Abstract
Objective
Considerable research examines fear conditioning in adult anxiety disorders but few studies examine youths. Adult data suggest that anxiety disorders involve elevated fear but intact differential conditioning. We used a novel paradigm to assess fear conditioning in pediatric anxiety patients.
Method
Sixteen individuals with anxiety disorders and 38 healthy comparisons viewed two photographs of actresses displaying neutral expressions. One picture served as the conditioned stimulus (CS), paired with a fearful expression and a shrieking scream (CS+), whereas the other picture served as a CS unpaired with the aversive outcome (CS−). Conditioning was indexed by self-reported fear. Subjects participated in two visits involving conditioning and extinction trials.
Results
Both groups developed greater fear of the CS+ relative to CS−. Higher fear levels collapsed across each CS characterized anxious relative to healthy subjects, but no significant interaction between group and stimulus type emerged. Fear levels at visit 1 predicted avoidance of visit 2. Fear levels to both CS types showed stability even after extinction.
Conclusions
Consistent with adult data, pediatric anxiety involves higher fear levels following conditioning but not greater differential conditioning. Extending these methods to neuroimaging studies may elucidate neural correlates of fear conditioning. Implications for exposure therapies are discussed.
Keywords: pediatric anxiety disorders, fear conditioning, extinction
Fear conditioning is the process by which a neutral stimulus (e.g., tone) acquires the fear-eliciting properties of an aversive unconditioned stimulus (e.g., shock) through repeated pairings of these stimuli. Associative learning mechanisms enable the neutral stimulus to become a conditioned stimulus (CS) that evokes fear independently of the unconditioned stimulus (UCS). Extinction, by comparison, is the process by which fear associated with the CS declines after repeated presentation of the CS in the absence of the UCS. Many theories have implicated fear conditioning and extinction in the pathogenesis of anxiety disorders.1–5 In particular, anxious individuals are hypothesized to show stronger fear learning that is more resistant to extinction. This focus has broad translational appeal, given parallels in the neural circuitry of clinical anxiety and fear conditioning.6,7 Furthermore, it carries clear clinical implications, given that effective treatments for anxiety disorders use desensitization techniques modeled on principles of extinction.
A recent meta-analysis documents fear-conditioning abnormalities in adult anxiety disorders,5 although the nature of these deficits appear relatively subtle. In simple conditioning paradigms, in which a neutral cue is paired repeatedly with the anxiety-provoking UCS, anxious patients exhibit greater fear responses than healthy subjects to the CS. Yet these patient-control differences do not typically emerge in discrimination conditioning paradigms, in which fear responses to the CS paired with an aversive UCS (CS+) are adjusted relative to responses to a CS unpaired with the UCS (CS−). Studies using this design suggest that although both anxiety patients and healthy controls exhibit comparable levels of differential conditioning, manifested as greater fear to the CS+ over the CS−, anxious subjects continue to show greater overall levels of fear to both types of stimuli (CS+ and CS−). Extinction data are fewer, but findings from the meta-analysis tentatively suggest results similar to those obtained at acquisition. Thus, patient-control differences in the resistance to extinction are greater following simple conditioning than in discrimination conditioning paradigms. Although conditioning-related abnormalities provide a plausible explanation for the enhanced fear response among anxious individuals,5 the results could also implicate nonassociative processes such as greater sensitization to both stimuli during fear states. These distinctions are important because different cognitive and chemical processes mediate conditioning and sensitization.8
From a neuroscience perspective, conditioning studies in pediatric anxiety disorders represent an important avenue for future research. Studies in rodents and nonhuman primates document strong developmental plasticity in neural circuits engaged during fear conditioning.9 Environmental and genetic influences produce more lasting effects on these circuits in immature organisms. Despite the wealth of clinical research in adults and the importance of developmental neuroscience approaches, only two studies have examined fear conditioning in pediatric anxiety disorders.10,11 Neither study documented any sign of enhanced anxiety-related responding during acquisition in patients relative to healthy peers to either the CS+ or CS−. Although one study reported greater fear responses to the CS+ following extinction relative to the CS− among anxious but not healthy subjects,10 the second study failed to find this group difference.11
The present study compares pediatric anxious and healthy individuals using a novel fear-conditioning paradigm that addresses two major limitations of previous research. First, ethical constraints complicate fear-conditioning research with children. The most common unconditioned stimulus in adult studies, electric shock, has not been used with children due to these constraints. Instead, UCSs that have been used with children, such as loud sounds, unpleasant photographs, and air puffs, typically provoke minimal anxiety levels12–14 that may compromise the degree of fear conditioning. We therefore developed a novel UCS for use with children, by pairing a facial photograph with a shrieking scream. Pilot work shows this UCS to be more aversive than other sounds or air puffs but less aversive than electric shocks,15 increasing its potential for ethically eliciting fear and related learning processes. Given the salience of human facial expressions as rapid conveyors of emotionally significant information, this UCS also represents an ecologically valid source of threat, salient among pediatric samples. The current study thus presents data from an innovative fear-conditioning paradigm using this UCS. A second novel feature of the present study is its use of subjective reports of anxiety to define fear conditioning and extinction. Although most studies of patients index conditioning via physiological reactions, anxiety disorders are typically defined by subjective reports. The persistence of distress reactions in anxiety patients is also consistent with greater resistance to extinction of conditioned subjective reports rather than physiological reactions.16 Thus, one would expect patients with anxiety disorders to differ from healthy subjects in the acquisition and extinction of self-reported distress reactions. The present study explores these processes in anxious and healthy subjects.
