Abstract
The purpose of this study was to validate serum creatinine (SCr) concentrations assayed in the Central Biochemistry Laboratory of the National Institutes of Health (NIH)-funded Chronic Kidney Disease in Children (CKiD) study utilizing an enzymatic assay (Siemens Advia 2400) against a method traceable to reference isotope dilution mass spectroscopy (IDMS) developed by the National Institute of Standards and Technology (NIST). High-performance liquid chromatography (HPLC) measured SCr after external validation utilizing IDMS-based standard reference materials. Sera from the first 201 subjects enrolled in CKiD were analyzed and compared for creatinine concentration by enzymatic and HPLC methods. Fifty “normal” pediatric sera were subsequently analyzed. Finally, a “pediatric” reference standard was prepared and examined for accuracy and precision. Enzymatic SCr concentrations (median 1.4 mg/dl) of CKiD subjects were well correlated with HPLC (r=0.984) but were slightly higher (+7%; p<0.001). Agreement was poorer at lower SCr (median 0.4 mg/dl) when using samples from normal children and the “pediatric” reference standard. However, the Roche enzymatic assay was comparable with HPLC in accuracy and precision. Referring physicians should be aware of the accuracy and reproducibility of their laboratory's SCr assay. Our enzymatic assay agreed well with HPLC in CKiD subjects with elevated SCr. We suggest that NIST develop a pediatric SCr standard reference material for use by assay manufacturers to improve accuracy and precision of assays at the low SCr levels observed in most pediatric patients.
Keywords: High-performance liquid chromatography, Glomerular filtration rate, Enzymatic creatinine assay, Jaffe creatinine assay, Isotope dilution mass spectroscopy, Pediatric standards
Introduction
Serum creatinine (SCr) is the most widely used endogenous marker of kidney function. Accurate SCr measurements are critical to estimating glomerular filtration rate (GFR) [1] and to the ongoing global public health efforts to diagnose and treat chronic kidney disease (CKD). The National Kidney Foundation's Kidney Disease Quality Outcomes Initiative (K/DOQI) has presented a five-stage classification system of CKD based primarily on measuring urine protein-to-creatinine ratios and estimating GFR from SCr using estimating equations [2]. It should be noted that the mildest stage of CKD may have abnormalities in urine composition or in imaging tests but without any obvious increase in SCr.
Reliable measurement of SCr is a key factor in estimating GFR, and efforts to standardize such determinations nationwide is a major function of the National Kidney Disease Education Program (NKDEP) [3]. With the effort to estimate GFR in adults from the Modification of Diet in Renal Disease Study [4] as part of the output from the basic chemistry profiles performed in laboratories throughout the United States, a comparable need has been realized to provide similar estimates in children. It is well known that SCr values in normal infants and children are substantially lower than those in adults [5], suggesting the possibility that the assays used presently may not be sensitive or precise enough to reliably determine changes in SCr superimposed on the much lower pediatric baseline values.
One of the goals of the National Institutes of Health (NIH)-supported Chronic Kidney Disease in Children (CKiD) observational cohort study is to develop an improved GFR-estimating equation for children with CKD. A crucial first step in the process is to validate SCr concentrations as assayed in the Central Biochemistry Laboratory utilizing an enzymatic method (Siemens Advia 2400) against a method traceable to a reference method such as isotope dilution mass spectroscopy (IDMS) utilizing reference materials developed by the National Institute of Standards and Technology (NIST). Importantly, we used high-performance liquid chromatography (HPLC) to accurately measure SCr in the same samples. This paper presents the validation of our SCr measurement compared with IDMS traceable standards in children with CKD and in healthy children with low SCr values.
Methods
Study populations
The CKiD study is a prospective, observational cohort of children who have an estimated GFR [1]of 30–90 ml/min per 1.73 m2 and therefore SCr generally greater than 1.0 mg/dl. These children were recruited from 43 sites in the United States and Canada, and the first 201 serum samples were measured for creatinine concentration by enzymatic and HPLC assays at the Central Biochemistry Laboratory in Rochester, NY.
