Abstract
Anxiety disorders, depression and animal models of vulnerability to a depression-like syndrome have been associated with dysregulation of serotonergic systems in the brain. To evaluate the effects of early life experience, adverse experiences during adulthood, and potential interactions between these factors on serotonin transporter (slc6a4) mRNA expression, we investigated in rats the effects of maternal separation (180 min/day from days 2–14 of life; MS180), neonatal handing (15 min/day from days 2–14 of life; MS15), or normal animal facility rearing control conditions (AFR) with or without subsequent exposure to adult social defeat on slc6a4 mRNA expression in the dorsal raphe nucleus (DR) and caudal linear nucleus. At the level of specific subdivisions of the DR, there were no differences in slc6a4 mRNA expression between MS15 and AFR rats. Among rats exposed to a novel cage control condition, increased slc6a4 mRNA expression was observed in the dorsal part of the DR in MS180 rats, relative to AFR control rats. In contrast, MS180 rats exposed to social defeat as adults had increased slc6a4 mRNA expression throughout the DR compared to both MS15 and AFR controls. Social defeat increased slc6a4 mRNA expression, but only in MS180 rats and only in the “lateral wings” of the DR. Overall these data demonstrate that early life experience and stressful experience during adulthood interact to determine slc6a4 mRNA expression. These data support the hypothesis that early life experience and major stressful life events contribute to dysregulation of serotonergic systems in stress-related neuropsychiatric disorders.
Keywords: anxiety, depression, maternal separation, raphe, serotonin, stress
1. Introduction
Genetic influences and adverse experience, either during early life or during adulthood, contribute to the vulnerability of individuals to anxiety and affective disorders (Caspi et al, 2003; Hettema et al, 2006; Kendler et al, 2004; Kendler et al, 2005; McCauley et al, 1997; Teicher et al, 2006). One mechanism through which these factors may influence vulnerability to stress-related neuropsychiatric disorders is through effects on brainstem monoaminergic systems, including serotonergic systems (Canli and Lesch, 2007; Owens and Nemeroff, 1994). Indeed, the low-expressing s allele of a serotonin transporter-linked polymorphic region (5-HTTLPR) has been associated with increased vulnerability to anxiety and depression following exposure to major stressful life events during adulthood (Caspi et al, 2003; Grabe et al, 2005), although a recent meta-analysis has challenged this view (Risch et al, 2009). Furthermore, depressed subjects reporting childhood abuse have lower serotonin transporter binding than non-abused depressed subjects (Miller et al, 2009). Together, these data suggest that genetic influences, adverse early life experiences, and major stressful events during adulthood contribute to vulnerability to depression and the associated changes in serotonergic systems during adulthood.
Exposure of rats to prolonged periods of maternal separation during the neonatal period has been suggested as an animal model of vulnerability to development of anxiety states and a depression-like syndrome (Hall, 1998; Ladd et al, 2000; Plotsky et al, 1998; Willner, 1990). Although the mechanisms involved in the effects of adverse early life experience on subsequent vulnerability to anxiety states and a depression-like syndrome in rats are not clear, adverse early life experiences have been shown to alter serotonergic systems within the brain, including altered stress-induced serotonergic neurotransmission during adulthood (Daniels et al, 2004; Gartside et al, 2003; van Riel et al, 2004), and altered responsiveness of serotonergic neuronal firing rates to selective serotonin reuptake inhibitors (Arborelius et al, 2004). In contrast, exposure of rats to short periods of neonatal maternal separation (MS15 procedure) has been suggested as an animal model of resilience to stress and stress-related pathology, effects that are dependent on epigenetic programming and altered limbic serotonergic function (Meaney and Szyf, 2005; Smythe et al, 1994). Recent studies in human suicides confirm that childhood abuse alters epigenetic regulation of stress-related genes, including the gene encoding the glucocorticoid receptor (McGowan et al, 2009).
Evidence suggests that allelic variants of the gene (solute carrier family 6 (neurotransmitter transporter, serotonin), member 4, slc6a4) encoding the serotonin transporter may be genetic predictors of anxiety (for review, see Hariri and Holmes, 2006; Hu et al, 2006; Lesch et al, 1996; Ozaki et al, 2003), and affective disorders (Caspi et al, 2003; Hoefgen et al, 2005; Ogilvie et al, 1996; Rotondo et al, 2002), genetic predictors of suicide risk among depressed patients (Caspi et al, 2003), as well as genetic predictors of responses to antidepressant treatment (Smits et al, 2004; Yu et al, 2002).
Although it is clear that genetic influences can alter slc6a4 expression and that allelic variants of slc6a4 are associated with anxiety and affective disorders, it is unknown if interactions between adverse early life experience or stressful experience during adulthood, important vulnerability factors for anxiety disorders and depression, can alter slc6a4 expression.
We hypothesized that early life experience and subsequent stressful experience during adulthood interact to regulate slc6a4 mRNA expression in serotonergic neurons. In order to test this hypothesis, we studied potential interactions between different early life experience and stressful experience (exposure to social defeat or a novel cage control condition) during adulthood on slc6a4 mRNA expression in rats.
2. Results
Analysis at the level of the DR
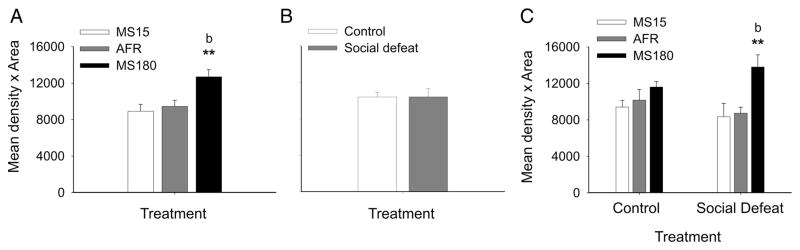
Analysis of slc6a4 mRNA expression across all 48 subregions of the DR studied (see Figure 1 for details) in each rat, using multifactor ANOVA with repeated measures, revealed significant interactions among early life experience, social defeat, and brain region (F(94, 1316) = 1.73; P < 0.001). Early life experience had a strong main effect on slc6a4 mRNA expression (F(2, 28) = 10.30; P < 0.001) as evidenced by the mean slc6a4 mRNA expression across all 48 subregions of the DR in rats exposed to either novel cage control conditions or social defeat as adults (Figure 2A; one-way ANOVA, F(2,32) = 7.75, P = 0.002). Analysis of slc6a4 mRNA expression without consideration of adult experience indicated that slc6a4 mRNA expression was increased 43% in MS180 rats relative to MS15 rats, and increased 34% in MS180 rats relative to AFR control rats. In contrast, analysis without consideration of differences in early life experience indicated that rats exposed to social defeat had comparable levels of slc6a4 mRNA expression across all 48 subregions of the DR relative to rats exposed to a novel cage (Figure 2B). This suggested that any effects of social defeat were evident only in rats with specific early life experience, only in specific subregions of the DR, or both. Collapsing the data for all 48 subregions of the DR indicated that MS180 rats exposed to social defeat, but not MS180 rats exposed to a novel cage, had higher slc6a4 mRNA expression relative to AFR and MS15 rats (Figure 2C). Among rats exposed to social defeat, the mean slc6a4 mRNA expression in all 48 subregions of the DR in MS180 rats was 65% higher than in MS15 rats and 58% higher than in AFR rats.
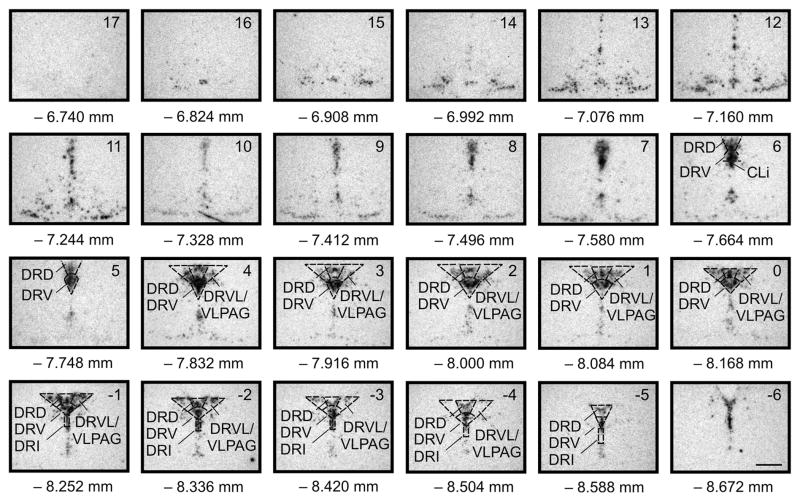
Figure 1.
Atlas of rat slc6a4 mRNA expression in the midbrain raphe complex (84 μm intervals) used for analysis of subregions of the DR with a high level of neuroanatomical resolution. Photographs are autoradiographic images of slc6a4 mRNA expression as indicated by in situ hybridization histochemistry. The levels chosen for analysis ranged from −7.664 mm Bregma (designated Level 6) though −8.588 mm Bregma (designated Level −5). Abbreviations; CLi, caudal linear nucleus of the raphe; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; VLPAG, ventrolateral periaqueductal gray. Scale bar, 1 mm. Adapted from Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A (2008) Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Serotonin and Sleep: Molecular, Functional and Clinical Aspects. J.M. Monti, S.R. Pandi-Perumal, B.L. Jacobs, and D.J. Nutt, Eds. Birkhauser Verlag AG, Basel, Switzerland, pp. 25–67. ISBN: 978-3-7643-8560-6, with permission.
