Abstract
Resveratrol has been known to possess various potent cardiovascular effects in animal, but there is little information on its functional effect on the secretion of catecholamines (CA) from the perfused model of the adrenal medulla. Therefore, the aim of the present study was to determine the effect of resveratrol on the CA secretion from the isolated perfused model of the normotensive rat adrenal gland, and to elucidate its mechanism of action. Resveratrol (10~100µM) during perfusion into an adrenal vein for 90 min inhibited the CA secretory responses evoked by ACh (5.32 mM), high K+ (a direct membrane-depolarizer, 56 mM), DMPP (a selective neuronal nicotinic Nn receptor agonist, 100µM) and McN-A-343 (a selective muscarinic M1 receptor agonist, 100µM) in both a time- and dose-dependent fashion. Also, in the presence of resveratrol (30µM), the secretory responses of CA evoked by veratridine 8644 (an activator of voltage-dependent Na+ channels, 100µM), Bay-K-8644 (a L-type dihydropyridine Ca2+ channel activator, 10µM), and cyclopiazonic acid (a cytoplasmic Ca2+-ATPase inhibitor, 10µM) were significantly reduced. In the simultaneous presence of resveratrol (30µM) and L-NAME (an inhibitor of NO synthase, 30µM), the CA secretory evoked by ACh, high K+ , DMPP, McN-A-343, Bay-K-8644 and cyclopiazonic acid were recovered to a considerable extent of the corresponding control secretion compared with the inhibitory effect of resveratrol alone. Interestingly, the amount of nitric oxide (NO) released from the adrenal medulla was greatly increased in comparison to its basal release. Taken together, these experimental results demonstrate that resveratrol can inhibit the CA secretory responses evoked by stimulation of cholinergic nicotinic receptors, as well as by direct membrane-depolarization in the isolated perfused model of the rat adrenal gland. It seems that this inhibitory effect of resveratrol is exerted by inhibiting an influx of both ions through Na+ and Ca2+ channels into the adrenomedullary cells as well as by blocking the release of Ca2+ from the cytoplasmic calcium store, which are mediated at least partly by the increased NO production due to the activation of NO synthase.
Keywords: Resveratrol, Catecholamine secretion, Adrenal medulla, Cholinergic receptors, Nitric oxide
INTRODUCTION
Resveratrol, 3,4',5-trihydroxystilbene, is a naturally occurring polyphenolic compound present in variety of plants, such as Yucca schidigera (Uenobe et al, 1997), a South Africa medicinal plant Erythrophleum lasianthum (Orisini et al, 1997), and grapes (Celotti et al, 1996; Sato et al, 1997). Interest in resveratrol has expanded in recent years, focusing on its potentially beneficial effects on the cardiovascular as well as neoplastic diseases. It has been shown that resveratrol mainly produced endothelium-dependent and nitric oxide-mediated vasodilation in human internal mammary arteries, and only partially in saphenous vein rings, and improved their endothelial reactivity (Rakici et al, 2005). Several epidemiological studies indicate an association between moderate consumption of red wine and reduced risk of coronary heart disease (Renaud and de Lorgeril, 1992; German and Walzem, 2000). It has also found that red wine polyphenolic compounds (PCRW) promote the endothelium-dependent relaxation, activate NO synthase, inhibit platelet aggregation, and prevent oxidation of LDL-cholesterol (Fitzpatrick et al, 1993; Frankel et al, 1993; Demrow and Slane, 1995; Andriambeloson et al, 1997; Flesh et al, 1998; Leikert et al, 2002).
The polyphenolic compound resveratrol contained in red wine is thought to be a responsible factor for its beneficial cardiovascular effects. Resveratrol has similar effects to PCRW, such as promotion of vasodilation, activation of nitric oxide synthase, inhibition of platelet aggregation and leukocyte activation, prevention of oxidation of LDL-cholesterol and reduction of cholesterol synthesis (Frankel et al, 1993; Pace-Asciak et al, 1995; Chen and Pace-Asciak, 1996; Rotondo et al, 1998; Wallerath et al, 2002).
On the other hand, it has been observed that these natural polyphenols, like a number of antidepressant drugs (Slotkin et al, 1986; Gareri et al, 2000; To et al, 2005), inhibit the uptake of 5-hydroxytryptamine (5-HT) by human platelets. In recent studies (Yáñez et al, 2006), both cis-resveratrol and trans-resveratrol (5~200µM) in a concentration- dependent manner inhibited the uptake of [3H]NA and [3H]5-HT by synaptosomes from rat brains and the uptake of [3H]5-HT by human platelets. Both cis-resveratrol and trans-resveratrol (5~200µM) in a concentration-dependent manner also inhibited the enzymatic activity of commercial (human recombinant) MAO (monoamine oxidase) isoform (MAO-A and MAO-B) activity.
Resveratrol has various potent cardiovascular effects in animals but there is so far no evidence about its functional effect on the CA secretion from the perfused model of the adrenal gland. Therefore, the aim of the present study was to examine the ability of resveratrol on the CA secretion in the isolated perfused model of the rat adrenal gland, and to elucidate its mechanism of action.
METHODS
Experimental procedure
Male Sprague-Dawley rats, weighing 180 to 300 grams, were anesthetized with thiopental sodium (40 mg/kg) intraperitoneally. The adrenal gland was isolated by the methods described previously (Wakade, 1981). The abdomen was opened by a midline incision, and the left adrenal gland and surrounding area were exposed by placing three hook retractors. The stomach, intestine and portion of the liver were not removed, but pushed over to the right side and covered by saline-soaked gauze pads and urine in bladder was removed in order to obtain enough working space for tying blood vessels and cannulations.
A cannula, used for perfusion of the adrenal gland, was inserted into the distal end of the renal vein after all branches of adrenal vein (if any), vena cava and aorta were ligated. Heparin (400 IU/ml) was injected into vena cava to prevent blood coagulation before ligating vessels and cannulations. A small slit was made into the adrenal cortex just opposite entrance of adrenal vein. Perfusion of the gland was started, making sure that no leakage was present, and the perfusion fluid escaped only from the slit made in adrenal cortex. Then the adrenal gland, along with ligated blood vessels and the cannula were carefully removed from the animal and placed on a platform of a leucite chamber. The chamber was continuously circulated with water heated at 37±1℃.
Perfusion of adrenal gland
The adrenal glands were perfused by means of ISCO pump (WIZ Co.) at a rate of 0.33 ml/min. The perfusion was carried out with Krebs-bicarbonate solution of following composition (mM): NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.18; NaHCO3, 25; KH2PO4, 1.2; glucose, 11.7. The solution was constantly bubbled with 95 % O2+5 % CO2 and the final pH of the solution was maintained at 7.4~7.5. The solution contained disodium EDTA (10µg/ml) and ascorbic acid (100µg/ml) to prevent oxidation of CA.
Drug administration
The perfusions of DMPP (10-4 M) and McN-A-343 (10-4 M) for 2 minutes and/or a single injection of ACh (5.32×10-3 M) and KCl (5.6×10-2 M) in a volume of 0.05 ml were made into perfusion stream via a three-way stopcock, respectively. Veratridine (10-4 M), Bay-K-8644 (10-5 M) and cyclopiazonic acid (10-5 M) were also perfused for 4 min, respectively.
