Abstract
The layers of keratinocytes form an acid mantle on the surface of the skin. Herein, we investigated the effects of acidic pH on the membrane current and [Ca2+]c of human primary keratinocytes from foreskins and human keratinocyte cell line (HaCaT). Acidic extracellular pH (pHe≤ 5.5) activated outwardly rectifying Cl- current (ICl,pH) with slow kinetics of voltage-dependent activation. ICl,pH was potently inhibited by an anion channel blocker 4,4'-diisothiocyanostilbene-2,2'-disulphonic acid (DIDS, 73.5% inhibition at 1 µM). ICl,pH became more sensitive to pHe by raising temperature from 24℃ to 37℃. HaCaT cells also expressed Ca2+-activated Cl- current (ICl,Ca), and the amplitude of ICl,Ca was increased by relatively weak acidic pHe (7.0 and 6.8). Interestingly, the acidic pHe (5.0) also induced a sharp increase in the intracellular [Ca2+] (Δ[Ca2+]acid) of HaCaT cells. The Δ[Ca2+]acid was independent of extracellular Ca2+, and was abolished by the pretreatment with PLC inhibitor, U73122. In primary human keratinocytes, 5 out of 28 tested cells showed Δ[Ca2+]acid. In summary, we found ICl,pH and Δ[Ca2+]acid in human keratinocytes, and these ionic signals might have implication in pathophysiological responses and differentiation of epidermal keratinocytes.
Keywords: Keratinocyte, Extracellular pH, Anion channel, Intracellular calcium, pH-activated Cl- current
INTRODUCTION
The human skin has an acidic surface; the acidity of stratum corneum reaches to pH 4.5 and becomes normal pH at deeper layer of epidermis. The major function of the 'acid mantle' has been suggested to be an antimicrobial one. However, recent studies suggested that the acid mantle is also critical for the permeability barrier homeostasis and normal integrity (Hachem et al, 2003; Fluhr et al, 2004). Multiple mechanisms have been suggested as the origin of the acid mantle; 1) fatty acids, urocanic acids, and lactic acids generated from various enzymes (Fluhr et al, 2001), and 2) active generation of proton by Na+/H+ exchangers in keratinocytes (Behne et al, 2002).
Although the acidity such as pH 5.0 is unlikely to be present in the normal internal environment of our body, the keratinocytes might be occasionally exposed to such an acidic pH. For example, 'Sting test' is applied for screening patients with 'sensitive skin'. A fraction of the population is believed to have sensitive skin showing stinging, itching, desquamation, papules, and whealing to various skin agents (Willis et al, 2001). To identify a 'sensitive' person, a variety of test methods have been used, and the Sting test among them is widely used (Frosch et al, 1977). For the Sting test, skin reactions to 10% (w/v) lactic acid are evaluated. Since the pKa of lactic acid is 3.85, it is supposed that the Sting test might elicit further and deeper acidification of epidermis in addition to the acidic mantle in stratum corneum.
In this background, the cellular responses of keratinocytes to the acidic pH are worthwhile to be investigated, and here we focused on the membrane ion channels and intracellular Ca2+ signals. Among various ion channels, the anion channels are becoming appreciated as important players controlling cell volume, cell cycle and apoptosis (Okada et al, 2004; Lang et al, 2005; Wang et al, 2007). In the present study, therefore, we investigated the effects of acidic extracellular pH (pHe) on the anionic currents and cytoplasmic concentration of Ca2+ ([Ca2+]c) in the primary human keratinocytes and immortalized human keratinocyte (HaCaT).
METHODS
Cell culture
HaCaT cells were cultured in Dulbecco's modified Eagle's media (DMEM) supplemented with penicillin (400 U/ml), streptomycin (50 µg/ml), and 10% FBS in a humidified 5% CO2 atmosphere at 37℃. Human primary keratinocytes obtained from foreskins were cultured in keratinocyte growth media (Clonetics, San Diego, CA) primarily as described previously (Boyce et al, 1983), which was composed of MCDB 153 medium supplemented with epidermal growth factor (10 ng/ml), bovine pituitary extract (70 µg/ml), hydrocortisone (0.5 µg/ml), penicillin (100 µg/ml), streptomycin (100 µg/ml), and fungizone (0.25 µg/ml). Third-passage keratinocytes were used.
