Chronic kidney disease (CKD) is a major public health concern, both in the US and worldwide, with rising incidence and prevalence. Recent worldwide initiatives have attempted to garner attention for chronic kidney disease by emphasizing that CKD is “Common, Harmful, and Treatable.”1 In the US, as many as 26 million adults may have CKD, an increase from approximately 10% of the US adult population between 1988 and 1994 to over 13% just one decade later.2, 3 Similar rates have been seen worldwide, with CKD prevalence of 13% in Beijing, China4 and 16% in Australia.5 In the US, the dramatic rise in the prevalence of CKD likely reflects similar increases in obesity and its sequelae – namely diabetes, hypertension and cardiovascular disease.3 The prevalence of CKD, and its associated costs, are expected to continue to increase.6
CKD is defined by either a reduced glomerular filtration rate (GFR <60 mL/min per 1.73m2) or by evidence of kidney damage. Early stages of CKD manifest with only kidney damage in the setting of overtly intact GFR; the most common marker of kidney damage is albumin in the urine (Table 1). Stage 3 CKD is defined by GFR between 30 and 59 mL/min per 1.73m2 and is the stage at which clinical sequelae of CKD are often first appreciated. Stage 4 CKD is defined by GFR of 15 to 29 mL/min per 1.73m2, and stage 5 by GFR <15 mL/min per 1.73m2 or requirement for kidney replacement therapy. Notably, only a small proportion of people with CKD develop kidney failure; reflecting higher rates of cardiovascular disease and cardiovascular risk factors, they often die prematurely of cardiovascular disease.
Table 1.
Stages, Description, Prevalence and Clinical Action Plan for CKD and Individuals at Increased Risk of CKD
| Stage | Description | GFR mL/min per 1.73 m2 | US Prevalence N [1,000s] | Clinical Action Plan | |
|---|---|---|---|---|---|
| --- | Increased risk for CKD | Not applicable | Risk factor prevalence | ||
| • Age ≥60 years | • Age >60: | 50,600 (23.2%) | Screening | ||
| • Hypertension | • Hypertension | 65,000 (32.3%) | Primary prevention and CKD risk reduction, including | ||
| • Diabetes | • Diabetes: | 20,600 (9.6%) | blood pressure and glycemic control | ||
| • Cardiovascular disease | • CVD: | 71,300 (34.2%) | |||
| • Family history of CKD | • Family History: | unknown | |||
| 1 | Kidney damage with normal or increased GFR |
≥90 | 3,600(1.8%) | Diagnosis of CKD cause Education about CKD Treatment of comorbid conditions Evaluation of risk for and assessment of rate of progression Treatment to slow progression CVD risk reduction Referral to nephrologist for rapid kidney disease progression |
|
| 2 | Kidney damage with mild decrease in GFR |
60–89 | 6,500 (3.2%) | ||
| 3 | Moderately decreased GFR | 30–59 | 15,500(7.7%) | Evaluate and treat complications, including bone and mineral disorder, anemia, and dyslipidemia Consider discussion of kidney replacement therapy options, particularly in late stage 3 or rapid progressors |
|
| 4 | Severely decreased GFR | 15–29 | 700 (0.4%) | Prepare for kidney replacement therapy Place vascular access or develop plan for peritoneal access or pre-emptive transplant Referral to nephrologist |
|
| 5 | Kidney failure | <15 or dialysis | ~400 (0.2%) | Kidney replacement therapy | |
Chronic kidney disease is pernicious, often recognizable only by laboratory abnormalities until its latest stages. The major causes of kidney disease and subsequent kidney failure in the US are diabetes (accounting for 44.4% of incident cases of kidney failure in 2006) and hypertension (accounting for 26.8%),7 both of which are increasingly common in an increasingly overweight US population.8, 9 Conditions accounting for the remaining 29% include primary glomerulopathies like focal glomerulosclerosis and IgA nephropathy, inherited conditions like polycystic kidney disease, and autoimmune conditions like lupus. The major outcomes of CKD include progression to kidney failure as well as the complications of decreased kidney function, such as cardiovascular disease, anemia and bone disease. With the rising prevalence of diabetes and hypertension, the incidences of both earlier stages of CKD as well as its associated outcomes, including progression to kidney failure, are expected to rise.7
Harms associated with CKD include both those that impact the health of an individual as well as those that impact society as a whole. The most obvious societal effect is the tremendous financial cost and loss of productivity associated with kidney failure. While individuals with CKD can pose substantial expense to the healthcare system, those with end-stage renal disease cost exponentially more. In the US, ESRD care accounted for $23 billion from Medicare alone in 2006 – or 6.4% of the entire Medicare budget dedicated to less than 1% of Medicare beneficiaries.7 These costs are likely unsustainable.
