Abstract
Purpose
Superoxide (O2.-) may function as a second messenger or regulator of signal transduction when produced at low concentrations in the proper locations within cells. The purpose of these studies was to determine whether human corneal stromal (HCS) fibroblasts are capable of producing O2.- via nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, a family of protein complexes believed to be responsible for the localized and limited production of O2.- with regulatory activity.
Methods
HCS cells, grown as primary and low-passage cultures of fibroblasts, were used as the sources of RNA for reverse transcriptase PCR, with primers specific for mRNAs encoding the proteins that comprise NADPH oxidases. Small interfering (si)RNAs were used to knockdown specific NOX mRNAs. Proteins composing the NADPH oxidase complexes were identified using western blots. The production of O2.- by whole cells and cell-free preparations was assessed by measurement of NADPH-dependent superoxide dismutase-inhibitable cytochrome c reduction.
Results
Whole cells and cell-free extracts of corneal stromal fibroblasts produced O2.- in an NADPH-dependent manner. These fibroblasts constitutively produced mRNAs encoding eight proteins known to comprise NADPH oxidase complexes. mRNAs encoding NOX1, NOX4, NOX5, p22 phox, p47 phox, p67 phox, and p40 phox as well as Rac were expressed. Treatment of HCS fibroblasts with siRNA pools specific for each of these three NOXs significantly reduced the steady state levels of the respective mRNAs. Western blots confirmed the existence of all the proteins required for O2.- production. Rac 1, a regulator of the activity of some forms of NADPH complexes was present in membranous cell fractions containing the oxidase proteins.
Conclusions
HCS fibroblasts produced O2.- in a NADPH-dependent manner via at least three isoforms of NADPH oxidase. These cells expressed NOX1, NOX4, NOX5, p22 phox, p47 phox, p67 phox, and p40 phox as well as Rac. SiRNAs directed against each of the three putative isoforms of NOX significantly reduced the steady state levels of the appropriate NOX mRNA pools, thus confirming the existence of the three isoforms. The O2.- produced by the NADPH oxidases in HCS fibroblasts is a potential contributor to signal transduction pathways and a regulator of gene expression as well as a potential participant in processes that occur during inflammation.
Introduction
Superoxide anion (O2.-) is one of a group of molecules referred to as reactive oxygen species (ROS). The production of low amounts of O2.- by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in a highly regulated and localized manner can play critical roles in cell survival and death [1-5]. O2.- may be produced directly by enzyme activity or by uncoupled enzymes (i.e., indirectly by enzymatic reactions where the reduction of molecular oxygen is no longer directly linked to product formation). Superoxide can be produced in cells by several enzyme systems, including xanthine oxidase, uncoupled nitric oxide synthase, complexes I or III of the mitochondrial electron transport system, uncoupled NADPH cytochrome P450 reductase, or NADPH oxidases [6-9].
The NADPH oxidase family currently contains seven members composed of multiple proteins that may exist in several isoforms. The NADPH oxidase of neutrophils is the classic membrane-bound complex that is assembled upon exposure of the cells to activating stimuli, such as bacterial lipopolysacchride. The complex in neutrophils contains the NOX2 protein and is capable of the production of large amounts of O2.- [10]. This oxidase consists of NOX2 complexed with p22 phox, p47 phox , p67 phox, p40 phox, and Rac2. The complex is assembled at the cytoplasmic membrane when the cell receives the proper stimulus. NADPH oxidase complexes are also responsible for the production of O2.- in several other cell types, including cardiac myocytes, dermal epithelial cells, and kidney epithelial cells [4,11,12]. Some cells contain homologues of the classic NADPH oxidase proteins. The proteins of these homologous complexes, as they exist in nonmyeloid cells, may contain any of several isoforms of NOX, p47 phox, or p67 phox. Cells containing complexes that are homologues of the NOX2 oxidase produce relatively small amounts of O2.- [13]. Cells other than leukocytes and macrophages may express NOX proteins (NOX1-5) as well as isoforms of p47 phox, p40 phox, and p67 phox and Rac1 rather than Rac2 [14-16]. Several functions have been proposed for the O2.- produced by these NADPH complexes, including roles in apoptosis, senescence, cell proliferation, oxygen sensing, signal transduction, and regulation of hormone synthesis [1,4,17].
In the human cornea multiple potential sources of O2.- exist not only during inflammation but also in healthy tissue. Enzymes, such as xanthine oxidase, uncoupled NADPH cytochrome P450 reductase, nitric oxide synthase 2 (NOS2), and complexes I and III of the mitochondrial electron transport chain are all capable of O2- production in corneal cells [8,18-20]. Cells derived from eyes, including rabbit corneal epithelial and stromal cells as well as human lens epithelial cells and retinal vascular endothelial cells, contain modified forms of NADPH oxidase complexes [20-23]. The expression of NADPH oxidases has not been previously demonstrated in human corneal stromal (HCS) cells, although O2.- has been hypothesized to contribute to keratoconus [24]. The results of the studies reported here document that HCS cells are capable of producing O2.- by the oxidation of NADPH via NADPH oxidases. The results demonstrate that the oxidases exist as an NOX5 oxidase as well as NOX1 and NOX4 complexed with p22 phox, p47 phox, p40 phox, p67 phox, or their homologues and Rac.
Methods
Cell culture
HCS cells were isolated, grown to confluence, and subcultured, using modifications to published procedures [25]. Corneal stromal cells were isolated from excised corneas after removal of the epithelium and endothelium by scraping. The stroma was digested for 16 h at 37 °C with 150 units/ml collagenase (Clostridium histolyticium, Invitrogen, Carlsbad, CA) in Hank’s balanced salts solution (HBSS) containing penicillin G (100 units/ml) and streptomycin sulfate (100 μg/ml; both from Sigma Aldrich, St. Louis, MO). The cells were recovered by centrifugation (800× g), suspended in growth medium, and grown at 34 °C in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5 g/l glucose (Invitrogen), 5% heat-inactivated defined fetal bovine serum (FBS; Hyclone/Thermo Scientific, Waltham, MA), 0.1% Mito+serum extender (BD Biosciences, San Jose, CA), and 10 μg/ml ciprofloxacin (Sigma Aldrich). Cells were passaged at a 1:3 split ratio using 0.05% trypsin and 0.53 mM EDTA. Cells were used in the first four passages.