Electrophysiology
HaCaT cells were transferred into a bath mounted on the stage of an inverted microscope (IX-70, Olympus, Osaka, Japan). The bath (0.15 ml) was superfused with K+-free Tyrode's solution at 5 ml/min, and voltage clamp experiments were performed at room temperature (22~25℃). Patch pipettes with a free-tip resistance of about 2.5 MΩ were connected to the head stage of a patch-clamp amplifier (EPC-9, HEKA elektronik, Germany). Liquid junction potentials were corrected with an offset circuit before each experiment. A conventional whole-cell clamp was achieved by rupturing the patch membrane after making a giga-seal. Voltage data were low pass filtered (5 kHz), stored in a Pentium-grade computer and analyzed using Origin version 7.0 (Microcal Software Inc., Northampton, MA, USA).
Measurement of cytosolic Ca2+ concentration ([Ca2+]c)
Keratinocytes were loaded with the Ca2+-sensitive fluorescent indicator fura-2-AM (2 µM, 15 min) in K+-free Tyrode's solution at room temperature, and then the unloaded indicators were washed out with fresh solution. The recordings of [Ca2+]c, was performed with a microfluorometric system consisting of an inverted fluorescence microscope (Eclipse-2000, Nikon, Tokyo, Japan) interfaced with DeltaRAM ratiometry system (Photon Technology International, Lawrenceville, NJ, USA) (IX-70, Olympus). Light was provided by a 75-W xenon lamp (Ushino, Tokyo, Japan). A chopper wheel alternated the light path to monochromators filtering specific wavelengths of light (340 and 380 nm). As a measure of [Ca2+]c, the ratio of the fluorescence emissions at 340 nm and 380 nm excitation (R340/380) is presented.
Measurement of intracellular pH (pHi)
For the measurement of pHi, keratinocytes were exposed to acetoxymethyl ester form of 2,7-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-AM, 2 µM, K+-free Tyrode's solution) for 15 min at room temperature to load the cells with the fluorescent dye. Unloaded dye was washed twice with fresh K+-free Tyrode's solution. BCECF-loaded cells were allowed to adhere to the base of a superfusion chamber mounted on the Eclipse-2000 inverted microscope interfaced with DeltaRAM ratiometry system. Cells were excited at 488 nm and 440 nm, and emitted fluorescence was measured at 530 nm. Intracellular pH was estimated by in situ calibration of the ratios of fluorescence at 488 nm to that at 440 nm (F488/F440) according to the nigericin-high K+ method (Silver, 1998).
Solutions and chemicals
The K+-free Tyrode's solution with the following composition [(in mM) 140 NaCl, 4 CsCl, 2 CaCl2, 1 MgCl2, 5 HEPES, 5 MES, 10 glucose and 10 sucrose at pH 7.4 (titrated with NaOH)] was superfused during all whole-cell patch clamp recordings. The CsCl pipette solution contained (in mM) 140 CsCl, 5 EGTA, 10 HEPES, 1 MgCl2 and 5 MgATP at pH 7.2 (titrated with CsOH). In preliminary studies and also in the experiments shown in Fig. 1A, the CsCl pipette solution contained 130 CsCl, 20 BAPTA, 10 HEPES, 1 MgCl2 and 5 MgATP at pH 7.2 (titrated with CsOH). The activation of ICl,pH was not different between these two conditions. For NMDG-Cl pipette solution, CsCl was totally replaced with equimolar NMDG-Cl. DIDS and Niflumic acid were purchased from Sigma (St. Louis, MO). Cell culture media, antibiotics and fetal bovine serum (FBS) were purchased from Gibco. The calcium-sensitive indicator Fura-2AM was obtained from Molecular Probes (Carlsbad, CA, USA).
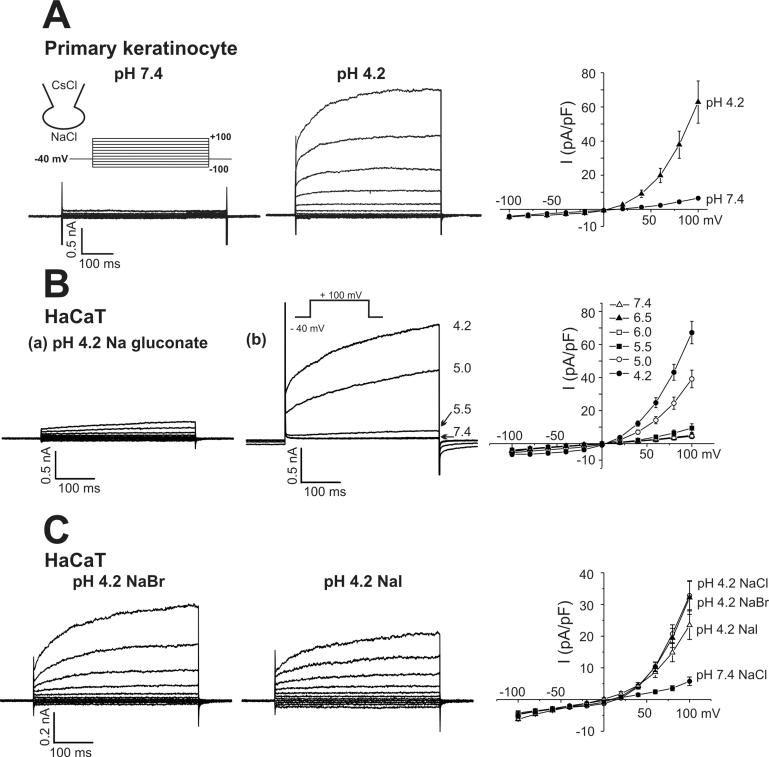
Fig. 1.