In the preliminary experiments, it was found that upon administration of the above drugs, secretory responses to ACh, KCl, McN-A-343, veratridine, Bay-K-8644 and cyclopiazonic acid returned to preinjection level in about 4 min, but the responses to DMPP returned in 8 min.
Collection of perfusate
As a rule, prior to stimulation with various secretagogues, the perfusate was collected for 4 min to determine the spontaneous secretion of CA (background sample). Immediately after the collection of the background sample, collection of the perfusates was continued in another tube as soon as the perfusion medium containing the stimulatory agent reached the adrenal gland. Stimulated sample's perfusate was collected for 4 to 8 min. The amounts secreted in the background sample have been subtracted from that secreted from the stimulated sample to obtain the net secretion value of CA, which is shown in all of the figures.
To study the effect of resveratrol on the spontaneous and evoked secretion, the adrenal gland was perfused with Krebs solution containing quinine for 20 min, then the perfusate was collected for a certain period (background sample). Then the medium was changed to the one containing the stimulating agent or along with resveratrol, and the perfusates were collected for the same period as that for the background sample. The adrenal gland's perfusate was collected in chilled tubes.
Measurement of catecholamines
CA content of perfusate was measured directly using the fluorometric method of Anton and Sayre (1962) without the intermediate purification alumina for the reasons described earlier (Wakade, 1981) using fluorospectrophotometer (Kontron Co., Milano, Italy).
A volume of 0.2 ml of the perfusate was used for the reaction. The CA content in the perfusate of stimulated glands by secretagogues used in the present work was high enough to obtain readings several folds greater than the reading of control samples (unstimulated). The sample blanks were also lowest for perfusates of stimulated and non-stimulated samples. The content of CA in the perfusate was expressed in terms of norepinephrine (base) equivalents.
Measurement of NO release
NO release was measured using a NO-selective microelectrode (amiNO-700, innovative Instruments Inc) and an amplifier (inNO meter, Innovative Instruments). Platelet NO production was quantified as the integrated signal detected by the microelectrode after platelet activation, as previously described (Freedman et al, 2000). The electrode was calibrated by producing standardized concentrations of NO in 0.5% (wt/vol) KI in 0.1 mol/L H2SO4 from NaNO2 standards. NO release was quantified as the current detected at the electrode 30 min after the presence of Provinol at room temperature. NO release was calculated as picomole. NO production was also measured indirectly by measuring nitrite content in the supernatant.
Statistical analysis
The statistical difference between the control and pretreated groups was determined by the Student's t and ANOVA tests. A p-value of less than 0.05 was considered to represent statistically significant changes unless specifically noted in the text. Values given in the text refer to the means and the standard errors of the mean (SEM). The statistical analysis of the experimental results was made using the computer program described by Tallarida and Murray (1987).
Drugs and their sources
The following drugs were used: resveratrol hydrochloride, 1.1-dimethyl-4 -phenyl piperazinium iodide (DMPP), acetylcholine chloride (ACh), norepinephrine bitartrate, potassium chloride (KCl), Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), methyl-1, 4-dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethyl-phenyl)-pyridine-5-carboxylate (BAY-K-8644), cyclopiazonic acid, veratridine hydrochloride (Sigma Chemical Co., USA), and (3-(m-chloro-phenyl-carbamoyl -oxy)-2-butynyltrimethyl ammonium chloride [McN-A-343] (RBI, U.S.A.). Drugs were dissolved in distilled water (stock) and added to the normal Krebs solution as required except Bay-K-8644, which was dissolved in 99.5% ethanol and diluted appropriately with Krebs-bicarbonate solution (final concentration of alcohol was less than 0.1%). Concentrations of all drugs used are expressed in terms of molar base.
RESULTS
Effect of resveratrol on CA secretion evoked by ACh, excess K+, DMPP and McN-A-343 from the perfused rat adrenal glands
After the perfusion with oxygenated Krebs-bicarbonate solution for 1 hr, basal CA release from the isolated perfused rat adrenal glands amounted to 23.1±2.2 ng/2 min (n=6). Since some papers have demonstrated that resveratrol relaxes isolated vascular arteries (Fitzpatrick et al, 1993; Jager and Nguyen-Duong, 1999; Naderali et al, 2000; Naderali et al, 2001), it was attempted initially to examine the effects of resveratrol itself on CA secretion from the perfused model of the rat adrenal glands. However, in the present study, resveratrol (10-5~10-4 M) itself did not produce any effect on basal CA output from perfused rat adrenal glands (data not shown). Therefore, it was decided to investigate the effects of resveratrol on cholinergic receptor stimulation- as well as membrane depolarization-mediated CA secretion. Secretagogues were given at intervals of 15 min. Resveratrol was perfused for 90 min after completion of control responses to various secretagogues.