Fortunately, while CKD typically is not reversible, the rate of CKD progression and other sequelae of CKD are often treatable. However, in order to treat CKD, it must first be recognized by patients and providers. Therefore, substantial emphasis has been placed on developing strategies to identify at-risk individuals in order to target screening and management strategies to achieve the greatest benefit.10 The need for targeted screening reflects findings that universal screening of the general US population, even with a test as simple as using a urine dipstick to estimate proteinuria, is likely not cost-effective.11 For example, in the US in persons with neither hypertension nor diabetes, the cost-effectiveness ratio for universal screening versus no screening is extremely unfavorable ($282,818 USD per quality adjusted life year (QALY) gained); however, if screening is directed to persons with hypertension, the ratio becomes highly favorable ($18,621 per QALY).11 This benefit reflects the presence of relatively inexpensive and safe medications to delay CKD progression, most notably angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), as well as the dramatic cost increase associated with kidney failure and dialysis compared to earlier stages of CKD.
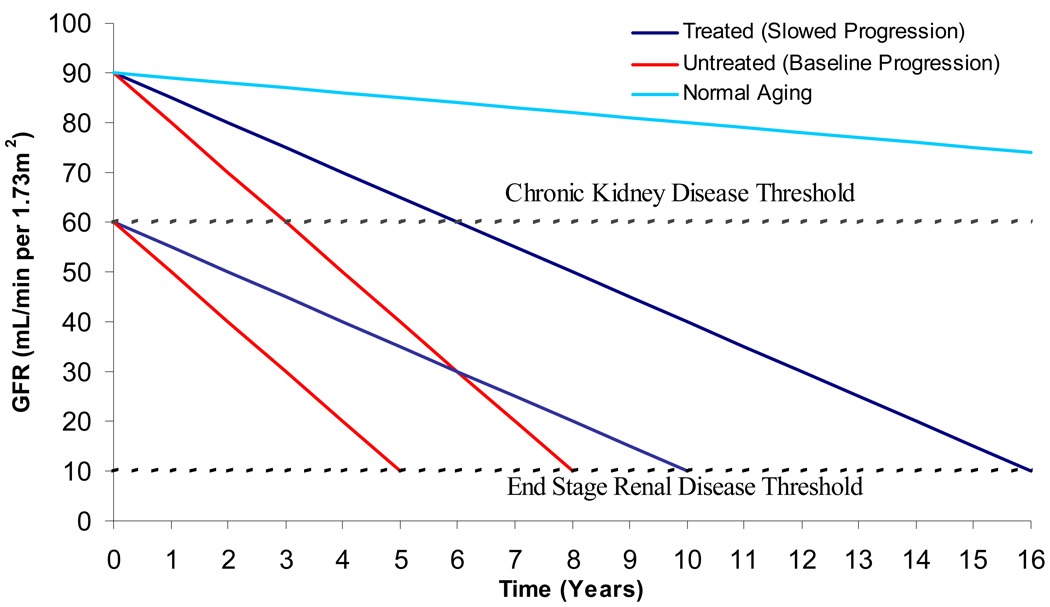
Accordingly, diagnosing CKD and initiating stage appropriate treatment can have substantial benefits. As demonstrated in Figure 1, early treatment of CKD to reduce the rate of progression of kidney disease can potentially double the amount of time to reach kidney failure. This is particularly true in proteinuric kidney disease, as is often most often the case in diabetic nephropathy, where urine protein excretion of greater than 1g per day is a powerful marker of risk for progressive loss of kidney function.12 Renin-angiotensin-aldosterone system (RAAS) blockade, most commonly with ACE inhibitors and ARBs, is central in preventing progressive kidney function loss. This has been illustrated in several trials. The RENAAL Study randomized 1513 individuals with proteinuric diabetic nephropathy and serum creatinine levels between 1.3 and 3 mg/dL to losartan plus standard antihypertensive therapy versus placebo plus standard antihypertensive therapy; the annualized median rate of decline in the treatment arm was 4.4 ml/min per 1.73 m2 versus 5.2 ml/min per 1.73 m2 in the placebo group (P=0.01).13 RENAAL followed up on earlier results from the Collaborative Study Group where 409 participants with type I diabetes and proteinuric diabetic nephropathy (mean baseline creatinine clearance of approximately 80 mL/min) were randomized to captopril versus placebo; among those receiving captopril, the annual decline in creatinine clearance was 11 ± 21% versus 17 ± 20% in the placebo group (P = 0.03).14 These data were extrapolated into Figure 1, where the effects of treating diabetic nephropathy on development of kidney failure are illustrated. Based on the captopril data, in the absence of ACE inhibitor therapy and tight blood pressure control, the rate of kidney function decline may be 10 mL/min per year or higher, while, in the presence of blood pressure control and RAAS blockade, this can conceivably be dropped to 4–5 mL/min per year. Accordingly, an individual treated beginning at a GFR of 90 mL/min per 1.73m2 could gain as much as 8 additional years without dialysis, while treatment beginning at a GFR of 60 mL/min per 1.73m2 could result in a gain of as much as 5 years without dialysis. Data from the AIPRD Study, evaluating the effects of