Abstract
Hematocrit (Hct) as an indicator of blood viscosity and mean arterial blood pressure (MAP) were assessed according to the season in adult participants of health screenings conducted throughout Kinshasa, Democratic Republic of Congo. Data was collected at the end of summer (April) and the end of winter (August) and identified by gender. Male Hcts in August were significantly higher (P < 0.0001) than in April (48.3% ± 4.2% and 45.7% ± 2.3%, respectively) while male MAP (85.0 ± 8.4 mm Hg) was identical to that recorded in April (85.4 ± 7.7 mm Hg). August female Hcts (41.4% ± 3.1%) were statistically higher than those recorded in April (39.6% ± 1.9%, P = 0.001), MAP being 82.3 ± 7.3 vs 87.9 ± 6.6 mm Hg, respectively (P = 0.0001). Systolic and diastolic blood pressures, heart rate, body mass indices, ages, and personal and familial medical histories of the August and April groups were not significantly different. This study offers further support for the assertion that the relationship between blood viscosity and pressure of a healthy population shows that increased Hct, and therefore increased blood viscosity is associated with lowered MAP, and presumably peripheral vascular resistance.
Keywords: blood pressure, African adults, blood viscosity, cardiovascular, shear stress
Introduction
Blood viscosity is a determinant of peripheral vascular resistance, and therefore cardiovascular function and blood pressure. The classic conceptualization indicates that increased blood viscosity leads to increased peripheral vascular resistance, leading in general to increased blood pressure.1,2 However, the nature of the functional relationship between blood viscosity and blood pressure is presently undergoing a major revision in view of studies showing that in some populations increased blood viscosity related to increased hematocrit (Hct) leads to decreased blood pressure.3,4 Furthermore, experimental studies show that acute, small increases of Hct, within the range of the normal variability of this parameter in the population leads to a significant lowering of blood pressure.5
Hct in the population presents variability due to genetics, environmental factors, diet, age, exercise, etc. It also presents significant seasonal variations.6–8 Furthermore, there are reports that significant departures from the Hct norm may constitute hidden cardiovascular risk factors for healthy individuals.9,10 These considerations prompted the examination of the results of health screenings conducted in voluntary adults (age ≥18 years) throughout Kinshasa, Democratic Republic of Congo (DRC), by the Group de Reflection, Actions et Etude de Culture (GRAEC), a nongovernmental organization. The results were scrutinized according to gender, Hct, and blood pressure values in the participating populations from the end of summer (April), and the end of winter (August) in order to determine their ability to compensate for the changes in blood viscosity and explore the associated potential health risk factors.
Research design and methods
Inclusion criteria
The health care screening database of participants during August 2008 and April 2009, constituting 365 and 160 mixed male and female individuals, respectively, was further characterized. The months of April and August were chosen due to availability of comprehensive data as well as having opposing seasonal climates. Furthermore, these months correspond to the end of the corresponding season, thus providing for a prolonged period of adaptation to the related environmental conditions. However, participants during August did not return in April. Inclusion of participant results followed a rigorous inspection of thoroughly completed examination records. Exclusion criteria were diabetes, hypertension, currently afflicted by malaria or other blood disease, pregnancy, lactation, smoking, and major surgery within six months prior to screening. Individuals were also required to have a fasting blood glucose (FBG) less than or equal to 110 mg/dL and a mean arterial pressure (MAP) equal to or below 100 mm Hg. MAP was determined using equation 1, where P stands for pressure:
| (1) |
Procedures for the current analysis were approved by the DRC Minister of Health as well as the Health Zone Chief of Kinshasa.
Participants with abnormal test results, according to the American Heart Association,11 were referred to their local physician for secondary testing and treatment. No further interventions were made following normal results.
Health care screening methodology and biochemistry
Blood pressures, taken prior to blood sample collection, were measured using battery-operated automatic blood pressure monitors, approved by the US Food and Drug Administration (FDA).
Antecubital vein blood samples were taken by experienced technical personnel, supervised by medical practitioners, using nonheparnized needles and syringes. FBG and Hct were directly determined from blood samples. FBG was measured using a standard blood glucose system, approved for personal glucose testing by the US FDA. Blood sample Hct was established following centrifugation of heparinzed capillary tubes (13,000 g for 3 min, at 20 °C with a Power-Spin BX Centrifuge; Unico, Dayton, NJ USA).