We tested one primary hypothesis based on conditioning and three secondary hypotheses based on extinction. Consistent with meta-analytic results of conditioning,15 we expect both anxiety patients and healthy controls to show comparable levels of differential learning, manifested as greater fear evoked by the CS+ versus CS− in both groups. However, we also expect anxiety patients to report greater fear to conditioned stimuli whether paired (CS+) or unpaired (CS−) with the aversive event (UCS), relative to healthy controls. For hypotheses based on extinction, previous findings are less clear. Adult data suggest similar between-group differences following extinction as observed for conditioning in discrimination-learning paradigms. Thus, we first anticipated that anxious and control subjects will show similar levels of differential fear to the CS+ and CS− after extinction, but with greater overall levels of fear among patients. Second, we hypothesized that reported reactions to the conditioning procedure are resistant to extinction. Specifically, level of reported fear in both patients and healthy subjects may predict refusal to return (attrition) for the second conditioning session, consistent with data linking experimentally induced fear reactions to behavioral avoidance.4 Third, we expected fear reactions after conditioning to strongly correlate with fear reactions after extinction.
METHOD
Participants
Sixteen medication-free adolescents with DSM-IV anxiety disorders and 38 healthy adolescents were recruited for the present study through local schools and newspapers (Table 1). This sample size has 75% power to detect group differences with effect sizes of at least 0.80. All of the anxious subjects completed comprehensive clinical assessments on the Schedule for Affective Disorders and Schizophrenia17) conducted by clinicians trained to exhibit acceptable reliability (κ > .75). All of the subjects and their parents reported subject anxiety symptoms using total scores from the Screen for Child Anxiety Related Emotional Disorders18 (Table 1). IQ was assessed through two subtests of the Wechsler Abbreviated Scale of Intelligence,19 and socioeconomic status was determined on a 9-point scale ranging from 1 (<$5,000) to 9 (>$180,000), based on parental income levels. Composition of different racial groups among the sample was as follows: white (70.4%), African American (14.8%), Latino (3.7%), Asian American (3.7%), and other (7.4%).
TABLE 1.
Demographic and Diagnostic Variables Across Participants
| Whole Sample (n = 64) | Patient Group (n = 15) | Healthy Group (n = 39) | |
|---|---|---|---|
| Subject anxiety diagnosis | |||
| Generalized anxiety disorder | 9 | 9 | |
| Social phobia | 9 | 9 | |
| Separation anxiety | 3 | 3 | |
| Anxiety symptom scores | |||
| Self-reported SCARED total | 16.59 (9.77) | 26.71 (10.68) | 12.76 (6.03) |
| Parent-reported SCARED total | 9.63 (10.58) | 24.36 (8.00) | 4.05 (4.02) |
| Demographics | |||
| Mean age | 13.64 (2.37) | 12.84 (2.47) | 13.98 (2.28) |
| No. of females (% of sample) | 30 (55.6) | 9 (56.3) | 21 (55.3) |
| SES | 7.15 (1.32) | 7.86 (0.86) | 6.84 (1.38) |
| Full Scale IQ | 113.38 (11.31) | 115.25 (11.05) | 112.56 (11.48) |
Note: SCARED = Screen for Child Anxiety-Related Emotional Disorders; SES = socioeconomic status.
Of the 16 anxiety patients, 9 met full criteria for current generalized anxiety disorder, 9 for social phobia, and 3 for separation anxiety (Table 1). Three individuals were diagnosed with two disorders, and one individual met criteria for all three diagnoses. Comorbidity of anxiety disorders resembled patterns in other study samples such as in the Research Units on Pediatric Psychopharmacology anxiety trial.20 All 38 healthy subjects were free from present or past psychiatric diagnoses. Other inclusion criteria for patients were clinically significant anxiety on the Pediatric Anxiety Rating Scale (score ≥10),20 significant impairment on the Children’s Global Assessment Scale21 (score <60); and persistent anxiety during 3 weeks of therapy. Exclusion criteria were current Tourette’s syndrome, obsessive-compulsive disorder, or conduct disorder; exposure to trauma; suicidal ideation; lifetime history of mania, psychosis, or pervasive developmental disorder; and IQ <70. Three patients with concurrent major depressive disorder were included because their anxiety disorder was considered their primary ailment. The study was approved by the NIMH Institutional Review Board, and all of the participants/parents provided written informed assent/consent.