ACE inhibitors on kidney function decline in individuals with predominantly non-diabetic stage 3–4 CKD, support a similar approach, again particularly true for individuals with more than 500mg to 1g of urine protein excretion daily.15 The socioeconomic effects of ACE inhibitor use in patients at risk of kidney failure can be dramatic; one study estimated the cost-effectiveness to Medicare of first-dollar coverage of ACE inhibitors for beneficiaries with diabetes and demonstrated 0.23 QALYs gained and $1606 (USD) saved per beneficiary.16
Figure 1.
Concept diagram illustrating the effect of altering the rate of kidney disease progression in individuals with CKD. The base cases are patients with diabetic nephropathy, where the rate of GFR decline may be 10 mL/min per 1.73m2 annually in the absence of treatment but can be slowed to 5 mL/min per 1.73m2 annually with treatment. Two hypothetical patients are presented – one whose diabetic kidney disease was detected at a GFR of 90 and the other whose diabetic kidney disease was detected at a GFR of 60 mL/min per 1.73m2. The light blue line illustrates expected age related decline in older individuals with normal kidney function.
People with CKD are also subject to additional risks, most notably a substantially increased risk of cardiovascular disease and death. This is most marked in the dialysis population, where the cardiovascular death risk for a 20 year-old receiving dialysis is identical to that of an 80 year-old in the general population.17 Even in earlier stages of CKD, there is a significantly increased risk of cardiovascular disease, for both de novo and recurrent cardiovascular events.18 This is particularly notable in African Americans, potentially reflecting longer duration or greater severity of traditional cardiovascular disease risk factors like hypertension and diabetes, decreased access to medical care, and/or decreased provision of primary and secondary preventative interventions.19 Once these individuals are receiving dialysis therapies, attempts to modify cardiovascular risk have largely been discouraging. This reflects several factors: 1) there are multiple competing risk factors for mortality in dialysis patients and modification of a single factor may have a limited impact on overall risk; 2) dialysis patients have been excluded from general population studies of cardiovascular interventions; and 3) few adequately powered trials have been conducted examining risk factor modification in dialysis patients. Illustrating these points are the German Diabetes and Dialysis Study (4D) and AURORA, the largest randomized medication trials in hemodialysis. 4D evaluated atorvastatin versus placebo in 1255 German hemodialysis patients with diabetes and mean LDL cholesterol level of 126 mg/dL and found no statistically significant effect of atorvastatin on the composite primary end point of cardiovascular death, nonfatal myocardial infarction, and stroke.20 Similarly, AURORA examined rosuvastatin in 2776 hemodialysis patients and found no significant difference in the primary composite endpoint of time to cardiovascular death, nonfatal myocardial infarction, or stroke.21 However, it should be noted that individuals with earlier stages of CKD who were included in general population trials of lipid lowering medications generally did gain benefit, particularly in reducing cardiovascular outcomes.22 In sum, these data suggest that intervention needs to be focused on patients with earlier stages of CKD, supporting earlier recognition of kidney disease in at-risk populations.