Reverse transcriptase PCR and real-time PCR
Total RNA was prepared using Trizol (Invitrogen) followed by DNA digestion with DNase I (Invitrogen). Total RNA concentration was determined by measuring the optical density at 260 nm. cDNA synthesis was performed using Superscript III (Invitrogen) and 1-2 μg of RNA. cDNA as amplified using AmpliTaq Gold (Applied Biosystems, Foster City, CA) and sequence-specific primers for standard reverse transcriptase (RT)-PCR (Table 1) and real-time PCR (Table 2). Approximately 4-10% of the RT reaction mixture was used in each PCR reaction containing 1-3 mM MgCl2, 200 µM each deoxynucleotide triphosphates (dNTPs), 0.2 to 0.4 µM primers, and 0.05 units/μl units AmpliTaq Gold in reaction buffer (10 mM Tris-HCl, pH 8.3, and 50 mM KCl). The PCR was run at 95 °C for 10 min then 40 cycles of 95 °C for 1 min, 54 to 62 °C for 1 min, and 72 °C for 1 min. Control reactions were run in the absence of the RT in order to detect the presence of amplifiable DNA that might be contaminating the RNA preparation. The reaction products were detected on ethidium bromide-stained NuSieve (2%) agarose gels (Cambrex Bioscience, East Rutherford, NJ).
Table 1. Primers for the detection of NADPH oxidase and other cDNAs in human corneal stromal fibroblasts.
| Primer name | Sequence 5’→3’ | Product (bp) | Reference |
|---|---|---|---|
| HuNOX1-S1 |
GCCTGTGCCCGAGCGTCTGC |
522 |
AJ438989 |
| HuNOX1-AS2 |
ACCAATGCCGTGAATCCCTAAGC |
||
| HuNOX2-S1 |
GGAGTTTCAAGATGCGTGGAAACTA |
549 |
[14] |
| HuNOX2-AS2 |
GCCAGACTCAGAGTTGGAGATGCT |
||
| HuNOX3-S1-2 |
CCATGGGACGGGTCGGATTGT |
376 |
NM_015718 |
| HuNOX3-AS2-2 |
GGGGGCAGAGGTAAGGGTGAAGG |
||
| HuNOX4-S1 |
GTCATAAGTCATCCCTCAGA |
797 |
[45] |
| HuNOX4-AS2 |
TCAGCTGAAAGACTCTTTAT |
||
| NOX5-S1 |
ATCAAGCGGCCCCCTTTTTTCAC |
238 |
[14] |
| NOX5-AS2 |
CTCATTGTCACACTCCTCGACAGC |
||
| P22PHOX-S1 |
GTTTGTGTGCCTGCTGGAGT |
316 |
[46] |
| P22PHOX-AS2 |
TGGGCGGCTGCTTGATGGT |
||
| P47PHOXR-S1 |
CTCCCGCTGTCCACACCTGCTGAA |
349 |
AF324409 |
| P47PHOXR-AS2 |
GGGCTCTGGGTCCTCTGGCTCGTC |
||
| P67PHOX-S1 |
TACTTCCAACGAGGGATGCTC |
714 |
[47] |
| P67PHOX-AS2 |
AGCTTTCCTCCTGGGCT |
||
| P67PHOXR-S1 |
CACGCCCGGATCTGCTTCAAC |
363 |
AF323789 |
| P67PHOXR-AS2 |
GTGGCGGGGCTCGACTTCAT |
||
| P40PHOXR-S1 |
TCATCTACCGCCGCTACCGCCAGTTC |
473 |
AF323790 |
| P40PHOXR-AS2 |
AGTGCCCTCCAGCCAGTCCTTGTTGA |
||
| P40PHOXR2-S1 |
CCGGAGGAAGATGACCCCACCAACTG |
249 |
AF323790 |
| P40PHOXR2-AS2 |
GCCTCGCCTGCCTCCACCAT |
||
| MuRAC1-S1 |
CCTACCCGCAGACAGTTGGAGACAC |
395 |
XM_132485 |
| MuRAC1-AS2 |
CTTGACAGGAGGGGGACAGAGAACC |
||
| MuRAC2-S1 |
GCCCCAGCACCCCCATCATCC |
252 |
NM_009008 |
| MuRAC2-AS2 |
AGGGGCGCTTCTGCTGTCGTGTG |
||
| MuRAC3-S1 |
GCTTCGGCCACTCTCCTATCCTCA |
202 |
AB040819 |
| MuRAC3-AS2 |
CTTCTTGTCCCGCAGCCGTTCA |
||
| MamGAPDH-S1 |
CCATGGAGAAGGCTGGGG |
196 |
[48] |
| MamGAPDH-AS2 | CAAAGTTGTCATGGATGACC |
Table 2. Real time PCR primers.