Climate
Kinshasa’s climate can be described as tropical. During the months of May through August, the so-called dry season, temperatures range from 18.3 to 26.7 °C, with low humidity. During the rainy season, extending from October through April, temperatures range from 29.4 and 37.8 °C, with high humidity. Data on monthly mean temperatures in Kinshasa were obtained for August 2008 and April 2009 from an international weather information website, Weather Underground (see http://www.wunderground.com/history/airport/FZAA).
Data analysis
Results are presented as mean ± standard deviation. Data comparisons made between and within groups were analyzed using the unpaired t- test or a correlation analysis. Differences were considered significant for P < 0.05. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software; San Diego, CA, USA).
Results
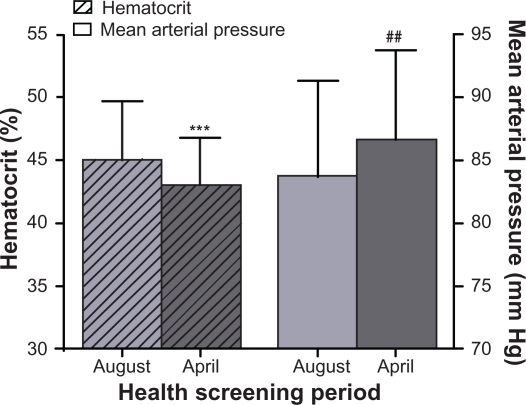
A total of 365 male and female individuals were examined during the month of August, with a final inclusion of 140 individuals who passed the inclusion criteria, yielding a mean Hct of 45.0% ± 4.5% and mean MAP of 83.7 ± 7.6 mm Hg. The same examinations were conducted in 200 male and female individuals during the month of April, with final inclusion of 92 individuals with mean Hct 43.1% ± 3.7% and MAP of 86.6 ± 7.1 mm Hg.
Kinshasa is located below the equator. In terms of seasonal variations of temperature, August is one of the colder months of the year and April is one of the warmer months. According to records, average monthly temperatures for August 2008 and April 2009 were 22.7 °C and 30.0 °C, respectively. The data shows that Hcts recorded in August were statistically higher than those recorded in the hotter month of April (P = 0.001; Figure 1). MAP values collected in August were 2.9 mm Hg lower when compared to those recorded in April, a difference that was also statistically significant (P = 0.004; Figure 1).
Figure 1.
Variation in hematocrit and mean arterial pressure due to seasonal differences between August and April, of the mixed male and female adult population in Kinshasa, Democratic Republic of Congo.
Notes: Data are presented as mean ± standard deviation. ***P ≤ 0.001 vs August hematocrit. ##, P < 0.01 vs August mean arterial pressure.
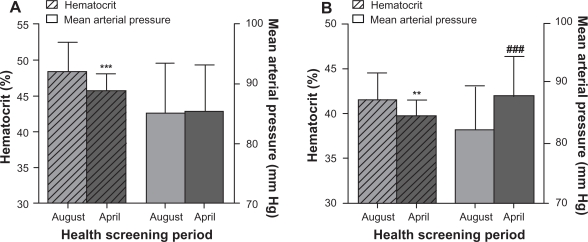
The seasonal variation in Hct in Kinshasa was also found when the data was separated according to gender. Male Hcts in the month of August were significantly higher (P < 0.0001) than those measured in April (48.3% ± 4.2% and 45.7% ± 2.3%, respectively), however, male MAP in August (85.0 ± 8.4 mm Hg) was identical to that recorded in April (85.4 ± 7.7 mm Hg, Figure 2A).
Figure 2.
Variation in hematocrit and mean arterial pressure due to seasonal differences between August and April, of separate male A) and female B) adult populations in Kinshasa, Democratic Republic of Congo.
Notes: Data are presented as mean ± standard deviation. ***P < 0.001 vs August hematocrit. **P < 0.01 vs August hematocrit. ###P < 0.0001 vs August mean arterial pressure.
Female Hcts during August (41.4% ± 3.1%) were statistically higher when compared to those recorded in April (39.6% ± 1.9%, P = 0.001). Conversely, female MAP levels recorded in August (82.3 ± 7.3 mm Hg) were significantly lower than those measured in April (87.9 ± 6.6 mm Hg), the difference being statistically significant (P = 0.0001; Figure 2B).
