S A. 2000;97(24):13120–13125. doi: 10.1073/pnas.240466597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firmbach-Kraft I, Stick R. The role of CaaX-dependent modifications in membrane association of Xenopus nuclear lamin B3 during meiosis and the fate of B3 in transfected mitotic cells. J Cell Biol. 1993;123(6 Pt 2):1661–1670. doi: 10.1083/jcb.123.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa K, Fritze CE, Gerace L. The major nuclear envelope targeting domain of LAP2 coincides with its lamin binding region but is distinct from its chromatin interaction domain. J Biol Chem. 1998;273(7):4213–4219. doi: 10.1074/jbc.273.7.4213. [DOI] [PubMed] [Google Scholar]
- 19.Ellis SR, Hopper AK, Martin NC. Amino-terminal extension generated from an upstream AUG codon is not required for mitochondrial import of yeast N2, N2-dimethylguanosine-specific tRNA methyltransferase. Proc Natl Acad Sci U S A. 1987;84(15):5172–5176. doi: 10.1073/pnas.84.15.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin NC, Hopper AK. How single genes provide tRNA processing enzymes to mitochondria, nuclei and the cytosol. Biochimie. 1994;76(12):1161–1167. doi: 10.1016/0300-9084(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 21.Rose AM, Belford HG, Shen WC, Greer CL, Hopper AK, Martin NC. Location of N2, N2-dimethylguanosine-specific tRNA methyltransferase. Biochimie. 1995;77(1–2):45–53. doi: 10.1016/0300-9084(96)88103-x. [DOI] [PubMed] [Google Scholar]
- 22.Murthi A, Hopper AK. Genome-wide screen for inner nuclear membrane protein targeting in Saccharomyces cerevisiae: roles for N-acetylation and an integral membrane protein. Genetics. 2005;170(4):1553–1560. doi: 10.1534/genetics.105.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbett AH, Koepp DM, Schlenstedt G, Lee MS, Hopper AK, Silver PA. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995;130(5):1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose AM, Joyce PB, Hopper AK, Martin NC. Separate information required for nuclear and subnuclear localization: additional complexity in localizing an enzyme shared by mitochondria and nuclei. Mol Cell Biol. 1992;12(12):5652–5658. doi: 10.1128/mcb.12.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis SR, Hopper AK, Martin NC. Amino-terminal extension generated from an upstream AUG codon increases the efficiency of mitochondrial import of yeast N2, N2-dimethylguanosine-specific tRNA methyltransferases. Mol Cell Biol. 1989;9(4):1611–1620. doi: 10.1128/mcb.9.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belgareh N, Doye V. Dynamics of nuclear pore distribution in nucleoporin mutant yeast cells. J Cell Biol. 1997;136(4):747–759. doi: 10.1083/jcb.136.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanford DR, Martin NC, Hopper AK. ADEPTs: information necessary for subcellular distribution of eukaryotic sorting isozymes resides in domains missing from eubacterial and archaeal counterparts. Nucleic Acids Res. 2000;28(2):383–392. doi: 10.1093/nar/28.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopper AK, Traglia HM, Dunst RW. The yeast RNA1 gene product necessary for RNA processing is located in the cytosol and apparently excluded from the nucleus. J Cell Biol. 1990;111(2):309–321. doi: 10.1083/jcb.111.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolerico LH, Benko AL, Aris JP, Stanford DR, Martin NC, Hopper AK. Saccharomyces cerevisiae Mod5p-II contains sequences antagonistic for nuclear and cytosolic locations. Genetics. 1999;151(1):57–75. doi: 10.1093/genetics/151.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krogan NJ, Peng WT, Cagney G, Robinson MD, Haw R, Zhong G, Guo X, Zhang X, Canadien V, Richards DP, Beattie BK, Lalev A, Zhang W, Davierwala AP, Mnaimneh S, et al. High-definition macromolecular composition of yeast RNA-processing complexes. Mol Cell. 2004;13(2):225–239. doi: 10.1016/s1097-2765(04)00003-6. [DOI] [PubMed] [Google Scholar]
- 31.Hetzer MW, Walther TC, Mattaj IW. Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annu Rev Cell Dev Biol. 2005;21:347–380. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 32.Lange A, Mills RE, Devine SE, Corbett AH. A PY-NLS nuclear targeting signal is required for nuclear localization and function of the Saccharomyces cerevisiae mRNA-binding protein Hrp1. J Biol Chem. 2008;283(19):12926–12934. doi: 10.1074/jbc.M800898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dingwall C, Laskey RA. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 34.Polizotto RS, Cyert MS. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J Cell Biol. 2001;154(5):951–960. doi: 10.1083/jcb.200104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82(3):463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 36.Fischer U, Huber J, Boelens WC, Mattaj IW, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82(3):475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 37.Michael WM, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83(3):415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 38.Hopper AK. Nucleocytoplasmic transport: Inside out regulation. Curr Biol. 1999;9(21):R803–806. doi: 10.1016/s0960-9822(99)80494-1. [DOI] [PubMed] [Google Scholar]