Activation of outwardly rectifying Cl- current by acidic pHe. Representative current traces obtained from primary keratinocytes (A) and HaCaT cells (B) by step-like pulses. The membrane voltage was held at -40 mV, and incremental step-like pulses from -100 to 100 mV (20 mV intervals, 400 ms duration, see inset). The currents (B-a) were obtained with Nagluconate solution in the bath (pH 4.2). (B-b) Representative current traces obtained by step-like pulses under different pHe. The current to voltage relations (I-V curves), measured at the end of each pulse, are shown in right panels. Step like pulses were applied in different conditions of extracellular anions (pH 4.2 Br-, and I-). Current traces presented in (C) were obtained from the same cell, respectively. I-V curves of ICl,pH obtained in NaCl, NaBr, and NaI solutions are shown in the right panel of (C) Each symbol represents mean±SEM of current amplitudes normalized to cell capacitance (pA/pF).
Statistics
Statistical significance was determined using the Student's t-test. Results are presented as means±SEM. All p-values quoted are two-tailed and significance was accepted when p<0.05.
RESULTS
Acidic pHe-activated anionic current in human primary keratinocytes
We first examined the effect of pHe on the membrane current of human primary keratinocyte using CsCl pipette solution in the whole-cell configuration. Currents were elicited by stepping from a holding potential of -40 mV to a series of test potentials from -100 mV to +100 mV in 20 mV increments. In control condition (pH 7.4), the membrane conductance was very small (7±0.97 pA/pF at 100 mV, n=6). By changing pHe from 7.4 to 4.2, an immediate increase of membrane conductance was observed. The acidic pHe-induced-current showed outwardly rectifying current-voltage relation (I-V curve) and time-dependent activation at depolarizing voltages (Fig. 1A). The same type of outwardly rectifying current was induced by acidic pHe in HaCaT cells (Fig. 1B). To elucidate the acid-sensitivity of ICl,pH in HaCaT cells, pHe was changed to 6.5, 6.0, 5.5, 5.0 and 4.2 (Fig. 1B (b)). A discernible activation of ICl,pH was observed from pHe 5.5 and below.
The acidic pHe-induced-current was largely abolished by replacing Cl- with 145 mM gluconate in the bath solution (Fig. 1B(a)) whereas unaffected by total replacement of intracellular and extracellular monovalent cations with NMDG+ (data not shown). Such results indicated that the acidic pHe activated Cl- current (ICl,pH). Hereafter, further characterization of ICl,pH was performed in HaCaT cells with K+-free Tyrode's bath solution and CsCl pipette solution. The ICl,pH channels were also permeable to other halide anions (Br- and I-) as well as to Cl-. The outwardly rectifying currents were similarly acivated when extracellular Cl- was replaced with Br- or I- (Fig. 1C). From the observed reversal potentials of I-V curves (-8.0±1.98 mV, 7.3±1.90 mV and 13.4±2.42 mV, for Cl- (n=8), Br- (n=8), I- (n=5), respectively), it could be concluded that the permeability for the ICl,pH channel is I-≥Br-≥Cl-.
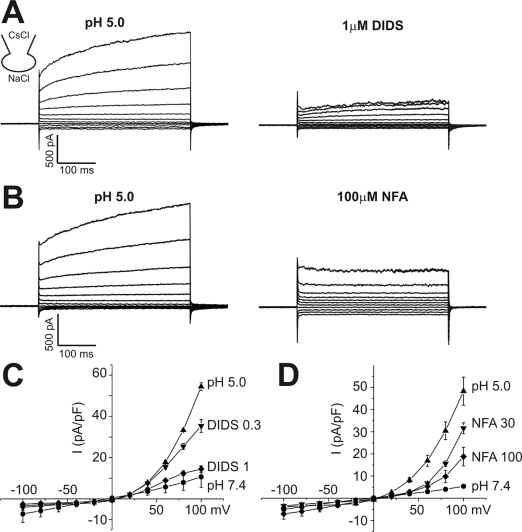
The effects of pharmacological blockers on ICl,pH was tested at pHe 5.0. ICl,pH was sensitively inhibited by 4,4'-diisothiocyanostilbene-2,2'-disulphonic acid (DIDS, 73.5% inhibition at 1 µM, n=5, Fig. 2). Niflumic acid, another class of anion channel blocker, showed only partial inhibition of ICl,pH (62.2% of inhibition at 100 µM, n=5, Fig. 2). The other type of anion channel blocker, NPPB, was not tested because NPPB became precipitated at pH 5.0.
