Abstract
Background
Mungbean is an important economical crop in Asia. However, genomic research has lagged behind other crop species due to the lack of polymorphic DNA markers found in this crop. The objective of this work is to develop and characterize microsatellite or simple sequence repeat (SSR) markers from genome shotgun sequencing of mungbean.
Result
We have generated and characterized a total of 470,024 genome shotgun sequences covering 100.5 Mb of the mungbean (Vigna radiata (L.) Wilczek) genome using 454 sequencing technology. We identified 1,493 SSR motifs that could be used as potential molecular markers. Among 192 tested primer pairs in 17 mungbean accessions, 60 loci revealed polymorphism with polymorphic information content (PIC) values ranging from 0.0555 to 0.6907 with an average of 0.2594. Majority of microsatellite markers were transferable in Vigna species, whereas transferability rates were only 22.90% and 24.43% in Phaseolus vulgaris and Glycine max, respectively. We also used 16 SSR loci to evaluate phylogenetic relationship of 35 genotypes of the Asian Vigna group. The genome survey sequences were further analyzed to search for gene content. The evidence suggested 1,542 gene fragments have been sequence tagged, that fell within intersected existing gene models and shared sequence homology with other proteins in the database. Furthermore, potential microRNAs that could regulate developmental stages and environmental responses were discovered from this dataset.
Conclusion
In this report, we provided evidence of generating remarkable levels of diverse microsatellite markers and gene content from high throughput genome shotgun sequencing of the mungbean genomic DNA. The markers could be used in germplasm analysis, accessing genetic diversity and linkage mapping of mungbean.
Background
Mungbean (Vigna radiata (L.) Wilczek) is an important food leguminous crop in Asia, with an annual production of around 3.5 - 4.0 million tons [1]. The crop is grown principally for its protein-rich dry seeds (24% protein) which is a major protein source for people in Asian countries as part of a nutritionally balance diet [2]. It is popularly grown as a component in various cropping systems because of its ability to fix nitrogen in association with soil bacteria, early maturity (ca. 60 days) and relatively drought tolerance. Mungbean belongs to the genus Vigna, in which several species such as azuki bean (Vigna angularis (Wild.) Ohwi & Ohashi), bambara groundnut (Vigna subterranea (L.) Verdc.), blackgram (Vigna mungo (L.) Hepper), cowpea (Vigna unguiculata (L.) Walp.), moth bean (V. aconitifolia (Jacq.) Maréchal) and rice bean (Vigna umbellata (Thunb.) Ohwi & Ohashi), are domesticated and utilized in a similar way to mungbean.
Mungbean is a self-pollinated diploid plant with 2n = 2x = 22 chromosomes and a genome size of 515 Mb/1C [3]. Genomic study in this crop is far behind other legume crops. Mungbean was among the primary crops that genetic linkage maps have been developed. However, the current linkage maps, based on RFLP and RAPD markers of mungbean, do not resolve 11 linkage groups [4]. Microsatellites or simple sequence repeats (SSRs) are markers of choice for crop improvement of many species because they are reliable and easy to score [5]. SSRs are clusters of short tandem repeated nucleotide bases distributed throughout the genome. SSR markers are co-dominant, multi-allelic and requiring small amount of DNA for scoring. The traditional method of SSR marker development involves construction of SSR-enriched library, cloning, and sequencing, which is costly and labor intensive. Nevertheless, significant efforts have been invested in development of SSR markers in recent years, but so far only 35 polymorphic SSR markers published for mungbean [6-10]. In a study by Somta et. al. (2008), more than 200 primer pairs amplifying SSRs were tested for polymorphism among 17 mungbean accessions, only 12 (5.7%) primer pairs were polymorphic. The authors suggested that the use of SSR markers has been limited due to the lack of polymorphism in this species [7].
Over the past few years, the introduction of a massively-parallel pyrosequencing technology developed by 454 Life Sciences Technology has opened new possibilities for high-throughput genome analysis [11]. This new technology has been applied to the sequencing of microbial genomes, genotyping, genome resequencing, transcriptome profiling and methylation studies. Although, sequences generated by this technique are relatively short, there are evidences suggesting that this technique can be used to sequence plant genomes that are complex and large [12-14]. Wicker et al. (2006) suggested that 454 sequencing technology could reveal almost complete assembly of the entire gene sequences in 4 barley BAC clones at only 9-folds coverage and concluded that the method is a rapid and cost effective way of sequencing the gene-containing portions of the genome. Low coverage shotgun sequencing using 454 sequencing technology has also been used to study functional genomics in soybean [12], repetitive DNA in the pea genome [14] and transcriptome from a normalized cDNA library of Medicago truncatula [15]. Here, we report genome shotgun sequencing of the mungbean genomic DNA using 454 Life Sciences sequencing technology for isolation of SSR markers and characterization of gene content.
Results and Discussion
Shotgun sequencing of Vigna radiata genome
Sequencing of Vigna radiata genomic DNA was carried out using 454 Life Sciences technology on the Genome Sequencer (GS) FLX System. A total of 470,024 quality filtered sequence reads was generated with the average read length of 216 bases covering 100.5 Mb. All reads were deposited in NCBI Short Read Archive (ID = SRA003681) http://www.ncbi.nih.gov/Traces/. Assembly of the obtained nucleotide sequence reads was performed using the Newbler, de novo sequence assembly software [11]. Redundant reads were reduced to 46,646 contigs with the average contig length of 297 bases covering 13.85 Mb. The contig sequence data were reported in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession number BABL01000001-BABL01046645. The contig length ranges from 89 bases to 44,462 bases. The average GC content of mungbean genomic DNA generated in this study is 34.69% which is consistent with the reports on GC contents in other plant genomes such as Arapbidopsis (36% [16]), grape (34.6% [17]), poplar (33.7% [18]), tomato (36.2% [19]) and potato (35.6% [19]). It is slightly higher than the mean of GC content for intergenic regions in the Arabidopsis genome (32.9%, Genome Indices 8/04: http://http//gi.kuicr.kyoto-u.ac.jp) [20]; but it is much lower than the average GC content of Arabidopsis coding sequences (44.5%) [21].
