Abstract
Background
Repeated antimalarial treatment for febrile episodes and self-treatment are common in malaria-endemic areas. The intake of antimalarials prior to participating in an in vivo study may alter treatment outcome and affect the interpretation of both efficacy and safety outcomes. We report the findings from baseline plasma sampling of malaria patients prior to inclusion into an in vivo study in Tanzania and discuss the implications of residual concentrations of antimalarials in this setting.
Methods and Findings
In an in vivo study conducted in a rural area of Tanzania in 2008, baseline plasma samples from patients reporting no antimalarial intake within the last 28 days were screened for the presence of 14 antimalarials (parent drugs or metabolites) using liquid chromatography-tandem mass spectrometry. Among the 148 patients enrolled, 110 (74.3%) had at least one antimalarial in their plasma: 80 (54.1%) had lumefantrine above the lower limit of calibration (LLC = 4 ng/mL), 7 (4.7%) desbutyl-lumefantrine (4 ng/mL), 77 (52.0%) sulfadoxine (0.5 ng/mL), 15 (10.1%) pyrimethamine (0.5 ng/mL), 16 (10.8%) quinine (2.5 ng/mL) and none chloroquine (2.5 ng/mL).
Conclusions
The proportion of patients with detectable antimalarial drug levels prior to enrollment into the study is worrying. Indeed artemether–lumefantrine was supposed to be available only at government health facilities. Although sulfadoxine–pyrimethamine is only recommended for intermittent preventive treatment in pregnancy (IPTp), it was still widely used in public and private health facilities and sold in drug shops. Self-reporting of previous drug intake is unreliable and thus screening for the presence of antimalarial drug levels should be considered in future in vivo studies to allow for accurate assessment of treatment outcome. Furthermore, persisting sub-therapeutic drug levels of antimalarials in a population could promote the spread of drug resistance. The knowledge on drug pressure in a given population is important to monitor standard treatment policy implementation.
Introduction
The intake of antimalarial drugs prior to inclusion in an in vivo study may interfere with the estimation of treatment outcomes (for both efficacy and safety) due to the presence of residual antimalarials. The standard World Health Organization (WHO) protocol for monitoring antimalarial drug efficacy does not exclude patients with a history of previous antimalarial drug use or the presence of antimalarial drugs in the urine or blood [1]. Nonetheless, it is customary in clinical studies to record the occurrence of previous drug intake at screening as reported by the patient, parent or guardian. Two studies in Africa investigated self-reporting of drug intake [2], [3], and both concluded that it is inaccurate. A more objective indication on the drug use in a study population would be obtained by screening the urine or blood for the presence of antimalarial drugs. There are studies on residuals of antimalarials that have been used in past policies, i.e. chloroquine (CQ) or sulfadoxine–pyrimethamine (SP), in urine or blood in the general population or patients [4]–[17]. However, to our knowledge, there is no study on the presence of lumefantrine in malaria patients seeking medical care.
Policy makers in malaria endemic countries are faced with the difficult problem of ensuring easy and early access to effective and high quality antimalarials, while preventing their uncontrolled and unnecessary use, which would increase drug pressure on the parasites and encourage parasite resistance. Thus, knowledge of drug use in a specific area could help decisions makers to assess how treatment policies are implemented.
Here we report the findings of the analysis of baseline samples from patients with Plasmodium falciparum malaria recruited in an in vivo study in Tanzania. Samples were analysed for the presence of 14 currently in-use antimalarials in a single run using a liquid chromatography-tandem mass spectrometry assay [18].
Methods
Ethics Statement
All the applied protocols and related documents were approved by the Ethikkommission beider Basel (EKBB), the Institutional Review Board of the Ifakara Health Institute and the National Institute for Medical Research Review Board. Blood samples were obtained after written informed consent in Swahili from the participants or their responsible guardians.
Study Area and Population
The study was performed in a rural area with moderate to high malaria transmission intensity (Kilombero district, Morogoro region, Tanzania) during the main rainy season from March to May 2008. At the time of the study, artemether–lumefantrine (AL) had recently been introduced as first-line treatment and was only available at government health facilities to ensure controlled prescription. Before 2006, the official first-line treatment in Tanzania was SP, which had in turn replaced CQ in 2001. In 2008, amodiaquine, SP and quinine were widely available in the private sector in the study area. Artesunate, dihydroartemisinin, and halofantrine could also be found sporadically in a few drug shops (Alba S et al., in preparation). In the private sector these drugs could be purchased over the counter without a doctor's prescription.