Fig. 2.
Effects of anion channel blockers on ICl,pH. (A, B) Representative current traces obtained by step-like pulses, same as in Fig. 1. ICl,pH was activated by pHe 5.0, and DIDS (1 µM) or niflumic acid (NFA, 100 µM) was added (right panels). (C, D) I-V curves of ICl,pH activated by pHe 5.0 and effects of DIDS (0.3 µM and 1 µM) or niflumic acid (30 µM and 100 µM). Each symbol represents mean±SEM (n=5, respectively) of current amplitudes normalized to the cell capacitance (pA/pF).
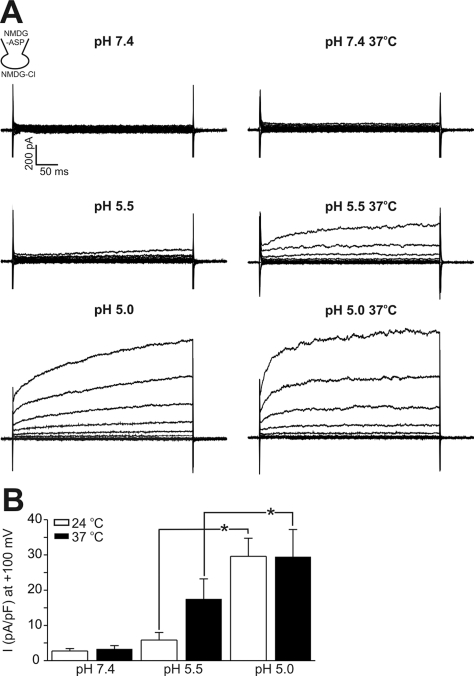
Although keratinocytes are lying at the outermost surface of human body, the epidermal temperature might increase close to the core temperature or above, depending on the environmental situation. Therefore, we tested whether the pH threshold of ICl,pH activation could be altered by raising the bath temperature (Fig. 3). To exclude the activation of other ionic conductance (e.g. thermosensitive nonselective cationic current), cations in the bath and pipette solutions were replaced with NMDG+ (symmetrical NMDG-Cl solution). Also, the pH of warmed NMDG-Cl solution was separately titrated to 5.5 at 37℃, because the pH could be changed by raising the bath temperature. Again, only small amplitudes of ICl,pH were induced by pHe 5.5 at room temperature (24℃), which was 17.7% of ICl,pH at pHe 5.0. In contrast, a substantial amplitude of ICl,pH was induced by pHe 5.5 at 37℃, which was 52.7% of the ICl,pH induced by pHe 5.0. The amplitude of ICl,pH induced by pHe 5.0 was not different between recordings under 24 and 37℃, although the activation kinetics on step depolarization became faster (Fig. 3).
Fig. 3.
Effects of bath temperature on ICl,pH activation in HaCaT cells. (A) Representative current traces obtained by step like pulses, same as in Fig. 1. Current traces at pH 7.4, 5.5, and 5.0 were recorded under room temperature (24℃, left panels) and 37℃ (right panels). The raise of temperature alone had insignificant effect on membrane conductance, whereas substantial increase was shown at pH 5.5. At pH 5.0, high temperature increased the activation kinetics with similar peak amplitude. All current trances shown were obtained from the same cell. (B) Mean amplitudes of normalized ICl,pH (pA/pF) measured at 100 mV under different pHe and temperature were compared (n=8) *indicate p-value<0.05.