Characterization of polymorphic microsatellite markers in Vigna radiata
We isolated 1,493 microsatellite regions using the Troll software. There were 889 dinucleotide repeats (DNPs), 282 trinucleotide repeats (TNPs), 123 tetranucleotide repeats (TTNPs), 124 pentanucleotide repeats (PNPs) and 75 SSRs with hexanucleotide repeats or more. The distribution of the number of motif repeat ranged from 4 - 30 repeats (Table 1). The most common motif type of DNPs was TA/AT (89.3% of DNPs) followed by TC/AG (7.1% of DNPs) and AC/TG (3.6% of DNPs). The GC/CG motif was not found in the data set. TNPs were found at 282 SSR loci (18.9%), which was three times lower than that of DNPs. The TAA repeat was the most common motif type found at 184 loci (65.24% of TNPs). The least frequent TNP motif was GC-rich (GCG/CGC) found at only 2 loci. The genomic SSRs with GC-rich motif repeats are rare in most plants as previously reported in rice, corn, soybean [22], wheat [23], Arabidopsis thaliana, apricot, peach [24], coffee [25] and rubber tree [26]. In contrast, the GC-rich motifs have been reported as frequent motifs in studies on development of SSR from expressed sequence tags and genomes with methylation filtration [27-30]. Thus, GC-rich SSR are most likely to be derived from the coding region of the genome. The frequency of identified SSR in mungbean was one SSR in every 67 kb (1,493 SSRs in 100.5 Mb) which is significantly lower than the SSR frequency in soybean (1/7.4 kb) [31]. Among plant species, the SSR frequencies range from 1/1.5 kb in coffee to 1/20 kb in cotton [25,31]. The observed low SSR frequency in this study is probably because a large proportion of reads from the low coverage sequencing (0.2x) of the mungbean genome were biased toward highly repetitive parts of the genome.
Table 1.
Distribution of identified SSRs using the Troll software according to SSR motif type and repeat number.
| Number of motif repeat | Di | Tri | Tetra | Penta | Hexa | Hepta | Octa |
|---|---|---|---|---|---|---|---|
| n = 4 | N/A | N/A | N/A | 97 | 45 | 14 | 2 |
| n = 5 | N/A | N/A | 89 | 20 | 4 | 2 | 0 |
| n = 6 | N/A | N/A | 23 | 4 | 3 | 1 | 0 |
| n = 7 | N/A | 142 | 9 | 2 | 0 | 0 | 0 |
| n = 8 | N/A | 75 | 1 | 0 | 0 | 0 | 0 |
| n = 9 | N/A | 36 | 0 | 0 | 0 | 1 | 1 |
| n = 10 | 137 | 17 | 1 | 1 | 1 | 0 | 1 |
| n = 11 | 115 | 4 | 0 | 0 | 0 | 0 | 0 |
| n = 12 | 80 | 3 | 0 | 0 | 0 | 0 | 0 |
| n = 13 | 50 | 1 | 0 | 0 | 0 | 0 | 0 |
| n = 14 | 59 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 15 | 50 | 2 | 0 | 0 | 0 | 0 | 0 |
| n = 16 | 46 | 1 | 0 | 0 | 0 | 0 | 0 |
| n = 17 | 45 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 18 | 49 | 1 | 0 | 0 | 0 | 0 | 0 |
| n = 19 | 43 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 20 | 48 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 21 | 32 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 22 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 23 | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 24 | 34 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 25 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 26 | 19 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 27 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 28 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 29 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| n = 30 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| total | 889 | 282 | 123 | 124 | 53 | 18 | 4 |
From 1,493 identified SSRs, 192 SSRs were identified from contigs and 1,301 SSRs were from singletons. Among 192 contigs containing SSR motifs, majority of contigs were assembled from 2 reads (87 contigs) followed by 3 reads (48 contigs) and 4 reads (16 contigs) (Table 2). By applying the Lander-Waterman model [32] to this dataset, there should be no contig assembled from more than 9 reads provided that all sequences were generated by chance from non repetitive DNA (Table 2). Therefore, 16 out of 192 contigs that were assembled from more than 9 reads are likely to represent repetitive sequences of the genome. It should be noted that loci present in multiple copies are not desirable for construction of genetic maps. Interestingly, there was a highly repetitive contig containing SSR (contig 44495) which was assembled from 3,174 raw reads. Sequence homology search revealed that contig 44495 is a fragment of the chloroplast genome. The number of chloroplast genome of higher plants can reach hundreds of copies per cell. Due to the deep sequencing nature of 454 technology, it is expected to obtain a large number of reads from sequences with multiple copies such as organellar genomes, transposons and ribosomal DNA [12]. The degree of sequencing over-representation in a repetitive genome can be estimated from the difference between the observed read coverage and the predictions from the Lander-Waterman model (Table 2) as suggested by [12]. It should be noted that the number of observed contigs with assembled reads = 2 was much lower than the prediction by the model. This was probably due to the effect of low sequencing coverage; thus it was not included in the calculation of the number of repetitive reads. In total, there were 241,410 reads (51%) present in multiple copies. We estimated that 51% of shotgun reads from 0.2× genome coverage represented repetitive DNA. This estimate is slightly more than the result from the DNA re-association kinetic study which estimated 46% of the total leaf DNA as repetitive sequences [33].
Table 2.
The table lists number of contigs containing SSRs, observed number of contigs from 454 data set, predicted number of contigs according to the Lander-Waterman model for sampling a completely non-repetitive genome and the repetitive sequences calculated using the differences between the observed number of contigs and the predictions.
