Abstract
Background
Secreted phospholipases A2 (sPLA2s) are released in plasma and other biologic fluids of patients with inflammatory, autoimmune, and allergic diseases.
Objective
We sought to evaluate sPLA2 activity in the bronchoalveolar lavage fluid (BALF) of asthmatic patients and to examine the expression and release of sPLA2s from primary human lung mast cells (HLMCs).
Methods
sPLA2 activity was measured in BALF and supernatants of either unstimulated or anti-IgE–activated HLMCs as hydrolysis of oleic acid from radiolabeled Escherichia coli membranes. Expression of sPLA2s was examined by using RT-PCR. The release of cysteinyl leukotriene (LT) C4 was measured by means of enzyme immunoassay.
Results
Phospholipase A2 (PLA2) activity was higher in the BALF of asthmatic patients than in the control group. BALF PLA2 activity was blocked by the sPLA2 inhibitors dithiothreitol and Me-Indoxam but not by the cytosolic PLA2 inhibitor AZ-1. HLMCs spontaneously released a PLA2 activity that was increased on stimulation with anti-IgE. This PLA2 activity was blocked by dithiothreitol and Me-Indoxam but not by AZ-1. HLMCs constitutively express mRNA for group IB, IIA, IID, IIE, IIF, III, V, X, XIIA, and XIIB sPLA2s. Anti-IgE did not modify the expression of sPLA2s. The cell-impermeable inhibitor Me-Indoxam significantly reduced (up to 40%) the production of LTC4 from anti-IgE–stimulated HLMCs.
Conclusions
sPLA2 activity is increased in the airways of asthmatic patients. HLMCs express multiple sPLA2s and release 1 or more of them when activated by anti-IgE. The sPLA2s released by mast cells contribute to LTC4 production by acting in an autocrine fashion. Mast cells can be a source of sPLA2s in the airways of asthmatic patients.
Keywords: Lung mast cells, secreted phospholipase A2, leukotriene C4, arachidonic acid
Phospholipases A2 (PLA2s) are key enzymes involved in the mobilization of arachidonic acid from membrane phospholipids.1 This is the initial step in the metabolic cascade, leading to the synthesis of eicosanoids (prostaglandins, leukotrienes [LTs], and other). PLA2s thought to play a role in arachidonic acid release are currently classified as high-molecular-weight cytosolic phospholipases A2 (cPLA2s) and low-molecular-weight secreted PLA2s (sPLA2s).1
Ten isoforms of sPLA2s have been identified in human cells and tissues.2 sPLA2s are released in extracellular fluids during local or systemic inflammation.3 In addition, it has been previously shown that sPLA2 activity is detectable in the bronchoalveolar lavage fluid (BALF) of healthy individuals.4 This activity is increased in the airways of patients with inflammatory lung diseases (pneumonia, adult respiratory distress syndrome, and sarcoidosis).3 Moreover, sPLA2 activity is also increased in the BALF of patients with bronchial asthma5,6 and in the nasal fluid of patients with allergic rhinitis after local allergen challenge.7 These observations indicate that sPLA2 enzymes can be released during allergic reactions in both the upper and lower airways. However, these studies did not provide information on the cellular sources of these enzymes.
The role of sPLA2 isoforms in airway inflammation has been investigated in rodents. For example, several sPLA2s, such as GIIA, GIID, GIIE, GV, and GX, are overexpressed in lung biopsy specimens in experimentally induced pulmonary inflammation.8,9 In particular, GX, the isoform with the highest phospholipolytic activity in mammalian cells in vitro,10,11 is constitutively expressed in the lung.8,9 Interestingly, GX expression did not change on LPS- or carrageenin-induced lung inflammation,8 whereas it was significantly increased in the airways of mice with ovalbumin-induced asthma.9 Moreover, knocking out GX reduced all the histologic and functional features associated with the inflammatory response and airway remodeling in this model of asthma. These studies demonstrated that certain sPLA2s play an important role in the pathogenesis of inflammatory and allergic diseases of the lung.
The expression of sPLA2 isoforms in the upper and lower airways has been examined in patients with chronic rhinosinusitis or pneumonia.12,13 Immunohistochemistry revealed that low levels of human GIIA (hGIIA) were expressed in the nasal epithelium and submucosal glands of healthy donors, whereas the expression of this sPLA2 was increased in patients with rhinosinusitis.12 In the lung only human GX (hGX) was detected in bronchial epithelial cells and subepithelial interstitium of both healthy donors and patients with pneumonia.13 In inflamed, but not normal, lung tissue hGIIAwas found in vascular smooth muscle cells and bronchial chondrocytes, whereas human GIID, GV, and GX were found in epithelial cells and macrophages.13 Immunostaining analysis of cells from induced sputum demonstrated that hGX was expressed by bronchial epithelial cells and macrophages in healthy donors and patients with asthma.14 However, in asthmatic patients the expression of hGX was significantly higher than in healthy subjects and was further increased during exercise-induced bronchoconstriction. These observations indicate that sPLA2 expression is upregulated in human airways during inflammatory and allergic disorders and suggest that cells resident in the lung might produce distinct sPLA2s.
Mast cells play a primary role in the pathogenesis of bronchial asthma and rhinitis.15 These cells can be activated by IgE– and non-IgE–mediated stimuli to release a variety of preformed and de novo synthesized proinflammatory mediators.16 Mast cells are particularly abundant at the body’s interface with the external environment, such as the mucosae of the respiratory and gastrointestinal tracts and the skin.17 This unique location justifies the important role of mast cells in allergic inflammation, as well as innate immunity and host defense against infections.16–18
Studies on the expression of sPLA2s in mast cells have been primarily carried out in mice. Enomoto et al19 showed that bone marrow–derived mast cells (BMMCs) from BALB/cJ and C57BL/6J mice express all members of the group II subfamily of sPLA2s, including GIIC, GIID, GIIE, GIIF, and GV. GIIA is expressed in BALB/cJ but not in C57BL/6J mast cells because the latter strain has a natural disruption of the gene encoding for GIIA. BMMCs from either strains do not express GIB and GX sPLA2s.19 This and other studies20 indicate that the expression pattern of sPLA2 isoforms differs in mast cells with different phenotypes and from different animal species.