| Primer name | Sequence 5'→3' | Product (bp) | Reference |
|---|---|---|---|
| GAPDH-S1 |
CTCCTGCTCCTCTGTTCG |
100 |
NM_002046 |
| GAPDH-AS-1 |
TAGCTCCGACCTTCACCTTC |
||
| HuNOX1-S1 |
CACAAGAAAAATCCTTGGGTCAA |
106 |
NM_013955 |
| HuNOX1-AS1 |
GACAGCAGATTGCGACACACA |
||
| HuNOX4-S1 |
GCAGGAGAACCAGGAGATTG |
125 |
NM_016931 |
| HuNOX4-AS1 |
CACTGAGAAGTTGAGGGCATT |
||
| HuNOX5-S1 |
GCAGGAGAAGATGGGGAGAT |
100 |
NM_024505 |
| HuNOX5-AS1 |
CGGAGTCAAATAGGGCAAAG |
||
| p22phox-S1 |
CGCTGGCGTCCGCCTGATCCTCA |
128 |
[26] |
| p22phox-AS1 | ACGCACAGCCGCCAGTAGGTAGAT |
Real-time quantitative amplifications were performed in an ABI 7500 (Applied Biosystems), using SYBR Green PCR Master Mix (Applied Biosystems) and 50-75 ng cDNA at 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, annealing for 1 min at 60 °C, then extension at 72 °C for 45 s. Primer concentrations for real-time PCR were 10 pM/20 μl. Melt curve analysis was performed by an additional dissociation step of 1 cycle at 95 °C for 15 s and ramping data collection at 60 °C for 1 min and 95 °C for 15 s. Data were normalized against the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal. Primers were either designed by us or as published by others (Table2) [26]. The increase in fluorescence of SYBR Green was measured after each extension step. CT values were calculated using ABI software. Relative expression values were obtained by normalizing CT values of the tested genes with CT values of the housekeeping genes, using the ΔΔCT method.
Sequencing
PCR products were prepared for cycle sequencing by gel extraction and purification with the QIAquick Gel Extraction Kit (QIAGEN, Valencia, CA). Cycle sequencing of the purified DNA was carried out with the ABI PRISM Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied BioSystems). The sequencing reactions were processed on an ABI PRISM 3130xl Genetic Analyzer (Applied BioSystems). The sequence analysis was done with the DNA Sequencing Analysis Software, Version 5.2 (Applied Biosystems) for Windows XP and Vista platforms. Sequence alignments were done with the SeqMan II sequence analysis software, version 5.06 (DNASTAR, Inc., Madison, WI).
Western blot analysis
Cells were removed from cell culture dishes in 15 mM HEPES buffer, pH 7.5, containing 145 mM NaCl, 0.1 mM MgCl2, 10 mM ethylene glycol bis (2- aminoethylether) N, N, N, N1–tetraacetic acid (EGTA), 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM phenylmethylsulfonylfluoride (PMSF), and 2 mM sodium orthovanadate (Na3VO4). Lysates were prepared by freeze thawing, Dounce homogenization, and sonication twice, each time for 15-s intervals at 100 W power. Cell debris was cleared from the lysates by centrifugation at 200× g for 15 min at 4 °C. Lysate fractions were separated into soluble and particulate fractions by centrifugation at 29,000× g for 30 min at 4 °C. The pellets were resuspended in modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Nonident P-40, 0.25% sodium deoxycholate, 150 mM sodium chloride, 1 mM ethylenediaminetetraacetic acid [EDTA], 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM Na3VO4, and 1 mM sodium fluoride, all purchased from Sigma Aldrich). Protein concentrations were determined with the BCA Protein Assay Reagent Kit (Pierce/Thermo Scientific, Rockford, IL). Both the supernatant and particulate fractions were stored at -80 °C.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed on NuPAGE NOVEX Bis- Tris–4-12% gels with MES SDS running buffer (50 mM Tris base 2-(N-morpholino)ethanesulfonic acid), 1 mm EDTA, 0.1% sodium dodecyl sulfate pH 7.3; Invitrogen). Proteins were detected on the immunoblots with the SuperSignal Femto West Chemiluminescent Substrate Kit (Pierce), according to the manufacturer’s directions. Development of the blots was done with CL-XPosure Film (Pierce), using Kodak GBX developer and fixer solutions (Pierce/Thermo Scientific). Blots were reprobed after removing the antibodies and substrates with the Restore Western Blot Stripping Buffer (Pierce), according to the manufacturer’s directions.
Antibodies used in these studies included: gp91 phox (611415) and p67 phox (69820; BD Biosciences); NOX5 (SC-34707), NOX4 (SC-21860), MOX1/NOX1 (SC-5821), p22 phox (SC-117212), p47 phox (SC-14015 and SC17845), p67 phox (SC-7663), Rac1 (SC-217), and Rac2 (SC-96; Santa Cruz Biotechnology, Santa Cruz, CA); p47 phox (05-540), p40 phox (05-039), and Rac (05-389; Millipore, Bellerica, MA) .
Assay of NADPH oxidase by superoxide dismutase-inhibitable cytochrome c reduction
NADPH oxidase activity was measured in a cell-free system in a manner similar to that used by others [27]. Cells were grown to confluence, rinsed in Delbeccos PBS without Ca+ or Mg++ (Gibco/Invitrogen, Carlsbad, CA), and harvested with trypsin and EDTA treatment. Trypsin was inhibited with Type I-S soybean trypsin inhibitor (Sigma Aldrich), and the harvested cells were washed with PBS at 4 °C and resuspended in buffer containing 20 mM 3-morpholino propane-1-sulfonic acid and potassium hydroxide (MOPS–KOH buffer, pH 7.4), containing 250 mM sucrose, 0.1 mM EDTA, 2 μM leupeptin, 1 μM aprotinin, and 2 μM pepstatin. The cells were disrupted after one freeze-thaw cycle by two 20-s cycles of Dounce homogenization and two 15-s cycles of sonication at 100 W. Whole cells were removed by centrifugation at 200× g for 5 min at 4 °C. The lysate was fractionated by centrifugation at 29,000g for 15 min at 4 °C. The pellet was then resuspended in 50 mM phosphate buffer at pH 7.4 and stored at -80 °C. Paired assays were conducted by incubation of 20-60 μg of protein with 10 mM phosphate buffer, 130 mM NaCl, 1 mM EGTA, 10 μM flavin adenine dinucleotide (FAD), 2 mM NaN3, 50 μM oxidized cytochrome c, and 10 μM guanosine 5' [γ-thio] triphosphate (GTPγS). One reaction of each pair contained 200 units of superoxide dismutase (SOD; Sigma Aldrich). Reactions were initiated by the addition of NADPH to a final concentration of 200 μM. After 1 h, activity was measured as the SOD-inhibitable increase in absorbance at 550 nm (ΕmM=21). Assay conditions were established documenting the linearity of superoxide production with time and protein concentrations.