Figure 2.
Early life experience and social defeat during adulthood interact to alter slc6a4 mRNA expression in adult midbrain serotonergic neurons. A) Mean slc6a4 mRNA expression levels in MS15, AFR, and MS180 rats, regardless of adult experience, based on the mean expression levels in forty-eight subdivisions of the DR. B) Mean slc6a4 mRNA expression levels in adult rats exposed to a novel cage (control condition) or social defeat, regardless of early life experience, based on the mean expression levels in forty-eight subdivisions of the DR. C) Mean slc6a4 mRNA expression levels in MS15, AFR, and MS180 rats exposed to a novel cage control condition (control, left) or social defeat (right) as adults. Slc6a4 mRNA expression was measured 4 h following exposure to a novel cage control condition or to social defeat. **P < 0.01, comparison of MS15 and MS180 groups. bP < 0.01, comparison of AFR and MS180 groups. Data are illustrated as means + SEM.
Analysis at the level of subdivisions of the DR
Serotonergic neurons in the DR are topographically organized, such that different subdivisions of the DR receive different afferents (Peyron et al, 1998), and give rise to unique patterns of efferents (Lowry et al, 2008a). Therefore, we analyzed slc6a4 mRNA expression in each of the major subdivisions of the DR and the CLi; this analysis, ignoring differences between the rostrocaudal levels of each subdivision, revealed that the effects of early life experience and social defeat were dependent on the specific subdivision of the DR studied (Figure 3; F(94, 1316) = 1.73; P < 0.001). Among either rats exposed to a novel cage control condition or rats exposed to social defeat, there were no differences in slc6a4 mRNA expression in MS15 rats, compared to AFR rats. In contrast, differences in slc6a4 mRNA expression in MS15 or AFR rats relative to MS180 rats were observed in several subdivisions of the DR and CLi, predominantly in rats exposed to social defeat. Among rats exposed to a novel cage control condition, slc6a4 mRNA expression was higher in MS180 rats, compared to AFR control rats, but only in the dorsal part of the DR (DRD; Figure 3A). In rats exposed to social defeat, however, slc6a4 mRNA expression was higher in MS180 rats, compared to either MS15 or AFR rats, in all subdivisions excluding the interfascicular part of the DR (DRI). Percent changes in slc6a4 mRNA expression within subdivisions of the DR and the CLi, due to early life experience, are reported in Table 1.
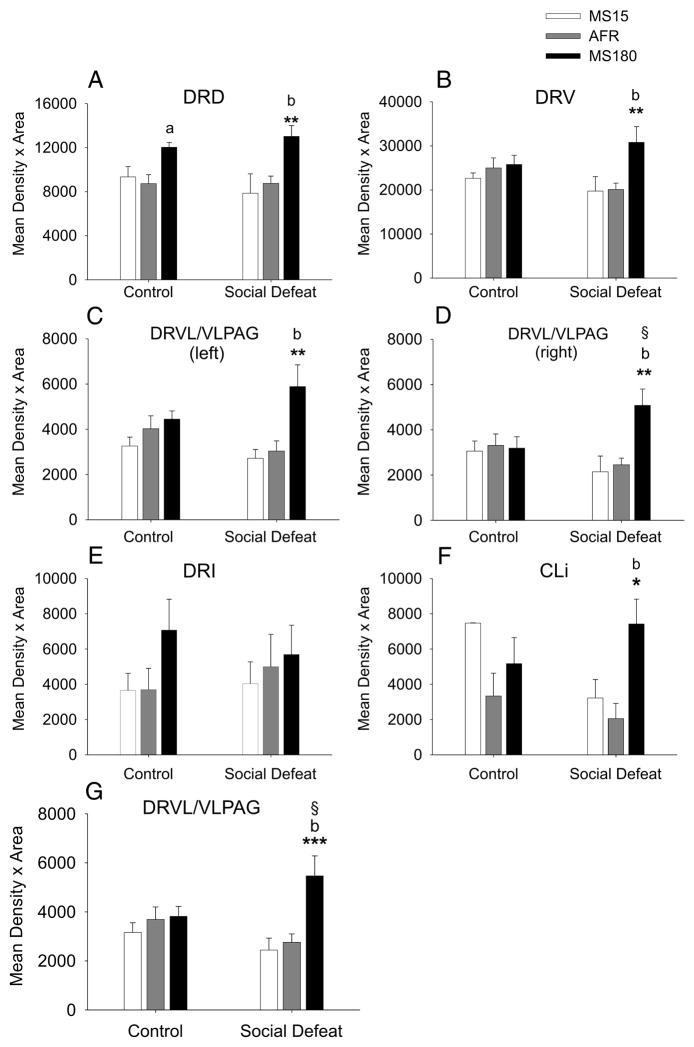
Figure 3.
Effects of social defeat on slc6a4 mRNA expression in specific subdivisions of the DR are dependent on early life experience. Graphs illustrate the effects of early life experience and social defeat on slc6a4 mRNA expression in each of the major subdivisions of the DR and the CLi. Data are collapsed across the rostrocaudal extent of each subdivision. A) DRD; B) DRV; C) left DRVL/VLPAG; D) right DRVL/VLPAG; E) DRI; F) CLi; G) combined left and right DRVL/VLPAG. For abbreviations, see Figure 1 legend. *P < 0.05, **P < 0.01, ***P < 0.001, comparison of MS15 and MS180 groups. aP < 0.05, bP < 0.01, comparison of AFR and MS180 groups. §P < 0.05, comparison of control and social defeat groups within the same early life experience treatment group. Data are illustrated as means + SEM.
Table 1.
Percent changes in slc6a4 mRNA expression due to early life experience in subdivisions of the DR and CLi.
| MS15 vs AFR | MS180 vs MS15 | MS180 vs AFR | ||
|---|---|---|---|---|
| DRD | CO | +6.9 | +28.9 | +37.8a |
| SD | −10.2 | +65.6** | +48.7b | |
| DRV | CO | −9.5 | +13.8 | +3.1 |
| SD | −1.8 | +56.1** | +53.3b | |
| DRVL left | CO | −19.0 | +36.5 | +10.7 |
| SD | −10.7 | +116.9** | +93.8b | |
| DRVL right | CO | −7.7 | +4.4 | −3.6 |
| SD | −12.5 | +136.7** | +107.1b | |
| DRI | CO | −1.3 | +93.9 | +91.4 |
| SD | −19.3 | +41.3 | +14.1 | |
| CLI | CO | +124.0 | −30.8 | +55.1 |
| SD | +56.9 | +130.6* | +261.9b | |
| DRVL | CO | −14.4 | +20.9 | +3.6 |
| SD | −11.5 | +123.9*** | +98.1b | |
Social defeat increased slc6a4 mRNA expression, but this effect was only significant in the right DRVL/VLPAG, or the combined right and left DRVL/VLPAG, and only in MS180 rats (Figure 3D, 3G). The comparison of slc6a4 mRNA expression in the left DRVL/VLPAG in MS180 rats exposed to a novel cage relative to MS180 rats exposed to social defeat approached statistical significance (P = 0.082). Percent changes in slc6a4 mRNA expression within subdivisions of the DR and the CLi, due to social defeat, are presented in Table 2. Together, these data suggest that the effects of social defeat, by itself, to increase slc6a4 mRNA expression were greatest in the DRVL/VLPAG region and furthermore that the effects of social defeat in the DRVL/VLPAG were only observed in MS180 rats. Indeed, when the mean slc6a4 mRNA expression was considered for the combined left and right DRVL, slc6a4 mRNA expression was higher in rats exposed to social defeat compared to rats exposed to the novel cage control condition, and this effect was only observed in MS180 rats (Figure 3G).
Table 2.
Percent changes in slc6a4 mRNA expression due to social defeat in subdivisions of the DR and CLi.
| SD vs CO | |||
|---|---|---|---|
| MS15 | AFR | MS180 | |
| DRD | −15.8 | +0.3 | +8.2 |
| DRV | −12.7 | −19.6 | +19.6 |
| DRVL left | −16.8 | −24.5 | +32.2 |
| DRVL right | −29.7 | −25.8 | +59.4§ |
| DRI | +10.5 | +35.1 | −19.5 |
| CLI | −56.9 | −38.5 | +43.6 |
| DRVL | −22.7 | −25.2 | +43. 2§ |
p < 0.05; for full analysis see figure 3.
Analysis at the level of different rostrocaudal levels of subdivisions of the DR
In addition to anatomical and functional differences among different subdivisions of the DR, there is anatomical and functional heterogeneity along the rostrocaudal axis of the DR (Abrams et al, 2004; Imai et al, 1986; Lowry et al, 2005; Lowry et al, 2008a). Separate analysis of the effects of early life experience and social defeat on slc6a4 mRNA expression at specific rostrocaudal levels of each of the major subdivisions of the DR revealed effects of early life experience and social defeat that were limited to specific rostrocaudal levels of the midbrain raphe complex (Figure 4, 5).