Marked biochemical and functional differences exist between murine and human mast cells, and in many cases information on cell activation and mediator production obtained in murine models was not confirmed in human mast cells.21 Data on sPLA2 expression in human mast cells are scarce because of the limited number of cells detectable in biopsy specimens or retrieved from biologic fluids. Immunohistochemistry studies demonstrated that human synovial22 and gut23 mast cells express hGIIA. However, there are no data on the expression and function of sPLA2s in mast cells purified ex vivo from human tissues. In this study we provide evidence that human lung mast cells (HLMCs) express mRNA for several sPLA2s and release a PLA2 activity with biochemical and pharmacologic characteristics similar to that of the PLA2s secreted in the airways of patients with bronchial asthma.
METHODS
Reagents
Percoll, dimethyl sulfoxide, L-glutamine, antibiotic-antimycotic solution (10,000 IU/mL penicillin, 10 mg/mL streptomycin, and 25 μg/mL amphotericin B), and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma (St Louis, Mo). Dithiothreitol (DTT) was from MP Biomedicals (Solon, Calif). Me-Indoxam and AZ-1 were prepared as previously described.11,24 Tritiated oleic acid (OA)–labeled Escherichia coli membranes were kindly provided by Dr Gianfrancesco Goracci (University of Perugia, Perugia, Italy). The rabbit anti-human Fce antibody was donated by Drs T. Ishizaka and K. Ishizaka (La Jolla Institute for Allergy and Immunology, La Jolla, Calif).
Study population
Bronchoalveolar lavage was performed in 14 patients with mild persistent asthma and 19 nonasthmatic subjects (see the Methods section in this article’s Online Repository at www.jacionline.org). The study protocol was approved by the Ethical Committee of the University of Naples Federico II, and informed consent was obtained from each subject before bronchoscopy.
Bronchoalveolar lavage procedure
Bronchoscopy and bronchoalveolar lavage were performed according to a standardized protocol based on current National Heart, Lung, and Blood Institute guidelines (see also the Methods section in this article’s Online Repository).25
Cell isolation
The study protocol involving the use of human lung tissue was approved by the Ethical Committee of the University of Naples Federico II, and informed consent was obtained from patients undergoing thoracic surgery. Human mast cells were obtained from the lungs of patients undergoing thoracic surgery and were purified (>98%) by means of immunomagnetic selection, as previously described (see also the Methods section in this article’s Online Repository).26
Cell incubation
Mast cells suspended in PCG buffer26 (106/mL) were incubated (at 37°C for 15–120 minutes) with anti-IgE (0.03–1 μg/mL). For LTC4 production, the cells (105/mL) were preincubated (at 37°C for 15 minutes) with increasing concentrations (0.01–10 μmol/L) of Me-Indoxam or AZ-1 before stimulation (at 37°C for 30 minutes). The reactions were stopped by means of centrifugation (at 800g for 5 minutes at 4°C), and the cell-free supernatant was stored at −80°C for determination of PLA2 activity, histamine release, LTC4 production, or β-hexosaminidase release. The cell pellets were lysed with freeze-thaw cycles in distilled water, and aliquots were stored at −80°C for determination of total content of histamine or β-hexosaminidase.
PLA2 assay
PLA2 activity in BALF and HLMC supernatants was measured as previously described27 by using tritiated OA–labeled E coli membranes. PLA2 activity was determined in 50 mmol/L Tris HCl (pH 7.5) and 10 mmol/L CaCl2 in a total volume of 1.0 mL. The reaction was initiated by the addition of 0.1 μCi of tritiated OA–labeled E coli membranes. At the end of incubation (90 minutes at 37°C), the reaction was stopped by adding 2 mL of methanol, 1 mL of chloroform, and 50 μL of 9% formic acid, and lipids were extracted and separated by means of thin-layer chromatography. Tritiated OA was measured by means of liquid scintillation counting (Tri-Carb 2800 TR; PerkinElmer, Waltham, Mass), and PLA2 activity was expressed as picomoles of tritiated OA released per minute per milliliter of BALF or HLMC supernatants. Aliquots of BALF or HLMC supernatants were incubated (for 1 hour at 37°C) with 10 mmol/L DTT, 10 μmol/L Me-Indoxam, 10 μmol/L AZ-1, or 2 mmol/L PMSF before PLA2 assay to examine the effect of various inhibitors on PLA2 activity.
Mediator release assays
Histamine was measured in duplicate determinations by using a commercially available enzyme immunoassay (Immunotech, Praha, Czech Republic). β-Hexosaminidase was measured in duplicate determinations by using a colorimetric assay.28 LTC4 was measured in mast cell supernatants in duplicate determinations with a commercially available enzyme immunoassay (GE Healthcare, Fairfield, Conn). The linearity range of this assay was 15 to 1,000 pg/mL. Inhibition of LTC4 production was expressed as a percentage of maximum response calculated as follows: (R−Rb)/(Rmax−Rb)×100, where R is the release in samples treated with the inhibitor, Rb is the release in unstimulated samples, and Rmax is the release in samples stimulated in the absence of the inhibitor.
RT-PCR for sPLA2s
Total RNA from HLMCs was extracted by using the SV total RNA isolation system (Promega, Madison, Wis), treated with RNase-free DNase I, and suspended in diethylpyrocarbonate-treated (DEPC) water. RNA concentration and quality were assessed by means of spectroscopy. One microgram of total RNA was reverse transcribed with 25 mmol/L MgCl2, 50 μmol/L oligo(dT), and 200 U of Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, Calif). Semiquantitative and real-time quantitative PCR (qPCR) were performed as previously described (see the Methods section and Table E1 in this article’s Online Repository at www.jacionline.org).29,30
Statistical analysis
Data are expressed as means ± SEs of the indicated number of experiments. P values were determined with the Student unpaired or paired t tests. Correlation was assessed by using the linear regression function of Microsoft (Redmond, Wash) Excel software.