An additional group of “pediatric” samples submitted for creatinine analysis was obtained from the clinical laboratory of the University of Rochester Medical Center. The group was selected purely by virtue of being from subjects 17 years of age or younger and who had provided a large enough volume to allow determination of SCr by HPLC in duplicate. Due to the potential interference of hemoglobin on the enzymatic measurement of creatinine [6], grossly hemolyzed sera were not used in this analysis.
Creatinine assays
Enzymatic assays of SCr are generally more specific and sensitive than the conventional Jaffe alkaline picrate method. Thus, Jaffe measurements tend to overestimate SCr, resulting in underestimation of GFR calculated from SCr [1]. Enzymatic methods have been successfully adapted to high throughput and generally show fewer interferences than the Jaffe method [1, 3]. The enzymatic creatinine method used in the Central Biochemistry Lab, run on the Advia 2400 Chemistry system (Siemens Diagnostics, Tarrytown, NY, USA), was based on the conversion of creatinine by creatinine deiminase to ammonia and N-methylhydantoin, and the ammonia combines with 2-oxoglutarate and nicotinamide adenosine dinucleotide phosphate (NADPH; reduced form), which in the presence of glutamate dehydrogenase yields glutamate and NADP (oxidized form). The reaction is monitored at 340 nm, and the inverse rate is proportional to the creatinine concentration. The manufacturer states an overall coefficient of variability of 10.3% in this range of SCr (Bayer Advia 2400 Performance Characteristics).
We also utilized the Roche/Hitachi Modular P800 SCr enzymatic assay at the Johns Hopkins Clinical Laboratory to further assess NIST calibration standards utilized in referencing the SCr methods. The Roche enzymatic assay, traceable to IDMS, is based on the determination of glycine after enzymatic conversion of creatinine to glycine. The liberated hydrogen peroxide reacts with 4-aminophenazone and 2,4,6-triiodo-3-hydroxybenzoic acid to form a quinone imine chromogen that is measured photometrically. Calibrations of both enzymatic instruments were performed as recommended by the manufacturers. Readout in milligrams per deciliter was normally given to one decimal place.
For HPLC measurement, serum samples were stored at –20°C until analysis. After thawing, protein was precipitated by the addition of five volumes of 1.25% zinc sulfate, followed by centrifugation. Fifty microliters of the supernatant were injected onto a strong cation exchange column (Allsphere SCX 5 μm, 4.6×250 mm, Alltech Associates, Deerfield, IL, USA) of an Agilent 1100 HPLC system with variable wavelength ultraviolet detector, according to the method of Ambrose et al. [7]. Creatinine was eluted isocratically with 40 mM potassium citrate, pH 5.5, + 1% methanol, flowing at 1 ml/min. The column was maintained at 50°C, and peaks were monitored at 234 nm.
Creatinine calibrators for HPLC were prepared at three levels (0.5, 1.25, and 3.0 mg/dl) by spiking 7.5% bovine serum albumin (BSA, Sigma, St. Louis, MO, USA) with creatinine (NIST Standard Reference Material 914a, creatinine clinical standard, 99.7% certified purity). Quality-control materials were prepared in sheep serum; the low level of 0.6 mg/dl was the endogenous creatinine level, whereas the high level was achieved by spiking sheep serum with creatinine to 2.0 mg/dl. Calibrators and controls were stored at –80°C until use.
The chromatographic peak, eluting in the citrate buffer system at ~6 min, was used to determine creatinine concentration (mg/dl). Quantification was via an external standards calculation based on peak area, using an unweighted linear calibration curve with the origin included. A BSA zero sample was extracted and run with each set of specimens to demonstrate the absence of any “blank” effect. Patient specimens were extracted and run in duplicate, with the mean value reported. The between-run assay imprecision was 3.7% and 3.0% at 0.76 and 2.00 mg/dl, respectively, and the assay was linear from 0.10 to 10.00 mg/dl of SCr. The limit of SCr quantitation was 0.06 mg/dl.