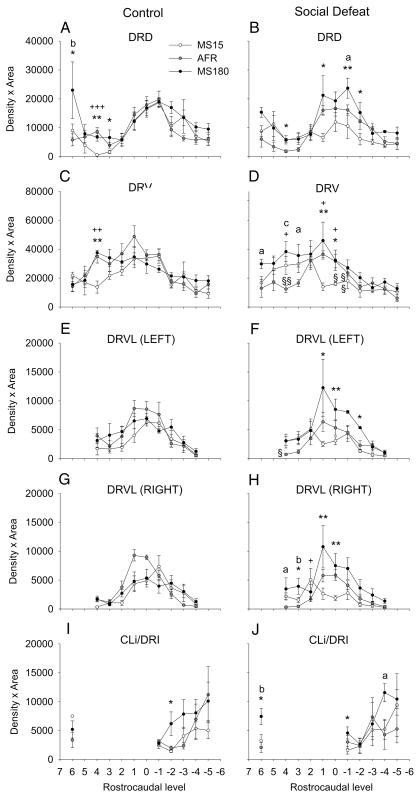
Figure 4.
Early life experience and social defeat during adulthood interact to regulate slc6a4 mRNA expression at specific rostrocaudal levels of subdivisions of the DR. Each graph illustrates the mean slc6a4 mRNA expression levels throughout the rostrocaudal extent of a different subdivision of the DR in MS15 and MS180 rats exposed to a novel cage control condition (control, left) or social defeat (right) as adults. A) DRD; B) DRV; C) left DRVL/VLPAG; D) right DRVL/VLPAG; E) CLi and DRI). For abbreviations, see Figure 1 legend. +P < 0.05, ++P < 0.01, +++P < 0.001, comparison of MS15 and AFR groups. *P < 0.05, **P < 0.01, comparison of MS15 and MS180 groups. aP < 0.05, bP < 0.01, cP < 0.001, comparison of AFR and MS180 groups. §P < 0.05, §§P < 0.01, comparison of control and social defeat groups within the same early life experience treatment group at the same rostrocaudal level. Data are illustrated as means ± SEM.
Figure 5.
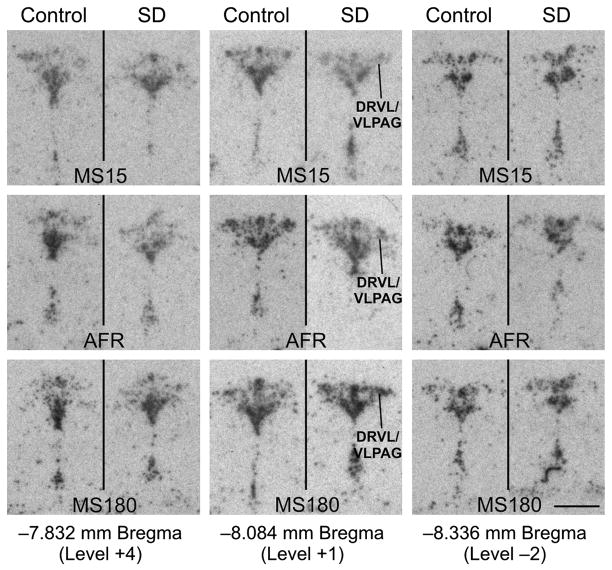
Social defeat increases slc6a4 mRNA expression in the DRVL/VLPAG region 4 h following exposure to the social defeat paradigm, but only in MS180 rats. Autoradiographic images illustrate slc6a4 mRNA expression at three anatomical levels (rostral, −7.832; mid-rostrocaudal, −8.084; and caudal, −8.336 mm Bregma) from each treatment group including rats exposed to control and social defeat conditions among MS15, AFR, and MS180 rats. In each panel, the DR is at the top and the median raphe nucleus and supralemniscal cell group (B9) are at the bottom. Slc6a4 mRNA expression within the DRVL/VLPAG region is indicated by a line in the social defeat images for MS15, AFR and MS180 at the midrostrocaudal anatomical level. Scale bar, 1 mm.
Among rats exposed to novel cage control conditions, increases in slc6a4 mRNA expression were observed in the rostral part of the DRD and DRV of MS180 rats and AFR rats, relative to MS15 rats (Figure 4, 5). Together, these data suggest that early life experience had modest effects on slc6a4 mRNA expression within subregions of the DR and CLi in rats exposed to a novel cage control condition as adults.
Effects of early life experience on slc6a4 mRNA expression were more widespread in rats subsequently exposed to social defeat, with effects at specific rostrocaudal levels of all subdivisions of the DR (DRD, DRV, left and right DRVL/VLPAG region, DRI) and CLi studied (Figure 4, 5). Within the DRD, among rats exposed to social defeat, rats exposed to maternal separation during early life had increases in slc6a4 mRNA expression, compared to either AFR control rats or MS15 rats, that were largely restricted to the mid-rostrocaudal DRD (Figure 4B). In contrast, MS15 rats had decreased slc6a4 mRNA expression, compared to AFR control rats, at one level of the mid-rostrocaudal DRD. Thus, maternal separation increased, while neonatal handling (MS15 condition) decreased, slc6a4 mRNA expression within the mid-rostrocaudal DR.
Within the DRV, among rats exposed to social defeat, rats exposed to maternal separation during early life had increases in slc6a4 mRNA expression, compared to MS15 rats, that were restricted to the mid-rostrocaudal DRV; meanwhile, increases in slc6a4 mRNA expression in MS180 rats, compared to AFR control rats, were restricted to the rostral DRV (Figure 4D, 5). In contrast, MS15 rats had decreased slc6a4 mRNA expression, compared to AFR control rats, within the mid-rostrocaudal DRV (Figure 4D). Thus, maternal separation increased slc6a4 mRNA expression while neonatal handling (MS15 condition) decreased slc6a4 mRNA expression within the rostral and mid-rostrocaudal DRV, respectively.
Within the DRVL/VLPAG region, among rats exposed to social defeat, maternal separation increased slc6a4 mRNA expression, relative to MS15 rats, in the left and right mid-rostrocaudal DRVL/VLPAG regions, and increased slc6a4 mRNA expression, relative to AFR control rats, in the most rostral pole of the right DRVL/VLPAG region (Figure 4G, 4H, 5). Thus, the most evident effects of early life experience on slc6a4 mRNA expression within the DRVL/VLPAG region were the effects of maternal separation to increase slc6a4 mRNA expression, relative to MS15 rats and AFR control rats, within the mid-rostrocaudal and rostral DRVL/VLPAG regions, respectively.
Within the DRI, among rats exposed to social defeat, rats exposed to maternal separation had elevated slc6a4 mRNA expression, relative to MS15 rats, within the rostral DRI, and relative to AFR control rats, within the mid-caudal DR (Figure 4J). Finally, within the CLi, among rats exposed to social defeat, rats exposed to maternal separation had increased slc6a4 mRNA expression compared to either MS15 or AFR control rats (Figure 5). Thus, within both the DRI and CLi, analysis of specific rostrocaudal levels suggests that maternal separation generally results in an increase in slc6a4 mRNA expression, relative to MS15 rats and AFR control rats.
Social defeat decreased slc6a4 mRNA expression in specific rostrocaudal regions of the DRV of MS15 and AFR rats, relative to MS15 and AFR rats exposed to a novel cage control condition, and these effects were only observed in the mid-rostrocaudal and rostral section of the DRV.
3. Discussion
An interaction between adverse early life experience and stress during adulthood led to increased slc6a4 mRNA expression in the DR. In addition, exposure to social defeat during adulthood, compared to exposure to a novel cage control condition, increased slc6a4 mRNA expression, but only in MS180 rats and only in the DRVL/VLPAG region. Analysis of specific rostrocaudal levels of each major subdivision of the DR revealed effects of early life experience and social defeat at specific rostrocaudal levels of several subdivisions, consistent with an anatomic and functional heterogeneity within the DR. These data suggest that adverse early life experience results in vulnerability to stress-induced increases in slc6a4 mRNA expression. These changes in slc6a4 mRNA expression may alter subsequent responses to aversive stimuli, but this will need to be determined experimentally.
Although we have not included behavioral analysis in this study, in a previous study conducted using rats from the same litters, tested under the same conditions (Gardner et al, 2005), MS180 rats displayed a higher ratio of passive-submissive behaviors (passive genital sniff, genital sniff, sniffing bedding, freezing, sideways submission, and full submission) versus proactive coping behaviors (attack, upright defensive behavior, rearing, and escape) compared with either MS15 or AFR rats, based on the frequencies or durations of behaviors during defeat. Consistent with these findings, based on analysis of the duration of behaviors, MS180 rats responded with more passive–submissive behavior and less proactive coping behavior compared with either MS15 or AFR rats. As serotonergic systems are thought to contribute to regulation of defensive behavior (Deakin and Graeff, 1991; Graeff et al, 1996), the differential vulnerability of MS180 rats to stress-induced effects on DR serotonergic systems may contribute to these behavioral differences.