Superoxide production in whole cells
HCS fibroblasts were grown to the fourth passage in DMEM without phenol red and containing Mito+ and 5% FBS. Cells were harvested using trypsin and EDTA and washed three times with HBSS without Ca and Mg. Cells (107) were resuspended in PBS, pH 7.2, containing 100 μg/ml bovine serum albumin and protease inhibitor cocktail (EDTA free complete protease mix; Roche Diagnostics, Indianapolis, IN). Reduced streptolysin O (5 units/ml; Sigma Aldrich) was added, and the mixture was incubated for 10 min at 37 °C [28]. The permeabilized cells were chilled to 4 °C, harvested by centrifugation, and washed with phosphate buffer, pH 7.2. To measure superoxide production permeabilized cells (1×105 to 1×106) were resuspended in duplicate tubes, with and without 200 units of SOD, containing 30 mM MOPS (pH 7.2), 100 μM FAD, and 100 μM oxidized cytochrome c, incubated at room temperature for 5 min, and NADPH added to a final concentration of 200 μM. The change in optical density at 550 nm was recorded every 5 min for 1 h. At the conclusion of the experiment, cells were lysed in 20 mM HEPES buffer containing 1% Triton X-100, and the protein quantitated using the BCA Protein Assay Reagent Kit. Superoxide production was expressed as SOD-inhibitable cytochrome c reduction/min/mg protein.
Small interfering RNA treatment of cells
NOX1, NOX4, and NOX5 ON-Target Plus siRNA pools were purchased from Dharmacon (Lafayette, CO). SiRNA complexes were formed in serum-free medium, using INTERFERIN (PolyPlus; Genesee Scientific, San Diego, CA), according to the manufacturer’s instructions. Optimal siRNA/INTERFERIN concentrations were established. Cells were plated at 50-60% confluence in DMEM containing 5% FBS, Mito+serum extender (Gibco, Carlsbad, CA), and ciprofloxacin and maintained at 34 °C. Twelve to 16 h post plating, the media was removed and the cells rinsed with HBSS. The cells were treated with a final concentration of 40 nM siRNA. Cells were transfected with complexes in media composed of INTERFERIN in DMEM containing 5% FBS Mito+ and ciprofloxacin ( in a 1 to 50 ratio) for 16 h. The media was removed and replaced with DMEM with 5% FBS and ciprofloxacin. After 96 h post transfection, the media was removed, the cells were rinsed in PBS, and the RNA was extracted with Trizol, according to the manufacturer’s instructions. The amount of mRNA was determined by real-time PCR, as described above.
Statistical analysis
Means were compared by one-way analysis of variance, and the significance of differences among means of treatment groups was determined by the Holm-Sidak method (Sigma Stat 3.0; SPSS Inc., Chicago, IL).
Results
NADPH-dependent superoxide production by whole cells and membranes
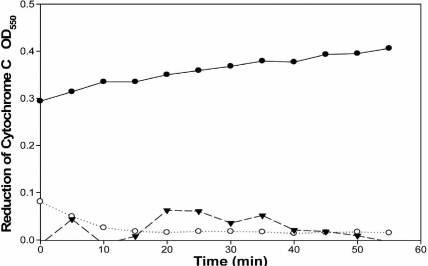
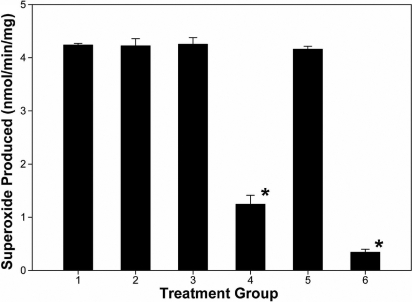
Cultures of HCS cells were grown in serum containing medium as fibroblasts. Cells were harvested, permeabilized, and O2.- production was measured as SOD-inhibitable cytochrome c reduction. Whole cells permeabilized with streptolysin O produced O2- in an NADPH-dependent manner (Figure 1). Membranes prepared from human stromal fibroblasts produced O2.- at a rate of 4.24±0.03 nmole/min/mg protein (Figure 2). The addition of inhibitors of nitric oxide synthase, L-NAME or 1400W, or an inhibitor of xanthine oxidase, allopurinol, did not significantly reduce the production of O2.-, thus indicating that NADPH oxidase was the source of the O2.- . NADH did not serve as an effective substrate so the source of the activity was likely not the electron transport chain (Figure 2). The oxidase inhibitor, diphenyliodonium (DPI), reduced activity by 90%, thus indicating that NADPH oxidase was the likely source of O2.- production (Figure 2).
Figure 1.
Nicotinamide adenine dinucleotide phosphate-dependent superoxide production by human corneal stromal fibroblasts. Cells were grown in cell culture, harvested, and permeabilized with streptolysin O. The permeabilized cells (2.5×105) were incubated in 3-morpholinopropane-1-sulfonic acid (MOPS) buffer in the presence and absence of 200 units of superoxide dismutase (as described in the Methods). Reactions were initiated by the addition of (●) 200 µM NADPH, (○) 200 µM NADH, or (▼) no substrate. The superoxide dismutase-inhibitable change in optical density at 550 nm (OD550) is plotted verses time for 2.5×105 cells.
Figure 2.