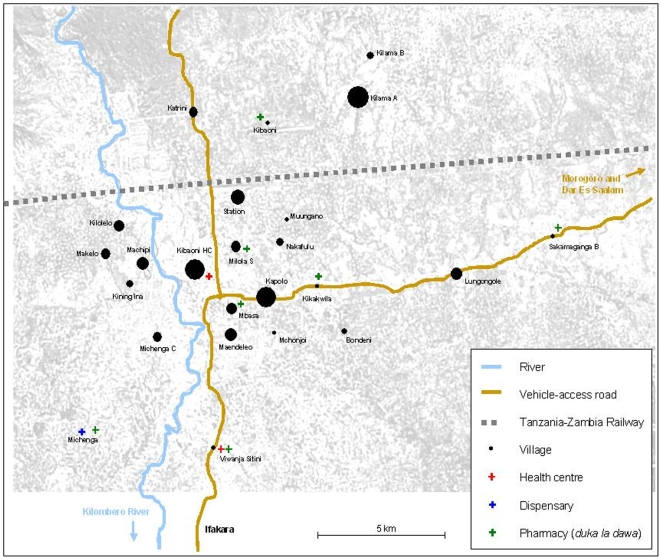
Febrile patients were recruited at the Kibaoni Health Center, 6 km from Ifakara down town, that serves a population of 26,261. The population lives in villages with good coverage of government health facilities and licensed drug stores (pharmacies, part II drug stores [duka la dawa baridi] and Accredited Drug Dispensing Outlets [ADDO; duka la dawa muhimu]) [19], [20]. A map of the villages of residence of the patients included in the study with location of health facilities and drug dispensing outlets is presented in Figure 1.
Figure 1. Villages of residence of the patients included in the study.
Clinical Procedures
The trial was designed to assess the effects of the individual pharmacogenetic profiles on the disposition of standard antimalarials (to be reported elsewhere). It was based on the standard WHO protocol for in vivo testing. Suspected malaria cases were screened by rapid diagnostic test (Paracheck Pf®, Orchid Biomedical Systems, India) for the presence of Plasmodium falciparum. Parasite count and specification of Plasmodium were done by microscopy. The patients with a positive result were then seen by a clinical officer, who invited them to participate in the study if they did not present with danger signs of complicated malaria or severe concomitant illness, and if they reported not having taken antimalarials in the previous 28 days. The latter information was checked against the patient's care log book when available. Consenting patients had a baseline sample (Day 0, 4.5 mL venous blood collected in an EDTA Vacutainer®; Becton, Dickinson and Company, USA) taken to check for potential residual antimalarials and correct pharmacokinetic analyses later on. Treatment with the standard first-line treatment AL was then initiated, according to body weight and age category.
Laboratory Procedures
Blood samples were kept on ice for no longer than 6 h after bleeding (venipuncture) and then aliquoted into whole blood, plasma and pellet and immediately stored at −80°C. Plasma concentrations of 14 antimalarial drugs and their metabolites, i.e. artemether, artesunate, dihydroartemisinin, amodiaquine, N-desethyl-amodiaquine, lumefantrine, desbutyl-lumefantrine, piperaquine, pyronaridine, mefloquine, chloroquine, quinine, pyrimethamine and sulfadoxine, were determined by liquid chromatography coupled to tandem triple stage mass spectrometry (LC-MS/MS) [18]. The lower limits of the calibration range (LLC) in our method were selected as the lowest levels of the calibration curves, which confidently provide a bias and CV% below ±20%, in accordance to FDA recommendations [21]. All samples were analyzed twice. First, quantitative measurement was performed using calibration and quality control samples; then, for confirmation, qualitative assessment was repeated using a new chromatographic column that had not been exposed to any antimalarial drugs,. In order to exclude contamination and false positive results, a large set of blank controls was analyzed prior to the clinical samples on the new column, checking for the absence of specific MS/MS signals of the antimalarials investigated.
Data Management and Analysis
Summary statistics, Chi-square tests, multivariate analysis and graphs of residual plasma concentrations of antimalarials found prior to treatment were produced using Stata® (version 10.1 “intercooled”, Stata Corporation, College Station, TX, USA). Logistic regression analysis was used to investigate the influence of body weight, sex and distance from health facilities or pharmacies on the presence of antimalarials at study entry. The distance between patient home and health facility or pharmacy was defined as “close” or “far” depending on the distance in kilometers, also taking into account ease of access, i.e. main road and river (according to Figure 1). In the first multivariate analysis we considered Bondeni, Kapolo, Katrini, Kibaoni, Kibaoni HC, Kikwawila, Kilolelo, Kining'ina, Machipi, Maendeleo, Makelo, Mbasa, Mchonjoi, Michenga C, Milola S, Muungano, Nakafulu, Sakamaganga B, Station, and Viwanja Stini as “close” and Lungongole, Kilama A, and Kilama B as “far”. In the second analysis we also classified Kilolelo, Kining'ina, Machipi, Makelo, and Michenga C as “far” because the flooding of the Lumemo river might have been an obstacle during the rainy season. We also evaluated the contribution of SP alone, AL alone or both, using likelihood ratio tests.