Rats exposed to maternal separation followed by exposure to a novel cage control condition during adulthood had slc6a4 mRNA expression levels in the DR that were equivalent to levels in MS15 and AFR control rats. This suggests that baseline levels of slc6a4 mRNA expression were equivalent among the groups of rats with different early life experience. These findings are consistent with a previous study demonstrating that there are no differences in slc6a4 binding or mRNA expression in adult MS15 and MS180 rats (Arborelius et al, 2004). However, these findings conflict with another report that slc6a4 mRNA expression is lower in rats exposed to maternal separation, compared to non-handled control rats under baseline conditions (Lee et al, 2007). These differences may be due to differences in the strain of rat used, differences in the methods for maternal separation, differences in housing conditions during adulthood, or the fact that rats in the current study were exposed to a novel cage control condition. The novel cage was located in the same room where social defeat occurred, out of sight of the social defeat cage; however, rats exposed to the novel cage control condition would have been exposed to any auditory or olfactory stimuli, including ultrasonic vocalizations, which itself may have been stressful.
Although previous studies (Arborelius et al, 2004), and the current study, have found no differences between MS15 and MS180 rats with respect to slc6a4 mRNA expression or serotonin transporter binding in the DR (Arborelius et al, 2004), or limbic forebrain structures (Vicentic et al, 2006), MS15 and MS180 rats differ in their responsiveness of DR serotonergic neuronal firing rates to the selective serotonin reuptake inhibitor citalopram (Arborelius et al, 2004). Since baseline firing rates of DR serotonergic neurons in MS15 and MS180 rats do not differ (Arborelius et al, 2004), differential sensitivity to citalopram might occur if tryptophan hydroxylase activity and serotonin release was elevated in MS180 rats. Consistent with this possibility, we have found in a previous study that MS180 rats have increased tryptophan hydroxylase 2 (tph2) mRNA expression in the DR, relative to MS15 rats (Gardner et al, 2009).
An interaction between adverse early life experience and stress during adulthood led to increased slc6a4 mRNA expression within specific subdivisions of the DR. Among rats exposed to a novel cage control condition, slc6a4 mRNA expression was for the most part equivalent in MS15, AFR, and MS180 rats. In contrast, all regions, excluding the DRI, had increased slc6a4 mRNA expression in MS180 rats compared to MS15 or AFR rats when rats were exposed to social defeat. One region, the DRD, responded with increased slc6a4 mRNA expression in MS180 rats exposed to either a novel cage control condition, or social defeat. This region also selectively responded to social defeat with increased c-Fos expression in serotonergic neurons, in MS15, AFR, and MS180 rats (Gardner et al, 2005), suggesting that this region is uniquely activated by social defeat. The DRD has been associated with facilitation of stress- or anxiety-related responses (Lowry et al, 2005; Lowry et al, 2008b; Maier and Watkins, 2005), and it is therefore possible that differences in slc6a4 mRNA expression among the different early life experience treatment groups are absent under baseline conditions, but the mild stress of exposure to a novel cage increased slc6a4 mRNA expression in the DRD of MS180 rats, but not in MS15 or AFR control rats. Future studies will be required to resolve this issue.
Among rats exposed to social defeat, MS180 rats, compared to rats exposed to neonatal handling (MS15 condition) or AFR control conditions, had increased slc6a4 mRNA expression in the mid-rostrocaudal part of the DRD. The mid-rostrocaudal and caudal parts of the DRD region are selectively activated following exposure of rats to a number of stress- and anxiety-related stimuli (Abrams et al, 2005; Lowry et al, 2005; Lowry et al, 2008b; Lowry et al, 2008a; Lowry and Hale, 2009; Staub et al, 2005; Staub et al, 2006), including social defeat (Gardner et al, 2005) and give rise to a dense innervation of the amygdala (Abrams et al, 2005). Human imaging studies reveal that the number of low-expressing s alleles of the serotonin transporter (5-HTT)-linked polymorphic region (5-HTTLPR) is positively correlated with activation of the amygdala in response to exposure to aversive, but not non-aversive pictures (Hariri and Holmes, 2006; Heinz et al, 2005), as well as to uncertain, presumably stressful, stimuli (Heinz et al, 2007). Studies in mice suggest that developmental influences could account for these differences and for vulnerability to psychiatric disorders in individuals with low-expressing slc6a4 promoter alleles (Ansorge et al, 2004). Here we demonstrate something quite different, that stressful experiences during adulthood can increase slc6a4 mRNA expression, at least in vulnerable individuals. The functional consequences of this increased expression, in amygdala circuits for example, would be dependent on the functional expression of the serotonin transporter protein and its cellular trafficking. If the serotonin transporter is preferentially trafficked to dendrites, then we would predict that serotonergic neurotransmission would be increased (due to decreased negative feedback at 5-HT1A autoreceptors). In contrast, if the serotonin transporter is preferentially trafficked to axon terminals, then we would predict that serotonergic neurotransmission would be decreased (due to more rapid clearance of 5-HT from synaptic sites) at a time point consistent with trafficking of serotonin transporter protein to the axon terminals. Future studies are required to determine the specific outcomes. Nevertheless, our studies provide evidence for increased stress-sensitivity of rats previously exposed to maternal separation, as measured by changes in gene expression.
Our data suggest that main effects of stress to increase slc6a4 mRNA expression were only observed in MS180 rats, and only in the DRVL/VLPAG region. The mechanisms underlying the regional specificity of the effects of stress are not certain, but may be related to alterations of context-dependent, specific afferent input to this region. The developmentally dependent, regionally specific effects of stress on slc6a4 mRNA expression, may be due to activation of specific neural circuits by social defeat, which are known to include the DRVL/VLPAG region (Gardner et al, 2005; Martinez et al, 1998; Matsuda et al, 1996; Miczek et al, 2004; Nikulina et al, 1998). The DRVL/VLPAG region plays an important role in passive emotional coping responses to different inescapable, stress-related stimuli, including social defeat (Johnson et al, 2005; Keay and Bandler, 2001). These stimuli often induce hypotension and bradycardia, responses associated with a passive emotional coping response consisting of disengagement from the environment and behavioral quiescence (Keay and Bandler, 2001). These functional properties of the DRVL/VLPAG region are consistent with the shift toward a passive-submissive behavioral response to social defeat in MS180 rats (Gardner et al, 2005). The DRVL/VLPAG region receives direct input from the prefrontal cortex in rats (Peyron et al, 1998) and the medial, orbito-insular, and cingulate/dorsomedial convexity prefrontocortical areas in primates (Keay and Bandler, 2001). Differential input from these regions regulating emotional behavior is one mechanism that may account for the site-specific effects of stress on slc6a4 mRNA expression.
It is possible that brainstem afferents to the DR also contribute to the selective actions of adverse early life experience and social defeat stress on slc6a4 mRNA expression in subpopulations of serotonergic neurons. A number of excellent studies have evaluated brainstem inputs to the DR (Braz et al, 2009; Lee et al, 2005; Lee et al, 2003; Peyron et al, 1996; Sakai et al, 1977). Included among caudal brainstem afferents to the DR are the brainstem catecholaminergic systems, which play an excitatory role in regulation of serotonergic neuronal firing rates (Vandermaelen and Aghajanian, 1983). Recently, studies by Braz and colleagues (2009), confirmed that A1, A2, A5, A6 (locus coeruleus), and A7 noradrenergic cells specifically innervate DR serotonergic neurons. A few studies have analyzed brainstem catecholamine afferents to specific subdivisions of the DR (Lee et al, 2005; Lee et al, 2003; Peyron et al, 1996). These studies reveal that the DRVL/VLPAG region (the “lateral wings” of the DR) has a comparable innervation by the A5 and A6 cell groups, but together with the rostral DRD, is selectively innervated by the rostral (C1 adrenergic cell group) and caudal (A1 noradrenergic cell group) ventrolateral medulla, and the A2 cell group in the commissural part of the nucleus of the solitary tract (Peyron et al, 1996). These findings are consistent with a high density of phenylethanolamine-N-methyltransferase- (PNMT) and dopamine-β-hydroxylase- (DBH) immunoreactive fibers specifically within the rostral dorsal part of the DR and the lateral wings of the DR (Herbert and Saper, 1992), and the finding that stress-induced noradrenaline release is elevated in MS180 rats (Liu et al, 2000). Thus, social defeat-induced activation of the C1, A1, and A2 catecholaminergic cell groups could contribute to the selective stress-induced activation of serotonergic neurons in the DRVL/VLPAG region in MS180 rats.
Previous studies suggest that repeated social defeat may induce a more widespread increase in slc6a4 mRNA expression in the midbrain raphe nuclei. Increased slc6a4 mRNA expression was evident in losers of aggressive encounters in mice exposed to chronic (10 days) social defeat, relative to either winners of the aggressive encounters or single housed controls, using brain microdissection and reverse transcription polymerase chain reaction (RT-PCR) (Filipenko et al, 2002). Similarly, chronic stress (5 days), but not acute stress, has been found to increase slc6a4 mRNA expression in the midbrain raphe nuclei of male and female rats using brain microdissection and northern blot (Pare et al, 1999). Together, these findings suggest that the overall trend for chronic stress-induced changes in slc6a4 mRNA expression during adulthood is an increase in expression. In addition, treatment of mice with interferon-α, which, like chronic stress, is known to have psychiatric consequences including depression in human patients (Raison et al, 2006), also increases slc6a4 mRNA expression (Morikawa et al, 1998). In contrast, repeated exposure to electroconvulsive shock (Shen et al, 2001), which has antidepressant effects in humans, or chronic treatment with the antidepressants imipramine, fluoxetine, or tianeptine (Kuroda et al, 1994; Lesch et al, 1993), decreases slc6a4 mRNA expression in the rat midbrain raphe complex. The findings with antidepressant drug treatment are not consistent, however, as other studies have found that antidepressants either increase slc6a4 mRNA expression (Lopez et al, 1994) or have no effect (Burnet et al, 1994; Koed and Linnet, 1997; Linnet et al, 1995; Spurlock et al, 1994). However, the latter studies measured slc6a4 mRNA expression in whole brain homogenates using multiprobe oligonucleotide solution hybridization (Spurlock et al, 1994), midbrain homogenates using northern blot (Koed and Linnet, 1997; Linnet et al, 1995), or undefined regions of the midbrain raphe complex using in situ hybridization histochemistry (Burnet et al, 1994), and therefore would not have detected regional differences in slc6a4 mRNA expression.