Procedures
The conditioning task was administered over two visits by trained psychologists not involved in diagnostic or treatment procedures. A mean interval of 16 days (range 1–42 days) separated visits. All of the procedures were completed before initiating treatment.
Conditioning Task
The present study used a differential fear-conditioning paradigm, in which subjects learn to associate an aversive UCS with the paired conditioned stimulus (CS+), but not the unpaired conditioned stimulus (CS−). We were concerned that exposure to and rating of the CS+ and the CS− before the task would influence between-group differences in conditioning by self-focusing on subjective fear levels. Such “instructed” fear paradigms may activate differential neural regions to those responsive to fear conditioning22 and thus are likely to involve different psychological processes. Based on these concerns and previous conditioning research among adults, subjects rated internal fear states after conditioning.4 Subjects were also not told about possible contingencies between a UCS and CS. Rather, they were told: “You will see pictures of two women on the screen. Please watch the pictures while they are on the computer screen. While you are watching the pictures, you will see a mildly unpleasant picture of a person and hear an unpleasant sound.” Subjects were told they could discontinue the study procedures at any point.
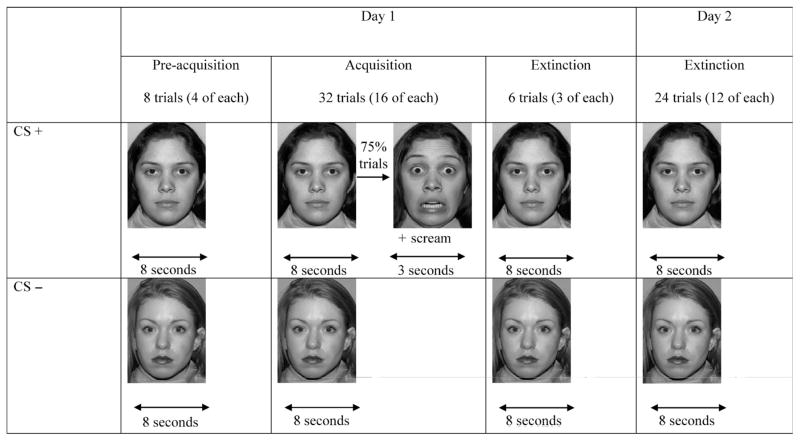
Two photographs of actresses displaying neutral facial expressions served as the CS+ and CS−, with one of the two actresses randomly selected to serve as the CS+ for each subject. A fearful facial expression presented simultaneously with a shrieking female scream served as the UCS. The four photographs (two neutral, two fearful) were selected from standard facial stimuli sets. Three trial types were collected over two visits: preacquisition, acquisition, and extinction (Fig. 1). The CS− actress displayed a neutral expression for 8 seconds across all of the trial types. The CS+ actress also presented a neutral expression for 8 seconds during preacquisition and extinction trials. However, at acquisition, 75% of the 8-second CS+ trials were immediately followed by the UCS: a 3-second presentation of a photograph of the same actress, depicting a fearful expression, co-occurring with a 3-second presentation of a 95-dB scream. Furthermore, a 75% contingency ratio was selected given evidence that partial reinforcement prevents habituation to the unconditioned stimulus (fearful expression and scream), leading to more resistance during subsequent extinction.23
Fig. 1.
A schematic depiction of the preacquisition, acquisition, and extinction phases of the conditioning paradigms. CS = conditioned stimulus.
Subjects received four exposures of each CS during preacquisition (8 trials), 16 of each during acquisition (32 trials), and 15 exposures of each during extinction (30 trials). Visit 1 consisted of subjects receiving all of the preacquisition and acquisition trials and 6 extinction trials over 2 blocks of 24 and 22 trials administered one after the other. Trials were separated over 2 blocks due to concerns that younger participants would find it difficult to focus throughout the session if trials were presented in one long sequence. The remaining 24 trials of extinction were presented in one block of trials at the second visit. We refer to visit 1 and visit 2 as conditioning and extinction sessions, respectively.
Measures
The primary dependent measures, reported fear of the CS+ and CS−, were assessed after completion of the conditioning and extinction visits. Subjects were asked to indicate on a 10-point Likert scale the degree to which they felt afraid of the actress depicted in the CS− photograph and in the CS+ photograph, both depicting neutral expressions.