The population as a whole showed that in general MAP decreased as Hct increased and vice versa, with some differences in the extent of the effects when men and women were analyzed separately. Correlation analysis between MAP and Hct within any given group did not show statistical significance, yielding positive as well as negative trends, with very low R2 values (<0.05), and low statistical significance with P > 0.05 (data not shown).
Systolic and diastolic blood pressures, heart rate (Table 1), body mass indices, ages, and personal and familial medical histories of the August and April groups were not found to be significantly different.
Table 1.
Blood pressures and heart rates of the adult population in Kinshasa, Democratic Republic of Congo
| Health screening period | Systolic pressure (mm Hg) | Diastolic pressure (mm Hg) | Heart rate (beats/min) |
|---|---|---|---|
| August 2008 | 114.7 ± 12.6 | 68.5 ± 8.2 | 73.3 ± 11.7 |
| April 2009 | 117.4 ± 14.0 | 70.0 ± 7.7 | 70.7 ± 12.1 |
Notes: Data are presented as mean ± standard deviation.
Discussion
Our principle finding is that the seasonal difference of Hct in the adult population of Kinshasa exhibits an inverse relationship to the corresponding MAP changes. During the cooler season (August) Hcts are higher, while MAP is lower and vice versa, where the variation is significantly different. This result differs from those in previous studies carried out in healthy patients12 and patients undergoing dialysis, where Hct increased during the warm period of the year;13 however, comparison of effects between healthy individuals and those undergoing dialysis may be problematic. Notably, in either case regardless of the season in which Hct changes occurred, MAP decreased when Hct increased. Seasonal variations of Hct were found in both males and females; however, changes of both Hct and MAP were only found in the female population.
The seasonal differences in temperature would not appear to be sufficient to explain the observed changes in Hct in the present study. However, these changes cannot be attributed to variation in the diet, which is minimally affected by the slight seasonal variation of the type of fruit, vegetable, and protein sources available in Kinshasa. Similarly, the daily activities of the general population throughout Kinshasa do not significantly vary from climactic season to season. Seasonal Hct fluctuations have been previously related to hemodilution due to increased environmental temperature. However, exposure to high environmental temperatures induces sweating, lowering total fluid availability, and may induce hemoconcentration.14 This was not apparent from the results from the April screenings. This may be in part due to the general increase in fluid intake due to increased temperatures.
In these studies we assume that Hct is the principal determinant of blood viscosity, as shown by Kameneva and colleagues.15 In ideal conditions we would measure blood viscosity directly; however, this option was not available for the purpose of this study. Consequently, other effects due to occult diseases or conditions not apparent at the time of blood sampling, that could influence blood viscosity could not be accounted for.
The inverse relationship between small, acute Hct increases and the corresponding change (decrease) in blood pressure was previously shown to be related to increased blood viscosity and the stimulation of nitric oxide (NO) production due to increased shear stress in the vasculature, leading to vasodilation. A similar effect was found in wild-type mice but not in eNOS knockout mice,16 suggesting that this response is related to the ability of the endothelium to sense shears stress stimuli due to changes in Hct and therefore blood viscosity. Therefore our study, showing that seasonally increased Hct leads to a lowered blood pressure in females suggests that this population presents a normal endothelial function. The male population, exhibiting the same mean arterial blood pressure independently of Hct and season, suggests that its endothelial function is equally responsive to the shear stress stimulus, however, to a lesser degree than in the female population.
The sampled male population was not hypertensive and did not show evidence of endothelial dysfunction. However, the comparatively lesser ability to respond to the increased Hct and, therefore, blood viscosity stimulus by comparison to women suggests a lower capacity for vascular regulation via the production of vasodilators in response to increased blood viscosity. This condition may lead to an increased risk of eventual development of hypertension and other vascular diseases. This contention is in part supported by the finding that healthy normotensive young males with intermediate to high Hcts, 43.3%–45.2% and >45.2% respectively, were reported to have statistically higher blood pressures, compared to those with low Hct (≤43.25%).10 Elevated Hct levels were previously associated with hypertension and other cardiovascular disease risk factors.9,10,17 Longo-Mbenza and colleagues also reported that male gender, Hct, and fibrinogen levels, along with other cardiovascular parameters, are significant predictors of ischemic stroke mortality for Africans.18,19
The relationship between Hct and blood pressure in healthy and diseased populations is yet to be defined, and reliable health standards for African countries are slowly emerging. Our findings showing a decreased response to Hct in males suggests the latent inability to regulate vascular tone with greater variation in blood viscosity and/or flow velocity. Therefore, monitoring blood pressures of high Hct normotensive African males, as with hypertensives, is advised.