Estimation of Time of Drug Intake for Lumefantrine
To estimate the probable timing of drug intake, we compared the plasma concentrations of lumefantrine at baseline (C0) and on Day 7 (C7) after a complete treatment with AL for the same patients. We included only patients for whom we had both samples and who complied with the three-day, six-dose AL treatment schedule. Assuming a terminal elimination half-life of t½ = 3.3 days for lumefantrine, an inter-individual variability of 40% [22] and a similar dosage on pre-study exposure and during the study, a back-calculation was done to estimate the intake time before baseline sampling:
The variability on t½ was used to estimate a 90% confidence interval around this intake time, considering plausible inter-individual variations in elimination rate [22].
Bayesian Back-Calculations for Sulfadoxine
A Bayesian estimation of the most likely drug intake time was also attempted from individual sulfadoxine plasma concentration data, with a minimization approach using the “Solver” implemented in Microsoft Excel®. Patients were assumed to have taken a single dose of sulfadoxine (combined with pyrimethamine) according to body weight: 1500 mg for >45 kg, 1000 mg for 31–45 kg, 750 mg for 21–30 kg, 500 mg for 11–20 kg and 250 mg for 5–10 kg. Approximate averages of pharmacokinetic parameters, inter-individual variability and intra-individual (residual) variability were derived from a literature review (Tables 1 and 2). These parameters were used to back-calculate the most likely time for dose intake, expected to produce the observed concentration according to a standard one-compartment model. The variability was used to estimate a 90% confidence interval around this intake time, considering plausible variations in clearance, distribution volume and measurement/modeling error [23]. Similar calculations were not attempted for other antimalarials, as their dosage forms are more heterogeneous (lumefantrine and quinine), their population pharmacokinetic parameters are less well characterized (lumefantrine) and their half-lives are shorter (pyrimethamine, quinine).
Table 1. Review of pharmacokinetic studies of sulfadoxine.
| Study | Number of subjects | Samples per subject | Sex | Age [y] | Body weight [kg] | Condition | Analytic method | Sample type | Dose [mg] | Regimen | PK approach | Compartments |
| Mansor et al. [32] | 10 | 18 | males | 22–30 | 51–60 | Healthy adults | GC | Plasma | 1000 | single dose | AUTOAN-NONLIN | 2 |
| Obua et al. [33] | 83 | 6 | both | 2.5 | 11.9±2.7 | Falciparum malaria | HPLC-UV | Whole blood on filter paper | 250 or 500 | one dose daily for three days | NONMEM | 2 |
| Green et al. [34] | 33 | 7 | females | 15–30 | 47.2–71.0 | Falciparum malaria, pregnancy, HIV positiv | HPLC-UV | Whole blood | 1500 | single dose | log-linear regression | 1 |
| Trenque et al. [35] | 89 | 3 | both | 0–14 | 3–59 | Congenital toxoplasmosis | HPLC-UV | Plasma | 25/kg | two or three times a month | NONMEM | 1 |
| Barnes et al. [36] | 290 | 8 | both | 14 | <103 | Falciparum malaria | HPLC-MS | Whole blood on filter paper | 25/kg, ≤1500 | single dose | WinNonlin | 1 |
| Edstein et al. [34] | 7 | both | Adults | Healthy adults | Whole blood | 500 | single dose | |||||
| Corvaisier et al. [38] | 32 | 5 | both | 0.2–2.1 | 5–13.4 | Congenital toxoplasmosis | HPLC-UV | Plasma | 25/kg | one dose every 10 days | NPEM | 1 |
| Obua et al. [39] | 9 | 14 | both | Healthy adults | HPLC-UV | Plasma | 1500 | single dose | WinNonlin | 1 |
Table 2. Review of pharmacokinetic studies of sulfadoxine (continued).
| Study | Volume of distribution (V) | Clearance (CL) | Absorption rate constant (ka) | Terminal half-life (t½) | Additive residual variability | Proportional residual variability | Comment | ||
| [L] | [L/kg] | [mL/h/kg] | [L/h] | [h−1] | [h] | [µg/L] | |||
| Mansor et al. [32] | 30.90a | 0.084±0.013 | 1.30±1.10 | 255±61 | Mean±S.D. | ||||
| Obua et al. [33] | Central: 0.13±47%; peripheral: 1.6±0% | 0.023±33% | 0.30 | 2.60 | 27% | Estimate±CV% | |||
| Green et al. [34] | 15.0 (12.1–16.0) | 0.24 (0.021–0.27) | 1.01 (0.82–1.61) | 0.066 (0.048–0.092) | 148 (121–193) | Median (IQR) | |||
| Trenque et al. [35] | 2.07±0% for 11 kg | 0.0108±16.3% for 11 kg | 0.055 | 139 c | 8.70 | 31% | Estimate±CV% | ||
| Barnes et al. [36] | 0.40 (0.29–0.56) | 1.85 (1.15–2.89) | 161 (105–218) | Median (IQR) | |||||
| Edstein et al. [34] | 0.25±0.03 | 0.79±0.15 | Mean±S.D. | ||||||
| Corvaisier et al. [38] | 0.393±85% | 2.07b | 1.659±94% | 132±40% | Mean±CV% | ||||
| Obua et al. [39] | 0.15 (0.12–0.18) | 0.39 (0.30–0.56) | 0.32 (0.29–0.62) | 229(139–272) | Median (range) | ||||
| Overall | 0.25±33% | 1±33% | 1.5±0% | 173 | 0 | 30% | |||
Derived from clearance and plasma half-life (t½).