Data Analysis
Study hypotheses were tested using analysis of variance (ANOVA), t tests, and correlation analyses. Given assumptions of sphericity and homogeneity of variance across patients and controls, Mauchly’s test of sphericity for CS ratings across sessions and Levene’s test of the equality of variance across patients and controls revealed no violation of these assumptions within our analyses. Our primary hypothesis was that patients with anxiety disorders would show similar levels of differential learning (manifested as higher ratings to CS+ relative to CS−) as those of healthy subjects but exhibit overall higher fear ratings to each CS following conditioning. This hypothesis was tested in a repeated-measure ANOVA with one between-subject factor (patient, healthy subject) and one within-subject factor (CS+, CS−), examining both main effects of patient status, stimulus type, and their interaction. Age and sex were included as covariates if significant main effects were demonstrated using Pearson correlations and independent-sample t tests. We also examined associations between fear ratings to each CS and continuous measures of anxiety symptoms in the entire sample, using self- and parent-reported anxiety symptoms. Although considerable work has established that self- and parent reports provide unique variance in predicting various aspects of adolescent anxiety,24 these reports were significantly correlated (r = 0.56, p < .001) in the present study. Moreover, the strength of associations between anxiety symptoms and fear ratings were similar for self-reports and parent reports. As such, we also reported results from averaged scores.
Secondary hypotheses relating to extinction were tested through three sets of analyses. First, we tested whether patients and healthy subjects would show similar levels of differential fear (manifested as higher ratings to the CS+ relative to the CS−) following extinction, but with higher overall ratings to both CS types among patients, using repeated-measures ANOVA. Age and sex were included as covariates if significant main effects were demonstrated using Pearson correlations and independent-sample t tests. We also computed correlations between fear ratings and parent-reported and self-reported anxiety symptoms. Next, we explored the hypothesis that higher fear ratings following conditioning would predict attrition at visit 2. To examine whether fear ratings are higher in subjects refusing to return for the second visit, we relied on a repeated-measure ANOVA with one between-subject factor (completer, noncompleter) and one within-subject factor (CS+, CS−) to assess main effects of subject and its interaction with stimulus type. We also used a binary logistic regression analysis to explore effects of fear ratings on refusal to return at visit 2 (completer, noncompleter), beyond other potential predictors, including demographic variables (age, sex, socioeconomic status), IQ, and diagnosis (patient, healthy). The final hypothesis examined stability of CS ratings across visits using Pearson correlations.
RESULTS
Sample Characteristics
Table 1 presents demographic characteristics of the 16 patients and 38 healthy subjects. Groups did not differ on age (t52 = 1.64; p = not significant (n.s.), sex (χ2 = .95; p = n.s.), socioeconomic status (t52 = 0.68; p = n.s.), or intelligence (t50 = 0.79; p = n.s.). No significant differences between males and females emerged in fear ratings to either the CS+ or CS− following visit 1 or 2. Older adolescents reported significantly lower fear ratings to the CS+ stimulus at visit 1 (r = −0.30; p < .05). Age was included as a covariate in the repeated-measures ANOVA of visit 1 data.
Conditioning Session
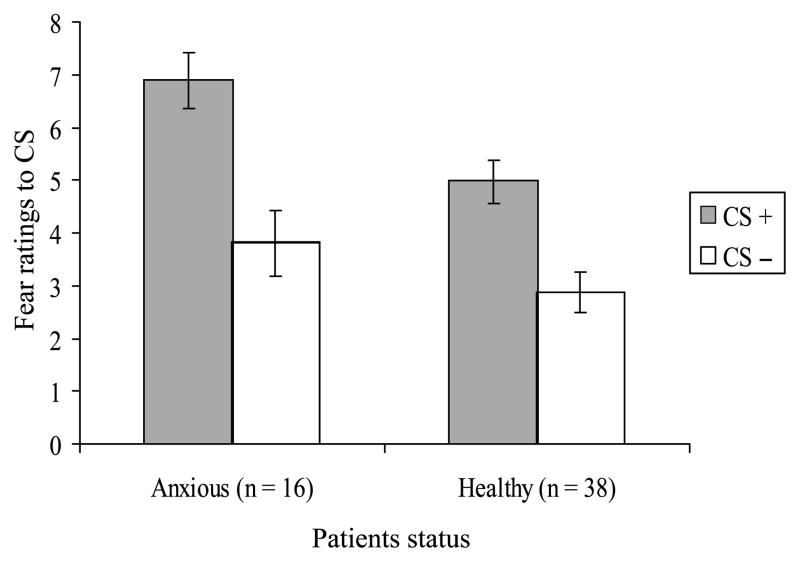
Figure 2 displays mean fear ratings to conditioned stimuli after visit 1 in patients and healthy subjects. Four findings emerged. First, the repeated-measures ANOVA revealed a significant effect of stimulus type (F1,51 = 6.38; p < .05) and a significant effect of group (F1,51 = 4.62; p < .05), with no group × stimulus type interaction (F1,51 = 0.61; p = n.s.) or effects of age (F1,51 = 1.39; p = n.s.). As shown in Figure 2, this indicates that both subject groups rated the CS+ as more fear-provoking than the CS−, with a large effect size (d = 0.96). Second, patient ratings collapsed across CSs were higher than those of healthy subjects, with a medium effect size (d = 0.60). Third, levels of differential fear conditioning (i.e., higher ratings to CS+ relative to CS−) were the same across groups. Nevertheless, follow-up tests revealed greater fear to the CS+ among patients with a large effect (t = 2.65; p < .05, d = 0.85) but no group differences in fear to the CS− (t = 1.31; p = n.s.). Finally, both self-reported and parent-reported anxiety symptoms correlated significantly with fear ratings to the CS+ (r = 0.29 and 0.33) but not the CS− (r = 0.12 and 0.22). Correlations based on average scores across self-reports and parent reports are shown in Table 2.