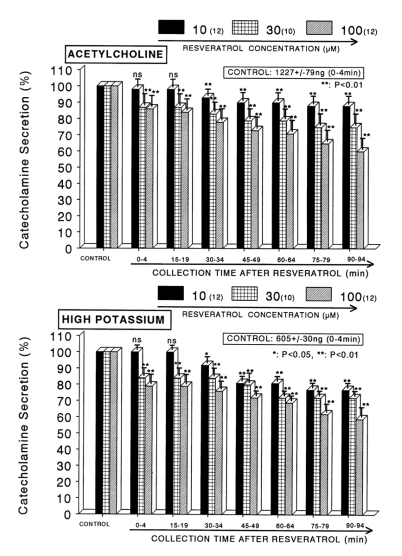
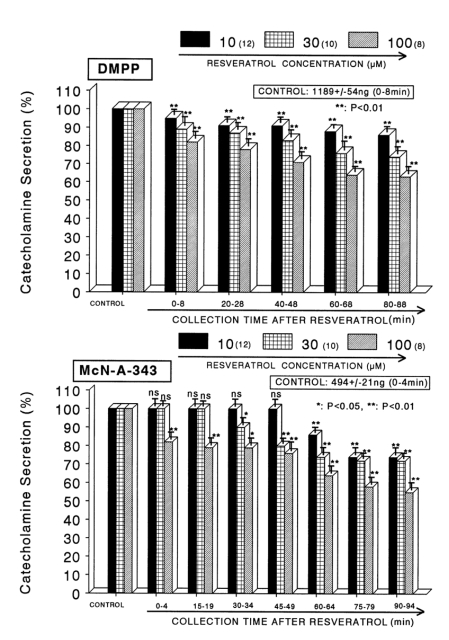
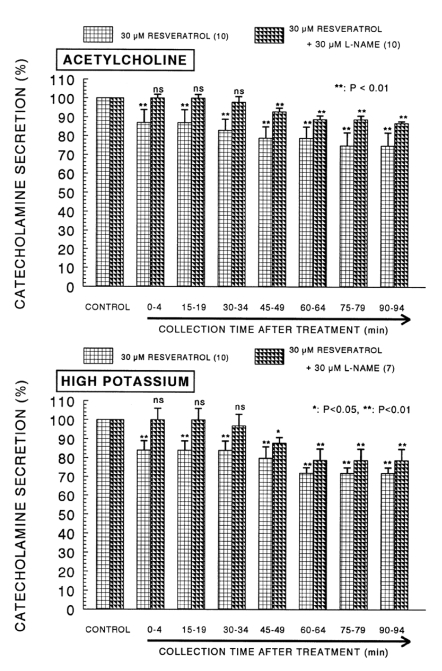
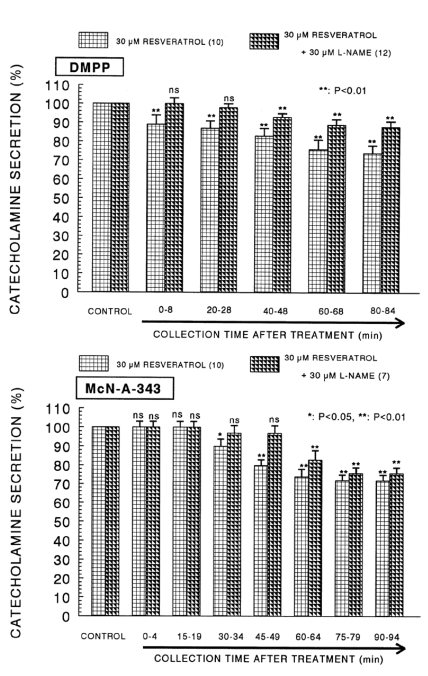
When ACh (5.32×10-2 M) in a volume of 0.05 ml was given into the adrenal vein, the amount of CA secreted was 1227±79 ng for 4 min. However, the pretreatment with resveratrol in the range of 10-5~10-4 M for 90 min concentration- and time-dependently inhibited ACh-evoked CA secretion. As shown in Fig. 1 (Upper), in the presence of resveratrol, ACh-evoked CA releasing responses were inhibited maximally by 60% of the corresponding control release. Also, it has been found that a depolarizing agent, high K+ stimulates CA secretion (605±30 ng for 0~4 min). However, in the presence of resveratrol (10-5 M~10-4 M), excess K+ (5.6×10-2 M)-evoked CA secretion was significantly inhibited by 59% of the control release (Fig. 1-Lower). When perfused through the rat adrenal gland, DMPP (10-4 M), which is a selective neuronal nicotinic receptor agonist, evoked a sharp and rapid increase in CA secretion (1189±54 ng for 0~8 min). However, as shown in Fig. 2 (Upper), DMPP-evoked CA secretion during treatment with resveratrol was greatly reduced to 63% of the control release. McN-A-343 (10-4 M), which is a selective muscarinic M1-agonist (Hammer and Giachetti, 1982), perfused into an adrenal gland for 4 min caused an increased CA secretion (4894±21 ng for 0~4 min). However, McN-A-343-evoked CA secretion in the presence of resveratrol was markedly depressed to 55% of the corresponding control secretion as depicted in Fig. 2 (Lower).
Fig. 1.
Dose-dependent effect of resveratrol on the secretory responses of catecholamines (CA) evoked by acetylcholine (upper) and high potassium (lower) from the isolated perfused rat adrenal glands. The CA secretion by a single injection of ACh (5.32×10-3 M) and K+ (5.6×10-2 M) in a volume of 0.05 ml was evoked at 15 min intervals during loading with 10, 30 and 100µM of resveratrol for 90 min as indicated by the arrow marks, respectively. The numbers in parentheses indicate the number of rat adrenal glands. Vertical bars on the columns represent the standard error of the mean (SEM). Ordinate: the amounts of CA secreted from the adrenal gland (% of control). Abscissa: collection time of perfusate (min). Statistical difference was obtained by comparing the corresponding control (CONTROL) with each concentration- treated group of resveratrol. ACh- and high K+-induced perfusates were collected for 4 minutes, respectively. **p<0.01. ns: not statistically significant.
Fig. 2.
Dose-dependent effect of resveratrol on the CA secretory responses evoked by DMPP (upper) and McN-A- 343 (lower) from the isolated perfused rat adrenal glands. The CA secretion by perfusion of DPPP (10-4 M) and McN-A-343 (10-4 M) for 2 min was induced at 20 and 15 min intervals during loading with 10, 30 and 100µM of resveratrol for 90 min, respectively. Statistical difference was obtained by comparing the corresponding control (CONTROL) with each concentration- pretreated group of resveratrol. DMPP- and McN-A-343-induced perfusates were collected for 8 and 4 minutes, respectively. Other legends are the same as in Fig. 1. *p<0.05, **p<0.01. ns: not statistically significant.
Effect of resveratrol on CA secretion evoked by veratridine, Bay-K-8644 and cyclopiazonic acid from the perfused rat adrenal glands
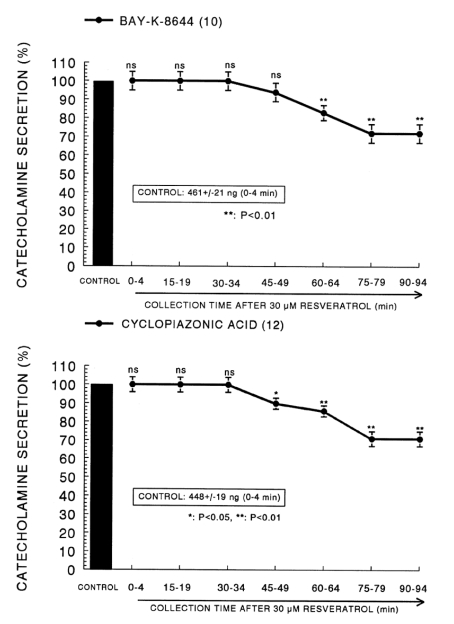
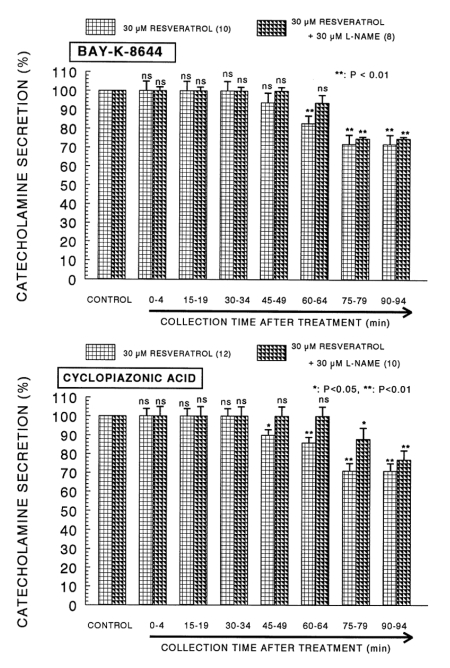
Since Bay-K-8644 is known to be a calcium channel activator, which enhances basal Ca2+ uptake (Garcia et al, 1984) and CA release (Lim et al, 1992), it was of interest to determine the effects of resveratrol on Bay-K-8644-stimulated CA secretion from the isolated perfused rat adrenal glands. Bay-K-8644 (10-5 M)-stimulated CA secretion in the presence of resveratrol (3×10-5 M) was greatly blocked to 72% of the control except for the early 45 min as compared to the corresponding control release (461±21 ng for 0~4 min) from 10 glands as shown in Fig. 3 (Upper).
Fig. 3.
The time-dependent effects of resveratrol on the CA release evoked by Bay-K-8644 (upper) and cyclopiazonic acid (lower) from the rat adrenal glands. Bay-K-8644 (10-5 M) and cyclopiazonic acid (10-5 M) were perfused into an adrenal vein for 4 min at 15 min intervals during loading with resveratrol (30µM) for 90 min. Other legends are the same as in Fig. 1. *p<0.05, **p<0.01. ns: not statistically significant.