Derived from clearance and elimination rate constant (λ).
Derived from V/CL × ln(2).
Corrected by a factor of (BW/11 kg)0.72 for other body weight (BW) values.
Corrected by a factor of (BW/11 kg)0..64 for other body weight (BW) values.
Results
A total of 1672 patients of all age were screened, of whom 389 (23%) had a positive malaria test and 150 were eligible and willing to participate in the in vivo study. Two patients (one from the Kibaoni HC area and one from Kining'ina) were excluded from the analyses (venipuncture unfeasible in one patient; treatment initiated before baseline sampling in the other one), leaving 148 patients with a valid baseline sample, of whom 64 (43.2%) were male and 84 (56.8%) female (3 (2.0%) pregnant in 3rd trimester). Patients' ages ranged from 1 to 78 years (median 9 years). 51 (34.5%) patients were children under the age of 5, and 94 (63.5%) were <12 years old.
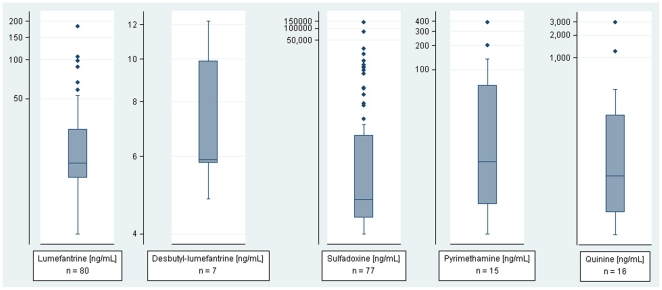
The presence of antimalarial drug was detected in the plasma of 111 (74.3%) patients: 80 (54.1%) had lumefantrine above the lower limit of calibration (LLC = 4 ng/mL), 7 (4.7%) desbutyl-lumefantrine (LLC = 4 ng/mL), 77 (52.0%) sulfadoxine (LLC = 0.5 ng/mL), 15 (10.1%) pyrimethamine (LLC = 0.5 ng/mL), 16 (10.8%) quinine (LLC = 2.5 ng/mL) and none chloroquine (LLC = 2.5 ng/mL) or any other antimalarials tested. Summary statistics are shown in Table 3, and box plots of residual plasma concentrations are represented in Figure 2. Among the 111 patients with residual drug concentrations, 57 (38.5%) had more than one drug (note that parent drug and metabolite or combined regimens such as SP are considered as one): 43 (29.1%) had both lumefantrine and SP, 6 (4.1%) had both lumefantrine and quinine, 1 (0.7%) had both SP and quinine, and 7 (4.7%) had all three agents. The presence of residual antimalarials in plasma was significantly more frequent among children under 5 years of age (86.3%, χ 2 = 5.82, P = 0.016) than among older children and adults (68.0% altogether).
Table 3. Residual plasma concentrations of antimalarials found prior to treatment in 148 malaria patients [ng/mL].
| Antimalarial | Patients (%) | Mean | Median | Minimum | Maximum |
| Lumefantrine | 80 (54.1) | 25.3 | 15.8 | 4.4 | 181.8 |
| Desbutyl-lumefantrine | 7 (4.7) | 7.3 | 5.9 | 4.8 | 12.2 |
| Sulfadoxine | 77 (52.0) | 4′480.2 | 4.4 | 0.6 | 138′887.5 |
| Pyrimethamine | 15 (10.1) | 56.8 | 7.1 | 0.9 | 391.3 |
| Quinine | 16 (10.8) | 318.0 | 26.3 | 4.4 | 2′947.2 |
No artemether, artesunate, dihydroartemisinin, amodiaquine, N-desethyl-amodiaquine, piperaquine, pyronaridine, mefloquine, or chloroquine was found.
Figure 2. Residual plasma concentrations of antimalarials found prior to treatment in 148 malaria patients.
Number of patients (n), median, 25th and 75th percentile, lower and upper adjacent values, and outside values are shown for lumefantrine, desbutyl-lumefantrine, sulfadoxine, pyrimethamine and quinine on a logarithmic scale [ng/mL].
For lumefantrine, among the 59 eligible patients the median plasma concentration (range) was 18.3 ng/mL (4.4–181.8 ng/mL) on Day 0 and 413 ng/mL (37.3–1402 ng/mL) on Day 7. This means that, to account for the levels observed on Day 0, a similar dosage level should have been administered at a median of 21 days (interquartile range 17–24 days, whole range 11–29 days) before study entry. In 2 patients (3%) this estimate was >28 days. The variability in t½ translates into 90% confidence intervals extending from 74% to 144% of estimates (median).