Augmentation of Ca2+-activated Cl- current by acidic pH
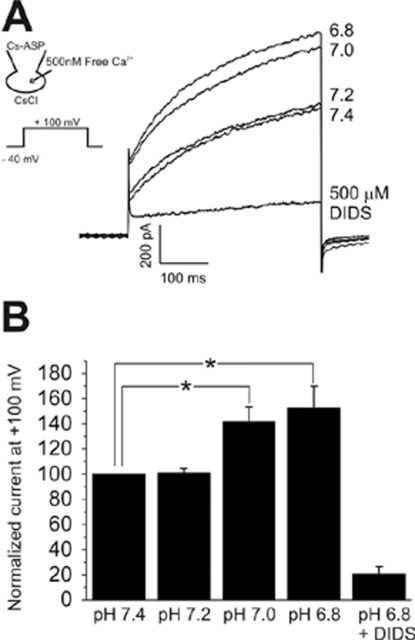
In keratinocytes, the expression of Ca2+-activated Cl- current (ICl,Ca) has previously been reported (Koegel and Alzheimer, 2001). Therefore, we also tested the effects of acidic pHe on ICl,Ca by using CsCl pipette solution with 500 nM free Ca2+. Under this condition, the step depolarizing pulses revealed outwardly rectifying currents with time-dependent activation kinetics, which was consistent with the known properties of ICl,Ca. The amplitudes of ICl,Ca was also increased by acidic pHe. The augmentation of ICl,Ca was observed from pH 7.0, which was significantly more sensitive than the activation of ICl,pH (Fig. 4). pH below 5.5 was not tested for ICl,Ca due to the additional activation of ICl,pH. Although the overall shapes of currents were similar between ICl,Ca and ICl,pH, the comparison of I-V curves showed that ICl,Ca has relatively larger inward current at negative voltages. As for the pharmacological sensitivity, ICl,Ca was much less sensitive to DIDS than ICl,pH; no inhibition by 5 µM DIDS, and 500 µM of DIDS was required to inhibit 83% of ICl,Ca (Fig. 4B, n=8). We also tested and found that niflumic acid had only a weak inhibitory effect on ICl,Ca (inhibition by 43% at 100 µM, n=3, data not shown).
Fig. 4.
Ca2+-activated Cl- current (ICl,Ca) of HaCaT cells and effects of acidic pHe. (A) Outward currents with slowly activating kinetics were induced by dialyzing cells against Ca2+-clamped CsCl solution (see inset). Lowering pHe to 7.0 and 6.8 increased the amplitude of outward current. After testing pHe effects, the inhibition of outward current by 500 µM DIDS was confirmed at pHe 6.8. (B) Summary of the effects of pHe on ICl,Ca of HaCaT cells. In each cell, the current amplitudes measured at the end of step-pulse were normalized to the control amplitude (pHe 7.4), and mean±SEM values were plotted (n=8). *indicate p-value<0.05.
Activation of PLC-mediated store Ca2+ release by acidic pHe
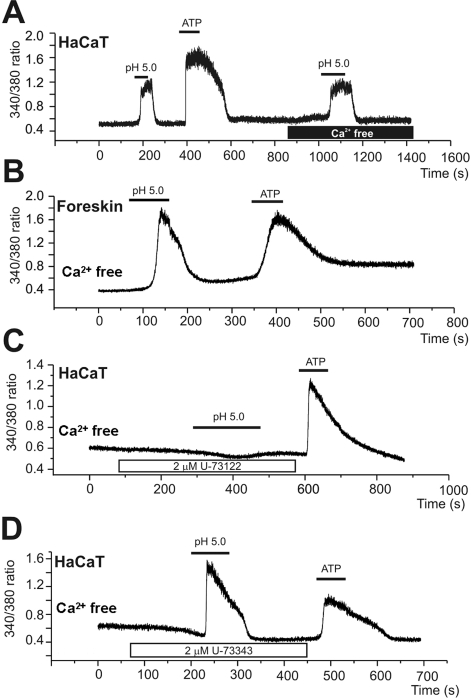
Finally, we spectrofluorimetrically examined the effects of acidic pHe on [Ca2+]c by using fura-2. The change of bath pH from 7.4 to 5.0 elevated [Ca2+]c in a reversible manner (Fig. 5). After returning to the control pH, the application of 100 µM ATP induced similar increase in [Ca2+]c (Fig. 5A). The acid-induced increase of [Ca2+]c (Δ[Ca2+]acid) was still observed in the absence of extracellular Ca2+, indicating the release from intracellular Ca2+ stores (Fig. 5A). The most common pathway of stored Ca2+ release is phospholipase C (PLC)-dependent generation of IP3 and subsequent activation of IP3 receptors in endoplasmic reticulum. In the present study, the Δ[Ca2+]acid was completely and reversibly suppressed by the pretreatment with 2 µM U73122, an inhibitor of PLC, whereas not by a negative analogue U73343 (Fig. 5B and C). The Δ[Ca2+]acid was consistently observed in HaCaT cells (19 out of total 20 tested HaCaT cells), whereas the proportion of cells showing [Ca2+]acid was only 18% in the primary human keratinocytes (5 out of 28 tested cells, Fig. 5B).