Cyclopiazonic acid, a mycotoxin from Aspergillus and Penicillium, has been described as a highly selective inhibitor of Ca2+-ATPase in skeletal muscle sarcoplasmic reticulum (Goeger and Riley, 1989; Seidler et al, 1989). The inhibitory action of resveratrol on cyclopiazonic acid-evoked CA secretory response was observed as shown in Fig. 3 (Lower). In the presence of resveratrol (3×10-5 M) in 12 rat adrenal glands, cyclopiazonic acid (10-5 M)-evoked CA secretion was significantly depressed by 71% of the control secretory response (448±19 ng for 0~4 min).
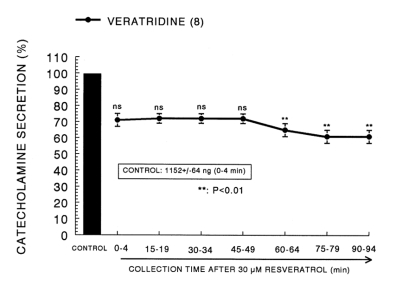
It has also been known that veratridine-induced Na+ influx mediated through Na+ channels increased Ca2+ influx via activation of voltage-dependent Ca2+ channels and produced the exocytotic secretion of CA in cultured bovine adrenal medullary cells (Wada et al, 1985). To characterize the pharmacological action of PCRW on voltage-dependent Na+ channels, the effect of PCRW on the CA secretion induced by veratridine was examined here. As shown in Fig. 4, veratridine greatly produced CA secretion (1,152±64 ng for 0-4 min). However, in the presence of resvertarol (30µM), veratridine (100µM)-evoked CA secretion was greatly inhibited to 61% of the corresponding control release in a time-dependent manner.
Fig. 4.
Time-course effects of resveratrol on the CA release evoked by veratridine from the rat adrenal glands. Veratridine (10-4 M) was perfused into an adrenal vein for 4 min at 15 min intervals during loading with resveratrol (30µM) for 90 min. Other legends are the same as in Fig. 1. **p<0.01.
Effect of resveratrol plus L-NAME on CA release evoked by ACh, high K+, DMPP, McN-A-343, BAY-K-8644 and cyclopiazonic acid from the perfused rat adrenal glands
In the present work, resveratrol caused inhibition in the CA secretion by cholinergic receptor stimulation from the perfused rat adrenal gland. Therefore, in order to establish the relationship between NO and resveratrol-induced inhibitory action on the CA release, we tried to examine the effect of L-NAME on resveratrol-induced inhibitory responses of CA secretion evoked by ACh, high K+, DMPP and McN-A-343.
In the simultaneous presence of resveratrol (30µM) and L-NAME (30µM) for 90 min, ACh-evoked CA release had recovered by 87~100% of the corresponding control release compared to the inhibitory effect of resveratrol-only treatment as illustrated in Fig. 5 (Upper). High K+ (56 mM)-evoked CA release in the simultaneous presence of resveratrol (30µM) and L-NAME (30µM) for 90 min had also recovered by 79~100% of the corresponding control release during all periods in comparison to the data of the treatment with resveratrol alone (Fig. 5-Lower).
Fig. 5.
Effect of resveratrol plus L-NAME on the CA secretory responses evoked by acetylcholine (upper) and high potassium (lower) from the perfused rat adrenal glands. The CA secretion by a single injection of ACh (5.32×10-3 M) and K+ (5.6×10-2 M) in a volume of 0.05 ml was evoked at 15 min intervals during simultaneous loading with resveratrol (30µM) plus L-NAME (30µM) for 90 min. Statistical difference was obtained by comparing the corresponding control (CONTROL) with resveratrol-treated group or group treated with resveratrol+L-NAME. Other legends are the same as in Fig. 1. *p<0.05, **p<0.01. ns: not statistically significant.
As shown in Fig. 6 (Upper), the simultaneous perfusion of resveratrol and L-NAME for 90 min got over the DMPP-evoked CA release to 88~100% of the control response compared to the corresponding control response in comparison to the inhibitory effect of the resveratrol-only treatment. Moreover, in the presence of resveratrol (30µM) and L-NAME (30µM), the CA secretory response evoked by McN-A-343 (10-4 M for 4 min) had recovered to 76~100% of the corresponding control release compared to results of the resveratrol-only treatment, as shown in Fig. 6 (Lower).
Fig. 6.
Effect of resveratrol plus L-NAME on the CA secretory responses evoked by DMPP (upper) and McN-A-343 (lower) from the perfused rat adrenal glands. The CA secretion by perfusion of DPPP (10-4 M) and McN-A-343 (10-4 M) for 2 min was induced at 20 and 15 min intervals after preloading with resveratrol (30µM) plus L-NAME (30µM) for 90 min, respectively. Other legends are the same as in Fig. 1 and 5. **p<0.01. ns: not statistically significant.
As shown in Fig. 7 (Upper), the simultaneous perfusion of resveratrol (30µM) and L-NAME (30µM) for 90 min had restored the CA release evoked by Bay-K-644 (10µM, an activator of voltage-dependent L-type calcium channel) to 75~100% of the corresponding control response compared to the inhibitory effect of the resveratrol-only treatment. After the simultaneous perfusion with resveratrol and L-NAME, cyclopiazonic acid (10µM, an inhibitor of Ca2+-ATPase of endoplasmic reticulum)-evoked CA release had also recovered by 77~100% of the control release in comparison to the results following the treatment with resveratrol alone (Fig. 7-Lower).
Fig. 7.
Effects of resveratrol plus L-NAME on the CA secretory responses evoked by Bay-K-8644 (upper) and cyclopiazonic acid (lower) from the rat adrenal glands. Bay-K-8644 (10-5 M) and cyclopiazonic acid (10-5 M) were perfused into an adrenal vein for 4 min at 15 min intervals during simultaneous loading with resveratrol (30µM) for 90 min. Other legends are the same as in Fig. 1 and 5. *p<0.05, **p<0.01. ns: not statistically significant.
Effect of resveratrol on the level of nitric oxide released from the perfused rat adrenal medulla
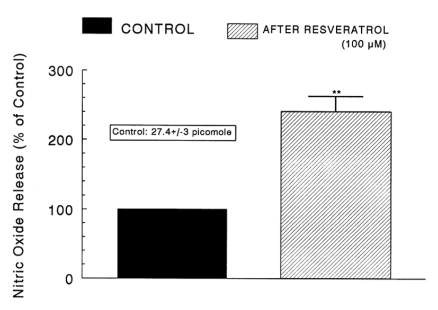
As shown in Fig. 5~7, it has been shown that, in the simultaneous presence of resveratrol and L-NAME (an inhibitor of NO synthase), the CA secretory responses evoked by ACh, high K+, DMPP, McN-A-343, BAY-K-8644 and cyclopiazonioc acid were greatly recovered to the extent of the corresponding control release compared to those effects of the resveratrol-only treatment. Therefore, we attempted to determine directly the amount of nitric oxide released from adrenal medulla following the perfusion of resveratrol-containing Krebs-bicarbonate solution. As shown in Fig. 8, the basal level of NO before the loading of resveratrol was 27.4±3 picomole. However, 30 min after the presence of resveratrol (100µM), it was greatly enhanced to 241% of the control release. Consequently, it was confirmed that resveratrol practically increase the release of NO from the rat adrenal medulla.