Back-estimation of the most likely times for sulfadoxine intake indicated a median of 108 days before blood sampling at study entry (interquartile range 67 to 121 days, whole range <1 to 130 days). Two patients had concentrations >78 mg/mL, compatible with same day intake. In 70 patients (91%), the estimate exceeded 28 days. The evaluation of uncertainty around individual dose intake times showed 90% confidence intervals extending from 49% to 202% of estimates (median).
The investigation of the influence of body weight, sex and distance to health facilities or pharmacies on the probability of residual antimalarials at study entry (details not presented) showed only a significant relationship between body weight and residual AL levels (likelihood ratio χ 2 = 9.06, P = 0.03 in the first analysis; likelihood ratio χ 2 = 9.60, P = 0.02 in the second analysis), patients with lower body weight being more likely to show residual AL levels. However, this was not the case for SP or SP and AL taken together. Furthermore, neither sex nor distance from health facilities or pharmacies showed a significant effect on residual levels of SP, AL or both at study entry.
Discussion
This is the first study investigating the presence of a range of antimalarials in the plasma of African malaria patients on enrollment into an in vivo study. The measurement of 14 antimalarial drugs currently in-use allowed a comprehensive assessment of drugs available in the community under study.
Artemether–Lumefantrine
Three in four patients had detectable plasma concentrations of antimalarials at the time of enrolment into the study, and in a majority of cases the agent detected was lumefantrine/desethyl-lumefantrine – indicating that they had taken AL, which was supposedly available only at government health facilities to ensure controlled prescription of first-line treatments. Assuming that the patients had taken a three-day, six-dose AL treatment regimen, most patients (97%) must have taken the drug within 28 days prior to treatment. Furthermore, it is also possible that patients might have taken a sub-therapeutic dose of AL more recently. However, as indicated by the wide variability in elimination half-lives, these values represent only rough estimates. Nevertheless, these findings suggest that at least half of the patients included into our study had taken AL, mostly within the previous month (one month corresponds roughly to the limit of evaluation of past exposure, considering assay LLC for lumefantrine). The Day 7 values observed in this study are comparable with those of a study in Thai patients [median plasma concentration (range) of lumefantrine was 528 ng/mL (49–5175 ng/mL) after 6 doses of AL over 60 h according to body weight [24]] and in patients from Bangladesh [860.3 ng/mL (53.8–6215.0 ng/mL) [25]]. Due to the short half-life of the artemisinin component, e.g. 45 min for dihydroartemisinin, it is likely that none of the patients had taken a co-formulated ACT (e.g AL) within the last 24 hours [26].
Sulfadoxine–Pyrimethamine
SP was still found in approximately half of the patients, although it had been officially abandoned as first-line treatment since 2006. Assuming that the patients with residual SP in their plasma had taken a single dose of sulfadoxine according to body weight, most patients must have taken the drug ∼3.5 months (median 108 days) prior blood withdrawal. However, 2 patients had sulfadoxine plasma concentrations indicating very recent exposure, and another 8 exposure during the last 4 weeks. Furthermore, it is also possible that patients might have taken a sub-therapeutic dose of SP more recently. These estimates are approximate, as indicated by the wide confidence interval explained by the fair degree of inter-individual variability in clearance, volume of distribution and residual error. The LC-MS/MS method used for the determination of sulfadoxine (LLC = 0.5 ng/mL) would theoretically make it possible to detect traces of sulfadoxine up to 4 months (127 days) after a single dose of 25 mg/kg of sulfadoxine. These findings are sufficient to conclude that a significant number of patients had taken SP for a previous febrile episode, which is against the standard treatment recommendations. Recent surveys on availability of antimalarials have shown that sick people were getting SP from drugs shops, public and private health facilities (Alba S et al., in preparation), especially so when clinicians were doubtful about the diagnosis of malaria.
Chloroquine and Quinine
This study tends to confirm that CQ, replaced by SP as first-line treatment in 2001, has been effectively withdrawn. On the other hand, one tenth of the patients were found with quinine in their plasma. Quinine has an elimination half-life of 16–18 h in malaria patients [27]. After a treatment with 10 mg/kg of quinine dihydrochloride administered 8-hourly orally for 7 days, the plasma quinine levels had fallen to below 0.4 µg/mL in almost all patients 40 h after the last dose on Day 7 [28]. Thus, we infer that most patients with quinine levels in our study must have taken the last quinine dose not more than 2 days before reporting at the health facility.