Fig. 2.
Mean fear ratings to CS+ and CS− in anxious and healthy subjects following visit 1. CS = conditioned stimulus.
TABLE 2.
Correlations Between Fear Ratings to Conditioned Stimuli (CS+, CS−) Within and Across Time and With SCARED Anxiety Symptom Scores
| Postconditioning |
Postextinction |
|||
|---|---|---|---|---|
| Fear Rating | CS+ | CS− | CS+ | CS− |
| Postconditioning | ||||
| CS+ | 1.00 | |||
| CS− | 0.33a | 1.00 | ||
| Postextinction | ||||
| CS+ | 0.57a | 0.26 | 1.00 | |
| CS− | 0.29 | 0.55a | 0.48a | 1.00 |
| Anxiety symptoms | 0.36a | 0.21 | −0.01 | −0.08 |
Note: Figures are symmetrical above and below the central diagonal of 1.00s, and SCARED scores were averaged across parent report and child self-report measures.
Correlation is significant at the .01 level.
Extinction Session
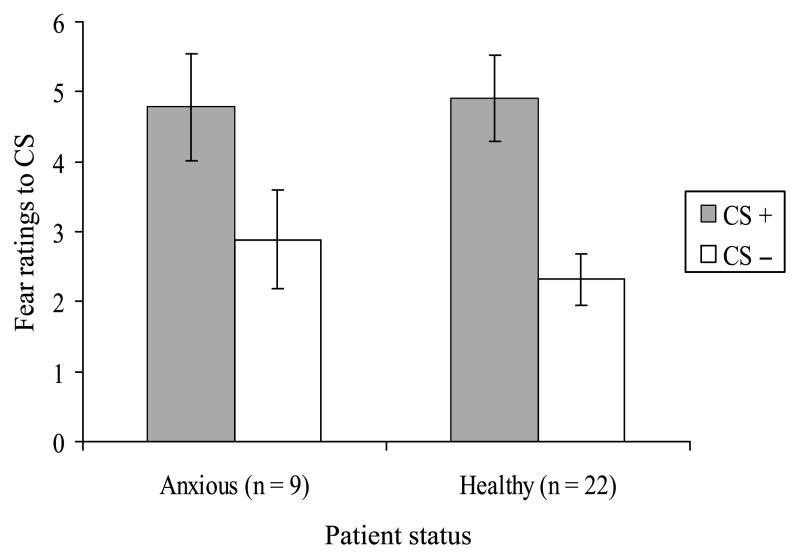
A repeated-measure ANOVA comparing patients and healthy subjects revealed a main effect of CS type (F1,29 = 18.75; p < .001) with a large effect size (d = 1.04) but neither a group effect (F1,29 = 0.08; p = n.s.) nor a group × CS type interaction (F1,29 = 0.46; p = n.s.; Fig. 3). Again, this implies that although both groups showed differential conditioning (higher ratings to CS+ relative to CS−), this was comparable across subjects. During extinction, patients did not rate CS− stimuli as more fear provoking than healthy subjects. Significant correlations did not emerge between fear ratings to CSs and either self-reported or parent-reported anxiety scores. Correlations based on average scores across self-reports and parent reports are shown in Table 2.
Fig. 3.
Mean fear ratings to CS+ and CS− in anxious and healthy subjects following visit 2. CS = conditioned stimulus.