Standard reference materials (SRMs) of creatinine in human serum were purchased from NIST (SRM 909b, Level I = 0.6355 mg/dl, Level II = 5.287 mg/dl) and used for validation of the HPLC method. In a final study, the Level I standard reference creatinine was diluted 1:1 with 7.5% BSA to make a 0.3178 mg/dl “pediatric” standard, which was examined by HPLC and both the Advia 2400 and Roche enzymatic assays. In this setting, the enzymatic instruments were enabled to read out to two decimal places, comparable with HPLC.
Statistical methods
Conventional reporting of SCr values is to one decimal place; however, both enzymatic analyzers utilized two decimal places for the diluted pediatric standard. HPLC determinations were consistently given to two decimal places. The term “recovery” is applied to the percent of the actual standard measured and indicates the ability of the HPLC assay to quantitatively retain 100% of the creatinine in the sample protein precipitation step. Theoretical recovery applies to a diluted sample in which the dilution is considered to be exactly 1:1.
Nonparametric statistics (e.g. medians and quartiles) were used to describe the demographics of the CKiD study population. Parametric statistics were used to characterize the pediatric samples and the variability of the methods. Standard regression methods for Gaussian data were used to characterize other comparisons between methods. Coefficient of variation (CV) is the mean divided by standard deviation (SD). Significance was asserted if p<0.05.
Log transformations of Scr values were utilized to reduce skewing of the raw data and characterize agreement between two measured values [X= log(Enzymatic Scr) and Y= log(HPLC Scr)] of Scr. To describe agreement between variables X and Y, we depicted the standard plot of X vs. Y and the corresponding Bland-Altman [8] plot of the average of X and Y vs. the difference of X from Y. The slope of the linear regression of the difference (X-Y) on the average [(X+Y)/2] from the Bland-Altman plot is directly related to the ratio of the SD of X and Y (i.e. the SD of X and Y are equal if and only if the slope is zero), and the residual variance is directly related to the correlation between X and Y. Furthermore, if the individuals’ averages (i.e. the dependent variable) are centered around their overall mean [i.e. (X+Y)/2 - m, where m is the mean of the individuals’ (X+Y)/2], the intercept of the regression corresponds to the mean of the differences between the two tests (i.e. the bias). Testing the null hypothesis of no bias (i.e. mean difference = 0) is accomplished by testing whether the intercept is zero.
Results
NIST standards
The NIST SRM 909b is human serum based and comes in two levels for creatinine: Level I = 0.6355 mg/dl ± 0.0062 and Level II = 5.287±0.060 mg/dl. In determining precision and recovery of the HPLC assay, 20 replicates of Level I and Level II materials were analyzed (Table 1). The mean values of creatinine were 0.6306±0.0251 and 5.2407±0.1602 mg/dl for Level I and Level II materials, respectively. Thus, the coefficients of variation of the HPLC method were 4.0% and 3.1% and the recoveries were 99.2% and 99.1% for Levels I and II, respectively.
Table 1.
National Institute of Standards and Technology (NIST) certified values determined by high-performance liquid chromatography (HPLC)
| Standard reference material (SRM) |
||
|---|---|---|
| Level 1 | Level 2 | |
| NIST certified value | 0.6355 | 5.287 |
| SD | 0.0062 | 0.060 |
| HPLC values | ||
| Mean | 0.6306 | 5.2407 |
| SD | 0.0251 | 0.1602 |
| CV | 3.97% | 3.06% |
| Recovery | 99.2% | 99.1% |
| Number | 20 | 20 |
SD standard deviation, CV coefficient of variation
Study populations
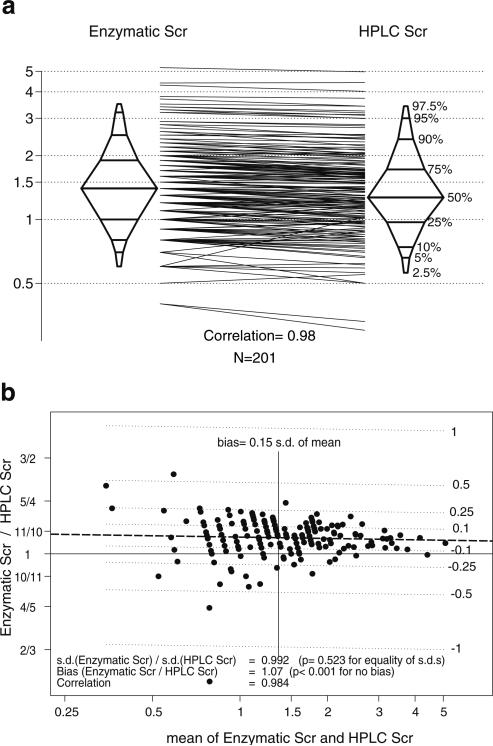
For the first 201 children enrolled in CKiD, the median enzymatic SCr was 1.4 mg/dl with interquartile range (IQR) of 1.0–1.9, compared with the median by HPLC of 1.27 with IQR 1.0–1.7 (Fig. 1a). The two measures of SCr were strongly correlated (r=0.98). Figure 1b shows the Bland-Altman plot of the log-transformed SCr measurements. The SDs were similar (enzymatic:HPLC ratio 0.991; p value for ratio being equal to one: 0.496). The enzymatic SCr was 7% higher (p<0.001) than HPLC values, and the bias did not significantly change over the observed range of SCr (0.4–4.5 mg/dl), but the scatter appeared larger at low SCr values.