Fig. 8.
Comparison of nitric oxide (NO) production before (CONTROL) and after administration of resveratrol in the isolated perfused rat adrenal medulla. Perfusate sample was taken for 8 min after loading the perfusion of resveratrol (100µM) at a rate of 0.31 ml/min. Ordinate: the amounts of NO released from the adrenal medulla (% of control). Abscissa: treatment (before and after resveratrol). Statistical difference was made by comparing the control with resveratrol-treated group. **p<0.01.
DISCUSSION
The present results demonstrate that resveratrol inhibits the CA secretory responses evoked by stimulation of cholinergic (both muscarinic and nicotinic) receptors as well as by direct membrane-depolarization from the isolated perfused rat adrenal gland. It seems that this inhibitory effect of resveratrol is mediated at least by inhibiting influx of both ions through Ca2+ and Na+ channels into the rat adrenomedullary cells as well as by blocking the Ca2+ release from the cytoplasmic calcium store, which are due to the increased NO production through the activation of neuronal NO synthase.
In support of this idea, it has been reported that PCRW from two different sources, with a similar pharmacological profile in different blood vessels, are able to produce endothelium-dependent relaxation of the rat thoracic aorta (Andriambeloson et al, l997; l999). This effect was mediated by an increase in NO content due to enhancement of NO synthesis rather than protection against its breakdown by oxygen radicals associated with the antioxidant properties of PCRW (Andriambeloson et al, I997). Furthermore, studies in bovine aortic endothelial cells (BAEC) have shown that an increase in NO-synthase activity is associated with an increase in intracellular calcium concentration ([Ca2+]i) and the activation of tyrosine kinases (Martin et al, 2002). It has been shown that oral treatment with Provinols™ (polyphenol-containing product) accelerated the regression of blood pressure and prevented the development of cardiovascular remodeling, myocardial fibrosis and aortic stiffness in NO-deficient hypertensive rats. Furthermore, it has been found that oral administration of Provinols™ produced a decrease of systolic blood pressure in normotensive rats, which was accompanied with an enhanced endothelium-dependent relaxation and induction of gene expression within the arterial wall (Diebolt et al, 200l). This effect probably involved an NO pathway inasmuch Nω-nitro-L-arginine-methyl-ester (L-NAME) treatment plus PCRW abolished the decrease in blood pressure and the improvement of endothelial function (Bernátová et al, 2002).
In the present study, in the simultaneous presence of resveratrol and L-NAME (NOS inhibitor), the CA secretory responses evoked by ACh, DMPP, high K+, McN-A-343, Bay-K-8644 and cyclopiazonic acid were considerably recovered to the corresponding control level compared to the inhibitory effect of resveratrol treatment alone. This result is well consistent with report that PCRW produced the endothelium-NO-dependent relaxation through an extracellular Ca2+-dependent mechanism (Andriambeloson et al, 1999). Amongst the different classes of polyphenolic compounds present in PCRW, anthocyanins and oligomeric condensed tannins had the same pharmacological profile as PCRW (Andriambeloson et al, 1998). Of different anthocyanins identified in wine, only delphinidin caused endothelium-dependent relaxation, although it was slightly less potent than PCRW (Andriambeloson et al, 1998).
Taking into account these findings, in this study, it is likely that resveratrol inhibits the CA secretory response evoked by various secretagogues through the increased NO production in adrenal chromaffin cells, but in the simultaneous presence of resveratrol and L-NAME, their secretory responses had recovered to the control level in comparison to the inhibition of resveratrol-only treatment. Furthermore, some epidemiological studies indicate that there is an association between moderate consumption of red wine and reduced risk of coronary heart disease (Renaud and de Lorgeril, 1992; German and Walzem, 2000). It has also been shown that PCRW promotes the endothelium-dependent relaxation, activates NO synthase, inhibits platelet aggregation, and prevents oxidation of LDL-cholesterol (Fitzpatrick et al, 1993; Andriambeloson et al, 1997; Flesh et al, 1998; Leikert et al, 2002; Demrow and Slane 1995; Frankel et al, 1993). The polyphenolic compound resveratrol present in red wine is thought to be the factor responsible for wine's beneficial cardiovascular effects. Since resveratrol has similar effects as PCRW such as promotion of vasodilation, activation of nitric oxide synthase, inhibition of platelet aggregation and leukocyte activation, prevention of oxidation of LDL-cholesterol and reduction of cholesterol synthesis (Chen and Pace-Asciak, 1996; Wallerath et al, 2002; Pace-Asciak et al, 1995; Rotondo et al, 1998; Frankel et al, 1993). These effects are in agreement with the present result that resveratrol inhibits the CA secretory responses evoked by cholinergic stimulation and membrane depolarization at least by the increased NO production through activation of nitric oxide synthase in the isolated perfused rat adrenal medulla, since this inhibitory effect of resveratrol on the CA secretory responses was significantly attenuated in the presence of L-NAME, an inhibitor of nitric oxide synthase.
Generally, the adrenal medulla possesses characteristic postganglionic sympathetic neurons, and the presence of neuronal NOS (nNOS) has been demonstrated (Marley et al, 1995; Oset-Gasque et al, 1994; Palacios et al, 1989; Schwarz et al, 1998). In vitro studies using NOS inhibitors and NO donors were performed to examine the role of NO in modulating CA secretion from the adrenal medulla but the results remain controversial. In the present work, in the simultaneous presence of resveratrol and L-NAME, the CA secretory responses evoked by cholinergic nicotinic and muscarinic stimulation and direct membrane-depolarization had recovered to the levels of the control secretion as compared with the inhibitory effects of resveratrol alone. This result demonstrates that resveratrol can inhibit the CA release at least partly by the inceased NO production through the activation of nNOS in the rat adrenal medulla. In the support of this finding, it has been reported that the NOS inhibitor, L-NAME enhances K+-stimulated CA secretion in cultured bovine chromaffin cells (Torres et al, 1994), and that sodium nitroprusside (SNP) inhibits ACh-induced CA secretion in bovine chromaffin cells (Rodriguez-Pascual, et al, 1996). These studies suggest that NO may play an inhibitory role in the control of CA secretion. Moreover, the presence of endothelial cells has been reported to inhibit the K+-induced or the nicotinic receptor agonist DMPP-induced CA secretion in cultured bovine chromaffin cells (Torres et al, 1994), suggesting that not only nNOS but also eNOS may play roles in modulating adrenal CA secretion. In contrast, it has been shown that L-NAME inhibits ACh-induced CA secretion in bovine chromaffin cells (Uchiyama et al, 1994), and that the NO donor SNP enhances nicotine-induced CA secretion in cultured bovine chromaffin cells (O'Sullivan and Burgoyne, 1990). These findings suggest that NO may facilitate cholinergic agonist-induced CA secretion. On the other hand, a few in-vivo studies have suggested that NO does not play a role in regulation of adrenal CA secretion (Breslow et al, 1992; Breslow et al, 1993). Based on these reports, the present studies suggest that resveratrol possesses the ability partly to activate nNOS in the rat adrenal medullary chromaffin cells, in addition to the direct inhibitory effects on the CA secretion. The present findings that resveratrol inhibited the CA secretory responses evoked by both nicotinic receptor stimulation as well as by membrane depolarization from the rat adrenal medulla also seem to support the fact that, in in vivo studies, PCRW lowers blood pressure in normotensive and hypertensive rats (Mizutani et al, 1999; Diebolt et al, 2001). It has been reported that red wines and grapes exhibit endothelium-dependent relaxation of blood vessels via enhanced generation and/or increased biological activity of NO, leading to the elevation of cGMP levels (Fitzpatrick et al, 1993; Fitzpatrick et al, 1995; Fitzpatrick et al, 2000; Zenebe et al, 2003). Therefore, these experimental results indicate that resveratrol-induced inhibitory activity of CA secretory response evoked by stimulation of nicotinic receptors may contribute at least partly to its hypotensive mechanism.