Social defeat decreased slc6a4 mRNA expression in specific rostrocaudal regions of the DRV of MS15 and AFR rats, relative to MS15 and AFR rats exposed to a novel cage control condition, and these effects were only observed in the mid-rostrocaudal and rostral sections of the DRV. The consequences of a stress-induced decrease in slc6a4 mRNA expression in the mid-rostrocaudal and rostral sections of the DRV are unclear, but may alter subsequent social behavior (see below). Elevations in slc6a4 mRNA expression in MS180 rats exposed to social defeat, relative to AFR and MS15 rats exposed to social defeat, were also restricted to the mid-rostrocaudal and rostral section of the DRV. As with the DRVL/VLPAG regions discussed above, the mechanisms underlying the regional specificity of the effects of stress and early life experience in the DRV are not certain, but may be related to alterations of context-dependent, specific afferent input to this region. The DRV is selectively innervated by the lateral orbital cortex, and medial and lateral parts of the preoptic area (Peyron et al, 1998). Activation of the lateral orbital cortex has been described following psychosocial and aggressive encounters in rats, and pyramidal cell activation in the lateral orbital cortex, together with the infralimbic, medial orbital, and agranular insular parts of the prefrontal cortex, predicts over 95% of variation in attack counts in general and violent attacks in particular (Halasz et al, 2006). Meanwhile, neural responses during response inhibition in behavioral impulsivity tasks were most prominent in the right lateral orbitofrontal cortex in human studies using functional magnetic resonance imaging (fMRI) (Horn et al, 2003). Consequently, activation of a lateral orbital cortex-DR pathway may be associated with the shift toward a passive-submissive emotional coping strategy in MS180 rats (Gardner et al, 2005). Consistent with these findings, a selective activation of the rostral part of the DRV has been described in losers in a resident-intruder paradigm in Syrian hamsters (Cooper et al, 2009). As with the lateral orbital cortex, the medial preoptic area is involved in regulation of inter-male aggressive behavior in rodents (Simon et al, 1998). The innervation of the medial preoptic area in turn arises predominantly from the rostral DRV, suggesting that these regions may have reciprocal projections (Rizvi et al, 1992), and that the rostral DRV may play a particularly important role in serotonergic regulation of aggressive behavior. Thus, activation of neural circuits involved in regulation of behavior during psychosocial and aggressive encounters may account for the site-specific effects of social defeat on slc6a4 mRNA expression within the rostral DRV observed in the present study. This could explain why other anxiety- or stress-related stimuli, such as anxiogenic drugs (Abrams et al, 2005), anxiety-related neuropeptides (Staub et al, 2005; Staub et al, 2006), inescapable shock (Grahn et al, 1999), and hypercapnia (Johnson et al, 2005), do not appear to activate the rostral DRV.
It is currently unclear if slc6a4 mRNA expression is increased, decreased, or normal in human depressed patients. Initial studies found no differences in slc6a4 mRNA expression in depressed suicides and controls (Little et al, 1997). One study has found that slc6a4 mRNA expression per cell is increased in depressed suicide patients (Arango et al, 2001). However, in the same study, there were 54% fewer DR neurons expressing slc6a4 mRNA, suggesting that some serotonergic neurons, but not others, have elevated slc6a4 mRNA expression in depressed patients. Several studies have measured brainstem serotonin transporter availability in depressed patients. Initial imaging studies demonstrated decreased brainstem serotonin transporter availability in depressed patients as measured by [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane ([123I]-β-CIT) or [123I]-nor-β-CIT and single photon emission computed tomography (SPECT) (Joensuu et al, 2007; Lehto et al, 2006; Malison and et al., 1998). However, a subsequent study using ([123I]-β-CIT) and SPECT combined with magnetic resonance imaging (MRI) found that the decrease in serotonin transporter availability was restricted to females, localized within the diencephalon, and not present at all in the midbrain (Staley et al, 2006). Meanwhile, using positron emission tomography (PET), Ichimiya and colleagues (2002) confirmed that there was no difference in the serotonin transporter binding potential in the midbrain of depressed patients compared with healthy controls, but revealed increased binding potential in the thalamus. Thus, there are reports of decreases, increases, or no change in serotonin transporter binding potential in depressed patients, depending on the area of interest. Taken together, it remains possible that slc6a4 mRNA expression is increased in a subpopulation of serotonergic neurons in depressed patients (Arango et al, 2001), similar to findings with tph2 mRNA expression in depressed patients (Bach-Mizrachi et al, 2008), which would be consistent with the present study.
In summary, an interaction between adverse early life experience and a stressful social defeat encounter during adulthood increased slc6a4 mRNA expression in the DR. Among rats exposed to social defeat, MS180 rats had increased slc6a4 mRNA expression compared to either MS15 or AFR control rats in all subdivisions of the DR studied, excluding the DRI. Social defeat increased slc6a4 mRNA expression, but only in the DRVL/VLPAG region, and only in rats exposed to adverse early life experiences (MS180 rats). Conversely, social defeat decreased slc6a4 mRNA expression, but only in the DRV and only in AFR and MS15 rats. Interactions among early life experience, stressful experiences during adulthood, and brain region in the transcriptional regulation of slc6a4 mRNA expression may explain some of the conflicting findings related to alterations in slc6a4 mRNA expression in depressed patients, as well as alterations in slc6a4 mRNA expression following antidepressant treatment in rats. These data are consistent with the hypothesis that early life experience interacts with stressful life events during adulthood to alter slc6a4 gene expression in subpopulations of serotonergic neurons. In addition, these data are consistent with the hypothesis that, in addition to genetic influences, early life experiences and major stressful life events interact to determine individual differences in slc6a4 gene expression in humans, as well as the vulnerability to stress-related psychiatric disorders.
4. Experimental Procedure
Animals
All animal care was conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (N.I.H. Publication No. 85-23) and approved by the Emory University Institutional Animal Care and Use Committee. Timed-pregnant Long Evans Crl:(LE)BR rats (Charles River, Portage, MI, USA) arrived at the Emory University vivarium on gestational day 12. Upon arrival, dams were individually housed and maintained on a 12:12 light dark cycle (lights on at 0700 h) with food and water available ad libitum.
Maternal separation
The animal care and maternal separation procedures used have been described in detail previously (Gardner et al, 2005; Huot et al, 2001; Plotsky and Meaney, 1993). The rats in this study belonged to the same cohort as rats used to determine the effect of early life experience and social defeat on behavior and c-Fos expression in serotonergic neurons in the midbrain raphe complex, and specific methods for animal handling and social defeat have been described (Gardner et al, 2005). Briefly, the day of birth was designated as postnatal day 0 (PND0). On PND2, dams were removed from their maternity cages to adjacent cages. There was a total of 72 male rat pups; 36 of which were used for behavioral analysis and immunohistochemical studies (Gardner et al, 2005) and 36 of which were used for in situ hybridization histochemistry studies; all rats were exposed to the same social defeat paradigm (see below). Female pups were culled from litters and male pups were pooled and standardized to foster litters consisting of eight rat pups each (9 foster litters in total). Previous studies using the MS15/MS180 rat models (PND 2–14) have used unmanipulated litters (Daniels et al, 2004; Jaworski et al, 2005; Vicentic et al, 2006), foster litters with male:female ratios of 1:1 (Boccia and Pedersen, 2001), litters culled to male:female ratios of 3:1 (Giachino et al, 2007; Huot et al, 2001; Lippmann et al, 2007), or male-only foster litters (Arborelius et al, 2004; Huot et al, 2002; Kalinichev et al, 2001; Kalinichev et al, 2002; McNamara et al, 2002; Plotsky et al, 2005). We chose to use male-only foster litters as studies using male-only foster litters have demonstrated that adult MS180 rats, although they have increased sensitivity to the inhibitory effect of the selective serotonin-reuptake inhibitor citalopram on the neuronal firing rates of serotonergic neurons, do not have altered numbers of serotonin transporter binding sites or altered slc6a4 mRNA expression within the DR, relative to MS15 rats (Arborelius et al, 2004).
The 9 foster litters were randomly assigned to one of three rearing conditions: (1) MS180—daily 180-min period of maternal separation from PND2 to 14 inclusive (“maternal separation”), (2) MS15—daily 15-min period of maternal separation from PND2 to 14 inclusive (“neonatal handling”), and (3) animal facility rearing (AFR) controls—handling of pups twice a week during routine cage changes.