Among the 16 patients and 38 healthy subjects tested in the conditioning session, only 9 patients and 22 healthy subjects returned for the extinction session (Fig. 3), with no differences in attrition between groups (χ2 = .01; p = n.s.). Of note, all of the subjects who completed the conditioning session continued to participate in other aspects of the research (with the exception of one subject who was unreachable). A repeated-measures ANOVA comparing CS ratings at conditioning in subjects who did and did not participate in visit 2 revealed main effects of ratings to CS− type (F1,52 = 40.06; p < .001), but neither a main effect of group (F1,52 = 2.56; p = n.s.) nor a group × CS− rating interaction (F1,52 = 2.41; p = n.s.). A logistic regression including demographic data, IQ, diagnosis, and fear-rating variables as predictors of participation in extinction revealed significant effects of sex (B = 2.97, df = 1; p < .05) and fear ratings to CS+ at visit 1 (B = −0.59, df = 1; p < .05). Males and individuals reporting higher levels of fear to the CS+ following visit 1 (6.39 versus 4.90, t = 2.21; p < .05) with a medium effect size (d = 0.61) were less likely to return.
Finally, Table 2 presents correlations among ratings to the CS+ and CS− across visits. As shown, the magnitude of correlations within stimulus type over time (r = 0.57 and 0.55 for CS+ and CS−, respectively) were somewhat stronger than those across stimulus type, either concurrently (r = 0.33 and 0.26 for postconditioning and postextinction, respectively) or over time (r = 0.29). Correlations between the degree of differential fear learning (fear ratings to CS+ minus fear ratings to CS−) at visits 1 and 2 also indicated high stability (r = 0.51; p < .001) even after controlling for variability in temporal interval between visits.
DISCUSSION
Four key findings emerged from our study. First, the current paradigm produced strong levels of differential conditioning, manifested as higher ratings to the CS+ relative to the CS− across all of the subjects. Second, in the context of similar levels of differential learning, overall fear collapsed across CS types was higher among anxious patients than healthy subjects. Furthermore, significant positive correlations between self-reported and parent-reported anxiety symptom scores and fear ratings to the CS+ following conditioning emerged, with similar sized correlations for the CS−. Third, among all of the subjects, fear following conditioning predicted avoidance of extinction session. Finally, fear ratings were resistant to extinction, showing strong stability across visits. These findings can be contextualized from the previous literature.
The present study used a novel fear-conditioning paradigm with CS− UCS pairings designed explicitly to address limitations in other paradigms used with juveniles. Air puffs, loud sounds, and aversive pictures have generally been ineffectual in eliciting fear or fear-related learning. Air puffs used by one study to examine children at risk of mood and anxiety disorders12,13 yielded inconsistent results across sexes and paradigms. Previous work with healthy subjects also found that this UCS elicits minimal fear in approximately 50% of subjects.14 Studies using loud sounds10,11 have struggled to demonstrate greater fear of the CS+ relative to the CS− among anxious children, whereas aversive pictures produce low levels of fear in children.25 In comparison, the present study showed higher fear levels to the CS+ relative to the CS−, with large effect sizes (d > 0.8) in both anxious and healthy subjects. Thus, levels of differential self-reported fear in this experiment appears greater than those of previous conditioning experiments among children and adolescents.
Ethical questions concerning the tolerability of these procedures should be considered. Three factors speak to this. First, although levels of fear were markedly higher to the CS+ relative to CS−, overall fear levels were moderate and comparable to levels reported in other experimental procedures shown to be safe, such as blood draws and exposure to CO2-enriched air.26 Second, fear levels appear commensurate with those experienced during everyday life experiences such as in various forms of media or entertainment. Third, all of the subjects in the present protocol (with one exception) continued to participate in our research and were followed over time by clinicians with no lasting impact noted.
These data comparing fear levels in anxious and healthy subjects can also be placed within a rich literature examining distress reactions in the laboratory among adult and pediatric anxious patients. At one level, as anxiety disorders are defined by high levels of reported distress, it is not surprising to observe greater fear in patients relative to healthy peers during conditioning. Yet work in other brain disorders, such as dementia, demonstrates the utility of paradigms evoking clinically relevant features of a disorder in controlled experimental settings.8 Previous experimental studies with both pediatric and adult anxiety disorders have not enjoyed consistent success in demonstrating expected patient-control differences using other methods, including fear-conditioning procedures.10 The present study documented large effect sizes of stimulus type within each subject group and in the comparison between anxious and healthy subjects. Furthermore, positive correlations emerged between concurrent anxiety symptoms and fear responses to the CS+ following conditioning. The effectiveness of the present procedure in eliciting disorder-relevant behaviors within remit of acceptable ethical standards makes it appealing to future investigations probing fear learning in pediatric anxiety.