Fig. 1.
Comparison of Siemens Advia enzymatic assay against high-performance liquid chromatography (HPLC) using sera from the Chronic Kidney Disease in Children (CKiD) subjects. a Histogram of results and comparison of each determination showing a correlation of 0.98. The y axis is in milligrams per deciliter (mg/dl) presented in log scale. b Bland-Altman plot showing mean of Advia and HPLC values on the x axis and ratio on the y axis (difference of logs), with a bias of 7% overestimation by the Advia enzymatic assay. The scatter clearly appears larger at low serum creatinine values
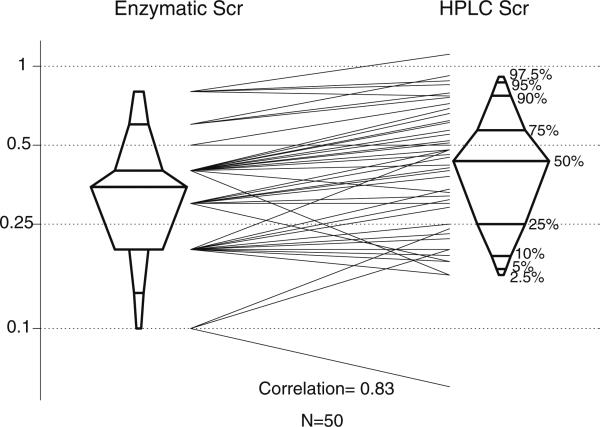
Because CKiD patients had elevated SCr, we focused subsequent studies on the lower range of SCr levels, wherein most pediatric blood samples fall. In 50 pediatric samples, Siemens enzymatic SCr values were moderately correlated with those of HPLC (r=0.83, Fig. 2) but the enzymatic SCr were lower than those by HPLC (median enzymatic = 0.35, IQR=0.2–0.4 mg/dl vs. median HPLC=0.44, IQR=0.25–0.57). Some of the loss of correlation may be due to the use of only one decimal output by the clinical laboratory (left side of the figure). The Bland-Altman plot showed that the enzymatic SCr mean value was 18.3% lower (p<0.001) than that of HPLC (not shown).
Fig. 2.
Comparison of Siemens Advia enzymatic assay against HPLC at low levels of creatinine. The y axis is in milligrams per deciliter (mg/dl) presented in log scale. Histogram of results and comparison of each determination showing a substantial negative bias compared with high-performance liquid chromatography (HPLC) values
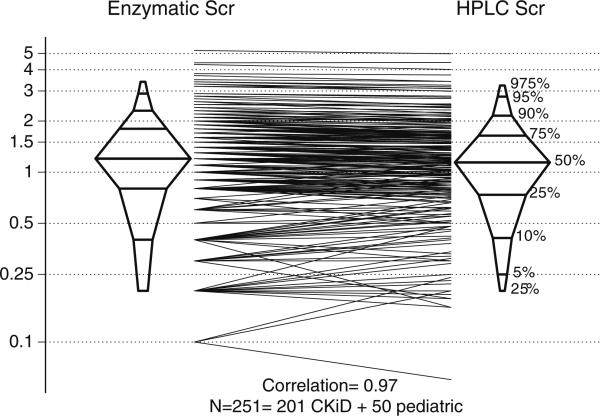
Overall, in the 251 determinations (Fig. 3), there was a very high correlation between Siemens enzymatic and HPLC SCr values (r=0.97). Most of the variability between the methods appeared at SCr levels below 1 mg/dl, whereas there was good agreement above that level.