- 39.Polevoda B, Sherman F. N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins. J Mol Biol. 2003;325(4):595–622. doi: 10.1016/s0022-2836(02)01269-x. [DOI] [PubMed] [Google Scholar]
- 40.Winzeler EA, Lee B, McCusker JH, Davis RW. Whole genome genetic-typing in yeast using high-density oligonucleotide arrays. Parasitology. 1999;118 (Suppl):S73–80. doi: 10.1017/s0031182099004047. [DOI] [PubMed] [Google Scholar]
- 41.Chen DC, Yang BC, Kuo TT. One-step transformation of yeast in stationary phase. Curr Genet. 1992;21(1):83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- 42.Moreland RB, Langevin GL, Singer RH, Garcea RL, Hereford LM. Amino acid sequences that determine the nuclear localization of yeast histone 2B. Mol Cell Biol. 1987;7(11):4048–4057. doi: 10.1128/mcb.7.11.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butterfield-Gerson KL, Scheifele LZ, Ryan EP, Hopper AK, Parent LJ. Importin-beta family members mediate alpharetrovirus gag nuclear entry via interactions with matrix and nucleocapsid. J Virol. 2006;80(4):1798–1806. doi: 10.1128/JVI.80.4.1798-1806.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones GM, Stalker J, Humphray S, West A, Cox T, Rogers J, Dunham I, Prelich G. A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat Methods. 2008;5(3):239–241. doi: 10.1038/nmeth.1181. [DOI] [PubMed] [Google Scholar]
- 45.Pringle JR, Adams AE, Drubin DG, Haarer BK. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- 46.Ellis SR, Morales MJ, Li JM, Hopper AK, Martin NC. Isolation and characterization of the TRM1 locus, a gene essential for the N2, N2-dimethylguanosine modification of both mitochondrial and cytoplasmic tRNA in Saccharomyces cerevisiae. J Biol Chem. 1986;261(21):9703–9709. [PubMed] [Google Scholar]
- 47.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 48.Kopp J, Schwede T. The SWISS-MODEL Repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004;32(Database issue):D230–234. doi: 10.1093/nar/gkh008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 51.Peitsch MC, Wells TN, Stampf DR, Sussman JL. The Swiss-3DImage collection and PDB-Browser on the World-Wide Web. Trends Biochem Sci. 1995;20(2):82–84. doi: 10.1016/s0968-0004(00)88963-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Endogenous Trm1 is not required for INM location of Trm1(73-151)-β–galactosidase. Top row: indirect immunofluorescence using wild-type (A) or trm1Δ (B) cells transformed with a gene encoding a fusion protein consisting of 1-14 amino acids of histone H2B, Trm1 amino acids 73-151, andβ–galactosidase. Top row: location of Trm1(73-151)-β–galactosidase Bottom row: overlay of DNA with DAPI staining with Cy3 detection of β–galactosidase. Bar = 1μm.
m22G metyltransferase activity of wild-type Trm1 and mutant versions with single amino acid substitutions. A trm1Δ strain contained plasmids encoding either wild-type (pGP54a-TRM1-II-GFP) or mutant versions generated by error-prone or site-directed mutagenesis. Extracts were generated and the assay conducted as described in the methods. Activities were measured at 0 (blue), 45 (magenta), and 90 (beige) min and the assays were repeated at least twice. Note that all mutations of the Trm1 region 133-151 destroy activity whether the mutations have no affect on INM location (R149A) or whether they cause Trm1 to fail to locate to the INM (I136S, R146A, A147D, A151D).
Intranuclear location of galactose-inducible Trm1-II-GFP mutants containing an overlapping series of 5 amino acid deletions. Data from each of seven mutant constructs missing Trm1-II-GFP amino acids as indicated. Top row: location of Trm1-II-GFP mutant proteins; middle row: the same cells showing DAPI staining of DNA; bottom row; overlay of cells in the top and middle rows. Bar = 5μm.
Predicted structure of S. cerevisiae Trm1 and location of the INM targeting/tethering motif. The same color scheme was used as for Fig. 8A. Magenta: sequence that is sufficient and necessary for Trm1 targeting/tethering to the INM; Green: ADEPT 2: Red: NLS motif within Trm1 ADEPT 2. All other Trm1 regions are colored blue. Note that the proposed structure for the green and red regions is not based on crystallography data because these regions are missing from Pyrococcus horikoshii Trm1. Also, the amino terminus of yeast Trm1-II is not modeled as the homology starts at amino acid 44 of S. cerevisiae Trm1.