In the present study, resveratrol also depressed the CA secretory response evoked by Bay-K-8644, which is known to activate L-type voltage-dependent Ca2+ channels, in a time-dependent manner (Garcia et al, 1984; Schramm et al, 1983). This result indicates that resveratrol may inhibit Ca2+ influx into the rat adrenomedullary cells. In support of this idea, in cultured bovine adrenal medullary cells, nicotinic (but not muscarinic) receptors mediate the Ca2+-dependent secretion of CA (Fisher et al, 1981; Yanagihara et al, 1979). It has also been known that the activation of nicotinic receptors stimulates the CA secretion by increasing Ca2+ entry through receptor-linked and/or voltage-dependent Ca2+ channels in both perfused rat adrenal glands (Wakade and Wakade, 1983; Lim and Hwang, 1991) and isolated bovine adrenal chromaffin cells (Kilpatrick et al, 1981; 1982; Knight and Kesteven, 1983). Wada and his coworkers (1985) have found that the adrenomedullary chromaffin cells have (i) nicotinic receptor-associated ionic channels, responsible for carbachol-induced Na+ influx, (ii) voltage-dependent Na+ channels, responsible for veratridine-induced Na+ influx and (iii) voltage-dependent Ca2+ channels, suggesting that the influx of Na+ caused either by carbachol or by veratridine leads to activate voltage-dependent Ca2+ channels by altering membrane potentials, whereas high K+ directly activates voltage-dependent Ca2+ channels without increasing Na+ influx. In the present study, the finding that high K+-induced CA secretory response was depressed by pretreatment with resveratrol indicates that this inhibitory effect of resveratrol is exerted by inhibiting Ca2+ influx into the adrenomedullary cells through the blockade of voltage-dependent Ca2+ channels. Furthermore, slight elevation in the extracellular potassium concentration increases both the frequency of spontaneous action potentials and the secretion of CA (Kidokoro and Ritchie, 1980), suggesting that the influx of Ca2+ that occurs during action potentials is directly linked to the rate of secretion. These findings that resveratrol inhibited the CA secretion evoked by Bay-K-8644 as well as by high K+ suggest that resveratrol directly inhibits the voltage-dependent Ca2+ channels. In the bovine chromaffin cells, stimulation of nicotinic, but not muscarinic ACh receptors is known to cause CA secretion by increasing Ca2+ influx largely through voltage-dependent Ca2+ channels (Burgoyne, 1984; Oka et al, 1979). Therefore, the finding that resveratrol inhibited the DMPP-evoked CA secretion seems to be associated with inhibition of Ca2+ influx through voltage-dependent Ca2+ channels.
The present study has also shown that resveratrol also inhibits the CA secretion evoked by cyclopiazonic acid. Cyclopiazonic acid is known to be a highly selective inhibitor of Ca2+-ATPase in skeletal muscle sarcoplasmic reticulum (Goeger and Riley, 1989; Seidler et al, 1989) and a valuable pharmacological tool for investigating intracellular Ca2+ mobilization and ionic currents regulated by intracellular Ca2+ (Suzuki et al, 1992). Therefore, in the present work, it can be speculated that the inhibitory effect of resveratrol on CA secretion evoked by McN-A-343 may be associated with the mobilization of intracellular Ca2+ from the cytoplasmic calcium store. This indicates that the resveratrol has an inhibitory effect on the release of Ca2+ from the intracellular pools induced by stimulation of muscarinic ACh receptors, which is weakly responsible for the secretion of CA. It has been shown that Ca2+-uptake into intracellular storage sites susceptible to caffeine (Ilno, 1989) is almost completely abolished by treatment with cyclopiazonic acid during the proceeding of Ca2+ load (Suzuki et al, 1992). This is consistent with the findings obtained in exposed smooth muscle fibers of the longitudinal layer of the guinea-pig ileum, where Ca2+-uptake was also inhibited by cylopiazonic acid (Uyama et al, 1992). Suzuki and his coworkers (1992) have shown that cyclopiazonic acid easily penetrates into the cytoplasm through the plasma membrane and reduces Ca2+-ATPase activity in sarcoplasmic/endoplasmic reticulum, resulting in increase in the subsequent Ca2+ release from those storage sites. Moreover, in bovine adrenal chromaffin cells, stimulation of muscarinic ACh receptors is also proposed to cause activation of phosphoinositide metabolism, resulting in the formation of inositol 1,4,5-trisphosphate, which induces the mobilization of Ca2+ from the intracellular pools (Cheek et al, 1989; Challis et al, 1991). The present results suggest that resveratrol-induced inhibition of the CA secretion evoked by McN-A-343 and cyclopiazonic acid may be due to the inhibition of Ca2+ release evoked by stimulation of muscarinic ACh receptors from the intracellular pools. Martin and his colleagues (2002) showed that PCRW stimulated a Ca2+-dependent release of nitric oxide (NO) from bovine aortic endothelial cells accounting for the relaxation of endothelium-denuded rat aortic rings. PCRW, ProvinolsTM and delphinidin also increased cytosolic free calcium ([Ca2+]i), by releasing Ca2+ from intracellular stores and by increasing Ca2+ entry. However, in the present study, it is uncertain whether the inhibitory effect of resveratrol on Ca2+ movement from intracellular pools is due to its direct effect on the PI response or the indirect effects.
On the other hand, the present findings disagree with those obtained by Yanez and his coworkers (2006), who reported that both cis-resveratrol and trans-resveratrol (5~200µM) concentration-dependently inhibited the uptake of [3H]NA and [3H]5-HT by synaptosomes from rat brain and the uptake of [3H]5-HT by human platelets. Both cis-resveratrol and trans-resveratrol (5~200µM) also inhibited the enzymatic activity of commercial (human recombinant) MAO isoform (MAO-A and MAO-B) activity in a concentration dependent manner.
It has also been observed that these natural polyphenols, like a number of antidepressant drugs (Slotkin et al, 1986; Gareri et al, 2000; To et al, 2005), inhibit the uptake of 5-HT by human platelets. It is also well known that other classes of drugs used to treat major depressive disorders are inhibitors of monoamine oxidase (MAO) (Gareri et al, 2000; To et al, 2005). To date, however, the effects of resveratrol isomers on MAO isoform (MAO-A and MAO-B) activity have not been studied. Only Zhou and his coworkers (2001), in a preliminary structure-activity relationship study of a number of stilbenoids, have previously shown that trans-resveratrol exhibits a selective inhibitory effect on MAO-A activity but not on MAO-B.