Nicotinamide adenine dinucleotide phosphate oxidase activity in membrane preparations of human corneal stromal fibroblasts as assayed by superoxide dismutase-inhibitable cytochrome c. Human corneal stromal fibroblasts were grown in cell culture, harvested, lysed, and the membranous fraction harvested as the 29,000× g pellet, as described in the Methods. Cell lysates were assayed for superoxide production under various conditions: (1) NADPH as substrate, (2) NADPH as substrate in the presence of 100 μM L-NAME, (3) NADPH as substrate in the presence of 50 µM 1400W, (4) NADPH as substrate in the presence of 10 µM diphenyliodonium, (5) NADPH as substrate in the presence of 100 µM allopurinol, and (6) NADH as substrate. Each bar represents the mean specific activity +/- standard deviation (n=3). An asterisk indicates a significant reduction in SOD inhibitable superoxide production, (p<0.001).
Detection of transcripts of NADPH oxidase and proteins
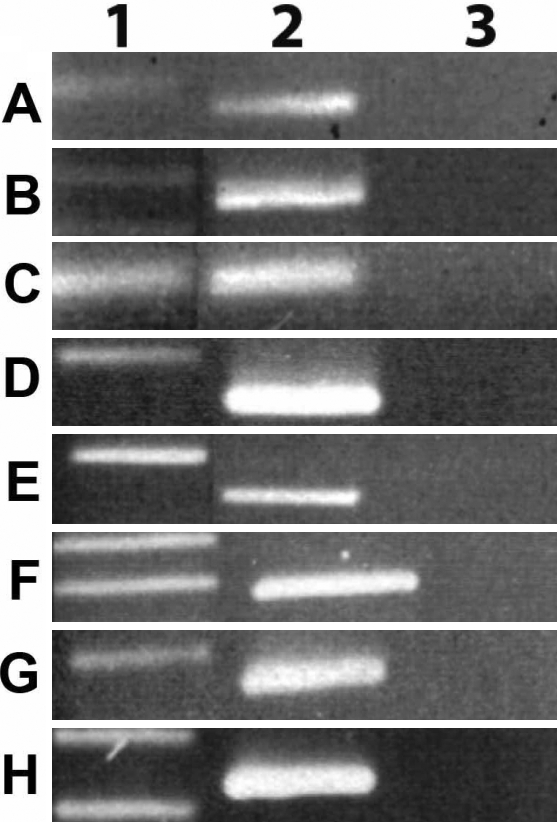
Cultures of human stromal fibroblasts expressed three NOX transcripts when grown in DMEM containing 5% FBS. PCR reactions containing cDNAs prepared from cultures of stromal cells produced the appropriate products from primers for NOX1, NOX4, and NOX5 (Figure 3A-C). RT PCR using NOX4 primers amplified a region of the NOX4 coding sequence, including bp 1686 to 1855, which was 98.0% identical to that originally discovered in cells from the kidney, NM_016931.2 [14]. The NOX5 RT PCR product was 99.0% identical to a region encoding a portion of the EF hand /Ca binding region of the NOX5 from human testes NM_024505 [29]. Western blots using antibodies to NOX1, NOX4, and NOX5 confirmed that these NOX proteins were expressed in HCS fibroblasts (Figure 4A-C).
Figure 3.
Reverse transcriptase PCR products of NADPH oxidase related genes. HCS fibroblasts were grown to confluence and the RNA extracted from cultures and amplified by RT PCR. Sequence specific primers produced the appropriate PCR products for the NADPH oxidase gene products and Rac including A: NOX1, B: NOX4, C: NOX5, D: p22phox, E: p47phox, F: p40phox, G: p67phox, and H: Rac. Row 1: molecular weight standards. Row 2: reverse transcriptase. (RT) PCR products from human corneal stromal fibroblasts; Row 3, no RT controls.
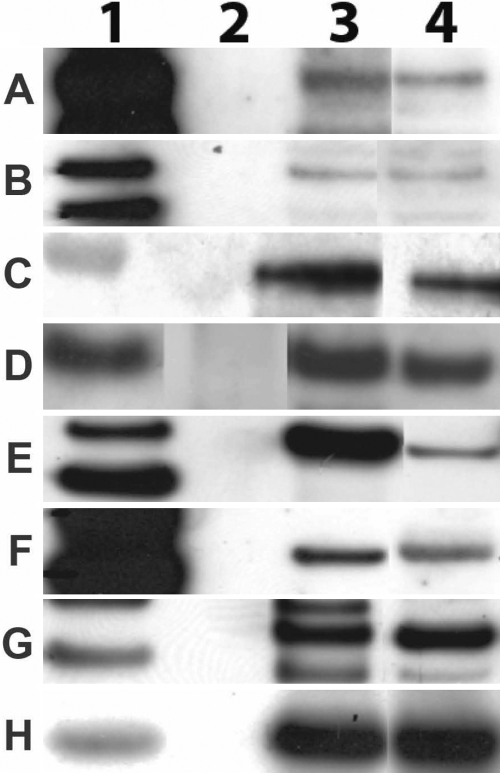
Figure 4.
Western blots of proteins from human corneal stromal fibroblasts. HCS fibroblasts were grown to confluence in DMEM containing 5% FBS. The cell membranes were prepared and lysed in RIPA. The aliquots of proteins were then analyzed by western blotting. The primary antibodies detected proteins of the correct molecular weight; Row 1, Magic Mark molecular weight standards; Row 2, empty; Row 3, positive control protein as detected protein as detected in HL60, HEK or HUVEC cells; Row 4, proteins as detected in HCS fibroblasts. Primary antibodies include A: NOX1, B: NOX4, C: NOX5, D: p22 phox, E: p47 phox, F: p40 phox, G: p67 phox, and H: Rac.