RESULTS
Characterization of PLA2 activity in BALF
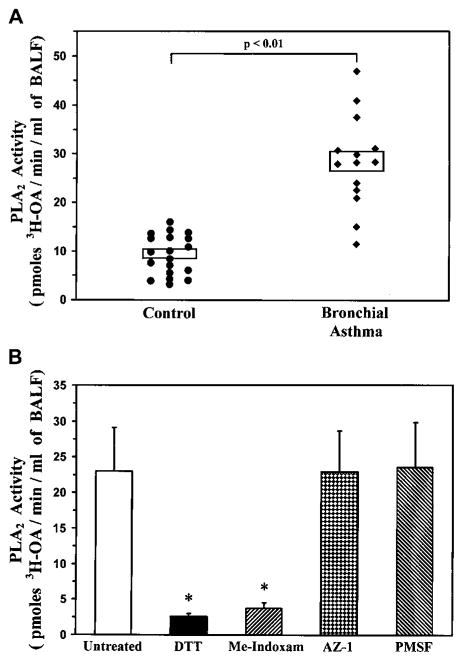
Initial experiments were performed to verify the presence of PLA2 activity in the airways of patients with bronchial asthma. PLA2 enzymatic activity was determined in the cell-free unconcentrated BALF of 14 asthmatic patients and 19 control subjects. Fig 1, A, shows that low levels of PLA2 activity were found in the BALF of control subjects (9.3 ± 0.9 pmol of tritiated OA/min/mL of BALF). PLA2 activity was significantly increased in patients with asthma (28.3 ± 2.2 pmol of tritiated OA/min/mL of BALF, P < .01 vs control subjects). There was no significant correlation between the PLA2 activity and the protein content of the BALF of asthmatic patients (see Fig E1, A, in this article’s Online Repository at www.jacionline.org), whereas a positive correlation was found in the control group (see Fig E1, B, in this article’s Online Repository).
FIG 1.
Characterization of PLA2 activity in human BALF. A, BALF from control subjects (n = 19) and asthmatic patients (n = 14) was assayed for PLA2 activity, as described in the Methods section. Boxes indicate means ± SEs. B, BALF was preincubated (for 1 hour at 37°C) with 10 mmol/L DTT, 10 μmol/L Me-Indoxam, 10 μmol/L AZ-1, or 2 mmol/L PMSF before the PLA2 activity assay. Data are presented as means ± SEs of BALF from 4 asthmatic patients. *P < .01 versus untreated.
To verify that the activity detected in the airways of asthmatic patients was due to the presence of sPLA2s, we incubated the BALF with 2 different inhibitors of sPLA2s (DTT and Me-Indoxam),11 an inhibitor of cPLA2s (referred to as AZ-1),31 or an inhibitor of platelet-activating factor acetylhydrolase (PMSF).32 In particular, DTT is a reducing agent that alters the secondary structure of all sPLA2s by reducing disulfide bonds, whereas Me-Indoxam is an active, site-directed, reversible, competitive inhibitor of sPLA2 enzymatic activity.11 The latter compound binds to sPLA2 catalytic sites, thereby preventing the interaction of the enzyme with its substrate. Because the various sPLA2 isoforms display structural differences in their catalytic sites,2 the affinity and inhibitory effect of Me-Indoxam are different for the various human sPLA2s. In particular, Me-Indoxam shows different potencies on the various sPLA2 isoforms in in vitro assays with an inhibitory concentration of 50% (IC50) of less than 100 nmol/L for hGIIA, hGIIE, and hGV; an IC50 of between 200 and 600 nmol/L for hGIB and hGX; and an IC50 of greater than 2 μmol/L for hGIID, hGIIF, hGIII, and hGXIIA.11 Fig 1, B, shows that the PLA2 activity in the BALF of asthmatic patients was almost completely suppressed by means of incubation with either DTT or Me-Indoxam, whereas it was not affected by AZ-1 or PMSF. These results indicate that 1 or more sPLA2s account for most of the enzymatic activity in the airways of asthmatic patients.
Release of sPLA2s from HLMCs
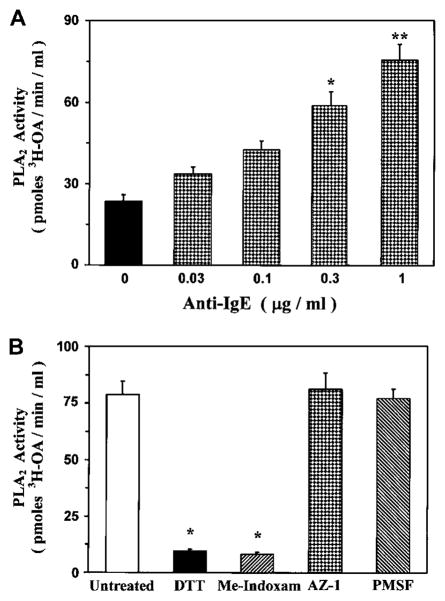
The detection of sPLA2 activity in the airways of patients with asthma led us to investigate the role of lung mast cells as a potential source of these enzymes. In this group of experiments, we explored the capacity of primary human mast cells purified from the lung parenchyma (HLMCs) to release sPLA2 activity on immunologic activation. Fig 2, A, shows that HLMCs spontaneously released PLA2 activity (23.6 ± 2.4 pmol of tritiated OA/min/mL of HLMC supernatant). Stimulation of HLMCs with anti-IgE (0.03–1 μg/mL) concentration-dependently increased the release of PLA2 activity, with a maximum at 1 μg/mL (75.6 ± 5.7 pmol of tritiated OA/min/mL of HLMC supernatant). To understand whether the PLA2 activity released by lung mast cells was from an sPLA2 and had the same biochemical properties as those present in the BALF of asthmatic patients, supernatants of anti-IgE–activated HLMCs were preincubated with the PLA2 inhibitors described in the previous section. Fig 2, B, shows that the PLA2 activity released by HLMCs was completely blocked by the reducing agent DTT and by the site-directed sPLA2 inhibitor Me-Indoxam at concentrations known to inhibit most of the human sPLA2s in vitro.11 In contrast, AZ-1 and PMSF had no effect on PLA2 activity released from mast cells. These data indicate that immunologically activated HLMCs released a PLA2 activity with biochemical and pharmacologic properties of an sPLA2.
FIG 2.
Characterization of PLA2 activity in supernatants of HLMCs. A, HLMCs were incubated (for 30 minutes at 37°C) with anti-IgE. PLA2 activity was determined in supernatants, as described in the Methods section. Data are presented as means ± SEs of 4 experiments. *P < .05 versus unstimulated; **P < .01 versus unstimulated. B, Supernatants of anti-IgE–stimulated HLMCs were preincubated (for 1 hour at 37°C) with 10 mmol/L DTT, 10 μmol/L Me-Indoxam, 10 μmol/L AZ-1, or 2 mmol/L PMSF before the PLA2 activity assay. Data are presented as means ± SEs of 4 experiments. * P < .01 versus untreated.