Protocols involving manipulation of the pups took place between 0800 and 1200 h daily. During separation, each dam was removed from its maternity cage and placed into an identical cage until the end of the separation period. Pups were then removed as complete foster litters from the nest, placed into an empty cage and transferred to an incubator in an adjacent room. The incubator was maintained at 32 ± 0.5 °C from PND2-PND5 and 30 ± 0.5 °C from PND6-PND14. At the end of the separation period, foster litters were returned to their maternity cages, followed by reunion with the dams. Bedding in the transfer cages and incubator was never changed. During PND4 PND14, half of the bedding in the maternity cages was changed once a week while the pups and dams were out of the cage. During PND15-PND18 foster litters were not disturbed. Beginning on PND18, bedding was completely changed twice a week. Pups were weaned on PND21, housed with their foster litter mates until PND30, then, using a completely randomized design, pair housed throughout adulthood with a member of the same early life treatment group. Of the 12 pairs of rats available for each early life experience treatment group, 6 pairs of rats were randomly selected for immunohistochemical and behavioral studies (Gardner et al, 2005) and 6 pairs were used for in situ hybridization studies described in this paper. Two days prior to the experiment adult rats were separated and individually housed.
Social Defeat
Procedures for the social defeat paradigm have been described previously (Gardner et al, 2005). For the social defeat protocol, at 10 weeks of age, half of the individually housed rats from each early life treatment group were randomly assigned to a novel cage control group; the other half were assigned to a social defeat group. Resident males were heavier and older than the male test subjects (intruders). Bedding in the resident cage was left unchanged the week prior to the experiment. The resident female was removed from the resident cage 15 min prior to the experiment. The social defeat paradigm consisted of both a pre-defeat phase and a defeat phase, each lasting 10 min (Martinez et al, 1998). During the pre-defeat phase, the resident’s cage (59.4 cm length × 30.8 cm width × 22.9 cm height) was fitted with a perforated poly(methyl methacrylate) (PMMA; Plexiglas®) plate to divide the length of the cage into a small (1/3) and a large (2/3) compartment with the resident in the large compartment, which prevented physical contact but allowed the rats to see and smell each other. Immediately following the initial 10 min pre-defeat phase, the Plexiglas® divide was removed and the rats were allowed to interact for 10 min. Control rats were placed in a clean novel cage (1042 cm3 floor space, identical to the home cage) for 20 min in the experimental room, out of visual sight of the resident’s cage, during the social defeat. Experiments were completed within the first 6 h of the light phase.
Behavior was recorded to confirm that all intruder rats were defeated during the social defeat exposure. Unfortunately, the recorded behavior of 7 rats (2 MS15 rats, 4 AFR rats, and 1 MS180 rat) was lost due to unrecoverable damage to a video tape. Among the remaining rats in the study, the resident male rat always attacked the intruder and the intruder always displayed full submission (the intruder lies on its back with its belly exposed to the resident; 10 of 11 rats) or sideways submission (the intruder crouches below the resident and turns to expose part of its belly; 9 of 11 rats) during the 10 min period; there were no group differences in the frequencies or durations of full submission, sideways submission, or attack behavior. These behavioral findings are consistent with a previous study of rats from the same litters exposed to the same behavioral paradigm, which found no group differences among full submission, sideways submission, or attack behavior (Gardner et al, 2005).
Tissue collection and sectioning
Following exposure to a novel cage control condition or to the social defeat protocol, rats were returned to their home cages and transferred back into the housing room for 4 h. Rats were then anesthetized with sodium pentobarbital (Fatal Plus, Vortech Pharmaceuticals Dearborn, MI, USA), transferred to a procedure room, and rapidly decapitated. We selected a 4 h survival period as we have shown previously that stress-induced increases in brain mRNA expression are evident at this time point (Harbuz et al, 1993; Harbuz and Lightman, 1989). The brains were removed, frozen using powdered dry ice and stored at −80 °C. Tissue sections (12 μm) were prepared using a cryostat and thaw-mounted onto gelatin-coated glass slides; after dehydrating slides for 2–3 min on a hotplate (40 oC) slides were stored at −80 °C until use. Alternate sets of seven sections were mounted on glass slides. One set of alternate sections from each rat was used for analysis; in other words, measurements were taken from one section every 84 μm from each rat throughout the rostrocaudal extent of the midbrain raphe complex analyzed (Figure 6). The brain was always blocked, mounted, and sectioned, and sections placed onto the slides in the same manner, ensuring the right and left hand sides of the brain were correctly placed.
Figure 6.
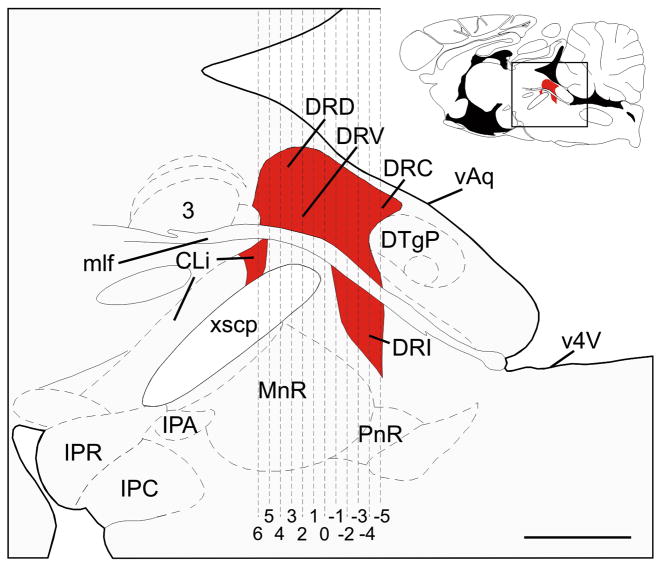
Schematic illustration of a midline sagittal section of the rat brainstem showing the subdivisions of the dorsal raphe nucleus (DR) and the caudal linear nucleus (CLi) that were selected for analysis (adapted from Paxinos and Watson, 1998). Red shading indicates regions of the DR and CLi containing slc6a4 mRNA expression. The full sagittal section is shown in the top right corner for reference; the box shows the area illustrated in the main figure. Dashed vertical lines correspond to the rostrocaudal levels selected for analysis, illustrated in Figure 1. Abbreviations: 3, oculomotor nucleus; CLi, caudal linear nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DTgP, dorsal tegmental nucleus, pericentral part; IPA, interpeduncular nucleus, apical subnucleus; IPC, interpeduncular nucleus, caudal subnucleus; IPR, interpeduncular nucleus, rostral subnucleus; mlf, medial longitudinal fasciculus; MnR, median raphe nucleus; PnR, pontine raphe nucleus; vAq, ventral surface of the cerebral aqueduct, v4V, ventral surface of the 4th ventricle; xscp, decussation of the superior cerebellar peduncle. Scale bar, 1mm.
Oligonucleotide probe preparation
Every seventh section (tissue cut at 12 μm) was used for in situ hybridization histochemistry to quantify slc6a4 mRNA expression. A 50 base oligonucleotide probe complementary to bases 207–256 (previously reported as bases 77–126 (Fujita et al, 1993; Hansen and Mikkelsen, 1998), based on the published sequence of the rat serotonin transporter cDNA clone (Blakely et al, 1991)) of slc6a4 mRNA (Rattus norvegicus solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 (slc6a4), mRNA GenBank Accession no., NM_013034.3) was used (5′-ACAGCGGACAGGGCAGAGCCTAGCCAAATATCCAATGGGTACTCTGCAGT-3′). The complimentary base sequence of the synthetic oligonucleotide probe was 5′-ACTGCAGAGTACCCATTGGATATTTGGCTAGGCTCTGCCCTGTCCGCTGT-3′.). No hybridization was detected using sense probes. The specificity of the oligonucleotide probe used for detection of slc6a4 mRNA expression has been confirmed previously (Fujita et al, 1993; Hansen and Mikkelsen, 1998). In addition, evaluation of the targeted mRNA sequence with the basic local alignment search tool (BLASTR) of National Center for Biotechnology Information (NCBI) databases indicated that the targeted mRNA sequence does not show any significant similarity with mRNAs other than the corresponding target in the rat genome. Antisense and sense (control) probes were labeled at the 3′ end with [α-35S]-labeled deoxyadenosine 5′-triphosphate (dATP, NEN, Brussels, Belgium) using terminal deoxynucleotidyl transferase (25 U/μl, Roche Molecular Biochemicals, Burgess Hill, UK). Briefly, autoclaved HPLC-grade water (28 μl), 10 μl 5x TdT tailing buffer, 5 μl CoCl2, 1 μl oligonucleotide (5 μM), 5 μl [35S]-dATP and 25 U TdT were added to two 1.5 μl eppendorf tubes labeled sense and antisense and then mixed and incubated for 1 h at 37 °C in a water bath. This procedure was predicted to add approximately 15 bases per probe. Unincorporated [35S]-dATP was removed using the QIAquick nucleotide removal kit (Quiagen, Crawley, UK).
Semi-quantitative in situ hybridization histochemistry
In situ hybridization histochemistry was performed as described previously (Harbuz et al, 1993; Harbuz and Lightman, 1989). All control and experimental sections were hybridized in the same hybridization reaction. Before hybridization, slides were equilibrated to room temperature and then immersed in 4% paraformaldehyde in 0.05 M phosphate buffered saline (PBS) for 10 min. Following two washes in 0.05 M PBS, slides were placed into freshly prepared 0.25% acetic anhydride in 0.9% NaCl containing 0.1 M triethanolamine (TEA) for 10 min. Sections were then dehydrated through a graded series of alcohols, delipidated in chloroform, rehydrated through a second series of alcohols, and then allowed to air dry.