Cultured HCS fibroblasts also produced transcripts of other proteins required to form functional NADPH complexes with NOX1 and NOX4 (Figure 3). HCS fibroblasts did express mRNAs for p22 phox, p47 phox, p40 phox, and p67 phox, as detected by RT-PCR reactions using sequence-specific primers (Figure 3D-H). HCS fibroblasts also constitutively expressed Rac1, as amplified with Rac1-specific primers (Figure 3H).
Western blots using antibodies to p22 phox did detect the production of a protein of the correct molecular weight for p22 phox (Figure 4D). A single dominant band of protein at about 47 kDa was detected with antibodies to p47 phox (Figure 4E). Western blots prepared with antibody to p40 phox and proteins extracted from HCS fibroblasts showed a band at 44 kDa when probed with the monoclonal antibody clone 1.9 (Millepore; Figure 4F). Blots prepared using mouse monoclonal antibody to p67 phox detected a dominant band of protein at 65-67 kDa, indicating that p67 phox was constitutively produced (Figure 4G). Western blots using antibodies to Rac produced a 21 kDa band in membrane containing fractions of HCS fibroblasts (Figure 4H). While these data do not prove that Rac is bound to the complex of NADPH oxidase proteins in the membrane, the data indicate that NOX1 and other oxidases that require Rac have the potential to be active.
Small interfering RNA suppression of NADPH oxidase activity
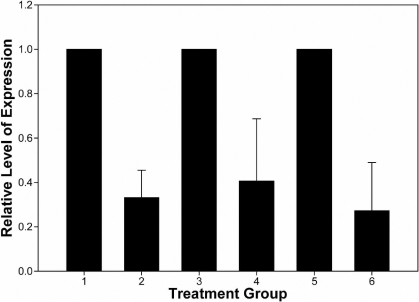
HCS fibroblasts were treated with siRNAs to NOX1, NOX4, or NOX5 to confirm the existence of these multiple forms of NOX. HCS fibroblasts were treated with siRNA smart pools or nontarget control siRNAs, as described in the Methods section. Real-time PCR documented reductions in the steady state mRNA pools by 67% for NOX1, 59% for NOX4, and 73% for NOX5 at 96 h post treatment (Figure 5).
Figure 5.
Relative mRNA content of cells treated with small interfering RNAs to NOX1, NOX4, or NOX5. Human corneal stromal fibroblasts were treated with 40 nM small intefering (si)RNAs to nontarget controls (lanes 1,3, and 5) or NOX1-specific (lane 2), NOX4-specific (lane 4), or NOX5-specific (lane 6) siRNAs. RNA was extracted and amplified in triplicate amplification by real-time reverse transcriptase PCR, using the SYBR Green. Each bar represents the mean and standard error of the mean of triplicate amplifications of RNAs extracted from triplicate cultures. A significant reduction (p<0.05) in the steady state level of RNA was observed for each specific mRNA relative to cultures treated with non-target control RNAs.
Discussion
HCS fibroblasts produced O2.-, as detected by NADPH-dependent, SOD-inhibitable, cytochrome c reduction. Membranes prepared from these cells retained the ability to produce O2.- in a manner that was inhibitable by DPI but that was insensitive to L-NAME and allopurinol. NADPH was the preferred substrate, while NADH was a poor substrate. These observations indicate that the source of the O2.- was not NOS, xanthine oxidase, or the mitochondrial electron transport chain. The rate of O2.- production, the dependence upon NADPH as the substrate, and the specific activity of O2.- production were indicative that NADPH oxidase was the source of O2.-.
HCS fibroblasts expressed three NOX genes as well as five genes whose products serve as accessory proteins that compose O2.--producing NADPH complexes. RT-PCR products generated with gene-specific primers and the proteins detected by western blots documented the expression of NADPH oxidase components. SiRNA knockdowns of mRNA pools confirm the expression of the NOX mRNAs. NOX1, NOX4, and NOX5 mRNAs were produced in a constitutive manner at detectable levels when cells were grown in serum containing medium. NOX2 mRNA was detected, but no protein was found despite the use of multiple NOX2 antibodies along with positive and negative controls. In addition, neither NOX3 mRNA nor protein was detected. The literature now indicates that it is not unusual for a given cell type to express multiple NOXs [4,30]. It has been hypothesized that each isoform of NOX may function in a specific manner by virtue of its localized production of O2.- [1]. Future studies will need to document the subcellular localization and function of these NADPH oxidases in corneal stromal cells.
Among the three isoforms of NOX present in HCS fibroblasts, NOX5 produces O2.- in a strictly NADPH-dependent manner when bound to the cytoplasmic membrane [31,32]. The accessory proteins are not required for activity of NOX5 [33]. NOX5 has been reported to exist in any of five isoforms (α, β, γ, δ, and ε) [34]. The literature indicates that the molecular weight of NOX5 may range from 65 to 85 kDa, depending on the isoform present and the extent of glycosylation [31]. The NOX5 gene encodes a protein with a molecular weight of 64.7 kDa, which is close to the estimated molecular weight of the protein that we detect with the NOX5 antibody [14]. The fact that NOX5 siRNA reduced the production of mRNA indicates that NOX5 is actively expressed in HCS fibroblasts.
Unlike NOX5, NOX4 requires p22 phox for stabilization and activity [5]. NOX4 does not require other accessory proteins for activity [4]. NOX4 appears to localize in membranes of cells, such as the cytoplasmic membrane and endoplasmic reticulum, but its localization appears cell-type dependent [35]. The data suggest that in some cells the activity of NOX4 is Rac dependent [36]. NOX4 can be active when complexed with p22 phox alone, but other studies indicate that accessory proteins present in the cytoplasm can be activated and complex with NOX4 and p22 phox to regulate NOX4 activity [33]. A variety of agents have been documented to activate NOX4, including lipopolysaccharides, cytokines, growth factors, angiotensin II, phorbol mynistic acid (PMA), and insulin [4]. Each activating stimuli has been observed to have its effects in a cell-type and sometimes species-dependent fashion. NOX4 has a predicted molecular weight of 66.9 kDa and is often detected as a protein of about 65 kDa by western blots, as we have detected [14]. NOX4-targeted siRNA treatment of HCS fibroblasts reduced the pools of mRNA, confirming the existence of the NOX4 message.