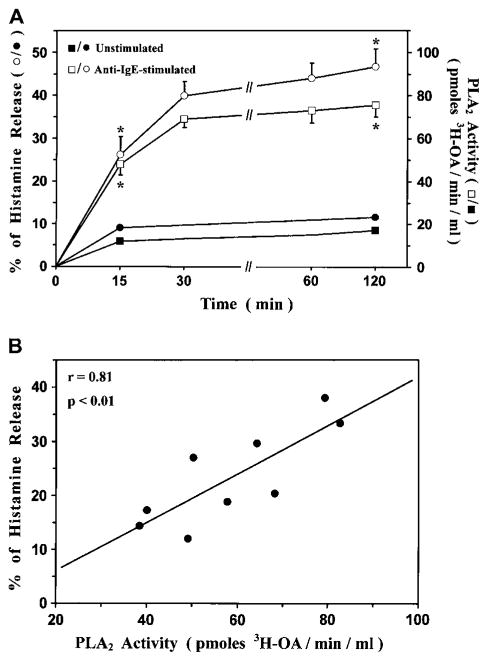
In the next group of experiments, we examined the kinetics of release of sPLA2 activity and histamine from anti-IgE–stimulated HLMCs. In 3 different preparations of HLMCs, the release of the sPLA2 activity was detectable already after 15 minutes of stimulation and peaked at 30 minutes (Fig 3, A). The kinetics of sPLA2 release were similar to those of histamine (half-time of sPLA2 release, 15.3 ± 3.2 minutes; half-time of histamine release, 12.8 ± 2.6 minutes). Data obtained with HLMCs from 9 different donors indicated that there was a significant correlation between maximal release of sPLA2s (expressed as biologic activity) and of histamine (expressed as the percentage of the total cellular content) when mast cells were stimulated with an optimal concentration of anti-IgE (1 μg/mL; Fig 3, B). These data suggest that the sPLA2 is stored as a preformed mediator within mast cells and is rapidly released on immunologic activation.
FIG 3.
Release of sPLA2s and histamine from anti-IgE–stimulated HLMCs. A, HLMCs were incubated with or without anti-IgE (1 μg/mL). Histamine release and PLA2 activity were determined in supernatants, as described in the Methods section. Data are presented as means ± SEs of 4 experiments. *P < .01 versus unstimulated. B, HLMCs were incubated (for 30 minutes at 37°C) with anti-IgE (1 μg/mL). Histamine release and PLA2 activity were determined as described above. Correlation between histamine release and sPLA2 activity was assessed by using the linear regression function of Microsoft Excel.
Expression of mRNA for sPLA2s in HLMCs
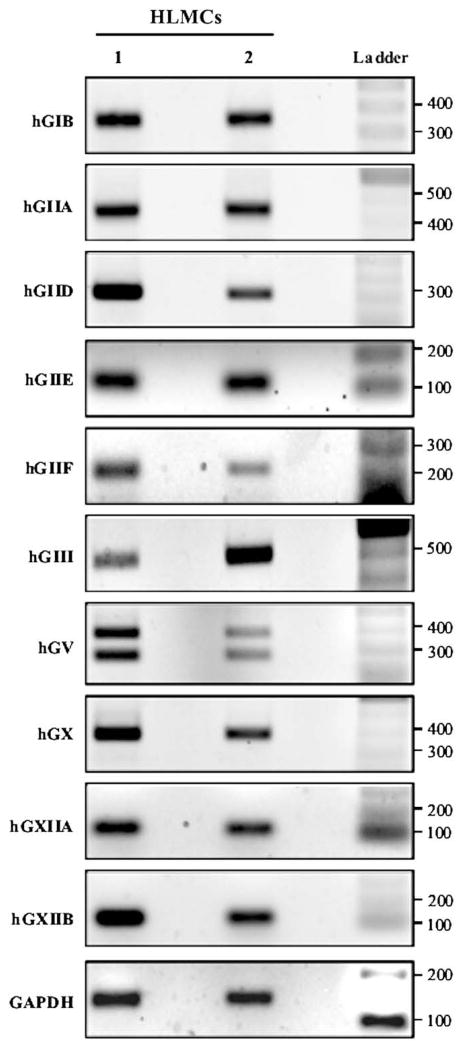
The results obtained thus far have indicated that human mast cells release 1 or more isoforms of sPLA2s. We therefore examined constitutive gene expression of the known human isoforms of sPLA2s in resting HLMCs by means of RT-PCR by using target-specific primers for the various sPLA2s (see Table E1 in this article’s Online Repository). Fig 4 shows the PCR amplification signals of 2 experiments representative of 4 different preparations of HLMCs. PCR fragments of the expected size encoding for hGIB (341 bp), hGIIA (434 bp), hGIID (294 bp), hGIIE (120 bp), hGIIF (211 bp), hGIII (500 bp), hGV (358 bp), hGX (370 bp), hGXIIA (105 bp), and hGXIIB (141 bp) were amplified in all HLMC preparations at subsaturating cycle numbers (35 cycles). As previously reported in human neutrophils, the primers used to evaluate hGV mRNA expression in HLMCs also generated a 251-bp PCR product that was identical to the hGV mRNA from nucleotides 24 to 381 but lacked the untranslated region corresponding to exon 4.24 These data indicate that primary HLMCs constitutively express mRNA for most human sPLA2s.
FIG 4.
Expression of sPLA2s in HLMCs. HLMCs from 2 different lung preparations were lysed, and total RNA was extracted. Expression of sPLA2s was evaluated by means of semiquantitative PCR, as described in the Methods section. RT-PCR amplification products were separated on 2% agarose gel, stained with ethidium bromide, and photographed.