High Numbers of Patients with Residual Antimalarials
Why was the number of patients with residual antimalarials so high? Through the demographic surveillance system (DSS) data and a treatment seeking survey in the Ifakara area it was found that approximately 8% of children had fever in the previous 2 weeks when seen between January and April 2008 (Alba S et al., personal communication). An epidemiological study which used the Explanatory Model Interview Catalogue (EMIC) reported that 87.5% (78.2–93.8%) of all fevers in children in our study area were treated with one of the recommended antimalarials (at that time SP, amodiaquine or quinine) [13]. Based on these data, one would expect 14% of children to have had an antimalarial treatment in the preceding month (i.e. (0.08×2)×0.875), which is much lower than the proportion of patients with residual antimalarial plasma levels in this study (86.3% for under 5 s, 68% for older children and adults). This large discrepancy could be either due to poor recall of the study subjects in the epidemiological survey or a selection bias. The latter could have arisen either because we captured (i) only patients preferentially seeking antimalarial treatment at a health facility instead at drug shops (∼76.3% of children under the age of 5 years receiving treatment according to the epidemiological study), (ii) patients who are more susceptible to repeated infections (and hence repeated treatments) or are more exposed to infection, or (iii) patients with easy access to treatment. However, similar results were found in two study sites in rural Cambodia (Hodel et al., in preparation).
Reliability of Medical History
Whatever the reason is for the large number of malaria patients with antimalarials in their blood at study baseline, the fact remains that these patients are the usual subjects investigated in in vivo studies and clinical trials. All patients included in the study reported not having taken antimalarials in the previous 28 days. Entry criteria based on self-reporting of previous drug intake (poor recall) or information recorded in the care log book (self-treatment not documented) are thus unreliable at least in this population and for lumefantrine.
Potential Bias in Drug Safety and Efficacy Assessment
Previous drug intake may affect the current treatment in several ways. Higher drug exposure resulting from cumulative levels may lead to better efficacy or more toxicity. The parasites causing the disease at the time of enrolment may be of a less sensitive population selected by the previous treatment. Thus, previous antimalarial intake may impact on the outcome of the treatment under investigation, and this study shows that only baseline drug concentration measurement in the blood can reliably be used to account for this effect. Our LC-MS/MS assay covers 14 antimalarials in a single run. We can confidently exclude a lack of specificity and false positives as we included blank plasma samples as negative controls and systematically repeated the measurement on a new chromatographic column. Furthermore, we have demonstrated that all drugs are stable for up to 48 h in plasma stored at 4°C [18]. Therefore, even in settings, where no LC-MS/MS instrument is available, samples can be collected in the field, and easily kept and transported to the nearest laboratory where they can be frozen and stored until assayed or further shipped to their final destination. The identification of patients with residual antimalarials allows distinguishing pre-treated from untreated subjects in a secondary analysis. This distinction should probably not aim at excluding pre-treated patients from enrollment in efficacy studies, but rather at addressing the question wether residual antimalarials affect subsequent treatment or not. Detractors may point out that the distinction between pre-treated and untreated patients (i) does not reflect clinical reality and (ii) will increase the number of participants required to maintain statistical power. We believe it could be an important information if some safety or efficacy issues arise from the observations.
High Drug Pressure as Risk Factor for the Spread of Drug Resistance
There is abundant literature on the effects of inadequate antimalarial treatment on the emergence and spread of resistance. Here, we do not know if the residual drug levels found were from a full or incomplete treatment, if the person had parasites at the time of the previous drug intake, and whether the parasites causing the current episode are the same or a new infection. Be it as it may, the residual levels were not enough to control parasite replication and clinical symptoms. This means that these parasites have been exposed to inadequate drug levels for some time. The chances of drug resistant parasites to be selected depends on several factors, and is higher for patients with no immunity (e.g. young children), drugs with long residence times and resistance being conferred through single point mutations, and for infections with a large parasite biomass [29]. These patients had a mean baseline parasite biomass of ∼9×1010 (ranging from ∼1×108 to ∼6×1011, data not shown), values which are in line with those reported for symptomatic cases in malaria-endemic areas [30], and were exposed to drug concentrations which were likely to be in the selective window [31].
The findings of this study must be confirmed in other settings as they have potential implications for both clinical research and surveillance (treatment efficacy and safety outcome) and control (pharmaco-epidemiology, adherence to policy).
Acknowledgments
We would like to thank the patients who participated in the study. Furthermore we would like to thank the staff from the Ifakara Health Institute in Tanzania, namely Dr. Hassan Mshinda and Fidelis Mbena, the staff of the Kibaoni Health Centre in Tanzania and all the drivers for their help in the sample collection.
Footnotes
Competing Interests: The authors have declared that no competing interests exist. PO is a staff member of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the WHO.
Funding: Support for this work was provided by grant No. 320000-112479 from the Swiss National Science Foundation (http://www.snf.ch/E/Pages/default.aspx). LAD received a REQUIP Grant N 326000-121314/1 from the Swiss National Science Foundation for the acquisition of a new LC-MS/MS instrumentation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. 2003. Available from http://apps.who.int/malaria/docs/ProtocolWHO.pdf.