The present data also form a developmental adjunct to research in adult anxiety.5 Both existing adult findings and the present data show comparable levels of differential conditioning to CS+ and CS− stimuli among anxious and healthy subjects, but with greater overall fear in patients collapsed across CS types. These between-group differences may arise from anxiety-related deficiencies in inhibiting fear responses, even in the presence of safety cues.27 As such, anxious individuals continue to exhibit high levels of fear to the stimulus unpaired with the UCS (CS−). In part, fear responses to the CS− may arise through a tendency to generalize fear across stimuli due to comparable visual properties (e.g., both CS + and CS− are photographs of female faces). However, as anxious individuals, like their healthy peers, report less fear of the CS−, some discrimination between these stimuli is present.
An alternative explanation for these results that does not rely on associative principles is that group differences may be due to enhanced sensitization among anxious relative to healthy subjects. That is, as a result of exposure to an anxiety-provoking UCS, patients exhibit heightened sensitivity to a range of stimuli. Because of a decision to collect ratings only following the conditioning procedures, no baseline indices of fear were available to verify this oversensitivity hypothesis. If preconditioning ratings to the CS+ and CS− were comparable in anxious patients and controls, then one could eliminate the possibility that enhanced fear responses were due to increased sensitization and attribute these directly to conditioning. Nevertheless, post hoc tests showed significant between-group differences in fear ratings to the CS+, but not to the CS−, following conditioning. Thus, although anxious individuals rate the CS+ as more fear provoking than healthy comparison subjects, this may not extend to the CS−. Future studies may establish baseline fear levels to the CS+ and CS− before conditioning to differentiate conditioning from sensitization accounts. Regardless, these data do show enhanced fear collapsed across CS− stimuli following conditioning in pediatric patients.
Findings that attrition rates between visits were not related to anxiety disorders per se but rather to acquired fear levels to the CS+ were also intriguing. These implied that subjects exhibiting higher distress to the CS+ following conditioning were less likely to attend visit 2. This finding is consistent with adult reports, showing higher avoidance rates (defined by refusal to return) among individuals who were unaware of the CS–UCS relationship during conditioning.4 Presumably for these individuals, as with our subjects, inability to predict occurrence of an aversive outcome was sufficiently fear provoking to warrant avoidance. Such instances of experimentally induced avoidance closely reflect clinically significant avoidance, a debilitating feature of anxiety disorders.
Finally, the present data on extinction extend previous findings. Recent work notes that extinction does not erase the memory of the CS+–UCS association but rather embeds this association in a new cognitive context.1 Thus, one would expect humans to show strong declarative memory for the CS+–UCS association, even after extinction. Consistent with this, previous studies using shock UCS in adults note strong persistence of reported fear.16 Similarly, the present data also show that fear of the CS+ after conditioning predicted fear of the CS+ after extinction, with stability comparable to that for clinical anxiety measures.
Four limitations of this study should be considered. First, the present study examined relatively small numbers of anxious and healthy subjects. Sample size may limit the ability to observe between-group differences in reactions to the CS− stimulus. Second, there was clear sample attrition across visits, biased among individuals reporting greater fear levels to the CS+ stimulus at visit 1. Although it is difficult to ascertain effects of attrition on stability of fear ratings, one might speculate underestimation of stability, given that subjects with higher fear ratings are expected to show consistently robust responses. Despite this, as it was our goal to design a procedure that elicits clinically meaningful fear among adolescents, significant levels of attrition are expected. A third caveat concerns a lack of baseline levels of fear to the CS+ and CS− before conditioning, precluding consideration of alternative explanations of the results. Finally, we obtained one set of fear ratings following sessions rather than continuous assessments. This is problematic because visit 1 included both acquisition and extinction trials, increasing ambiguity as to which process group differences are attributed to—visit 1 ratings may reflect fear associated with pure extinction trials toward the end of this session rather than fear during acquisition. Rating fear at the end of each session was driven by findings that self-focused evaluation of fear to facial stimuli yields differences between adolescent patients and healthy controls.7 To minimize confounds associated with self-focused evaluation of fear on group differences in fear generated by conditioning, we reduced the number of time points at which fear was assessed. A pertinent issue to arise is whether group differences in fear ratings at visit 1 can be attributed solely to acquisition. Two issues are relevant. First, given partial reinforcement rates during acquisition, in which only 75% of trials associated with the CS+ stimulus were followed by the UCS, participants may be less likely to attribute safety to the CS+ in the absence of the UCS during extinction. Thus, fear associated with extinction trials immediately following acquisition may be similar to the 25% of acquisition trials in which the CS+ is not followed by the UCS. Second, nonsignificant differences between anxious and healthy subjects in fear provocation following the extinction session makes it unlikely that greater fear provocation to CSs at visit 1 are driven by differences in fear to extinction. Future studies should derive cleaner indices of fear to distinguish these different processes.