To investigate whether anti-IgE challenge of HLMCs modifies the expression of sPLA2s, we next examined mRNA for the major sPLA2 isoforms (hGIIA, hGIID, hGIIE, hGIIF, hGIII, hGV, and hGX) in both resting and anti-IgE–activated HLMCs. To this end, we carried out qPCR in 3 different preparations of HLMCs incubated (at 37°C for 3 hours) in the absence (unstimulated) or presence of anti-IgE (1 μg/mL). These experiments allowed an accurate quantification of the sPLA2s constitutively expressed by HLMCs. Table I shows that human mast cells express high levels of hGIII, hGV, and hGX; intermediate levels of hGIID and hGIIF; and low levels of hGIIA and hGIIE. Stimulation with anti-IgE did not enhance the expression of any sPLA2s examined. We rather observed a tendency toward a reduction in the expression of all sPLA2s, but these results did not reach statistical significance.
TABLE I.
Expression of sPLA2s in human lung mast cells
| mRNA expression (ΔCt)* |
|||
|---|---|---|---|
| sPLA2s | Unstimulated† | Anti-IgE† | P value |
| GIIA | 15.73 ± 1.11 | 16.34 ± 1.17 | .179 |
| GIID | 9.36 ± 1.03 | 9.90 ± 0.47 | .225 |
| GIIE | 12.06 ± 0.94 | 13.46 ± 0.64 | .155 |
| GIIF | 9.09 ± 0.96 | 9.36 ± 0.54 | .322 |
| GIII | 4.84 ± 0.38 | 5.29 ± 0.29 | .222 |
| GV | 6.81 ± 0.78 | 7.54 ± 0.99 | .211 |
| GX | 7.43 ± 1.03 | 7.99 ± 1.28 | .119 |
mRNA expression is based on qPCR, and data are expressed as ΔCt (see the Methods section in this article’s Online Repository). A ΔCt of less than 10 means high to medium expression, a ΔCt of 10 to 15 means medium to low expression, and a ΔCt of greater than 15 means low expression. Data are presented as the means ± SEs of 3 different donors. P values were determined by using the Student paired t test.
The cells were incubated (at 37°C for 3 hours) in the absence (unstimulated) or presence of anti-IgE (1 μg/mL). At the end of incubation, mRNA expression of sPLA2s was evaluated as described above.
Role of endogenous sPLA2s in the generation of LTC4 from HLMCs
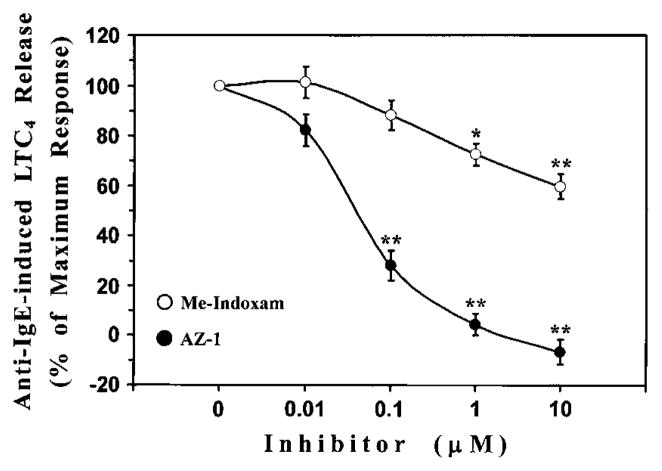
sPLA2s contribute to the generation of eicosanoids in murine mast cells19,27,33,34 and macrophages.10,35 However, it is still debated whether this contribution is due to intracellular or extracellular actions of sPLA2s. Our experiments demonstrated that human mast cells secrete 1 or more sPLA2s based on results obtained with Me-Indoxam. It is worth noting that, being cell impermeable, Me-Indoxam is able to inhibit the activity of sPLA2s only when they are secreted in the extracellular space.36 Thus we were able to evaluate the role of endogenous PLA2s on LTC4 production once these enzymes have been released by immunologically activated mast cells. In these experiments mast cells were stimulated with anti-IgE in the presence of increasing concentrations (0.1–10 μmol/L) of Me-Indoxam or AZ-1, a potent and cell-permeable inhibitor of GIV-cPLA2. At the end of incubation, LTC4 production was determined in the supernatants. Fig 5 shows that the GIV inhibitor AZ-1 caused a complete suppression of LTC4 synthesis (IC50, 40.3 ± 7.9 nmol/L). However, Me-Indoxam also inhibited, in a concentration-dependent fashion, up to 40% of anti-IgE–induced LTC4 release. Neither Me-Indoxam nor AZ-1 significantly influenced anti-IgE–induced degranulation of mast cells, as assessed by means of β-hexosaminidase release (data not shown). These results indicate that LTC4 synthesis in mast cells is primarily due to the GIV-cPLA2. However, sPLA2s, once secreted, significantly contribute to this process by augmenting IgE-mediated cysteinyl LT production.
FIG 5.
Effect of sPLA2 and cPLA2 inhibitors on LTC4 production from anti-IgE–stimulated HLMCs. The cells were preincubated (for 15 minutes at 37°C) with increasing concentrations of Me-Indoxam or AZ-1 before stimulation (for 30 minutes at 37°C) with anti-IgE (1 μg/mL). LTC4 production was determined in supernatants by using an enzyme immunoassay. Inhibition of LTC4 production was expressed as a percentage of maximum response. Data are presented as means ± SEs of 4 experiments. *P < .05 versus anti-IgE; **P < .01 versus anti-IgE.
DISCUSSION
Patients with mild persistent asthma have higher levels of sPLA2 activity in BALF than nonasthmatic control subjects. Primary lung mast cells constitutively express mRNA for several sPLA2s and release, on immunologic activation, sPLA2 activity with biochemical characteristics similar to those of the sPLA2s detected in the BALF of asthmatic patients. Endogenous sPLA2s released by mast cells significantly contribute to IgE-mediated production of cysteinyl LTs.
Our results indicate that low levels of sPLA2s are detectable in the airways of control subjects and that this activity is increased in patients with mild asthma. A significant correlation between sPLA2 activity and protein content exists in the BALF of control subjects. This correlation is lost in asthmatic patients, suggesting that sPLA2 enzymes are selectively released in the airways of these patients. These results are in line with previous studies showing that bronchial antigen challenge in asthmatic patients increases sPLA2 activity in the BALF 3- to 5-fold during the late-phase reaction (ie, 4–20 hours after challenge).5,6
Several cells involved in the pathogenesis of asthma, including eosinophils, basophils, TH2 cells, epithelial cells, macrophages, and fibroblasts, express sPLA2s.20 Our work provides the first characterization of sPLA2s in human mast cells and indicates that they might be a source of these proinflammatory molecules in patients with asthma. An interesting observation of this study is that, in contrast to other inflammatory cells involved in asthma, mast cells not only express sPLA2s but also release 1 or more of these enzymes on stimulation. Epithelial cells, macrophages, and eosinophils in induced sputum of asthmatic patients express various sPLA2s14; the ability of these cells to release sPLA2s was not investigated. This is a relevant issue because eosinophils,37 basophils,38 and macrophages29 synthesize 1 or more sPLA2s but fail to release them, at least in vitro. Thus mast cells are rather unique among effector cells in bronchial asthma because they secrete sPLA2s when activated by IgE.