- 2.Nwanyanwu O, Redd S, Ziba C, Luby S, Mount D, et al. Validity of mother's history regarding antimalarial drug use in Malawian children under five years old. Trans R Soc Trop Med Hyg. 90:66–68. doi: 10.1016/s0035-9203(96)90482-4. [DOI] [PubMed] [Google Scholar]
- 3.Mahomva A, Peterson D, Rakata L. Does an antimalarial drug history mean anything? Cent Afr J Med. 1996;42:345–346. [PubMed] [Google Scholar]
- 4.Wichmann O, Eggelte T, Gellert S, Osman M, Mylius F, et al. High residual chloroquine blood levels in African children with severe malaria seeking healthcare. Trans R Soc Trop Med Hyg. 2007;101:637–642. doi: 10.1016/j.trstmh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Quashie N, Akanmori B, Goka B, Ofori-Adjei D, Kurtzhals J. Pretreatment blood concentrations of chloroquine in patients with malaria infection: relation to response to treatment. J Trop Pediatr. 2005;51:149–153. doi: 10.1093/tropej/fmh104. [DOI] [PubMed] [Google Scholar]
- 6.Evans J, May J, Tominski D, Eggelte T, Marks F, et al. Pre-treatment with chloroquine and parasite chloroquine resistance in Ghanaian children with severe malaria. QJM. 2005;98:789–796. doi: 10.1093/qjmed/hci121. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhardt S, Mockenhaupt F, Eggelte T, Agana-Nsiire P, Stollberg K, et al. Chloroquine blood concentrations and molecular markers of chloroquine-resistant Plasmodium falciparum in febrile children in northern Ghana. Trans R Soc Trop Med Hyg. 97:697–701. doi: 10.1016/s0035-9203(03)80106-2. [DOI] [PubMed] [Google Scholar]
- 8.Ofori-Adjei D, Commey J, Adjepon-Yamoah K. Serum chloroquine levels in children before treatment for malaria. Lancet. 1984;1:1246. doi: 10.1016/s0140-6736(84)91736-7. [DOI] [PubMed] [Google Scholar]
- 9.Talisuna A, Langi P, Bakyaita N, Egwang T, Mutabingwa T, et al. Intensity of malaria transmission, antimalarial-drug use and resistance in Uganda: what is the relationship between these three factors? Trans R Soc Trop Med Hyg. 96:310–317. doi: 10.1016/s0035-9203(02)90108-2. [DOI] [PubMed] [Google Scholar]
- 10.Verhoef H, Hodgins E, Eggelte T, Carter J, Lema O, et al. Anti-malarial drug use among preschool children in an area of seasonal malaria transmission in Kenya. Am J Trop Med Hyg. 1999;61:770–775. doi: 10.4269/ajtmh.1999.61.770. [DOI] [PubMed] [Google Scholar]
- 11.Schwick P, Eggelte T, Hess F, Tueumuna T, Payne D, et al. Sensitive ELISA dipstick test for the detection of chloroquine in urine under field conditions. Trop Med Int Health. 1998;3:828–832. doi: 10.1046/j.1365-3156.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 12.Mockenhaupt F, May J, Bergqvist Y, Ademowo O, Olumese P, et al. Concentrations of chloroquine and malaria parasites in blood in Nigerian children. Antimicrob Agents Chemother. 2000;44:835–839. doi: 10.1128/aac.44.4.835-839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellgren U, Ericsson O, Kihamia C, Rombo L. Malaria parasites and chloroquine concentrations in Tanzanian schoolchildren. Trop Med Parasitol. 1994;45:293–297. [PubMed] [Google Scholar]
- 14.Tippawangkosol P, Na-Bangchang K, Ubalee R, Congpuong K, Sirichaisinthop J, et al. Investigations of incidence of pretreatment, drug sensitivity in vitro, and plasma levels of pyrimethamine in patients with multidrug resistant falciparum malaria following the three combination regimens of artemether/pyrimethamine. Southeast Asian J Trop Med Public Health. 1999;30:220–224. [PubMed] [Google Scholar]
- 15.Nsimba S, Rimoy G. Self-medication with chloroquine in a rural district of Tanzania: a therapeutic challenge for any future malaria treatment policy change in the country. J Clin Pharm Ther. 2005;30:515–519. doi: 10.1111/j.1365-2710.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 16.Pfeiffer K, Some F, Müller O, Sie A, Kouyaté B, et al. Clinical diagnosis of malaria and the risk of chloroquine self-medication in rural health centres in Burkina Faso. Trop Med Int Health. 2008;13:418–426. doi: 10.1111/j.1365-3156.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- 17.Rombo L, Kihamia C, Mahikwano L, Ericsson O, Sjöqvist F. Concentrations of chloroquine and desethylchloroquine in capillary blood dried on filter paper during and after treatment of Tanzanian children infected with Plasmodium falciparum. Trop Med Parasitol. 1986;37:237–240. [PubMed] [Google Scholar]
- 18.Hodel E, Zanolari B, Mercier T, Biollaz J, Keiser J, et al. A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:867–886. doi: 10.1016/j.jchromb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Hetzel M, Alba S, Fankhauser M, Mayumana I, Lengeler C, et al. Malaria risk and access to prevention and treatment in the paddies of the Kilombero Valley, Tanzania. Malar J. 2008;7:7. doi: 10.1186/1475-2875-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hetzel M, Iteba N, Makemba A, Mshana C, Lengeler C, et al. Understanding and improving access to prompt and effective malaria treatment and care in rural Tanzania: the ACCESS Programme. Malar J. 2007;6:83. doi: 10.1186/1475-2875-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) Guidance for Industry: Bioanalytical Method Validation. 2001. Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf.