The NOX1 homologue has been detected in a wide variety of cell types and tissues. NOX1 has been observed in three isoforms, but the full-length gene encodes a protein in the range of 55-60 kDa, as we have detected in HCS fibroblasts [4]. The subcellular localization of NOX1 is not well defined as it seems to vary in a cell-type-dependent manner. O2.- produced as a result of NOX1 activity has been observed to enhance proliferation of some cell types while inhibiting the growth of other cells [37,38]. Unlike NOX3 and 5, NOX1 gene expression and activity is regulated by growth factors and cytokines [4]. NOX1 mRNA was detected, and a NOX1 antibody-reactive protein of about 65 kDa was detected in membrane fractions of HCS fibroblasts. NOX1 requires p22 phox and an organizer protein, like NOXO1 or p47 phox, as well as a NOX activator protein like NOXA1 or p67 phox, and Rac for efficient O2.- production [39]. Treatment of cells with NOX1 siRNA suppressed NOX1 mRNA accumulation, documenting the existence of NOX1 mRNA pools within HCS fibroblasts. It remains to be determined how NOX1 activity is regulated in HCS fibroblasts and what the biological activity is of the O2.- it produces.
HCS fibroblasts also expressed mRNAs encoding p22 phox, p47 phox, p67 phox, and Rac. These proteins are either required or regulate the activities of NOX1, 2, 3, and 4 oxidases [13,33]. Each of these proteins has specific functions. P22 phox interacts with the NOX proteins and becomes part of a membrane-bound electron transfer complex know as cytochrome b558 [13]. HSC fibroblast constitutively expressed p22 phox mRNA and western blots with two different antibodies, indicate that the protein was present in the cytosol and membrane fractions. A second accessory protein required for NOX2 and NOX1 activity is p47 phox, the organizer protein [33]. P47 phox binds to the p22 phox/ NOX complex via the Src homology domain and to membranes by a PX domain [40,41]. It has been reported that in some cells p47 phox can be replaced functionally by a p47 phox homologue, NOXO1 (NOX organizer1) [42]. Based on the primers that we used and the antibodies available to us, it is not clear that we could distinguish between p47phox and its homologue. It appears that HCS fibroblasts produce p47 phox. A third protein that is known to activate the classic NOX2 complex and may modify the activity of NOX1, NOX3, and NOX4 complexes is p67 phox. A p67 phox homologue, NOXA1 (NOX activator) contributes to the activation of NOX1 in some cells [40]. P67 phox is expressed in HCS fibroblasts. A fourth protein believed to contribute to the activity of some NADPH oxidases is p40 phox. P40 clearly influences the activity of NOX2 NADPH oxidases, but it has not been clearly established to what extent it affects the activity of NOXs 1 and 3 [4]. P40 phox binds to p47 phox and p67 phox in NOX1 and NOX2 NADPH oxidase complexes. Our data clearly demonstrated its expression in HCS fibroblasts. While p40 phox is contained within membranous fractions, it does not appear to be required for activity of NOX1, based on the work of others [4]. Rac, another protein that appears to be required for full activity of NOX1, 2, and 3 and which may influence the activity of NOX4 oxidases was produced and was bound to membrane fractions in HCS fibroblasts [39,43,44].
HCS fibroblasts produce O2.- in a constitutive manner consistent with NADPH oxidase activity. mRNAs and proteins required to form functional NOX1, NOX4, and NOX5 NADPH oxidases were detected in these cells. Membranous fractions prepared from HCS fibroblasts produced O2.- in an NADPH-dependent manner that was consistent with NADPH oxidase activity. Both RT PCR and western blotting indicate that the accessory proteins p22 phox , p47 phox, p67 phox, p40 phox, and and Rac are all expressed in HCS fibroblasts. Treatment of cells with siRNAs for NOX1, NOX4, and NOX5 reduced the amount of each respective mRNA in cells. NOX and accessory proteins were present in membranous fractions prepared from cells, as we have described. The functions of the O2.- produced by each of the NOX oxidases remain to be discovered.
Acknowledgments
This work was supported by NIH grants R01 EY017079 and P30 EY01931 and an unrestricted grant from Research to Prevent Blindness.