The release of sPLA2s from stimulated mast cells is rapid and coincident with that of histamine. However, stimulation with anti-IgE does not modify the mRNA expression of any sPLA2s. These findings suggest that sPLA2s are synthesized and stored as pre-formed mediators. The correlation between the release of sPLA2 activity and histamine by activated mast cells supports the hypothesis that these mediators are stored together within secretory granules. Early studies demonstrated that sPLA2s are contained within granules of rodent mast cells.19,39 Moreover, unpublished data from our laboratory also indicate that sPLA2s of the group II subfamily, identified by means of immunohistochemistry, colocalize with tryptase in skin mast cells from patients with mastocytosis.
Another unique feature of human mast cells is the expression of mRNA for a number of sPLA2s. This is at variance with most human inflammatory cells, which express a restricted profile of sPLA2 isoforms.20 The amount of mRNA for the various sPLA2s is rather different because HLMCs constitutively express high levels of hGIII, hGV, and hGX; medium levels of hGIID and hGIIF; and low levels of hGIIA and hGIIE. These results suggest that these cells might synthesize different quantities of the various sPLA2s. The detection of sPLA2 proteins in mast cells by using conventional techniques (eg, Western blotting) is limited by the low number of cells retrieved from specimens of lung tissue. Thus although our data indicate that mast cells produce messages for all sPLA2s, they do not define which isoforms are translated into proteins, secreted, or both. However, information on the sPLA2s secreted by stimulated mast cells can be inferred from the data obtained with Me-Indoxam. This compound inhibits hGIB, hGIIA, hGIIE, hGV, and hGX with an IC50 of less than 600 nmol/L and hGIID, hGIIF, hGIII, hGXIIA, and hGXIIB with an IC50 of greater than 2 μmol/L. Thus it is conceivable that HLMC supernatants contain those sPLA2s that can be blocked by Me-Indoxam (hGIB, hGIIA, hGIIE, hGV, and hGX) rather than those sPLA2s that are poorly sensitive or nonsensitive to the inhibitory effect of this compound (hGIID, hGIIF, hGIII, hGXIIA, and hGXIIB). Further studies with more sensitive and specific techniques for sPLA2 detection will define which sPLA2 proteins are synthesized and released by human mast cells.
The role of sPLA2s in asthma is still under investigation. Some of these molecules, such as hGX,9,14 can participate in airway inflammation and remodeling through at least 3 mechanisms.
First, sPLA2s can contribute to prostaglandin and LT biosynthesis by potentiating the effect of cPLA2s. The results shown in Fig 5 indicate that LTC4 production in stimulated HLMCs is primarily dependent on GIV-cPLA2. Nevertheless, the observation that the cell-impermeable sPLA2 inhibitor Me-Indoxam reduces LTC4 production by 40% indicates that the sPLA2s released by HLMCs contribute to LT production by cross-talking with GIV-cPLA2. These data are reminiscent of those obtained with murine mast cells and macrophages showing that sPLA2s alone do not initiate LTC4 production but potentiate the eicosanoid-forming capacity of GIV-cPLA2.27,33,35 The mechanisms of the cross-talk between cPLA2s and sPLA2s are still unclear, but it is currently believed that sPLA2-induced intracellular signals might increase the activation of the GIV-cPLA2.34 Several isoforms of sPLA2s (GIB, GIIA, GV, and GX) bind to a specific M-type receptor, which generates intracellular signals leading to proinflammatory responses in target cells.40 Me-Indoxam blocks not only the catalytic activity of sPLA2s11 but also the receptor-mediated activation of inflammatory cells30,40 by preventing the binding of sPLA2s to the M-type receptor.41 Of relevance to the present study, GIB, GIIA, and GV activate the M-type receptor expressed on murine BMMCs, thereby activating GIV-cPLA2 and promoting arachidonate mobilization and eicosanoid production.42 Therefore the enhancement of LT production by sPLA2s in HLMCs might be due to an autocrine effect on the M-type receptor expressed on these cells.
A second mechanism by which sPLA2s might promote inflammation in asthma is through their nonenzymatic, receptor-mediated activation of inflammatory cells.40 We and others demonstrated that several sPLA2s (GIB, GIIA, and GX) activate cytokine and chemokine production30,43–45 by interacting with the M-type or other receptors expressed on human inflammatory cells.
Finally, sPLA2s might contribute to the pathogenesis of asthma in vivo through the degradation of surfactant phospholipids. Alterations of the physicochemical properties of surfactant occur in asthma and are associated with airway obstruction and hyperreactivity.46 sPLA2s hydrolyze surfactant phospholipids, generating lysophospholipids that, in turn, alter surfactant properties and induce proinflammatory effects.47 Together, these observations help explain why knocking out just 1 sPLA2 isoform (GX) dramatically reduces allergic inflammation.9
The capacity of mast cells to secrete sPLA2s might also be relevant to the role of these cells in innate immunity.16–18 Several sPLA2s have potent bactericidal activity.48,49 In addition, GIII sPLA2 inhibits HIV replication by blocking viral entry into the cells.50 Our results raise the interesting hypothesis that sPLA2s are mediators supporting the role of mast cells in innate immunity.
In conclusion, sPLA2s released by immunologically activated mast cells have biochemical properties similar to those of the enzymes secreted in the airways of asthmatic patients, indicating that mast cells might be a major source of sPLA2s in asthma. The demonstration that sPLA2s are released by mast cells further reinforces the concept that these molecules have an important role in inflammation and tissue remodeling in asthma.
Supplementary Material
Acknowledgments
Supported in part by grants from the Ministero dell’Istruzione, dell’Università e della Ricerca (M. T., G. M.), the Regione Campania (M. T.), the Istituto Superiore di Sanità (AIDS Project 40D.57; G. M.), and the National Institutes of Health (HL50040 and HL36235 to M. H. G.).