Increased cardiovascular related deaths during winter months20,21 have been causally linked with the seasonal influences, winter peak and summer through blood pressure observations13,21–24 in Australia, Asia, Europe, and North and South America. Parry and colleagues also reported a seasonal increase in cardiac failure in a mixed Nigerian population. However, peaks were due to hot and humid weather and cardiac failure was linked only to elevated female blood pressures, during the same period,25 but Hct was not reported in this study. Although Cheung and colleagues reported their study population of 64.5% African Americans showed the trend of blood pressure increase as temperatures drop,13 the other studies cited did not report inclusion of Africans in the study populations. Therefore, it is likely that African populations have distinct traits, leading to approaches for administering health care that should be different from those for westernized and/or better investigated populations, including westernized populations with African ancestry.
In conclusion our study further supports the contention that the relationship between Hct/blood viscosity and blood pressure in the healthy population is counterintuitive, since the increased blood viscosity due to seasonal factors leads to lowered or unaffected blood pressure. It is likely that other factors also influence this outcome, however, this result conforms to the hypothesis that increased blood viscosity leads to increased shear stress, which in turn acts on the functional endothelium results in the increased release of NO and other vasoactive mediators, causing vasodilation and lowering peripheral vascular resistance. This mechanism determines that the circulation as a whole, in the healthy organism, does not respond to changes in blood viscosity as expected from a system of rigid conduits where blood flows according to Poiseuille’s law because tube diameters are flow sensitive.26,27 Finally, the MAP/Hct relationship and seasonal Hct variation demonstrated may serve to indicate impaired vascular regulation and the potential for increased cardiovascular risk.
Acknowledgments
This work was supported in part by Group de Reflection, Actions et Etude de Culture (GRAEC), NIH grant T32 HL 007089 (CMH) and NIH grant R01 HL62354 (MI). The authors report no conflicts of interest in this work.
References
- 1.Devereux RB, Case DB, Alderman MH, Pickering TG, Chien S, Laragh JH. Possible role of increased blood viscosity in the hemodynamics of systemic hypertension. Am J Cardiol. 2000;85:1265–1268. doi: 10.1016/s0002-9149(00)00744-x. [DOI] [PubMed] [Google Scholar]
- 2.Letcher RL, Chien S, Pickering TG, Laragh JH. Elevated blood viscosity in patients with borderline essential hypertension. Hypertension. 1983;5:757–762. doi: 10.1161/01.hyp.5.5.757. [DOI] [PubMed] [Google Scholar]
- 3.de Simone G, Devereux RB, Chinali M, Best LG, Lee ET, Welty TK. Association of blood pressure with blood viscosity in American Indians. The Strong Heart Study. Hypertension. 2005;45:625–630. doi: 10.1161/01.HYP.0000157526.07977.ec. [DOI] [PubMed] [Google Scholar]
- 4.Salazar Vázquez BY, Salazar Vázquez MA, Venzor VC, et al. Increased hematocrit and reduced blood pressure following control of glycemia in diabetes. Clin Hemorheol Microcirc. 2008;38:57–64. [PubMed] [Google Scholar]
- 5.Martini J, Carpentier B, Chavez Negrete A, Frangos JA, Intaglietta M. Paradoxical hypotension following increased hematocrit and blood viscosity. Am J Physiol Heart Circ Physiol. 2005;289:H2136–H2143. doi: 10.1152/ajpheart.00490.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sebok MA, Notari EP, Chambers LA, Benjamin RJ, Eder AF. Seasonal temperature variation and the rate of donor deferral for low hematocrit in the American Red Cross. Transfusion. 2007;47:890–894. doi: 10.1111/j.1537-2995.2007.01206.x. [DOI] [PubMed] [Google Scholar]