| Number of reads in contigs | Predicted number of contigs by model | Observed number of contigs | repetitive reads (observed-predicted) | Observed number of contigs containing SSR |
|---|---|---|---|---|
| 2 | 59398 | 19622 | n/a | 87 |
| 3 | 10350 | 13576 | 9678 | 48 |
| 4 | 1803 | 6020 | 16868 | 16 |
| 5 | 314 | 2491 | 10885 | 6 |
| 6 | 55 | 1270 | 7290 | 10 |
| 7 | 10 | 765 | 5285 | 2 |
| 8 | 2 | 518 | 4128 | 3 |
| 9 | 1 | 363 | 3258 | 4 |
| 10 | 0 | 262 | 2620 | 1 |
| 11 | 0 | 216 | 2376 | 2 |
| 12 | 0 | 169 | 2028 | 1 |
| 13 | 0 | 149 | 1937 | 1 |
| 14 | 0 | 116 | 1624 | 1 |
| 15 | 0 | 115 | 1725 | 3 |
| ≥ 16 | 0 | 994 | 171708 | 7 |
| total | 241410 | 192 | ||
To evaluate these SSR loci in further detail, we designed 192 primer pairs to amplify all SSR loci identified from the contig data set. Among the 192 primer pairs evaluated in 17 mungbean accessions, 179 (93.23%) primer pairs were amplifiable and 127 (66.14%) primer pairs produced scorable bands. Of these, 58 primer pairs targeting 60 loci revealed polymorphism because 2 primer pairs, VR257 and VR400, were able to target 2 independent loci for each primer pair. Characteristics of all 60 loci are summarized in Additional File 1. These primer pairs were able to detect a range of 2 to 6 alleles with a mean of 2.6833 alleles per locus. Polymorphic information content (PIC) values ranged from 0.0555 to 0.6907 with an average of 0.2594 which is similar to the previous studies [7,34]. In this study, there were 33 pair-wise combinations that significantly deviated from linkage disequilibrium (LD). Genetic variation at a given locus in a population is measured by the observed heterozygosity (HO). The HO values varied from 0 to 0.6471 with the average HO of 0.0289; while the expected heterozygosity (HE) values ranged from 0.0571 to 0.7356 with the average HE of 0.2908. Tests for Hardy-Weinberg equilibrium (HWE) of the polymorphic loci revealed that all loci, except VR400, were significantly deviated from HWE (P < 0.05). This is in agreement with the previous studies in mungbean which have shown that most if not all of the loci deviated from HWE [6,7,34]. The low level of heterozygosity and significant deviation from HWE are probably because mungbean is a highly self-pollinated species with an estimated outcrossing rate of only 1.1% [35].
We also tested the SSR locus in the highly repetitive contig 44495, which was a fragment on the chloroplast genome. The VR0453 locus, located in the non-coding region near the atpB gene in the chloroplast genome, had 2 alleles and showed relatively low PIC value of 0.1046 (see Additional File 1). Chloroplast microsatellites have been used in ecological and evolutionary studies, especially at the intraspecific level, because they are nonrecombinant, uniparentally inherited and effectively haploid [36]. However, the major barrier for utilization of chloroplast microsatellites is the low mutation rates associated with the chloroplast genome [37] leading to low polymorphism level of markers in the chloroplast genome.
Sequence homology search of other loci against the Genbank non-redundant protein database and the TIGR plant repeat databases [38] revealed that there were 5 loci (VR029, VR073, VR216, VR256 and VR323) matched unknown proteins, 1 locus (VR390) matched beta-glucosidase and 1 locus (VR102) matched pectinesterase (see Additional File 1). Note that there was no sequence matched against known repeat sequences in the TIGR plant repeat databases.
Cross-species transferability of Vigna radiata microsatellite markers
With the exception of azuki bean (V. angularis), SSR markers are very limited for other Vigna species. Therefore, novel markers with high cross-species transferability rates are desirable. Cross-species amplification of the 127 microsatellite markers was assessed in 24 taxa of legumes in the tribe Phaseoleae including genus Vigna (African and Asian Vigna), Phaseolus and Glycine. One hundred and twenty five primer pairs successfully amplified DNA from more than one legume. Five primer pairs were able to amplify DNA of all legume taxa tested; while VR339 amplified only 1 legume species, V. aconitifolia. In most cases, mungbean microsatellite primers were able to amplify DNA of other Vigna species (Figure 1). The transferability rates of mungbean primers were between 45.80% (V. subterranean) and 91.60% (V. angularis). However, the amplification rate was reduced in Phaseolus vulgaris and Glycine max to 22.90% and 24.43%, respectively (Figure 1). Transferability rate of mungbean genomic microsatellite markers to other Vigna species appeared to be more or less similar to previous studies. Somta et al. (2009) reported that amplification of genic microsatellite markers in 19 taxa of Vigna species was between 80% (V. aconitifolia) to 95.3% (V. reflex-pilosa) [39]. Whereas, Chaitieng et al. (2006) reported that amplification of azuki bean (V. angularis) microsatellite markers in V. mungo, V. radiata, V. aconitifolia and V. umbellata was between 68.8 to 90.2% [40]. The high amplification rates of both mungbean and azuki bean microsatellite markers in Vigna species indicate high genome homology among species in this genus and are useful for genetics and genomics studies, especially genome mapping and comparative genomics.
Figure 1.
Cross-species amplification of 127 mungbean microsatellite markers in various species from genus Vigna, Phaseolus and Glycine. Abbreviations are as followed: Vac = V. aconitifolia, Van = V. angularis var. angularis, Van (wild) = V. angularis var. nipponensis, Var = V. aridicola, Vex = V. exilis, Vgr = V. grandiflora, Vhi = V. hirtella, Vmi = V. minima, Vmu = V. mungo var. mungo, Vum(wild) = V. mungo var. sylvestris, Vna = V. nakashimae, Vne = V. nepalensis, Vra = V. radiata var. radiate, Vra(wild) = V. radiata var. sublobata, Vst = V. stipulacea, Vsu = V. subramaniana, Vte = V. tenuicaulis, Vtr = V. trilobata, Vum = V. umbellate, Vsn = V. subterranean, Vun-Ung = V. unguiculata cv-gr. Unguiculata, Vun-Ses = V. unguiculata cv-gr. Sesquipedalis, Pha = P. vulgaris and Gly = G. max.