We thank Dr Vincenza Nardicchi (University of Perugia, Perugia, Italy) who prepared the tritiated OA–labeled E coli membranes.
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- BMMC
Bone marrow–derived mast cell
- cPLA2
Cytosolic phospholipase A2
- Ct
Cycle threshold
- DTT
Dithiothreitol
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- hGIIA
Human GIIA
- hGX
Human GX
- HLMC
Human lung mast cell
- IC50
Inhibitory concentration of 50%
- LTC4
Leukotriene C4
- OA
Oleic acid
- PLA2
Phospholipase A2*
- PMSF
Phenylmethylsulfonyl fluoride
- qPCR
Real-time quantitative PCR
- sPLA2
Secreted phospholipase A2
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
The Roman numeral after the letter G indicates the group, and the uppercase letter after the numeral indicates the subgroup (eg, GIB indicates group IB PLA2).
Clinical implications: HLMCs can be a source of sPLA2s in the airways of asthmatic patients. PLA2s secreted by mast cells are implicated in LT synthesis and might provide a novel therapeutic target in asthma.
References
- 1.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 3.Granata F, Balestrieri B, Petraroli A, Giannattasio G, Marone G, Triggiani M. Secretory phospholipases A2 as multivalent mediators of inflammatory and allergic disorders. Int Arch Allergy Immunol. 2003;131:153–63. doi: 10.1159/000071481. [DOI] [PubMed] [Google Scholar]
- 4.Samet JM, Madden MC, Fonteh AN. Characterization of a secretory phospholipase A2 in human bronchoalveolar lavage fluid. Exp Lung Res. 1996;22:299–315. doi: 10.3109/01902149609031777. [DOI] [PubMed] [Google Scholar]
- 5.Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997;155:421–5. doi: 10.1164/ajrccm.155.2.9032172. [DOI] [PubMed] [Google Scholar]
- 6.Chilton FH, Averill FJ, Hubbard WC, Fonteh AN, Triggiani M, Liu MC. Antigen-induced generation of lysophospholipids in human airways. J Exp Med. 1996;183:2235–45. doi: 10.1084/jem.183.5.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadel JM, Hoyle K, Naclerio RM, Roshak A, Chilton FH. Characterization of phospholipase A2 from human nasal lavage. Am J Respir Cell Mol Biol. 1994;11:108–13. doi: 10.1165/ajrcmb.11.1.8018333. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi K, Kuwata H, Yoshihara K, Masuda S, Shimbara S, Ohishi S, et al. Induction of distinct sets of secretory phospholipase A2 in rodents during inflammation. Biochim Biophys Acta. 2003;1635:37–47. doi: 10.1016/j.bbalip.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Henderson WR, Jr, Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, et al. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–77. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiga A, Morioka Y, Ono T, Nakano K, Ishimoto Y, Arita H, et al. Group X secretory phospholipase A2 induces potent productions of various lipid mediators in mouse peritoneal macrophages. Biochim Biophys Acta. 2001;1530:67–76. doi: 10.1016/s1388-1981(00)00167-0. [DOI] [PubMed] [Google Scholar]
- 11.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:48535–49. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Lu X, Wang H, You XJ, Gao QX, Cui YH. Group II subfamily secretory phospholipase A2 enzymes: expression in chronic rhinosinusitis with and without nasal polyps. Allergy. 2007;62:999–1006. doi: 10.1111/j.1398-9995.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 13.Masuda S, Murakami M, Mitsuishi M, Komiyama K, Ishikawa Y, Ishii T, et al. Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells. Biochem J. 2005;387:27–38. doi: 10.1042/BJ20041307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR., Jr Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007;176:1072–8. doi: 10.1164/rccm.200707-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marone G, Lichtenstein LM, Galli SJ. Mast cells and basophils. San Diego: Academic Press; 2000. [Google Scholar]
- 16.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–40. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enomoto A, Murakami M, Valentin E, Lambeau G, Gelb MH, Kudo I. Redundant and segregated functions of granule-associated heparin-binding group II subfamily of secretory phospholipases A2 in the regulation of degranulation and prostaglandin D2 synthesis in mast cells. J Immunol. 2000;165:4007–14. doi: 10.4049/jimmunol.165.7.4007. [DOI] [PubMed] [Google Scholar]
- 20.Triggiani M, Granata F, Giannattasio G, Marone G. Secretory phospholipases A2 in inflammatory and allergic diseases: not just enzymes. J Allergy Clin Immunol. 2005;116:1000–6. doi: 10.1016/j.jaci.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 22.Jamal OS, Conaghan PG, Cunningham AM, Brooks PM, Munro VF, Scott KF. Increased expression of human type IIa secretory phospholipase A2 antigen in arthritic synovium. Ann Rheum Dis. 1998;57:550–8. doi: 10.1136/ard.57.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilja I, Gustafson-Svard C, Franzen L, Sjodahl R, Andersen S, Johansen B. Presence of group IIA secretory phospholipase A2 in mast cells and macrophages in normal human ileal submucosa and in Crohn’s disease. Clin Chem Lab Med. 2000;38:1231–6. doi: 10.1515/CCLM.2000.194. [DOI] [PubMed] [Google Scholar]
- 24.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, et al. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–73. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 25.Busse WW, Wanner A, Adams K, Reynolds HY, Castro M, Chowdhury B, et al. Investigative bronchoprovocation and bronchoscopy in airway diseases. Am J Respir Crit Care Med. 2005;172:807–16. doi: 10.1164/rccm.200407-966WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triggiani M, Giannattasio G, Balestrieri B, Granata F, Gelb MH, de Paulis A, et al. Differential modulation of mediator release from human basophils and mast cells by mizolastine. Clin Exp Allergy. 2004;34:241–9. doi: 10.1111/j.1365-2222.2004.01851.x. [DOI] [PubMed] [Google Scholar]