Fig. 3.
Comparison of Siemens Advia enzymatic assay against high-performance liquid chromatography (HPLC) using 251 samples from the 201 CKiD subjects and the 50 healthy pediatric cases. Histogram of results and comparison of each determination showing a correlation of 0.97. The y axis is in milligrams per deciliter (mg/dl) presented in log scale. There is more scatter in the Advia assay at levels of serum creatinine below 1.0 mg/dl
In 34 determinations of pediatric samples run in duplicate by the HPLC assay, the means and standard deviations of each run were not significantly different (run a: 0.41±0.22; run b: 0.41±0.21; mean difference 0.00±0.01, paired t test p=0.19).
“Pediatric” standard reference material
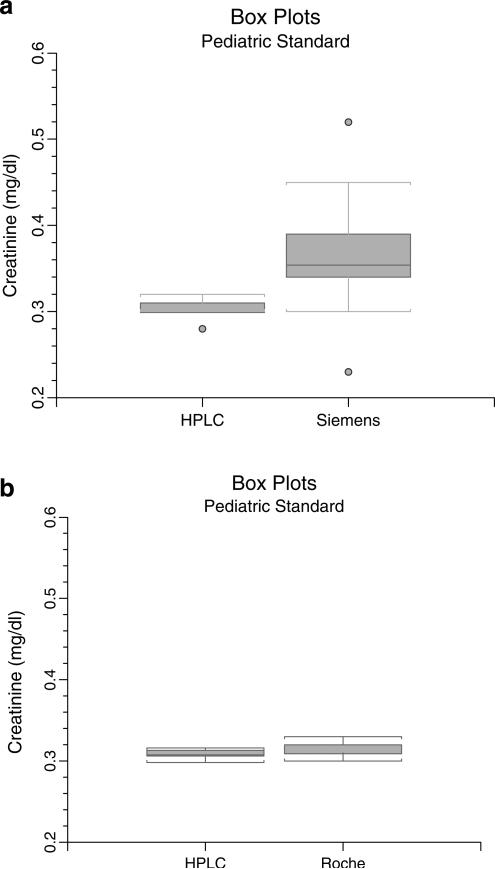
To further examine the finding that in pediatric samples with low levels of SCr the enzymatic assay did not agree so well with the HPLC assay, a low-range pediatric sample was prepared by diluting the human-serum-based NIST SRM 909b Level I material with 1:1 7.5% BSA to yield a target value of 0.3178 mg/dl. In 20 determinations, the Siemens enzymatic assay yielded a mean of 0.36, a median of 0.36, and a SD of 0.058 mg/dl (Fig. 4a), resulting in an apparent overestimation of the diluted standard by 13%. In contrast, the mean, median, and SD of the HPLC assay were 0.30, 0.30, and 0.006, respectively, indicating 96% (theoretical) recovery of the sample by HPLC. Both the means and the SDs by the two methods were significantly different by t test (p<0.01) using unequal variances and F test, respectively. The CV by Siemens enzymatic assay was 16% compared with 2% by HPLC.
Fig. 4.