Aside from slowing the rate of progression, perhaps the most important intervention that can occur in patients with CKD is planning for kidney failure. Multiple studies have shown that early referral to a nephrologist (more than 3–4 months prior to needing kidney replacement therapy) is associated with better health outcomes.23–25 This also is reflected in socioeconomic costs. Earlier referral allows time for careful consideration of and planning kidney replacement modality (hemodialysis, peritoneal dialysis, preemptive transplantation) and creation of hemodialysis access prior to need should that modality be selected.
In conclusion, CKD is a major and growing public health issue of considerable socioeconomic and medical importance. Although more adequately powered and well-designed studies are urgently needed to explore interventions to optimally manage kidney disease, including pharmaceutical combinations, varying blood pressure targets, and non-pharmacologic interventions, either individually or in concert, CKD is a treatable condition. Critically, with appropriate recognition and screening of high risk individuals, targeted therapy to slow progression and treat complications before the onset of kidney failure, and adequate planning for kidney failure as it approaches, the individual and societal impacts of CKD can be lessened.
REFERENCES
- 1.Levey AS, Andreoli SP, DuBose T, Provenzano R, Collins AJ. CKD: common, harmful, and treatable--World Kidney Day 2007. Am. J. Kidney Dis. 2007;49:175–179. doi: 10.1053/j.ajkd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am. J. Kidney Dis. 2008;51:373–384. doi: 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Chadban SJ, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J. Am. Soc. Nephrol. 2003;14 Suppl 2:S131–S138. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Schoolwerth AC, Burrows NR, Williams DE, Stith KR, McClellan W. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53:522–535. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Collins AJ, et al. United States Renal Data System 2008 Annual Data Report [abstract] Am. J. Kidney Dis. 2009;53 Suppl:vi–vii. S8–S374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 8.State-specific prevalence of obesity among adults--United States, 2007. MMWR. 2008;57:765–768. [PubMed] [Google Scholar]
- 9.State-specific incidence of diabetes among adults--participating states, 1995–1997 and 2005–2007. MMWR. 2008;57:1169–1173. [PubMed] [Google Scholar]
- 10.Powe NR, Boulware LE. Population-based screening for CKD. Am. J. Kidney Dis. 2009;53 Suppl 3:S64–S70. doi: 10.1053/j.ajkd.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR. Screening for proteinuria in US adults: a cost-effectiveness analysis. JAMA. 2003;290:3101–3114. doi: 10.1001/jama.290.23.3101. [DOI] [PubMed] [Google Scholar]
- 12.Jafar TH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann. Intern Med. 2003;39:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 14.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 15.Jafar TH, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann. Intern. Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 16.Rosen AB, Hame MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-effectiveness of full medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Ann. Intern. Med. 2005;143:89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- 17.Sarnak MJ, Levey AS. Cardiovascular disease and chronic renal disease: a new paradigm. Am. J. Kidney Dis. 2000;35 Suppl 1:S117–S131. doi: 10.1016/s0272-6386(00)70239-3. [DOI] [PubMed] [Google Scholar]
- 18.Weiner DE, et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am. J. Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Weiner DE, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J. Am. Soc. Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 20.Wanner C, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 21.Fellstrom BC, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 22.Strippoli GF, et al. Effects of statins in patients with chronic kidney disease: meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avorn J, et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch. Int. Med. 2002;162:2002–2006. doi: 10.1001/archinte.162.17.2002. [DOI] [PubMed] [Google Scholar]
- 24.Kinchen KS, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann. Int. Med. 2002;137:479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 25.Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am. J. Kidney Dis. 2003;41:310–318. doi: 10.1053/ajkd.2003.50038. [DOI] [PubMed] [Google Scholar]
- 26.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39 Suppl 1:S1–S266. [PubMed] [Google Scholar]