- 22.White NJ, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics of artemether-lumefanrine. Clin Pharmacokinet. 1999;37:105–25. doi: 10.2165/00003088-199937020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Jackson P, Tucker G, Woods H. Backtracking booze with Bayes–the retrospective interpretation of blood alcohol data. Br J Clin Pharmacol. 1991;31:55–63. doi: 10.1111/j.1365-2125.1991.tb03857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price R, Uhlemann A, van Vugt M, Brockman A, Hutagalung R, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman M, Dondorp A, Day N, Lindegardh N, Imwong M, et al. Adherence and efficacy of supervised versus non-supervised treatment with artemether/lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Bangladesh: a randomised controlled trial. Trans R Soc Trop Med Hyg. 2008;102:861–867. doi: 10.1016/j.trstmh.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Newton P, van Vugt M, Teja-Isavadharm P, Siriyanonda D, Rasameesoroj M, et al. Comparison of oral artesunate and dihydroartemisinin antimalarial bioavailabilities in acute falciparum malaria. Antimicrob Agents Chemother. 2002;46:1125–1127. doi: 10.1128/AAC.46.4.1125-1127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White N, Looareesuwan S, Warrell D, Warrell M, Bunnag D, et al. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated Falciparum malaria. Am J Med. 1982;73:564–572. doi: 10.1016/0002-9343(82)90337-0. [DOI] [PubMed] [Google Scholar]
- 28.Babalola C, Bolaji O, Ogunbona F, Sowunmi A, Walker O. Pharmacokinetics of quinine in African patients with acute falciparum malaria. Pharm World Sci. 1998;20:118–122. doi: 10.1023/a:1008699022244. [DOI] [PubMed] [Google Scholar]
- 29.White N. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White N, Pongtavornpinyo W. The de novo selection of drug-resistant malaria parasites. Proc Biol Sci. 2003;270:545–554. doi: 10.1098/rspb.2002.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepniewska K, White N. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother. 2008;52:1589–1596. doi: 10.1128/AAC.00903-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansor S, Navaratnam V, Mohamad M, Hussein S, Kumar A, et al. Single dose kinetic study of the triple combination mefloquine/sulphadoxine/pyrimethamine (Fansimef) in healthy male volunteers. Br J Clin Pharmacol. 1989;27:381–386. doi: 10.1111/j.1365-2125.1989.tb05381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obua C, Hellgren U, Ntale M, Gustafsson L, Ogwal-Okeng J, et al. Population pharmacokinetics of chloroquine and sulfadoxine and treatment response in children with malaria: suggestions for an improved dose regimen. Br J Clin Pharmacol. 2008;65:493–501. doi: 10.1111/j.1365-2125.2007.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green M, van Eijk A, van Ter Kuile F, Ayisi J, Parise M, et al. Pharmacokinetics of sulfadoxine-pyrimethamine in HIV-infected and uninfected pregnant women in Western Kenya. J Infect Dis. 2007;196:1403–1408. doi: 10.1086/522632. [DOI] [PubMed] [Google Scholar]
- 35.Trenque T, Simon N, Villena I, Chemla C, Quereux C, et al. Population pharmacokinetics of pyrimethamine and sulfadoxine in children with congenital toxoplasmosis. Br J Clin Pharmacol. 2004;57:735–741. doi: 10.1111/j.1365-2125.2004.02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes K, Little F, Smith P, Evans A, Watkins W, et al. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin Pharmacol Ther. 2006;80:582–596. doi: 10.1016/j.clpt.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Edstein M. Pharmacokinetics of sulfadoxine and pyrimethamine after Fansidar administration in man. Chemotherapy. 1987;33:229–233. doi: 10.1159/000238499. [DOI] [PubMed] [Google Scholar]
- 38.Corvaisier S, Charpiat B, Mounier C, Wallon M, Leboucher G, et al. Population pharmacokinetics of pyrimethamine and sulfadoxine in children treated for congenital toxoplasmosis. Antimicrob Agents Chemother. 2004;48:3794–3800. doi: 10.1128/AAC.48.10.3794-3800.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obua C, Ntale M, Lundblad M, Mahindi M, Gustafsson L, et al. Pharmacokinetic interactions between chloroquine, sulfadoxine and pyrimethamine and their bioequivalence in a generic fixed-dose combination in healthy volunteers in Uganda. Afr Health Sci. 2006;6:86–92. doi: 10.5555/afhs.2006.6.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]