In conclusion, the results of the present study have demonstrated that resveratrol inhibits the CA secretion by stimulation of cholinergic nicotinic receptors as well as by membrane depolarization in the isolated perfused rat adrenal glands. It seems that this inhibitory effect of resveratrol is exerted by blocking influx of both ions through Ca2+ and Na+ channels into the adrenomedullary cells as well as by blocking the release of Ca2+ from the cytoplasmic calcium store, which are at least partly due to the increased NO production through the activation of nitric oxide synthase. These experimental results may contribute partly to the hypotensive effect of resveratrol, through inhibition of the CA secretion from adrenomedullary chromaffin cells and consequent reduction of the CA level in the circulation.
ACKNOWLEDGEMENT
This study was supported partly by Chosun University (2007).
ABBREVIATIONS
- CA
catecholamines
- NO
nitric oxide
- DMPP
1.1-dimethyl-4-phenyl piperazinium iodide, methyl-1,4-dihydro-2
- ACh
acetylcholine
- McN-A-343
3-(m-chlloro-phenyl-carbamoyl-oxy-2-butynyl-trimethyl ammonium chloride
- BAY-K8644
6-dimethyl-3-nitro-4-(2-trifluoromethyl-phenyl)-pyridine-5-carboxylate
Footnotes
This paper was presented at the 22nd Scientific Meeting of International Society of Hypertension held in Berline, Germany, June 13-19, 2008.
References
- 1.Andriambeloson E, Kleschyov AL, Muller B, Beretz A, Stoclet JC, Andriantsitohaina R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br J Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriambeloson E, Magnier C, Haan-Archipoff G, Lobstein A, Anton R, Beretz A, Stoclet JC, Andriantsitohaina R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 1998;128:2324–2333. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- 3.Andriambeloson E, Stoclet JC, Andriantsitohaina R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J Cardiovasc Pharmacol. 1999;33:248–254. doi: 10.1097/00005344-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide trihydroxy indole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962;138:360–375. [PubMed] [Google Scholar]
- 5.Bernatova I, Pecháòová O, Babál P, Kyselá S, Stvrtina S, Andriantsitohaina R. Wine polyphenols improve cardiovascular remodelling and vascular function in NO-deficient hypertension. Am J Physiol Heart Circ Physiol. 2002;282:H942–H948. doi: 10.1152/ajpheart.00724.2001. [DOI] [PubMed] [Google Scholar]
- 6.Breslow MJ, Tobin JR, Bredt DS, Ferris CD, Snyder SH, Traystman RJ. Nitric oxide as a regulator of adrenal blood flow. Am J Physiol. 1993;264:H464–H469. doi: 10.1152/ajpheart.1993.264.2.H464. [DOI] [PubMed] [Google Scholar]
- 7.Breslow MJ, Tobin JR, Bredt DS, Ferris CD, Snyder SH, Traystman RJ. Role of nitric oxide in adrenal medullary vasodilation during catecholamine secretion. Eur J Pharmacol. 1992;210:105–106. doi: 10.1016/0014-2999(92)90659-r. [DOI] [PubMed] [Google Scholar]
- 8.Burgoyne RD. Mechanism of secretion from adrenal chromaffin cells. Biochem Biophys Acta. 1984;779:201–216. doi: 10.1016/0304-4157(84)90009-1. [DOI] [PubMed] [Google Scholar]
- 9.Celotti E, Ferrarini R, Zironi R, Conte LS. Resveratrol content of some wines obtained from dried Valpolicella grapes: recioto and Amarone. J Chromatogr A. 1996;730:47–52. doi: 10.1016/0021-9673(95)00962-0. [DOI] [PubMed] [Google Scholar]
- 10.Challiss RA, Jones JA, Owen PJ, Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 1991;56:1083–1086. doi: 10.1111/j.1471-4159.1991.tb02033.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheek TR, O'Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells: Distinct nicotinic and muscarinic patterns. FEBS Lett. 1989;247:429–434. doi: 10.1016/0014-5793(89)81385-7. [DOI] [PubMed] [Google Scholar]
- 12.Chen CK, Pace-Asciak CR. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- 13.Demrow HS, Slane PR. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–1188. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 14.Diebolt M, Bucher B, Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001;38:159–165. doi: 10.1161/01.hyp.38.2.159. [DOI] [PubMed] [Google Scholar]
- 15.Fisher SK, Holz RW, Agranoff BW. Muscarinic receptors in chromaffin cell culture mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981;37:491–487. doi: 10.1111/j.1471-4159.1981.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick DF, Fleming RC, Bing B, Maggi DA, O'Malley R. Isolation and characterization of endothelium-dependent vasorelaxing compounds from grape seeds. J Agric Food Chem. 2000;48:6384–6390. doi: 10.1021/jf0009347. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick DF, Hirschfield SL, Ricci T, Jantzen P, Coffey RG. Endothelium-dependent vasorelaxation caused by various plant extracts. J Cardiovasc Pharmacol. 1995;26:90–95. doi: 10.1097/00005344-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Flesch M, Schwarz A, Bolun M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998;275:H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- 20.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human DL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 21.Freedman JE, Li L, Sauter R, Keaney JF., JR Alpha-Tocopherol and protein kinase C inhibition enhance platelet-derived nitric oxide release. FASEB J. 2000;14(15):2377–2379. doi: 10.1096/fj.00-0360fje. [DOI] [PubMed] [Google Scholar]
- 22.Garcia AG, Sala F, Reig JA, Viniegra S, Frias J, Fonteriz R, Gandia L. Dihydropyridine Bay-K-8644 activates chromaffin cell calcium channels. Nature. 1984;309:69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- 23.Gareri P, Falconi U, De Fazio P, De Sarro G. Conventional andnew antidepressant drugs in the elderly. Prog Neurobiol. 2000;61:353–396. doi: 10.1016/s0301-0082(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 24.German JB, Walzem RL. The health benefits of wine. Annu Rev Nutr. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- 25.Goeger DE, Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989;38:3995–4003. doi: 10.1016/0006-2952(89)90679-5. [DOI] [PubMed] [Google Scholar]
- 26.Hammer R, Giachetti A. Muscarinic receptor subtypes: M1 and M2biochemical and functional characterization. Life Sci. 1982;31:2992–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- 27.Ilno M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989;94:363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jager U, Nguyen-Duong H. Relaxant effect of trans-resveratrol onisolated porcine coronary arteries. Arzneimitlelforschung. 1999;49:207–211. doi: 10.1055/s-0031-1300403. [DOI] [PubMed] [Google Scholar]
- 29.Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982;38:427–435. doi: 10.1111/j.1471-4159.1982.tb08647.x. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick DL, Slepetis RJ, Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981;36:1245–1255. doi: 10.1111/j.1471-4159.1981.tb01724.x. [DOI] [PubMed] [Google Scholar]
- 32.Knight DE, Kesteven NT. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond Biol Sci. 1983;218:177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- 33.Leikert JF, Räthel TR, Wohlfart PV, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide release from endothelial cells. Circulation. 2002;106:1614–1617. doi: 10.1161/01.cir.0000034445.31543.43. [DOI] [PubMed] [Google Scholar]