Box plots of multiple analyses (20) of diluted pediatric standard by enzymatic and high-performance liquid chromatography (HPLC) assays. The rectangle describes the interquartile range (IQR, 25th and 75th percentiles) and any horizontal line through the rectangle denotes the median. The horizontal portion of the “T” depicts upper and lower adjacent values, respectively, defined as the largest observation that is ≤75th percentile + 1.5 × IQR and the smallest observation that is ≥25th percentile minus 1.5 × IQR. Points above or below the upper and lower adjacent values are outliers. a Box plot of HPLC against Siemens Advia showing that the Siemens assay has a positive bias of 19% and a much larger range of values compared with HPLC. The CV of HPLC was 2% compared with 16% of the Advia. b Box plot of HPLC against the Roche enzymatic analyzer showing close agreement and narrow interquartile ranges. The CVs were 1.6% for HPLC and 2.3% for the Roche
In trying to assess whether other enzymatic creatinine assays commonly used in clinical laboratories would also have accuracy and precision problems at low creatinine levels, a separate comparison of 20 determinations of the diluted Level I sample was performed using the Roche Modular P analyzer (Fig. 4b). The Roche analyzer yielded a mean value of 0.31 mg/dl, median 0.31, and SD 0.007; the theoretical recovery of this diluted standard was 99%. On the same sample, HPLC mean was 0.31, median 0.31, and SD 0.005, indicating that the Roche and HPLC methods differed on the same standard by less than 2% (p<0.05); this difference was not clinically important. The CVs by Roche enzymatic and HPLC assays were 2.3% and 1.6%, respectively.
Discussion
The Laboratory Working Group of the NKDEP suggests that HPLC provides a fairly sensitive and analytically specific method for measuring SCr [3]. Sample deproteinization improves the specificity of creatinine measurement by HPLC by removing many protein-bound endogenous and exogenous compounds without altering the quantification of creatinine [3]. Data from CKiD subjects indicate that the Siemens creatinine enzymatic assay used by the Central Biochemistry Laboratory compares favorably with HPLC. This assay is reliable and more than adequate for the level of SCr encountered in children with moderate to severe CKD. With statistically significant numerical differences of only 7%, the enzymatic SCr values were slightly higher than those determined by HPLC, but the differences in the methods were within the manufacturers’ stated variability. Importantly, when the bias was examined along the entire range of SCr, there was no significant change. When a separate group of SCr samples from apparently normal infants and children with low SCr levels was examined, however, there was not a high agreement between the two assays, and the Siemens enzymatic assay had a negative bias against the HPLC assay by 18%.
The poor agreement at the low range of SCr led us to examine the accuracy at the low concentration ranges associated with pediatric patients. Since NIST does not have a SRM at this low concentration (Level I was at 0.6355 mg/dl), the only recourse, though less than ideal, was to dilute the NIST SRM Level 1 to achieve a target value of approximately 0.32 mg/dl. Even if the exact value were uncertain due to introduction of errors associated with the dilution step and the use of BSA as the diluent, it was still possible to obtain an assessment of accuracy and precision of the commercial enzymatic assays. HPLC and Roche enzymatic assays gave means of 0.31 mg/dl, which agreed very well with the estimated target value of 0.32 mg/dl. Siemens enzymatic method mean was 0.36 mg/dl, overestimating the target value by 16%. More importantly, the imprecision of the Siemens enzymatic assay was concerning, with a CV of 16% compared with 2.3% for the Roche and 1.6–2% for the HPLC assay.
Difficulties in measuring SCr at the low range have been previously encountered using the Jaffe colorimetric reaction [9], and efforts to improve precision at this level included initial dialysis steps, adsorption phases, and increasing signal to noise ratios (reviewed in [1]). NIST provides a “low-level” standard of 0.6355 mg/dl, which is twice the expected value of SCr for a healthy infant or toddler. Although HPLC assay linearity ranges extends to 0.15 mg/dl, accurate determination at the critical pediatric SCr range of 0.3 mg/dl is not possible due to the lack of a NIST SRM at that concentration range. Our resort to using a low “pediatric” standard prepared by dilution of the low SRM (Level I) made the HPLC method not commutable to an IDMS reference method due to the error introduced by the dilution process and the use of creatinine-free BSA instead of human serum as the diluent [10]. Nevertheless, our data showed that one of the two commercial assays for SCr had good agreement with HPLC, whereas the other had a positive bias of 13%, and was 19% higher than HPLC values, a bias that could have clinically significant consequences.