Fig. 5.
Acidic pHe-induced increase of [Ca2+]c (Δ[Ca2+]acid). (A, B) Representative traces of the fluorescence ratio (F340/380) and the responses of HaCaT (A) and primary keratinyocyte (B) to pHe 5.0 and ATP (100 µM). The Δ[Ca2+]acid was persistent in the absence of extracellular Ca2+ (A). (C, D) Inhibition of Δ[Ca2+]acid by PLC inhibitor (U73122, 2 µM)), whereas no effect of the negative analogue (U73343, 2 µM).
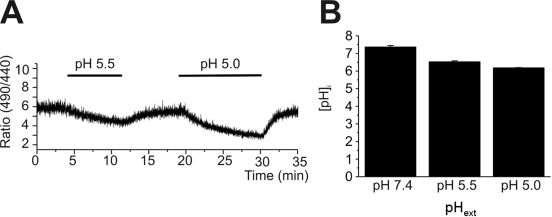
From the above results, it was asked whether the acute responses, such as ICl,pH and Δ[Ca2+]acid, might have been due to changes in intracellular pH. Therefore, we tested how fast the acidic pHe changes the intracellular pH (pHi), by using BCECF fluorescence ratio (R490/440). In contrast with the almost immediate activation of ICl,pH and Δ[Ca2+]acid, the change of pHi in response to acidic pHe was slow and did not reach a steady-state until 10 min after changing pHe (Fig. 6).
Fig. 6.
Slow decrease of intracellular pH (pHi) in response to extracellular acidification. (A) A representative trace of BCECF emission ratio (F490/440) measured in HaCaT cell. pHe was changed from 7.4 to 5.5. or to 5.0 as indicated. (B) Summary of calibrated pHi (mean±SEM, n=8) of control (pHe 7.4) and 10 min after pHe changes (5.5 and 5.0).
DISCUSSION
In this study, we examined the effects of acidic pHe on the anion channels and [Ca2+]c in human keratinocytes. Relatively strong acidic pHe (<5.5) directly activated ICl,pH, and the pH threshold was lowered by raising bath temperature. Although the less acidic conditions (pHe 7.0 and 6.8) did not directly activate ICl,pH, they nevertheless increased the amplitude of ICl,Ca. Interestingly, the strong acidic pH (5.0) also activated PLC-dependent store Ca2+ release in HaCaT cells, although the Δ[Ca2+]acid was not consistently found in the human primary keratinocytes.
Acidic pH-activated Cl- currents
The acidic pH-activated outward rectifying anion current similar to ICl,pH was firstly reported in rat Sertoli cells (Auzanneau et al, 2003) and subsequently in various types of cell lines (HEK-293, CHO, HeLa, PC-12, Caco-2 and HBE), hippocampal astrocytes and cardiac myocytes (Nobles et al, 2004; Lambert et al, 2005; Yamamoto et al, 2006; Wang et al, 2007). The properties of ICl,pH in human keratinocytes are very similar to those reported in the above literatures; activation at pHe below 5.5 (room temperature), time-dependent kinetics, outward rectification, and high sensitivity to DIDS blocking (Nobles et al, 2004; Lambert et al, 2005; Yamamoto et al, 2006; Wang et al, 2007). In this respect, ICl,pH appears to be broadly expressed in mammalian cells, like volume-activated Cl- current (ICl,vol). Actually, ICl,pH and ICl,vol seem to share some properties and to interconvert at combined hypotonicity and acidic pH; i.e. different manifestations of the same channel (Nobles et al, 2004). However, more recent study by Lambert & Oberwinkler (2005) showed that ICl,pH and ICl,Vol are distinct populations of anion channels with different properties such as Mg2+ sensitivity, steepness of I-V relation, voltage-dependent inactivation and some pharmacological sensitivity. Unfortunately, the properties of ICl,pH do not match with any of the cloned Cl- channels, and the molecular identity of this widely expressed channel remains to be elucidated.
Although widely expressed, ICl,pH seems to have physiological implication only under limited situations since the in vitro activation of ICl,pH occurs at very acidic pH such as 5.0 or below. Even in the inflammatory sites where local accumulation of lactic acid and short chain fatty acids produce acidic environment, the pH of exudates is above 6.0 (Menkin, 1958). Therefore, the in vivo activation of ICl,pH would be possible only at extreme inflammation and ischemia that lead to cell death. In this respect, the presence of ICl,pH in keratinocytes could have intriguing physiological implication. As mentioned in Introduction, human skin forms 'acid mantle' near the surface of epidermis, and skin is frequently exposed to chemical substances with acidic pH like cosmetics with preservatives and lactic acid solution (pH, ~4.0) used for 'Sting test' (Frosch et al, 1977). Although it is not certain whether the pH of deeper epidermis can be actually lowered to below pH 5.0, it is likely that keratinocytes in vivo might be exposed to the threshold pH to activate ICl,pH, especially when combined with raised temperature.