- 34.Lim DY, Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 1991;27:53–67. [Google Scholar]
- 35.Lim DY, Kim CD, Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 1992;15:115–125. [Google Scholar]
- 36.Marley PD, McLeod J, Anderson C, Thomson KA. Nerves containing nitric oxide synthase and their possible function in the control of catecholamine secretion in the bovine adrenal medulla. J Auton Nerv Syst. 1995;54:184–194. doi: 10.1016/0165-1838(95)00013-n. [DOI] [PubMed] [Google Scholar]
- 37.Martin S, Andriambeloson E, Takeda K, Andriantsitohaina R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br J Pharmacol. 2002;135:1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Extract of wine phenolics improves aortic biomechanical properties in stroke-prone spontaneously hypertensive rats (SHRSP) J Nutr Sci Vitaminol. 1999;45:95–106. doi: 10.3177/jnsv.45.95. [DOI] [PubMed] [Google Scholar]
- 39.Naderali EK, Doyle PJ, Wlliams G. Resveratrol induces vasorelaxation of mesenteric and uterine ateries from female guinea-pigs. Clin Sci. 2000;98:537–543. [PubMed] [Google Scholar]
- 40.Naderali EK, Smith SL, Doyle PJ, Wlliams G. The mechanism of resveratrol-induced vasorelaxation differs in the mesenteric resistance arteries of lean and obese rats. Clin Sci. 2001;100:55–60. [PubMed] [Google Scholar]
- 41.Oka M, Isosaki M, Yanagihara N. Isolated bovine adrenal medullary cells: studies on regulation of catecholamine synthesis and release. In: Usdin E, Kopin IJ, Brachas J, editors. Catecholamines: Basic and Clinical frontiers. Oxford: Pergamon Press; 1979. pp. 70–72. [Google Scholar]
- 42.Orsini F, Pelizzoni F, Verotta L, Aburjai T. Isolation, synthesis, and antiplatelet aggregation activity of resveratrol 3-O-b-D-Glucopyranosideand related compounds. J Nat Prod. 1997;60:1082–1087. doi: 10.1021/np970069t. [DOI] [PubMed] [Google Scholar]
- 43.Oset-Gasque MJ, Parramon M, Hortelano S, Bosca L, Gonzalez MP. Nitric oxide implication in the control of neurosecretion by chromaffin cells. J Neurochem. 1994;63:1693–1700. doi: 10.1046/j.1471-4159.1994.63051693.x. [DOI] [PubMed] [Google Scholar]
- 44.O'Sullivan AJ, Burgoyne RD. Cyclic GMP regulates nicotine-induced secretion from cultured bovine adrenal chromaffin cells: effects of 8-bromo-cyclic GMP, atrial natriuretic peptide, and nitroprusside (nitric oxide) J Neurochem. 1990;54:1805–1808. doi: 10.1111/j.1471-4159.1990.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 45.Pace-Asciak CR, Hahn SE, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implication for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 46.Palacios M, Knowles RG, Palmer RM, Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989;165:802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- 47.Rakici O, Kiziltepe U, Coskun B, Aslamaci S, Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J Cardiol. 2005;105:209–115. doi: 10.1016/j.ijcard.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Renaud S, de Lorgeril M. Wine alcohol, platelet and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Pascual F, Miras-Portugal MT, Torres M. Effect of cyclic GMP-increasing agents nitric oxide and C-type natriuretic peptide on bovine chromaffin cell function: inhibitory role mediated by cyclic GMP-dependent protein kinase. Mol Pharmacol. 1996;49:1058–1070. [PubMed] [Google Scholar]
- 50.Rotondo S, Rajtar G, Manarinis S. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato M, Suzuki Y, Okuda T, Yokotsuka K. Content of resveratrol, piceid and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci Biotechnol Biochem. 1997;61:1800–1805. doi: 10.1271/bbb.61.1800. [DOI] [PubMed] [Google Scholar]
- 52.Schramm M, Thomas G, Towart R, Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983;303:535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz PM, Rodriguez-Pascual F, Koesling D, Torres M, Forstermann U. Functional coupling of nitric oxide synthase and soluble guanylyl cyclase in controlling catecholamine secretion from bovine chromaffin cells. Neuroscience. 1998;82:255–265. doi: 10.1016/s0306-4522(97)00274-1. [DOI] [PubMed] [Google Scholar]
- 54.Seidler NW, Jona I, Vegh N, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- 55.Slotkin TA, Whitmore WL, Dew KL, Kilts CD. Uptake of serotonin into rat platelets and synaptosomes: comparative structure-activity relationships, energetics and evaluation of the effects of acute and chronic nortriptyline administration. Brain Res Bull. 1986;17:67–73. doi: 10.1016/0361-9230(86)90162-0. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992;107:134–140. doi: 10.1111/j.1476-5381.1992.tb14475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tallarida RJ, Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed. New York: Speringer-Verlag; 1987. p. 132. [Google Scholar]
- 58.To SE, Zepf RA, Woods AG. The symptoms, neurobiology, and current pharmacological treatment of depression. J Neurosci Nurs. 2005;37:102–107. [PubMed] [Google Scholar]
- 59.Torres M, Ceballos G, Rubio R. Possible role of nitric oxide in catecholamine secretion by chromaffin cells in the presence and absence of cultured endothelial cells. J Neurochem. 1994;63:988–996. doi: 10.1046/j.1471-4159.1994.63030988.x. [DOI] [PubMed] [Google Scholar]
- 60.Uchiyama Y, Morita K, Kitayama S, Suemitsu T, Minami N, Miyasako T, Dohi T. Possible involvement of nitric oxide in acetylcholine-induced increase of intracellular Ca2+ concentration and catecholamine release in bovine adrenal chromaffin cells. Jpn J Pharmacol. 1994;65:73–77. doi: 10.1254/jjp.65.73. [DOI] [PubMed] [Google Scholar]
- 61.Uenobe F, Nakamura S, Miyazawa M. Antimutagenic effects of resveratrol against Trp-P-1. Mutat Res. 1997;373:197–200. doi: 10.1016/s0027-5107(96)00191-1. [DOI] [PubMed] [Google Scholar]
- 62.Uyama Y, Imaizumi Y, Watanabe M. Effects of cyclopiazonic acid, a novel Ca2+-ATPase inhibitor on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992;106:208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wada A, Takara H, Izumi F, Kobayashi H, Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medullary cells. Neuroscience. 1985;15:283–292. doi: 10.1016/0306-4522(85)90135-6. [DOI] [PubMed] [Google Scholar]
- 64.Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981;313:463–480. doi: 10.1113/jphysiol.1981.sp013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983;10:973–978. doi: 10.1016/0306-4522(83)90235-x. [DOI] [PubMed] [Google Scholar]
- 66.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 67.Yanagihara N, Isosaki M, Ohuchi T, Oka M. Muscarinic receptor-mediated increase in cyclic GMP level in isolated bovine adrenal medullary cells. FEBS Lett. 1979;105:296–298. doi: 10.1016/0014-5793(79)80633-x. [DOI] [PubMed] [Google Scholar]
- 68.Yanez M, Fraiz N, Cano E, Orallo F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biophys Res Commun. 2006;344:688–695. doi: 10.1016/j.bbrc.2006.03.190. [DOI] [PubMed] [Google Scholar]
- 69.Zenebe W, Pechaoova O, Andriantsitohaina R. Red wine polyphenols induce vasorelaxation by increased nitric oxide bioactivity. Physiol Res. 2003;52:425–432. [PubMed] [Google Scholar]
- 70.Zhou CX, Kong LD, Ye WC, Cheng CH, Tan RX. Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense. Planta Med. 2001;67:158–161. doi: 10.1055/s-2001-11500. [DOI] [PubMed] [Google Scholar]