The use of IDMS-based SRMs in the pediatric range should bring accuracy to the measurement of low SCr levels (achieving trueness in creatinine results), eliminate methodological bias among assay manufacturers, and ultimately standardize the creatinine measurement. Thus, accurate knowledge of normal SCr values could be assured prior to the use of nephrotoxic agents, chemotherapy, or total body irradiation.
Whereas the availability of SRM at low SCr levels will help improve accuracy, assay manufacturers should also focus on improving the precision of their assays at low SCr levels. Our data showed that one commonly used SCr assay had a CV of 16% at 0.32 mg/dl. Whereas the low SCr values of infants and young children are seemingly reassuring, a true increase of SCr from 0.3 to 0.4 with acute pyelonephritis is of major clinical significance.
In summary, at the SCr levels encountered in CKiD study patients (median 1.4 mg/dl), the Central Biochemistry Laboratory Siemens assay yielded results comparable with the HPLC assay, which was commutable to IDMS for the concentration range bracketed by the two levels of serum SRMs (Level I = 0.6355 mg/dl, Level II = 5.287 mg/d). We extended this study to investigate whether the Siemens assay could accurately measure low SCr levels associated with normal infants and children. The results proved that it could not. We showed that another commonly used analyzer (Roche) was comparable with HPLC in accuracy and precision for the diluted low standard.
Therefore, we suggest that physicians ordering SCr on infants and children be aware of the accuracy and reproducibility of the creatinine assay used in the clinical laboratories of their choice, especially for evaluating changes in low SCr values. Further, we suggest that NIST develop a human “pediatric” SCr standard reference material for use by assay manufacturers to improve the accuracy and precision of their assays at the low SCr expected in most pediatric patients. In the absence of such development, the ability of the physician to monitor the progression of mild CKD in infants and children and safely prescribe therapies in which the dose is correlated to kidney function would be compromised.
Acknowledgments
The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, UO1-DK-66116). The CKiD Web site is located at http://www.statepi.jhsph.edu/ckid. The clinical coordinating centers (principal investigators) are at Children's Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, M.D.) and Johns Hopkins School of Medicine (Susan Furth, M.D., Ph.D.), and the data coordinating center (principal investigator) is at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.), with the Central Biochemistry Laboratory at the University of Rochester (George J. Schwartz, M.D.). We are grateful to Ms. Paula Maier of the CBL for her excellent assistance in coordinating this study.
Contributor Information
George J. Schwartz, University of Rochester Medical Center, Rochester, NY, USA Pediatric Nephrology, University of Rochester Medical Center, Box 777, 601 Elmwood Avenue, Rochester, NY 14642, USA George_Schwartz@urmc.rochester.edu.
Tai Kwong, University of Rochester Medical Center, Rochester, NY, USA.
Brian Erway, University of Rochester Medical Center, Rochester, NY, USA.
Bradley Warady, Children's Mercy Hospital, Kansas City, MO, USA.
Lori Sokoll, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Stanley Hellerstein, Children's Mercy Hospital, Kansas City, MO, USA.
Vikas Dharnidharka, University of Florida, Gainesville, FL, USA.
Susan Furth, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Alvaro Muñoz, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
References
- 1.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, Balk E, Lau J, Levin A, Kausz AT, Eknoyan G, Levey AS. National Kidney Foundation's kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416–1421. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 3.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers NL, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GJ, Haycock GB, Chir B, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88:828–830. doi: 10.1016/s0022-3476(76)81125-0. [DOI] [PubMed] [Google Scholar]
- 6.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- 7.Ambrose RT, Ketchum DF, Smith JW. Creatinine determined by “high-performance” liquid chromatography. Clin Chem. 1983;29:256–259. [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 9.Huang YC, Chiou WL. Creatinine XII: comparison of assays of low serum creatinine levels using high-performance liquid chromatography and two picrate methods. J Pharm Sci. 1983;72:836–837. doi: 10.1002/jps.2600720736. [DOI] [PubMed] [Google Scholar]
- 10.Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006;52:553–554. doi: 10.1373/clinchem.2005.063511. [DOI] [PubMed] [Google Scholar]