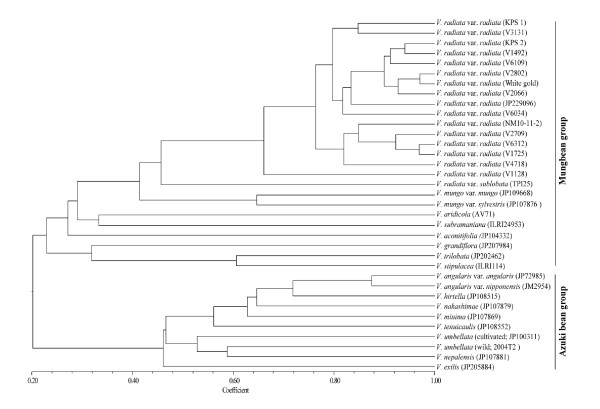
Phylogenetic relationship
To determine the genetic diversity structure and relationships between 35 genotypes of 20 taxa of Asian Vigna, polymorphism scores at 16 microsatellite loci without missing data were used (see Additional File 2). UPGMA cluster analysis was conducted using software NTSYSpc 2.2 [41]. Results from the cluster analysis revealed that all the genotypes of Asian Vigna could be clearly differentiated and classified into two groups; mungbean group and azuki bean group (Figure 2). The results were in agreement with previous studies using non-coding sequences of trnT-F [42,43]. In contrast, studies using AFLP [44], rDNA-ITS and atpB-rbcL sequences [45] recognized three groups within the Asian Vigna. In addition, it is worth noting that V. nepalensis, which has similar morphology [46] and close genetic relationship with V. angularis [43,45], was found to be highly distinct in our study. V. grandiflora previously shown to have high morphological and genetic similarity to V. radiata [46,47] was found to have closer genetic relationship with V. trilobata and V. stipulacea than V. radiate in this study. Also, V. subramaniana that was reported to be closely related to mungbean [45] appeared to be more distant from mungbean but more closely related to V. aridicola in our study. It should be noted that V. subramaniana has a complex taxonomic history, controversy in the literature and classification concerning the taxonomy of this species still remains [48]. The differences in the phylogenetic relationship of Asian Vigna may be explained by the differences in the methods used in the previous studies. Morphological traits [46], rDNA and cpDNA sequences [43,45] were used in previous studies to demonstrate phylogenetic relationship, while our study used SSR markers for demonstration. The use of PCR-based SSR markers may possibly result in size homoplasy of PCR products between/among species [49]. The same allele size of an SSR locus may contain different sequence variants; thus species sharing the same SSR allelic size include species that are identical by descent and species that have originated from convergent evolution.
Figure 2.
A dendrogram depicting genetic diversity and relationships among 35 genotypes from 20 taxa of Asian Vigna as revealed by the polymorphism of 16 mungbean microsatellite markers. Accession codes from the AVRDC-The world vegetable center and the National Institute of Agrobiological Sciences (Japan) are provided in brackets.
Sequence annotation and gene ontology
The contigs were analyzed by GeneMark.hmm eukaryotic version 3.3 [50] to predict Open Reading Frame (ORF) using Medicago trunculata as a model organism and default parameter conditions. Results from GeneMark predicted a total of 44,112 ORFs. For functional annotation, the potential coding regions were analyzed by BLAST2GO [51] leading to consistent gene annotations, assigning gene names, gene products, EC numbers and Gene Ontology (GO) numbers. Gene Ontology provides a system to categorize description of gene products according to three ontologies: molecular function, biological process and cellular component. Sequence homology search revealed that there were 1,542 ORFs matches with non-redundant protein database with an E-value cut-off at E-6. Nine hundreds and fifty sequences were mapped to one or more ontologies with multiple assignments possible for a given protein within a single ontology. There were 647 assignments made to the molecular function ontology, with a large proportion of these in catalytic (42.72%) and binding activities (44.17%) categories (Figure 3a). Under the biological process ontology, 555 assignments were made with a large proportion of assignments fell into metabolic process and cellular process (such as secretory pathway, transcription and translation) categories (Figure 3b).
Figure 3.
Gene Ontology classification of the predicted mungbean ORFs according to molecular function (a) and biological process (b) using BLAST2GO [51]with E-6 cutoff.
Similarity of mungbean predicted ORFs with other plant ESTs
To identify gene functions, the mungbean contig set was blasted (TBLASTX) to identify ESTs encoding similar proteins, at an e-value cutoff at E-6, against other plant gene indices collected in The Gene Index Databases, Dana Farber Cancer Institute, such as soybean (GMGI, 13.0), Arabidopsis (AGI, 13.0), rice (OGI, 17.0), M. truncatula (MTGI, 9.0) and Vitis vinifera (VVGI, 6.0) [52]. The number of sequences that showed similarity to encoding sequences is shown in Figure 4. Comparison between the mungbean dataset and the Glycine max gene index gave the highest number of matched sequences (7,940 sequences). V. radiata and G. max are grouped together as tropical season legumes or Phaseoloid exhibiting extensive genome conservation based on previous comparative genetic mapping [53,54]. The other Papilionoideae legume, M. truncatula, which is a cold season legume, also shares a large number of homologous sequences (5,759 sequences) with the mungbean dataset. A. thaliana and V. vinifera gave lower number of matched sequences to the mungbean dataset; 4872 and 4,949 sequences respectively. The lowest number of matched sequences (1,971 sequences) was observed when the mungbean dataset was blasted against the Oryza sativa gene index, the only monocot plant used in the comparison.
Figure 4.

Comparison of mungbean ORFs with 8 other plant gene indices by tBLASTX (e-value cutoff = E-6). Blue bars represent mungbean contigs with similar homology search against other plant gene index databases including soybean (GMGI, 13.0), Arabidopsis (AGI, 13.0), rice (OGI, 17.0), M. truncatula (MTGI, 9.0) and Vitis vinifera (VVGI, 6.0).
Discovery of microRNA
To predict functional non-coding RNA, such as microRNA, in the mungbean dataset, we made computational prediction of potential microRNA using MiRFinder to search for the potential hairpin-loop structure in their sequences [55]. Next we calculated the minimal folding free energy (MFE) using Sfold [56]. There were 2,247 microRNA candidates with MFE < -25 kcal/mol which were selected for further analysis. Then we blasted the mungbean microRNA candidates against previously known microRNAs from Arabidopsis, rice, and other plant species to search for potentially conserved microRNAs. A total of 4 miRNA candidates had sequence homology with miR171, miR408, miR1171 and miR414, which have been shown to target genes coding for SCARECROW-like proteins implicated in radial root pattern [57], plantacyanin [58], copper chaperone [59] and translation initiation factor [60], respectively (Table 3).
Table 3.