References
- 1.Ushio-Fukai M. Localizing NADPH Oxidase-Derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 2.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–80. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 3.Duerrschmidt N, Stielow C, Muller G, Pagano PJ, Morawietz H. NO-mediated regulation of NAD(P)H oxidase by laminar shear stress in human endothelial cells. J Physiol. 2006;576:557–67. doi: 10.1113/jphysiol.2006.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedard K, Krause K-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cejkova J, Stipek S, Crkovska J, Ardan T. Changes of superoxide dismutase, catalase and glutathione peroxidase in the corneal epithelium after UVB rays. Histochemical and biochemical study. Histol Histopathol. 2000;15:1043–50. doi: 10.14670/HH-15.1043. [DOI] [PubMed] [Google Scholar]
- 7.Miller AA, Megson IL, Gray GA. Inducible nitric oxide synthase-derived superoxide contributes to hypereactivity in small mesenteric arteries from a rat model of chronic heart failure. Br J Pharmacol. 2000;131:29–36. doi: 10.1038/sj.bjp.0703528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol. 2000;88:1880–9. doi: 10.1152/jappl.2000.88.5.1880. [DOI] [PubMed] [Google Scholar]
- 9.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–59. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babior BM. The leukocyte NADPH oxidase. Isr Med Assoc J. 2002;4:1023–4. [PubMed] [Google Scholar]
- 11.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 12.Takeya R, Ueno N, Sumimoto H. Regulation of superoxide-producing NADPH oxidases in nonphagocytic cells. Methods Enzymol. 2006;406:456–68. doi: 10.1016/S0076-6879(06)06034-4. [DOI] [PubMed] [Google Scholar]
- 13.Nauseef WM. Biological Roles for the NOX Family NADPH Oxidases. J Biol Chem. 2008;283:16961–5. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–40. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 15.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–12. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Carnevale KA, Cathcart MK. Human monocytes use Rac1, not Rac2, in the NADPH oxidase complex. J Biol Chem. 2003;278:40788–92. doi: 10.1074/jbc.M302208200. [DOI] [PubMed] [Google Scholar]
- 17.Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal. 2006;8:1443–5. doi: 10.1089/ars.2006.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cejkova J, Stipek S, Crkovska J, Ardan T, Midelfart A. Reactive oxygen species (ROS)-generating oxidases in the normal rabbit cornea and their involvement in the corneal damage evoked by UVB rays. Histol Histopathol. 2001;16:523–33. doi: 10.14670/HH-16.523. [DOI] [PubMed] [Google Scholar]
- 19.Mastyugin V, Mosaed S, Bonazzi A, Dunn MW, Schwartzman ML. Corneal epithelial VEGF and cytochrome P450 4B1 expression in a rabbit model of closed eye contact lens wear. Curr Eye Res. 2001;23:1–10. doi: 10.1076/ceyr.23.1.1.5422. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien WJ, Krema C, Heimann T, Zhao H. Expression of NADPH Oxidase in Rabbit Corneal Epithelial and Stromal Cells in Culture. Invest Ophthalmol Vis Sci. 2006;47:853–63. doi: 10.1167/iovs.05-1063. [DOI] [PubMed] [Google Scholar]
- 21.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem Sci. 2003;28:502–8. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 22.Rao PV, Maddala R, John F, Zigler JS., Jr Expression of nonphagocytic NADPH oxidase system in the ocular lens. Mol Vis. 2004;10:112–21. [PubMed] [Google Scholar]
- 23.Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, Caldwell RW, Caldwell RB. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–8. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chwa M, Atilano SR, Reddy V, Jordan N, Kim DW, Kenney MC. Increased Stress-Induced Generation of Reactive Oxygen Species and Apoptosis in Human Keratoconus Fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:1902–10. doi: 10.1167/iovs.05-0828. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien WJ, Heimann T, Tsao LS, Seet BT, McFadden G, Taylor JL. Regulation of nitric oxide synthase 2 in rabbit corneal cells. Invest Ophthalmol Vis Sci. 2001;42:713–9. [PubMed] [Google Scholar]
- 26.Li L, Frei B. Prolonged Exposure to LPS Increases Iron, Heme, and p22phox Levels and NADPH Oxidase Activity in Human Aortic Endothelial Cells: Inhibition by Desferrioxamine. Arterioscler Thromb Vasc Biol. 2009;29:732–8. doi: 10.1161/ATVBAHA.108.183210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–60. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 28.Brown GE, Stewart MQ, Liu H, Ha VL, Yaffe MB. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara T, Lambeth JD, Kawahara T, Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell. 2008;19:4020–31. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambeth JD. Nox enzymes, ROS, and chronic disease: An example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–47. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie. 2007;89:1159–67. doi: 10.1016/j.biochi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Demaurex N, Schlegel W, Krause KH. Mechanism of Ca2+ Activation of the NADPH Oxidase 5 (NOX5). J Biol Chem. 2004;279:18583–91. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 33.Lambeth JD, Kawahara T, Diebold B, Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–69. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 35.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorin Y, Ricono JM, Kim N-H, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–29. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 37.Ibi M, Katsuyama M, Fan C, Iwata K, Nishinaka T, Yokoyama T, Yabe-Nishimura C. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic Biol Med. 2006;40:1785–95. doi: 10.1016/j.freeradbiomed.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y-S, Morgan MJ, Choksi S, Liu Z-g. TNF-Induced Activation of the Nox1 NADPH Oxidase and Its Role in the Induction of Necrotic Cell Death. Mol Cell. 2007;26:675–87. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–26. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 40.Cheng G, Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–42. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 41.Stahelin RV, Burian A, Bruzik KS, Murray D, Cho W. Membrane binding mechanisms of the PX domains of NADPH oxidase p40phox and p47phox. J Biol Chem. 2003;278:14469–79. doi: 10.1074/jbc.M212579200. [DOI] [PubMed] [Google Scholar]
- 42.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 43.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–29. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 44.Kao YY, Gianni D, Bohl B, Taylor RM, Bokoch GM. Identification of a conserved Rac-binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J Biol Chem. 2008;283:12736–46. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiose A, Kuroda J, Tsuruya K, Hirai M, Naito S, Hattori M, Sakai Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–23. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 46.Jones SA, Wood JD, Coffey MJ, Jones OT. The functional expression of p47-phox and p67-phox may contribute to the generation of superoxide by an NADPH oxidase-like system in human fibroblasts. FEBS Lett. 1994;355:178–82. doi: 10.1016/0014-5793(94)01201-6. [DOI] [PubMed] [Google Scholar]
- 47.Pagano PJ, Chanock SJ, Siwik DA, Colucci WS, Clark JK, Angiotensin II. Induces p67phox mRNA Expression and NADPH Oxidase Superoxide Generation in Rabbit Aortic Adventitial Fibroblasts. Hypertension. 1998;32:331–7. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 48.Dveksler GS, Basile AA, Diffenbach CW. Analysis of gene expression: use of ologonucleotide primers for glyceraldehyde-3-phosphate dehydrogenase. PCR Methods Appl. 1992;1:283–5. doi: 10.1101/gr.1.4.283. [DOI] [PubMed] [Google Scholar]