Oligonucleotide probe hybridization solution (50% formamide, 20 × standard saline citrate (SSC), 25 mg/ml yeast tRNA, 10 mg/ml sheared salmon sperm DNA, 50X Denhardt’s solution, 50% dextran sulphate, 10 mM dithiothreitol (DTT) and 1 × 105 cpm total radiolabeled probe) was placed on each slide (90 μl), covered with parafilm coverslips, and incubated overnight in a humidified 37 °C chamber. The next day coverslips were removed in 1X SSC and each slide was washed with agitation 4 × 15 sec in 1X SSC. Slides were then put through 4 × 15 min washes in 1X SSC at 55 °C in a shaking water bath, 1X SSC at room temperature for 2 × 30 min and then briefly (1–2 sec) in distilled water at room temperature. Slides were allowed to air dry and were then apposed to autoradiography film (Hyperfilm-MP, GE Healthcare UK, Ltd, Little Chalfont, UK) for 2 weeks.
Imaging and densitometry of in situ hybridization autoradiograms
Autoradiographic images of the probe bound to slc6a4 mRNA together with 14C-labeled standards (to compensate for the non-linear response of the film to radioactivity) were measured using a computer-assisted image analysis system. Analysis was performed on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Measurements were taken by an observer (KLG) blind to the treatment of each rat. Each subdivision of the DR, or the CLi, was outlined and a measurement of the density × area of each DR subdivision was taken using the threshold function, which was constant throughout the analysis. All slides from the entire study were apposed to a single film, allowing us to use a single set of 14C-labeled standards. Rostrocaudal levels of the DR were determined by comparing the image of the tissue section with illustrations in a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998) and with atlases of tryptophan hydroxylase immunostaining (Abrams et al, 2004) and serotonin transporter mRNA expression (Lowry et al, 2008a) (Figure 5) in rat brain. At each anatomical level, raphe nuclei were further subdivided according to the descriptions of a stereotaxic atlas of the rat brain (Paxinos and Watson, 1998).
Statistics
To determine the effects of and potential interactions among early life experience, social defeat, and brain region on slc6a4 mRNA expression, a single multifactor ANOVA with repeated measures was used with early life experience and social defeat as between-subjects factors and brain region as a within-subjects factor. A total of 48 different brain regions were studied from the rostral (−7.66 mm Bregma) to caudal DR (−8.59 mm Bregma). The following subdivisions of the DR were studied at the following rostrocaudal levels: caudal linear nucleus (CLi; −7.66 mm Bregma), dorsal raphe nucleus, dorsal part (DRD; −7.75, −7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50, −8.59 mm Bregma), dorsal raphe nucleus, interfascicular part (DRI; −8.25, −8.34, −8.42, −8.50, −8.59 mm Bregma), dorsal raphe nucleus, ventral part (DRV; −7.66, −7.75, −7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50, −8.59 mm Bregma), left dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray region (left DRVL/VLPAG region or “lateral wings”; −7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50 mm Bregma), right DRVL/VLPAG region (−7.83, −7.92, −8.00, −8.08, −8.17, −8.25, −8.34, −8.42, −8.50 mm Bregma).
The mean slc6a4 mRNA expression in each subdivision (including all rostrocaudal levels) for each rat was also calculated as the mean slc6a4 mRNA expression (density × area) for each subdivision and analyzed as described above. Finally, the mean slc6a4 mRNA expression in all subdivisions (including all rostrocaudal levels of all subdivisions) for each rat was also calculated as the mean slc6a4 mRNA expression (density × area) of the DR. The mean slc6a4 mRNA expression values were then analyzed using two-factor ANOVA using early life experience and social defeat as between-subjects factors.
Two rats were removed from analysis due to missing data. Prior to the multifactor ANOVA with repeated measures, missing values (10.1% of the 1728 total data points) were replaced using the Petersen method (Petersen, 1985). These replacement values were used for the multifactor ANOVA with repeated measures only. If the multifactor ANOVA with repeated measures indicated an effect of early life experience, social defeat, or interactions among these factors and brain region, the topographical distribution of the effect was determined using Fisher’s Protected Least Significant Difference (LSD) tests. All statistics were performed using SPSS (Version 16 for Windows, SPSS Inc., Chicago, IL, USA).
Acknowledgments
We gratefully acknowledge Dr. Leonie Welberg and Dr. K.V. Thrivikraman for technical assistance. This study was supported by the BBSRC (Grant BBS/B/06806 to SLL), NIMH (Grants MH50113 and the Silvio O. Conte Center for the Neuroscience of Mental Disease MH58922 Project 1 to PMP), and the Neuroendocrinology Charitable Trust (Grant PMS/VW-01/02-808 to CAL). CAL was supported by a Wellcome Trust Research Career Development Fellowship (RCDF 068558/Z/02/Z), and is currently supported by a 2007 NARSAD Young Investigator Award and an NSF CAREER Award (NSF-IOS #0845550).
Abbreviations
- 5-HT
5-hydroxytryptamine; serotonin
- 5-HTT
serotonin transporter
- AFR
animal facility rearing
- ANOVA
analysis of variance
- CLi
caudal linear nucleus of the raphe
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL
dorsal raphe nucleus, ventrolateral part
- MS15
neonatal handling, separation from the dam for 15 min/day from postnatal day 2–14
- MS180
maternal separation, separation from the dam for 180 min/day from postnatal day 2–14
- PND
postnatal day
- VLPAG
ventrolateral periaqueductal gray region
Literature references
- 1.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomical and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- 3.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 4.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 5.Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB. Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology (Berl) 2004;176:248–255. doi: 10.1007/s00213-004-1883-x. [DOI] [PubMed] [Google Scholar]
- 6.Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 8.Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 9.Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J Comp Neurol. 2009;514:145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnet PW, Michelson D, Smith MA, Gold PW, Sternberg EM. The effect of chronic imipramine administration on the densities of 5-HT1A and 5-HT2 receptors and the abundances of 5-HT receptor and transporter mRNA in the cortex, hippocampus and dorsal raphe of three strains of rat. Brain Res. 1994;638:311–324. doi: 10.1016/0006-8993(94)90664-5. [DOI] [PubMed] [Google Scholar]
- 11.Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 12.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 13.Cooper MA, Grober MS, Nicholas CR, Huhman KL. Aggressive encounters alter the activation of serotonergic neurons and the expression of 5-HT1A mRNA in the hamster dorsal raphe nucleus. Neuroscience. 2009;161:680–690. doi: 10.1016/j.neuroscience.2009.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 15.Deakin JFW, Graeff FG. 5-HT and mechanisms of defence. Journal of Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 16.Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Repeated experience of social defeats increases serotonin transporter and monoamine oxidase A mRNA levels in raphe nuclei of male mice. Neurosci Lett. 2002;321:25–28. doi: 10.1016/s0304-3940(01)02495-8. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Shimada S, Maeno H, Nishimura T, Tohyama M. Cellular localization of serotonin transporter mRNA in the rat brain. Neurosci Lett. 1993;162:59–62. doi: 10.1016/0304-3940(93)90559-4. [DOI] [PubMed] [Google Scholar]
- 18.Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky P, Lowry CA. Adverse experience during early life and adulthood ineract to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.07.055. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 20.Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD. Early life adversity programs changes in central 5-HT neuronal function in adulthood. Eur J Neurosci. 2003;17:2401–2408. doi: 10.1046/j.1460-9568.2003.02668.x. [DOI] [PubMed] [Google Scholar]
- 21.Giachino C, Canalia N, Capone F, Fasolo A, Alleva E, Riva MA, Cirulli F, Peretto P. Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 2007;145:568–578. doi: 10.1016/j.neuroscience.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 22.Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10:220–224. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- 23.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 24.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 25.Halasz J, Toth M, Kallo I, Liposits Z, Haller J. The activation of prefrontal cortical neurons in aggression--a double labeling study. Behav Brain Res. 2006;175:166–175. doi: 10.1016/j.bbr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 27.Hansen HH, Mikkelsen JD. Long-term effects on serotonin transporter mRNA expression of chronic neonatal exposure to a serotonin reuptake inhibitor. Eur J Pharmacol. 1998;352:307–315. doi: 10.1016/s0014-2999(98)00349-5. [DOI] [PubMed] [Google Scholar]
- 28.Harbuz MS, Chalmers J, De SL, Lightman SL. Stress-induced activation of CRF and c-fos mRNAs in the paraventricular nucleus are not affected by serotonin depletion. Brain Res. 1993;609:167–173. doi: 10.1016/0006-8993(93)90870-s. [DOI] [PubMed] [Google Scholar]
- 29.Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- 30.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 32.Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, Mann K, Schumann G, Buchel C, Hariri AR, Weinberger DR. Serotonin transporter genotype (5-HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry. 2007;61:1011–1014. doi: 10.1016/j.biopsych.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Herbert J, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J Comp Neurol. 1992;315:34–52. doi: 10.1002/cne.903150104. [DOI] [PubMed] [Google Scholar]
- 34.Hettema JM, Kuhn JW, Prescott CA, Kendler KS. The impact of generalized anxiety disorder and stressful life events on risk for major depressive episodes. Psychol Med. 2006;36:789–795. doi: 10.1017/S0033291706007367. [DOI] [PubMed] [Google Scholar]