Results from homology search of the mungbean microRNA candidates against the microRNA database.
| Read name | Contig | miRNA family | MFE (kcal/mol) | Target | Ref |
|---|---|---|---|---|---|
| E4UUDJH02I4UG8 | contig25352 | miR171 | -37 | SCARECROW-like protein | Reinhart et al., 2002 |
| E4UUDJH02HINHM | contig16040 | miR408 | -29 | plantacyanin | Sunkar and Zhu, 2004 |
| E4UUDJH01ECGTX | contig11544 | miR1171 | -25 | putative copper chaperone3 | Molnar et al., 2007 |
| E4UUDJH01AVSEX | contig25342 | miR414 | -26 | TIF3H1 | Fattash et al., 2007 |
microRNA families and their target genes are also present in the table.
Conclusion
The results provided by the present study highlight a reliable and efficient way in obtaining polymorphic microsatellite markers and characterization of putative genes using shotgun genome sequences of Vigna radiata. A significance of the results from this study is that high-throughput shotgun sequences of mungbean can be useful not only for marker development, construction of linkage map, mungbean genetic improvement, phylogenetic relationship, but also for gene discovery as the paucity of DNA markers in cultivated mungbean has precluded detailed genetic research on this crop.
Methods
Plant materials, DNA extraction and 454 Life Sciences Sequencing
Seventeen accessions of mungbean (Vigna radiata) and the other 23 taxa of legumes in the tribe Phaseoleae including genus Vigna (African Vigna and Asian Vigna), Phaseolus and Glycine as listed in Additional File 3 were used in this study. For sequencing, DNA was extracted from young leaf tissue of mungbean cultivar "Kamphaeng Saen 1" using DNAeasy Plant Mini Kit (Qiagen). For SSR analysis, DNA of all plant materials was extracted from young fresh leaves using CTAB method [61]. The concentration of each sample was calculated from OD measurement and the samples were separated by gel electrophoresis on 0.8% agarose gels. The sequencing was performed using the GS-FLX instrument (454 Life Sciences, Branford, CT) and yielded 470,024 quality filtered sequence reads with the average length of 216 bp. The reads were deposited into NCBI Short Read Archive.
Prediction of sequencing coverage in contigs from a completely non repetitive genome was calculated according to the Lander and Waterman model [32]. The number of contigs expected containing a number of reads j is given by equation 1.
 |
Where N is the number of reads, L is the read length, G is the haploid genome size in base pairs, and T is the base pair overlap required for contig formation (in this case T = 40).
Isolation, amplification and transferability of SSR markers
In order to identify microsatellite markers, non-redundant sequences were screened for SSRs using TROLL software http://wsmartins.net/webtroll/troll.html. For the searches, we defined SSRs as being DNP ≥ 14 bases; TNP ≥ 15 bases; TTNP ≥ 16 bases; HNP (and more) ≥ 16 bases [31]. For comparison of SSRs in plant genomic sequences, we used the criteria of SSR motif of ≥ 20 bases [31,62,63]. Primer pairs were designed to amplify microsatellite regions using PRIMER3 [64]. PCR was carried out in a total volume of 10 μL containing 2 ng of DNA template, 1× Taq buffer, 2 mM MgCl2, 0.2 mM dNTPs, 1 U Taq DNA polymerase (Fermentas) and 0.5 μM each of forward and reverse primers. Amplification was performed in a GeneAmp PCR 9700 System thermocycler (Applied Biosystems) programmed as follow: 94°C for 2 min followed by 35 cycles of 94°C for 30 s, 50-65°C for 30 s, 72°C for 1 min, and a final extension step at 72°C for 10 min. Amplified products were separated on 5% denaturing polyacrylamide gels and visualized by silver-staining.
Analysis of polymorphic loci
Seventeen mungbean genotypes as listed in Additional File 3 were used for polymorphism analysis of SSR markers. Details of primer pairs for SSR markers are listed in Additional File 4. Scoring data from polymorphic loci were used to calculate Polymorphism Information Content (PIC) [65], Hardy-Weinberg equilibrium (HWE) [66], pairwise linkage disequilibrium (LD) using chi-square test, and observed heterozygosity and expected heterozygosity using the PowerMarker 3.25 software [67].
Cross taxa transferability and phylogenetic relationship
The cross taxa transferability of all scorable 127 SSR loci was evaluated using 17 accessions of mungbean (Vigna radiata) and the other 23 taxa of legumes in the tribe Phaseoleae including genus Vigna (African Vigna and Asian Vigna), Phaseolus and Glycine (see Additional File 3). The percentage of transferability was calculated for each taxon (23 taxa) in which the detected fragment/the total number of loci analyzed. A genetic similarity matrix (see Additional File 2) was prepared for 35 genotypes from 20 taxa at 16 SSR loci (as listed in Additiional File 4). UPGMA (unweighted pair group method with arithmetic mean) cluster analysis was conducted using software NTSYSpc 2.2 [41].
Analysis of gene content and annotation
The mungbean contig set was analyzed in two parts which are 1) gene prediction/Gene Ontology (GO) term annotation and 2) functional gene identification. GeneMark.hmm eukaryotic version 3.3 [50] based on Hidden Markov Models was used to predict coding sequence (cds) of the contig set using Medicago trunculata as a model organism and default parameter conditions. For the functional annotation, the potential coding sequences were analyzed by BLAST2GO [51]. To identify gene functions, sequence similarity search program-BLAST was used to identify ESTs encoding similar proteins of the mungbean contig set. All 46,646 contigs were blasted (TBLASTX) with the threshold E-value cutoff at 1e-6 against 580,213 assembled Unique Transcripts sequences from various plant species from The Plant Genome DataBase (PlantGDB) [52], which included Arabidopsis thaliana (324,630), Glycine max (105,862), Medicago truncatula (57,231), Oryza sativa (44,644), and Vitis vinifera (47,846).
Authors' contributions
ST conceived of the study together with the other authors, carried out the major part of the experiments, analyzed the results and drafted the manuscript. PS, WS and WM prepared plant materials and performed genetic analysis. DS participated in library construction and sequencing. PU and JC participated in analysis of the results. PS and ST participated in coordination and analysis of the results. All authors participated in writing the final manuscript. All authors read and approved the final manuscript.