- 27.Fonteh AN, Bass DA, Marshall LA, Seeds M, Samet JM, Chilton FH. Evidence that secretory phospholipase A2 plays a role in arachidonic acid release and eicosanoid biosynthesis by mast cells. J Immunol. 1994;152:5438–46. [PubMed] [Google Scholar]
- 28.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–50. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannattasio G, Lai Y, Granata F, Mounier CM, Nallan L, Oslund R, et al. Expression of phospholipases A2 in primary human lung macrophages Role of cytosolic phospholipase A2-alpha in arachidonic acid release and platelet activating factor synthesis. Biochim Biophys Acta. 2009;1791:92–102. doi: 10.1016/j.bbalip.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granata F, Petraroli A, Boilard E, Bezzine S, Bollinger J, Del Vecchio L, et al. Activation of cytokine production by secreted phospholipases A2 in human lung macrophages expressing the M-type receptor. J Immunol. 2005;174:464–74. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- 31.Connolly S, Bennion C, Botterell S, Croshaw PJ, Hallam C, Hardy K, et al. Design and synthesis of a novel and potent series of inhibitors of cytosolic phospholipase A2 based on a 1,3-disubstituted propan-2-one skeleton. J Med Chem. 2002;45:1348–62. doi: 10.1021/jm011050x. [DOI] [PubMed] [Google Scholar]
- 32.Triggiani M, De Marino V, Sofia M, Faraone S, Ambrosio G, Carratu L, et al. Characterization of platelet-activating factor acetylhydrolase in human bronchoalveolar lavage. Am J Respir Crit Care Med. 1997;156:94–100. doi: 10.1164/ajrccm.156.1.9608084. [DOI] [PubMed] [Google Scholar]
- 33.Murakami M, Yoshihara K, Shimbara S, Lambeau G, Singer A, Gelb MH, et al. Arachidonate release and eicosanoid generation by group IIE phospholipase A2. Biochem Biophys Res Commun. 2002;292:689–96. doi: 10.1006/bbrc.2002.6716. [DOI] [PubMed] [Google Scholar]
- 34.Kikawada E, Bonventre JV, Arm JP. Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse mast cells through amplification of ERK and cPLA2alpha activation. Blood. 2007;110:561–7. doi: 10.1182/blood-2006-10-052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, et al. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J Biol Chem. 2004;279:16488–94. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mounier CM, Ghomashchi F, Lindsay MR, James S, Singer AG, Parton RG, et al. Arachidonic acid release from mammalian cells transfected with human groups IIA and X secreted phospholipase A2 occurs predominantly during the secretory process and with the involvement of cytosolic phospholipase A2-alpha. J Biol Chem. 2004;279:25024–38. doi: 10.1074/jbc.M313019200. [DOI] [PubMed] [Google Scholar]
- 37.Blom M, Tool AT, Wever PC, Wolbink GJ, Brouwer MC, Calafat J, et al. Human eosinophils express, relative to other circulating leukocytes, large amounts of secretory 14-kD phospholipase A2. Blood. 1998;91:3037–43. [PubMed] [Google Scholar]
- 38.Hundley TR, Marshall LA, Hubbard WC, MacGlashan DW., Jr Characteristics of arachidonic acid generation in human basophils: relationship between the effects of inhibitors of secretory phospholipase A2 activity and leukotriene C4 release. J Pharmacol Exp Ther. 1998;284:847–57. [PubMed] [Google Scholar]
- 39.Chock SP, Schmauder-Chock EA, Cordella-Miele E, Miele L, Mukherjee AB. The localization of phospholipase A2 in the secretory granule. Biochem J. 1994;300:619–22. doi: 10.1042/bj3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triggiani M, Granata F, Frattini A, Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta. 2006;1761:1289–300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Boilard E, Rouault M, Surrel F, Le Calvez C, Bezzine S, Singer A, et al. Secreted phospholipase A2 inhibitors are also potent blockers of binding to the M-type receptor. Biochemistry. 2006;45:13203–18. doi: 10.1021/bi061376d. [DOI] [PubMed] [Google Scholar]
- 42.Fonteh AN, Atsumi G, LaPorte T, Chilton FH. Secretory phospholipase A2 receptor-mediated activation of cytosolic phospholipase A2 in murine bone marrow-derived mast cells. J Immunol. 2000;165:2773–82. doi: 10.4049/jimmunol.165.5.2773. [DOI] [PubMed] [Google Scholar]
- 43.Triggiani M, Granata F, Balestrieri B, Petraroli A, Scalia G, Del Vecchio L, et al. Secretory phospholipases A2 activate selective functions in human eosinophils. J Immunol. 2003;170:3279–88. doi: 10.4049/jimmunol.170.6.3279. [DOI] [PubMed] [Google Scholar]
- 44.Jo EJ, Lee HY, Lee YN, Kim JI, Kang HK, Park DW, et al. Group IB secretory phospholipase A2 stimulates CXC chemokine ligand 8 production via ERK and NF-kappaB in human neutrophils. J Immunol. 2004;173:6433–9. doi: 10.4049/jimmunol.173.10.6433. [DOI] [PubMed] [Google Scholar]
- 45.Beck G, Yard BA, Schulte J, Haak M, van Ackern K, van der Woude FJ, et al. Secreted phospholipases A2 induce the expression of chemokines in microvascular endothelium. Biochem Biophys Res Commun. 2003;300:731–7. doi: 10.1016/s0006-291x(02)02920-0. [DOI] [PubMed] [Google Scholar]
- 46.Hohlfeld JM. The role of surfactant in asthma. Respir Res. 2002;3:4. doi: 10.1186/rr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graler MH, Goetzl EJ. Lysophospholipids and their G protein-coupled receptors in inflammation and immunity. Biochim Biophys Acta. 2002;1582:168–74. doi: 10.1016/s1388-1981(02)00152-x. [DOI] [PubMed] [Google Scholar]
- 48.Koduri RS, Gronroos JO, Laine VJ, Le Calvez C, Lambeau G, Nevalainen TJ, et al. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:5849–57. doi: 10.1074/jbc.M109699200. [DOI] [PubMed] [Google Scholar]
- 49.Piris-Gimenez A, Paya M, Lambeau G, Chignard M, Mock M, Touqui L, et al. In vivo protective role of human group IIa phospholipase A2 against experimental anthrax. J Immunol. 2005;175:6786–91. doi: 10.4049/jimmunol.175.10.6786. [DOI] [PubMed] [Google Scholar]
- 50.Fenard D, Lambeau G, Valentin E, Lefebvre JC, Lazdunski M, Doglio A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J Clin Invest. 1999;104:611–8. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.