- 35.Hoefgen B, Schulze TG, Ohlraun S, von WO, Hofels S, Gross M, Heidmann V, Kovalenko S, Eckermann A, Kolsch H, Metten M, Zobel A, Becker T, Nothen MM, Propping P, Heun R, Maier W, Rietschel M. The power of sample size and homogenous sampling: association between the 5-HTTLPR serotonin transporter polymorphism and major depressive disorder. Biol Psychiatry. 2005;57:247–251. doi: 10.1016/j.biopsych.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 37.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder, Am. J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 39.Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- 40.Ichimiya T, Suhara T, Sudo Y, Okubo Y, Nakayama K, Nankai M, Inoue M, Yasuno F, Takano A, Maeda J, Shibuya H. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+)McN5652. Biol Psychiatry. 2002;51:715–722. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- 41.Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- 42.Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- 43.Joensuu M, Tolmunen T, Saarinen PI, Tiihonen J, Kuikka J, Ahola P, Vanninen R, Lehtonen J. Reduced midbrain serotonin transporter availability in drug-naive patients with depression measured by SERT-specific [(123)I] nor-beta-CIT SPECT imaging. Psychiatry Res. 2007;154:125–131. doi: 10.1016/j.pscychresns.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol. 2005;19:327–341. doi: 10.1177/0269881105053281. [DOI] [PubMed] [Google Scholar]
- 45.Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of Long-Evans rats results in long-lasting changes in morphine tolerance and dependence. Psychopharmacology (Berl) 2001;157:305–312. doi: 10.1007/s002130100806. [DOI] [PubMed] [Google Scholar]
- 46.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 47.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 48.Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- 49.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 50.Koed K, Linnet K. The serotonin transporter messenger RNA level in rat brain is not regulated by antidepressants. Biol Psychiatry. 1997;42:1177–1180. doi: 10.1016/s0006-3223(97)00345-4. [DOI] [PubMed] [Google Scholar]
- 51.Kuroda Y, Watanabe Y, McEwen BS. Tianeptine decreases both serotonin transporter mRNA and binding sites in rat brain. Eur J Pharmacol. 1994;268:R3–R5. doi: 10.1016/0922-4106(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 52.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 53.Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- 54.Lee HS, Kim MA, Waterhouse BD. Retrograde double-labeling study of common afferent projections to the dorsal raphe and the nuclear core of the locus coeruleus in the rat. J Comp Neurol. 2005;481:179–193. doi: 10.1002/cne.20365. [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Kim HJ, Kim JG, Ryu V, Kim BT, Kang DW, Jahng JW. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 2007;58:32–39. doi: 10.1016/j.neures.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Lehto S, Tolmunen T, Joensuu M, Saarinen PI, Vanninen R, Ahola P, Tiihonen J, Kuikka J, Lehtonen J. Midbrain binding of [123I]nor-beta-CIT in atypical depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1251–1255. doi: 10.1016/j.pnpbp.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 57.Lesch KP, Aulakh CS, Wolozin BL, Tolliver TJ, Hill JL, Murphy DL. Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Mol Brain Res. 1993;17:31–35. doi: 10.1016/0169-328x(93)90069-2. [DOI] [PubMed] [Google Scholar]
- 58.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 59.Linnet K, Koed K, Wiborg O, Gregersen N. Serotonin depletion decreases serotonin transporter mRNA levels in rat brain. Brain Res. 1995;697:251–253. doi: 10.1016/0006-8993(95)00906-7. [DOI] [PubMed] [Google Scholar]
- 60.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 61.Little KY, McLauglin DP, Ranc J, Gilmore J, Lopez JF, Watson SJ, Carroll FI, Butts JD. Serotonin transporter binding sites and mRNA levels in depressed persons committing suicide. Biol Psychiatry. 1997;41:1156–1164. doi: 10.1016/s0006-3223(96)00301-0. [DOI] [PubMed] [Google Scholar]
- 62.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 63.Lopez JF, Chalmers DT, Vazquez DM, Watson SJ, Akil H. Serotonin transporter mRNA in rat brain is regulated by classical antidepressants. Biol Psychiatry. 1994;35:287–290. doi: 10.1016/0006-3223(94)91262-9. [DOI] [PubMed] [Google Scholar]
- 64.Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti JM, Pandi-Perumal BL, Jacobs BL, Nutt DL, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhauser; Basel: 2008a. pp. 25–68. [Google Scholar]
- 65.Lowry CA, Hale MW. Serotonin and the neurobiology of anxious states. In: Muller CP, Jacobs BL, editors. The Behavioural Neurobiology of Serotonin. Elsevier; Amsterdam: 2009. in press. [Google Scholar]
- 66.Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008b doi: 10.1196/annals.1410.004. in press. [DOI] [PubMed] [Google Scholar]
- 67.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 68.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 69.Malison RT, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carboxymethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- 70.Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- 71.Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–170. [PubMed] [Google Scholar]
- 72.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 73.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McNamara RK, Huot RL, Lenox RH, Plotsky PM. Postnatal maternal separation elevates the expression of the postsynaptic protein kinase C substrate RC3, but not presynaptic GAP-43, in the developing rat hippocampus. Dev Neurosci. 2002;24:485–494. doi: 10.1159/000069359. [DOI] [PubMed] [Google Scholar]
- 75.Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Miczek KA, Covington HE, III, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse. 2009;63:565–573. doi: 10.1002/syn.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morikawa O, Sakai N, Obara H, Saito N. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol. 1998;349:317–324. doi: 10.1016/s0014-2999(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 79.Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- 80.Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwim GM, Smith CA. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–733. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- 81.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 82.Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 83.Pare WP, Blair GR, Kluczynski J, Tejani-Butt S. Gender differences in acute and chronic stress in Wistar Kyoto (WKY) rats. Integr Physiol Behav Sci. 1999;34:227–241. doi: 10.1007/BF02688691. [DOI] [PubMed] [Google Scholar]
- 84.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- 85.Petersen RG. Design and Analysis of Experiments. Marcel Dekker, Inc; New York: 1985. [Google Scholar]
- 86.Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol. 1996;364:402–413. doi: 10.1002/(SICI)1096-9861(19960115)364:3<402::AID-CNE2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 87.Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- 88.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 89.Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- 90.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 91.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- 94.Rotondo A, Mazzanti C, Dell’Osso L, Rucci P, Sullivan P, Bouanani S, Gonnelli C, Goldman D, Cassano GB. Catechol o-methyltransferase, serotonin transporter, and tryptophan hydroxylase gene polymorphisms in bipolar disorder patients with and without comorbid panic disorder. Am J Psychiatry. 2002;159:23–29. doi: 10.1176/appi.ajp.159.1.23. [DOI] [PubMed] [Google Scholar]
- 95.Sakai K, Salvert D, Touret M, Jouvet M. Afferent connections of the nucleus raphe dorsalis in the cat as visualized by the horseradish peroxidase technique. Brain Res. 1977;137:11–35. doi: 10.1016/0006-8993(77)91010-1. [DOI] [PubMed] [Google Scholar]
- 96.Shen H, Numachi Y, Yoshida S, Toda S, Awata S, Matsuoka H, Sato M. Electroconvulsive shock regulates serotonin transporter mRNA expression in rat raphe nucleus. Psychiatry Clin Neurosci. 2001;55:75–77. doi: 10.1046/j.1440-1819.2001.00788.x. [DOI] [PubMed] [Google Scholar]
- 97.Simon NG, Cologer-Clifford A, Lu SF, McKenna SE, Hu S. Testosterone and its metabolites modulate 5HT1A and 5HT1B agonist effects on intermale aggression. Neurosci Biobehav Rev. 1998;23:325–336. doi: 10.1016/s0149-7634(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 98.Smits KM, Smits LJ, Schouten JS, Stelma FF, Nelemans P, Prins MH. Influence of SERTPR and STin2 in the serotonin transporter gene on the effect of selective serotonin reuptake inhibitors in depression: a systematic review. Mol Psychiatry. 2004;9:433–441. doi: 10.1038/sj.mp.4001488. [DOI] [PubMed] [Google Scholar]
- 99.Smythe JW, Rowe WB, Meaney MJ. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Dev Brain Res. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 100.Spurlock G, Buckland P, O’Donovan M, McGuffin P. Lack of effect of antidepressant drugs on the levels of mRNAs encoding serotonergic receptors, synthetic enzymes and 5HT transporter. Neuropharmacology. 1994;33:433–440. doi: 10.1016/0028-3908(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 101.Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, Vythilingam M, Kugaya A, Baldwin RM, Seibyl JP, Charney D, Innis RB. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 102.Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 103.Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 104.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 105.van Riel E, van Gemert NG, Meijer OC, Joels M. Effect of early life stress on serotonin responses in the hippocampus of young adult rats. Synapse. 2004;53:11–19. doi: 10.1002/syn.20033. [DOI] [PubMed] [Google Scholar]
- 106.Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- 107.Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Willner P. Animal models of depression: an overview. Pharmacol Ther. 1990;45:425–455. doi: 10.1016/0163-7258(90)90076-e. [DOI] [PubMed] [Google Scholar]
- 109.Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry. 2002;7:1115–1119. doi: 10.1038/sj.mp.4001141. [DOI] [PubMed] [Google Scholar]