Supplementary Material
Characteristics of 58 primer pairs targeting 60 polymorphic microsatellite loci analyzed in 17 accessions of mungbean (16 cultivated and 1 wild mungbean) as listed in Additional File 3. PCR conditions and electrophoresis were described in Somta et al. (2008) [7]. Polymorphism information content (PIC), observed heterozygosity, expected heterozygosity, Hardy-Weinberg equilibrium (HWE), pair-wise and linkage disequilibrium (LD) of polymorphic loci were calculated using software POWERMARKER 3.25 [67].
Dice's Similarity Matrix. This additional file contains a table expressing the Dice's Similarity Matrix.
Plants used in this study. This additional file contains a table listing all of the plants used in the study.
SSRs used in the phylogenetic study. This additional file contains a table showing the SSRs used in the phylogenetic study.
Contributor Information
Sithichoke Tangphatsornruang, Email: sithichoke.tan@biotec.or.th.
Prakit Somta, Email: pksomta@gmail.com.
Pichahpuk Uthaipaisanwong, Email: pichahpuk.uth@biotec.or.th.
Juntima Chanprasert, Email: juntima.cha@biotec.or.th.
Duangjai Sangsrakru, Email: duangjai@biotec.or.th.
Worapa Seehalak, Email: wseehalak@hotmail.com.
Warunee Sommanas, Email: som.warunee@gmail.com.
Somvong Tragoonrung, Email: somvong.tra@biotec.or.th.
Peerasak Srinives, Email: agrpss@yahoo.com.
Acknowledgements
We acknowledge Dr. Piyanot Wirachsilp and the support by the Genome Institute, the National Center for Genetic Engineering and Biotechnology (Thailand), the National Science and Technology Development Agency (Thailand) and the Center for Agricultural Biotechnology, Kasetsart University, Kamphaeng Saen Campus through the Project on Biotechnology for Varietal Development of Thai Mungbean. We are thankful to Dr. Norihiko Tomooka of the National Institute of Agrobiological Sciences, Japan for providing Vigna germplasm (JP number) used in this study and Dr. Darin Kongkasuriyachai for reviewing the manuscript.
References
- Weinberger K. Impact analysis on mungbean research in south and southeast Asia. AVRDC Processing No 9991175. Shanhua, Taiwan. 2003.
- Poehlman JM. The mungbean. New Delhi: Oxford & IBH Publishing Co. PVT. Ltd; 1991. [Google Scholar]
- Parida A, Raina SN, Narayan RKJ. Quantitative DNA variation between and within chromosome complements of Vigna species (Fabaceae) Genetica. 1990;82:125–133. doi: 10.1007/BF00124642. [DOI] [Google Scholar]
- Humphry E, Konduri V, Lambrides J, Magner T, McIntyre L, Aitken B, Liu J. Development of a mungbean (Vigna radiata) RFLP linkage map and its comparison with lablab (Lablab purpureus) reveals a high level of colinearity between the two genomes. Theor Appl Genet. 2002;105(1):160–166. doi: 10.1007/s00122-002-0909-1. [DOI] [PubMed] [Google Scholar]
- Gupta P, Varshney R. The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat. Euphytica. 2000;113:163–185. doi: 10.1023/A:1003910819967. [DOI] [Google Scholar]
- Gwag JG, Chung JW, Chung HK, Lee JH, Ma KH, Dixit A, Park YJ, Cho EG, Kim TS, Lee SH. Characterization of new microsatellite markers in mung bean, Vigna radiata (L.) Molecular Ecology Notes. 2006;6(4):1132–1134. doi: 10.1111/j.1471-8286.2006.01461.x. [DOI] [Google Scholar]
- Somta P, Musch W, Kongsamai B, Chanprame S, Nakasathien S, Toojinda T, Sorajjapinun W, Seehalak W, Tragoonrung S, Srinives P. New microsatellite markers isolated from mungbean (Vigna radiata (L.) Wilczek) Mol Ecol Resource. 2008;8:1155–1157. doi: 10.1111/j.1755-0998.2008.02219.x. [DOI] [PubMed] [Google Scholar]
- Kumar SV, Tan SG, Quah SC, Yusoff K. Isolation of microsatellite markers in mungbean, Vigna radiata . Molecular Ecology Notes. 2002;2(2):96–98. doi: 10.1046/j.1471-8286.2002.00158.x. [DOI] [Google Scholar]
- Kumar SV, Tan SG, Quah SC, Yusoff K. Isolation and characterization of seven tetranucleotide microsatellite loci in mungbean, Vigna radiata . Molecular Ecology Notes. 2002;2(3):293–295. doi: 10.1046/j.1471-8286.2002.00239.x. [DOI] [Google Scholar]
- Miyagi M, Humphry M, Ma ZY, Lambrides CJ, Bateson M, Liu CJ. Construction of bacterial artificial chromosome libraries and their application in developing PCR-based markers closely linked to a major locus conditioning bruchid resistance in mungbean (Vigna radiata L. Wilczek) Theor Appl Genet. 2004;110(1):151–156. doi: 10.1007/s00122-004-1821-7. [DOI] [PubMed] [Google Scholar]
- Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437(7057):376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan K, Varala K, Hudson ME. Global repeat discovery and estimation of genomic copy number in a large, complex genome using a high-throughput 454 sequence survey. BMC Genomics. 2007;8:132. doi: 10.1186/1471-2164-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Schlagenhauf E, Graner A, Close TJ, Keller B, Stein N. 454 sequencing put to the test using the complex genome of barley. BMC Genomics. 2006;7:275. doi: 10.1186/1471-2164-7-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macas J, Neumann P, Navratilova A. Repetitive DNA in the pea (Pisum sativum L.) genome: comprehensive characterization using 454 sequencing and comparison to soybean and Medicago truncatula. BMC Genomics. 2007;8:427. doi: 10.1186/1471-2164-8-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung F, Haas BJ, Goldberg SM, May GD, Xiao Y, Town CD. Sequencing Medicago truncatula expressed sequenced tags using 454 Life Sciences technology. BMC Genomics. 2006;7:272. doi: 10.1186/1471-2164-7-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature. 2000;408(6814):796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449(7161):463–467. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313(5793):1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- Zhu W, Ouyang S, Iovene M, O'Brien K, Vuong H, Jiang J, Buell CR. Analysis of 90 Mb of the potato genome reveals conservation of gene structures and order with tomato but divergence in repetitive sequence composition. BMC Genomics. 2008;9:286. doi: 10.1186/1471-2164-9-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BC, Rapaka L, Lyons E, Pedersen B, Freeling M. Arabidopsis intragenomic conserved noncoding sequence. Proc Natl Acad Sci USA. 2007;104(9):3348–3353. doi: 10.1073/pnas.0611574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Hickey DA. Rapid divergence of codon usage patterns within the rice genome. BMC Evol Biol. 2007;7(Suppl 1):S6. doi: 10.1186/1471-2148-7-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LF, Tang JF, Li HW, Jia JZ. Analysis of microsatellites in major crops assessed by computational and experimental approaches. Molecular Breeding. 2003;12(3):245–261. doi: 10.1023/A:1026346121217. [DOI] [Google Scholar]
- Nicot N, Chiquet V, Gandon B, Amilhat L, Legeai F, Leroy P, Bernard M, Sourdille P. Study of simple sequence repeat (SSR) markers from wheat expressed sequence tags (ESTs) Theoretical and Applied Genetics. 2004;109(4):800–805. doi: 10.1007/s00122-004-1685-x. [DOI] [PubMed] [Google Scholar]
- Jung S, Abbott A, Jesudurai C, Tomkins J, Main D. Frequency, type, distribution and annotation of simple sequence repeats in Rosaceae ESTs. Funct Integr Genomics. 2005;5(3):136–143. doi: 10.1007/s10142-005-0139-0. [DOI] [PubMed] [Google Scholar]
- Aggarwal RK, Hendre PS, Varshney RK, Bhat PR, Krishnakumar V, Singh L. Identification, characterization and utilization of EST-derived genic microsatellite markers for genome analyses of coffee and related species. Theoretical and Applied Genetics. 2007;114(2):359–372. doi: 10.1007/s00122-006-0440-x. [DOI] [PubMed] [Google Scholar]
- Feng SP, Li WG, Huang HS, Wang JY, Wu YT. Development, characterization and cross-species/genera transferability of EST-SSR markers for rubber tree (Hevea brasiliensis) Molecular Breeding. 2009;23(1):85–97. doi: 10.1007/s11032-008-9216-0. [DOI] [Google Scholar]
- Yonemaru J, Ando T, Mizubayashi T, Kasuga S, Matsumoto T, Yano M. Development of genome-wide simple sequence repeat markers using whole-genome shotgun sequences of sorghum (Sorghum bicolor (L.) Moench) DNA Res. 2009;16(3):187–193. doi: 10.1093/dnares/dsp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell JA, Budiman MA, Nunberg A, Citek RW, Robbins D, Jones J, Flick E, Rholfing T, Fries J, Bradford K. Sorghum genome sequencing by methylation filtration. PLoS Biol. 2005;3(1):e13. doi: 10.1371/journal.pbio.0030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JK, Dake TM, Singh S, Benscher D, Li W, Gill B, Sorrells ME. Development and mapping of EST-derived simple sequence repeat markers for hexaploid wheat. Genome. 2004;47(5):805–818. doi: 10.1139/g04-057. [DOI] [PubMed] [Google Scholar]
- Asp T, Frei UK, Didion T, Nielsen KK, Lubberstedt T. Frequency, type, and distribution of EST-SSRs from three genotypes of Lolium perenne, and their conservation across orthologous sequences of Festuca arundinacea, Brachypodium distachyon, and Oryza sativa. BMC Plant Biol. 2007;7:36. doi: 10.1186/1471-2229-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardle L, Ramsay L, Milbourne D, Macaulay M, Marshall D. Computational and experimental characterization of physically clustered simple sequence repeats in plants. Genetics. 2000;156:847–854. doi: 10.1093/genetics/156.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Waterman MS. Genomic mapping by fingerprinting random clones: a mathematical analysis. Genomics. 1988;2(3):231–239. doi: 10.1016/0888-7543(88)90007-9. [DOI] [PubMed] [Google Scholar]
- Murray MG, Palmer JD, Cuellar RE, Thompson WF. Deoxyribonucleic acid sequence organisation in the mungbean genome. Biochemistry. 1979;18:5259–5266. doi: 10.1021/bi00590a034. [DOI] [PubMed] [Google Scholar]
- Seehalak W, Somta P, Sommanas W, Srinives P. Microsatellite markers for mungbean developed from sequence database. Mol Ecol Resour. 2009;9:862–864. doi: 10.1111/j.1755-0998.2009.02655.x. [DOI] [PubMed] [Google Scholar]
- Sangsiri C, Kaga A, Tomooka N, Vaughan D, Srinives P. Genetic diversity of the mungbean (Vigna radiata, Leguminosae) genepool based on microsatellite analysis. Aust J Bot. 2007;55:837–847. doi: 10.1071/BT07105. [DOI] [Google Scholar]
- Provan J, Powell W, Hollingsworth PM. Chloroplast microsatellites: new tools for studies in plant ecology and evolution. Trends Ecol Evol. 2001;16:142–147. doi: 10.1016/S0169-5347(00)02097-8. [DOI] [PubMed] [Google Scholar]
- Wolfe KH, Li WH, Sharp PM. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA. 1987;84(24):9054–9058. doi: 10.1073/pnas.84.24.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang S, Buell CR. The TIGR plant repeat databases: a collective resource for the identification of repetitive sequences in plant. Nucleic Acids Research. 2004;32:360–363. doi: 10.1093/nar/gkh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somta P, Seehalak W, Srinives P. Development, characterization and cross-species amplification of mungbean (Vigna radiata) genic microsatellite markers. Conserv Genet. 2009. http://www.springerlink.com/content/01738786115x452g/
- Chaitieng B, Kaga A, Tomooka N, Isemura T, Kuroda Y, Vaughan DA. Development of a black gram [Vigna mungo (L.) Hepper] linkage map and its comparison with an azuki bean [Vigna angularis (Willd.) Ohwi and Ohashi] linkage map. Theor Appl Genet. 2006;113(7):1261–1269. doi: 10.1007/s00122-006-0380-5. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ NTSYS-pc: numerical taxonomy and multivariate analysis system version 2.2 2005NewYork: Exeter Publishing Ltd; 16270455 [Google Scholar]