- 7.Thirup P. Haematocrit: Within-subject and seasonal variation. Sports Med. 2003;33:231–243. doi: 10.2165/00007256-200333030-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda M, Watanabe T, Koizumi A, et al. Seasonal variation in hemoglobin concentration in non-agricultural populations under various climatic conditions. Hum Biol. 1986;58:189–196. [PubMed] [Google Scholar]
- 9.Gobel BO, Schulte-Gobel A, Weisser B, Glanzer K, Vetter H, Dusing R. Arterial blood pressure Correlation with erythrocyte count, hematocrit, and hemoglobin concentration. Am J Hypertens. 1991;4:14–19. [PubMed] [Google Scholar]
- 10.Smith S, Julius S, Jamerson K, Amerena J, Schork N. Hematocrit levels and physiologic factors in relationship to cardiovascular risk in Tecumseh, Michigan. J Hypertens. 1994;12:455–462. [PubMed] [Google Scholar]
- 11.American Heart Association 2009. Available from: http://www.americanheart.org/presenter.jhtml?identifier=1200000 Accessed on October 10, 2009 [Google Scholar]
- 12.Rosenthal T. Seasonal variations in blood pressure. Am J Geriatr Cardiol. 2004;13:267–272. doi: 10.1111/j.1076-7460.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AK, Yan G, Greene T, et al. Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol. 2002;13:2345–2352. doi: 10.1097/01.asn.0000026611.07106.a7. [DOI] [PubMed] [Google Scholar]
- 14.Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ. Blood volume: Importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32:332–348. doi: 10.1097/00005768-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Kameneva MV, Watach MJ, Borovetz HS. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1999;21:357–363. [PubMed] [Google Scholar]
- 16.Martini J, Cabrales P, Tsai AG, Intaglietta M. Mechanotransduction and the homeostatic significance of maintaining blood viscosity in hypotension, hypertension and haemorrhage. J Intern Med. 2006;259:364–372. doi: 10.1111/j.1365-2796.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F, Stoltz JF. Blood rheology in arterial hypertension. J Hypertens Suppl. 1992;10:S69–S78. [PubMed] [Google Scholar]
- 18.Longo-Mbenza B, Lelo Tshinkwela M, Mbuilu Pukuta J. Rates and predictors of stroke-associated case fatality in black central African patients. Cardiovasc J Afr. 2008;19:72–76. [PMC free article] [PubMed] [Google Scholar]
- 19.Longo-Mbenza B, Tonduangu K, Muyeno K, et al. Predictors of stroke-associated mortality in Africans. Rev Epidemiol Sante Publique. 2000;48:31–39. [PubMed] [Google Scholar]
- 20.Boulay F, Berthier F, Sisteron O, Gendreike Y, Gibelin P. Seasonal variation in chronic heart failure hospitalizations and mortality in France. Circulation. 1999;100:280–286. doi: 10.1161/01.cir.100.3.280. [DOI] [PubMed] [Google Scholar]
- 21.Brennan PJ, Greenberg G, Miall WE, Thompson SG. Seasonal variation in arterial blood pressure. Br Med J (Clin Res Ed) 1982;285:919–923. doi: 10.1136/bmj.285.6346.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenner DA, English DR, Vandongen R, et al. Environmental temperature and blood pressure in 9-year-old Australian children. J Hypertens. 1987;5:683–686. [PubMed] [Google Scholar]
- 23.Sposito M, Nieto FJ, Ventura JE. Seasonal variations of blood pressure and overhydration in patients on chronic hemodialysis. Am J Kidney Dis. 2000;35:812–818. doi: 10.1016/s0272-6386(00)70249-6. [DOI] [PubMed] [Google Scholar]
- 24.Tozawa M, Iseki K, Iseki C, Morita O, Yoshi S, Fukiyama K. Seasonal blood pressure and body weight variation in patients on chronic hemodialysis. Am J Nephrol. 1999;19:660–667. doi: 10.1159/000013538. [DOI] [PubMed] [Google Scholar]
- 25.Parry EH, Davidson NM, Ladipo GO, Watkins H. Seasonal variation of cardiac failure in northern Nigeria. Lancet. 1977;1:1023–1025. doi: 10.1016/s0140-6736(77)91257-0. [DOI] [PubMed] [Google Scholar]
- 26.Melkumyants AM, Balashov SA, Khayutin VM. Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res. 1989;23:741–747. doi: 10.1093/cvr/23.9.741. [DOI] [PubMed] [Google Scholar]
- 27.Forconi S, Gori T. The evolution of the meaning of blood hyperviscosity in cardiovascular physiopathology: Should we reinterpret Poiseuille. Clin Hemorheol Microcirc. 2009;42:1–6. doi: 10.3233/CH-2009-1186. [DOI] [PubMed] [Google Scholar]