Facilitation of ICl,Ca by acidic pH
In contrast to the pH threshold for ICl,pH, the augmentation of ICl,Ca was observed at less acidic pHe. Previous studies in other cells also showed that alkaline pHe decreases ICl,Ca [18] and acidic pHe enhances ICl,Ca (Hirayama et al, 2002). Therefore, when combined with the [Ca2+]c activating stimuli (e.g. ATP), the acidic pHe would facilitate the anionic conductance to increase, subsequently evoking various cellular responses such as volume changes. The enhancement of ICl,Ca by less acidic pHe would contribute to the increase of anionic conductance of keratinocytes in broad ranges of pHe.
Acidic pH induces release of stored Ca2+
The recruitment of stored Ca2+ by acidic pHe (Fig 5) might have physiological implication with regard to the interplay between epidermal pHe gradient and the Ca2+-mediated differentiation of keratinocytes. It is well known that the proliferation and differentiation of keratinocytes in epidermis are regulated by Ca2+ signals. For examples, changes in the concentration of extracellular calcium affect the balance between proliferation and differentiation in epidermal keratinocytes; elevation of the extracellular calcium concentration (calcium switch) inhibits proliferation and induces the onset of terminal differentiation (Yuspa et al, 1989; Menon et al, 1992). Ca2+ sensing receptors (CaSR) coupled with PLC/InsP3 pathway and Ca2+-selective TRPV6 channels in keratinocytes have been suggested as candidate mechanisms mediating the 'calcium switch' into the increase of [Ca2+]c (Tu, 2001; Lehen'kyi et al, 2007). Although still remains controversial, it is suggested that the epidermis has a calcium gradient, with the lowest concentrations in the stratum basal and the highest in the stratum granulosum (Elias et al, 2002).
The inhibition of Δ[Ca2+]acid by U73122 suggests that PLC/IP3 pathway is activated during acidic stimulus. However, it is still unclear whether a specific type of G protein-coupled receptor is activated or the pHe change somehow directly activates PLC in keratinocytes. The decrease of pHi by external acidification occurs very slowly and incompletely (Fig. 6). Therefore, it is not likely than acidic pHe activates the PLC/InsP3 by intracellular acidification. The phenomenon of InsP3-mediated Ca2+ release evoked by acidic pHe has previous been reported in human fibroblasts, coronary endothelium, neuroblastoma and umbilical smooth muscle cells (Smith et al, 1989). Recently, a group of proton-activated GPCRs, called OGR1, G2A and TDAG8, has been reported (Ludwig et al, 2003; Murakami et al, 2004; Ishii et al, 2005). Among them, OGR1 and G2A are coupled with PLC/InsP3-mediated Ca2+ release pathway (Ludwig et al, 2003; Murakami et al, 2004). However, compared with the pH-dependence of Δ[Ca2+]acid in keratinocytes, the activation of OGR1 and G2A occurs at much higher pHe ranges (7.2~6.8), where the [Ca2+]c of keratinocytes does not change. Therefore, different type of pHe-sensing mechanism(s) or GPCR seems to be involved in Δ[Ca2+]acid of keratinocytes.
In summary, we found functional expression of ICl,pH in human keratinocytes and confirmed the enhancement of ICl,Ca by acidic pHe. Also, the strong acidic pH evokes PLC-dependent store Ca2+ release in human keratinocytes. Differing from other tissues in the body, epidermal keratinocytes are likely to be exposed to strong acidic environment. The specific roles of ICl,pH and Δ[Ca2+]acid in keratinocytes physiology would be interesting topics of future study.