- Yano A, Yasuda K, Yamaguchi H. A test for molecular identification of Japanese archaeological beans and phylogenetic relationship of wild and cultivated species of subgenus Ceratotropis (Genus Vigna, Papilionaceae) using sequence variation in two non-coding regions of the trnL and trnF genes. Economic Botany. 2004;58:S135–S146. doi: 10.1663/0013-0001(2004)58[S135:ATFMIO]2.0.CO;2. [DOI] [Google Scholar]
- Ye TT, Yamaguchi H. Phylogenetic relationship of wild and cultivated Vigna (subgenus Ceratotropis, Fabaceae) from Myanmar based on sequence variations in non-coding regions of trnT-F. Breed Sci. 2007;57:271–280. doi: 10.1270/jsbbs.57.271. [DOI] [Google Scholar]
- Tomooka N, Yoon MS, Doi K, Kaga A, Vaughan D. AFLP analysis of diploid species in the genus Vigna subgenus Ceratotropis. Genet Resour Crop Ev. 2002;49(5):521–530. doi: 10.1023/A:1020954800107. [DOI] [Google Scholar]
- Doi K, Kaga A, Tomooka N, Vaughan DA. Molecular phylogeny of genus Vigna subgenus Ceratotropis based on rDNA ITS and atpB-rbcL intergenic spacer of cpDNA sequences. Genetica. 2002;114(2):129–145. doi: 10.1023/A:1015158408227. [DOI] [PubMed] [Google Scholar]
- Tomooka N, Vaughan DA, Moss H, Maxted N. The Asian Vigna: Genus Vigna Subgenus Ceratotropis Genetic Resources. Kluwer, Dordrecht; 2002. [Google Scholar]
- Seehalak W, Tomooka N, Waranyuwat P, Thipyapong P, Laosuwan P, Kaga A, Vaughan DA. Genetic diversity of the Vigna germplasm from Thailand and neighboring regions revealed by AFLP analysis. Genet Resour Crop Evol. 2006;53:1043–1059. doi: 10.1007/s10722-004-7939-2. [DOI] [Google Scholar]
- Tomooka N, Kaga A, Vaughan D. Plant Genome Diversity and Evolution. Enfield: Science Publishers; 2006. The Asian Vigna (Vigna subgenus Ceratotropis) biodiversity and evolution. [Google Scholar]
- Garza JC, Slatkin M, Freimer NB. Microsatellite allele frequencies in humans and chimpanzees, with implications for constraints on allele size. Mol Biol Evol. 1995;12(4):594–603. doi: 10.1093/oxfordjournals.molbev.a040239. [DOI] [PubMed] [Google Scholar]
- Lukashin AV, Borodovsky M. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res. 1998;26(4):1107–1115. doi: 10.1093/nar/26.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Duvick J, Fu A, Muppirala U, Sabharwal M, Wilkerson MD, Lawrence CJ, Lushbough C, Brendel V. PlantGDB: a resource for comparative plant genomics. Nucleic Acids Res. 2008. pp. D959–965. [DOI] [PMC free article] [PubMed]
- Boutin SR, Young ND, Olson TC, Yu ZH, Vallejos CE, Shoemaker RC. Genome conservation among three legume genera detected with DNA markers. Genome. 1995;38(5):928–937. doi: 10.1139/g95-122. [DOI] [PubMed] [Google Scholar]
- Choi HK, Mun JH, Kim DJ, Zhu H, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss GB. Estimating genome conservation between crop and model legume species. Proc Natl Acad Sci USA. 2004;101(43):15289–15294. doi: 10.1073/pnas.0402251101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TH, Fan B, Rothschild MF, Hu ZL, Li K, Zhao SH. MiRFinder: an improved approach and software implementation for genome-wide fast microRNA precursor scans. Bmc Bioinformatics. 2007;8:341. doi: 10.1186/1471-2105-8-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Chan CY, Lawrence CE. Sfold web server for statistical folding and rational design of nucleic acids. Nucleic Acids Res. 2004;32:W135–W141. doi: 10.1093/nar/gkh449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes Dev. 2002;16(13):1616–1626. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Zhu JK. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16(8):2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Schwach F, Studholme DJ, Thuenemann EC, Baulcombe DC. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii . Nature. 2007;447(7148):1126–1129. doi: 10.1038/nature05903. [DOI] [PubMed] [Google Scholar]
- Fattash I, Voss B, Reski R, Hess WR, Frank W. Evidence for the rapid expansion of microRNA-mediated regulation in early land plant evolution. BMC Plant Biol. 2007;7:13. doi: 10.1186/1471-2229-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Weber JL, Zhong G, Tanksley SD. Survey of plant short tandem repeats. Theor Appl Genet. 1994;88:1–6. doi: 10.1007/BF00222386. [DOI] [PubMed] [Google Scholar]
- Lagercrantz U, Ellegren H, Andersson L. The abundance of various polymorphic microsatellite motifs differs between plants and vertebrates. Nucleic Acids Res. 1993;21(5):1111–1115. doi: 10.1093/nar/21.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Botstein D, White RL, Skalnick MH, Davies RW. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48(2):361–372. doi: 10.2307/2532296. [DOI] [PubMed] [Google Scholar]
- Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21(9):2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of 58 primer pairs targeting 60 polymorphic microsatellite loci analyzed in 17 accessions of mungbean (16 cultivated and 1 wild mungbean) as listed in Additional File 3. PCR conditions and electrophoresis were described in Somta et al. (2008) [7]. Polymorphism information content (PIC), observed heterozygosity, expected heterozygosity, Hardy-Weinberg equilibrium (HWE), pair-wise and linkage disequilibrium (LD) of polymorphic loci were calculated using software POWERMARKER 3.25 [67].
Dice's Similarity Matrix. This additional file contains a table expressing the Dice's Similarity Matrix.
Plants used in this study. This additional file contains a table listing all of the plants used in the study.
SSRs used in the phylogenetic study. This additional file contains a table showing the SSRs used in the phylogenetic study.