Several aspects of the findings of this study may be relevant to clinical treatments. First, anxious patients reported higher overall fear to CSs compared with healthy individuals. Such greater fear provocation was also associated with subsequent patterns of avoidance, persisting after extinction. Interventions should therefore target reduction of fear to both the threat cue (CS+) and the safety cue (CS−) to attenuate later negative outcomes of avoidance and persistence of fear. Considerable work in basic science also provides clues concerning extinction factors that may reduce fear in humans. For example, a recent study demonstrated that extinction training after a short interval to acquisition is often associated with reduced reinstatement of fear at a later time point.28 Therapists may thus consider using exposure therapies soon after an anxiety-provoking event. The utility of such therapies may be further enhanced if patients are trained to discriminate actual threat cues (CS+) from safety cues (CS−). Although fear reduction is necessary to both cues, it may be beneficial for patients to learn specifically to reduce their fear to the latter. Finally, medications facilitating extinction in animal models may contribute to parallel efforts in humans.29
The current fear conditioning paradigm using novel CS and UCS stimuli induced strong, consistent distress reactions in both patients and healthy subjects. Moreover, these effects were large, both for within-subject and between-subject comparisons. Finally, initial distress reactions predicted avoidance and showed strong stability even after extinction. Given the suitability of this paradigm for engaging fear-related processes among patients and controls, application to neuroimaging studies, in which anxiety-linked differences in fear circuit engagement can be examined, represents a promising avenue.
Acknowledgments
This research was supported by the NIMH/NIH Intramural Research Program. The authors thank the participants and the research staff who facilitated this work.
Footnotes
Disclosure: The authors have no financial relationships to disclose.
References
- 1.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 2.Chorpita BF, Barlow DH. The development of anxiety: the role of control in the early environment. Psychol Bull. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 4.Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- 5.Lissek S, Powers AS, McClure EB. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 7.McClure E, Monk C, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Squire LRK, Kendal ER. Memory: From Mind to Molecules. New York: Scientific American; 1999. [Google Scholar]
- 9.Gross CR, Hen R. The developmental origins of anxiety. Nat Rev Neurosci. 2004;5:545–552. doi: 10.1038/nrn1429. [DOI] [PubMed] [Google Scholar]
- 10.Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behav Res Ther. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Pliszka SR, Hatch JP, Borcherding SH, Rogeness GA. Classical conditioning in children with attention deficit hyperactivity disorder (ADHD) and anxiety disorders: a test of Quay’s model. J Abnorm Child Psychol. 1993;21:411–423. doi: 10.1007/BF01261601. [DOI] [PubMed] [Google Scholar]
- 12.Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biol Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- 13.Grillon C, Warner V, Hille J, Merikangas KR, et al. Families at high and low risk for depression: a three-generation startle study. Biol Psychiatry. 2005;57:953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 14.Monk CS, Grillon CL, Baas JM, et al. A neuroimaging method for the study of threat in adolescents. Dev Psychobiol. 2003;43:359–366. doi: 10.1002/dev.10146. [DOI] [PubMed] [Google Scholar]
- 15.Lissek S, Baas JM, Pine DS, et al. Airpuff startle probes: an efficacious and less aversive alternative to white-noise. Biol Psychol. 2005;68:283–297. doi: 10.1016/j.biopsycho.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Schell AM, Dawson ME, Marinkovic K. Effects of potentially phobic conditioned stimuli on retention, reconditioning, and extinction of the conditioned skin conductance response. Psychophysiology. 1991;28:140–153. doi: 10.1111/j.1469-8986.1991.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Psychological Corporation. WASI Manual. San Antonio, TX: Harcourt Brace; 1999. [Google Scholar]
- 20.RUPP ASG. The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2003;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer D, Gould MS, Brasic J, et al. A Children’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 22.Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 23.Mackintosh NJ. The Psychology of Animal Learning. London: Academic; 1974. [Google Scholar]
- 24.Dadds MR, James RC, Barrett PM, Verhulst FC, Ollendick TH, March JS. Diagnostic Issues in Phobic and Anxiety Disorders in Children and Adolescents: A Clinician’s Guide to Effective Psychosocial and Pharmacological Interventions. New York: Oxford University Press; 2004. [Google Scholar]
- 25.McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001;38:222–231. [PubMed] [Google Scholar]
- 26.Pine DS, Klein RG, Roberson-Nay R, et al. Response to 5% carbon dioxide in children and adolescents: relationship to panic disorder in parents and anxiety disorders in subjects. Arch Gen Psychiatry. 2005;62:73–80. doi: 10.1001/archpsyc.62.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Davis M, Falls WA, Gewirtz J. Neural systems involved in fear inhibition: extinction and conditioned inhibition. In: Myslobodsky M, Weiner I, editors. Contemporary Issues in Modeling Psychopathology. Boston: Kluwer Academic; 2000. pp. 113–142. [Google Scholar]
- 28.Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]