ACKNOWLEDGEMENT
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R11-2007-040-01003-0) and by a research agreement with AMOREPACIFIC Corporation, Korea
ABBREVIATIONS
- ICl,pH
acidic pH-activated Cl- current
- Δ[Ca2+]acid
acidic pH-induced intracellular Ca2+ increase
- ICl,Ca
Ca2+-activated Cl- current
- pHe
extracellular pH
References
- 1.Auzanneau C, Thoreau V, Kitzis A, Becq F. A novel voltage-dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J Biol Chem. 2003;278:19230–19236. doi: 10.1074/jbc.M301096200. [DOI] [PubMed] [Google Scholar]
- 2.Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, Clegg RW, Gratton E, Holleran WM, Elias PM, Mauro TM. NEH1 regulates the stratum corneum permeability barrier homeostasis. J Biol Chem. 2002;277:47399–47406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- 3.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 4.Elias PM, Ahn SK, Brown BE, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002;119:1269–1274. doi: 10.1046/j.1523-1747.2002.19622.x. [DOI] [PubMed] [Google Scholar]
- 5.Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117:44–51. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 6.Fluhr JW, Mao-Qiang M, Brown BE, Hachem JP, Moskowitz DG, Demerjian M, Haftek M, Serre G, Crumrine D, Mauro TM, Elias PM, Feingold KR. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 2004;123:140–151. doi: 10.1111/j.0022-202X.2004.22726.x. [DOI] [PubMed] [Google Scholar]
- 7.Frosch P, Kligman AM. Method for appraising the sting capacityof topically applied substances. J Soc Cosmetic Chem. 1977;28:197–209. [Google Scholar]
- 8.Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirayama Y, Kuruma A, Hiraoka M, Kawano S. Calcium-activated Cl- current is enhanced by acidosis and contributes to the shortening of action potential duration in rabbit ventricular myocytes. Jpn J Physiol. 2002;52:293–300. doi: 10.2170/jjphysiol.52.293. [DOI] [PubMed] [Google Scholar]
- 10.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 2005;280:9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 11.Koegel H, Alzheimer C. Expression and biological significance of Ca2+-activated ion channels in human keratinocytes. FASEB J. 2001;15:145–154. doi: 10.1096/fj.00-0055com. [DOI] [PubMed] [Google Scholar]
- 12.Lambert S, Oberwinkler J. Characterization of a proton-activated, outwardly rectifying anion channel. J Physiol. 2005;567:191–213. doi: 10.1113/jphysiol.2005.089888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang F, Föller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- 14.Lehen'kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, Prevarskaya N. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 2007;282:22582–22591. doi: 10.1074/jbc.M611398200. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker W, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 16.Menkin V. Chemical mediators in relation to cytologic constituents in inflammation. Am J Pathol. 1958;34:921–941. [PMC free article] [PubMed] [Google Scholar]
- 17.Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992;270:503–512. doi: 10.1007/BF00645052. [DOI] [PubMed] [Google Scholar]
- 18.Murakami N, Yokomizo T, Okuno T, Shimizu T. G2A is a proton-sensing G-protein-coupled receptor antagonized by lysophosphatidylcholine. J Biol Chem. 2004;279:42484–42491. doi: 10.1074/jbc.M406561200. [DOI] [PubMed] [Google Scholar]
- 19.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 20.Nobles M, Higgins CF, Sardini A. Extracellular acidification elicits a chloride current that shares characteristics with ICl(swell) Am J Physiol Cell Physiol. 2004;287:C1426–C1435. doi: 10.1152/ajpcell.00549.2002. [DOI] [PubMed] [Google Scholar]
- 21.Okada Y, Maeno E, Shimizu T, Manabe K, Mori S, Nabekura T. Dual roles of plasmalemmal chloride channels in induction of cell death. Pflugers Arch. 2004;448:287–295. doi: 10.1007/s00424-004-1276-3. [DOI] [PubMed] [Google Scholar]
- 22.Silver RB. Ratio imaging: practical considerations for measuring intracellular calcium and pH in living tissue. Methods Cell Biol. 1998;56:237–251. doi: 10.1016/s0091-679x(08)60429-x. [DOI] [PubMed] [Google Scholar]
- 23.Smith JB, Dwyer SD, Smith L. Lowering extracellular pH evokes inositol polyphosphate formation and calcium mobilization. J Biol Chem. 1989;264:8723–8728. [PubMed] [Google Scholar]
- 24.Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001;276:41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 25.Wang HY, Shimizu T, Numata T, Okada Y. Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflugers Arch. 2007;454:223–233. doi: 10.1007/s00424-006-0193-z. [DOI] [PubMed] [Google Scholar]
- 26.Willis CM, Shaw S, Lacharriere ODE, Baverel M, Reiche L, Jourdain R, Bastien P, Wilkinson J. Sensitive skin: an epidemiological study. Br J Dermatol. 2001;145:258–263. doi: 10.1046/j.1365-2133.2001.04343.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Ehara T. Acidic extracellular pH-activated outwardly rectifying chloride current in mammalian cardiac myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H1905–H1914. doi: 10.1152/ajpheart.00965.2005. [DOI] [PubMed] [Google Scholar]
- 28.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]