Abstract
The influence of alpha-fetoprotein (AFP) on the bone marrow (BM) natural suppressor (NS) cells of intact Ehrlich carcinoma -bearing CBA mice was studied. Bone marrow NS cells were fractionated into three fractions by isopycnic centrifugation on percoll gradients: NS1 (ρ=1.080 g/ml), NS2 (ρ=1.090 g/ml) and NS3 (1.100>ρ>1.090 g/ml). These fractions were highly different in their sensitivity to known NS cell inductors (interleukin (IL)-2, IL-3 or histamine). None of the NS fractions isolated from the intact mice spontaneously produced antiproliferative activity, however, they showed a high level of NS (antiproliferative and natural killer cell inhibitory) activity under the influence of AFP. A single injection of AFP to intact mice led to an increase of spontaneous NS activity and the inhibition of natural killer cell activity. NS activity, especially NS2, was increased in when tumor cells were subcutaneously inoculated three days after AFP injection. In the AFP-treated mice, the tumor mass at 14 days was 60% larger than that in the untreated mice. Our data confirmed that AFP is a tumor marker that can inhibit cancer immunity and plays a role in cancer pathogenesis.
Keywords: Alpha-fetoprotein (AFP), Natural suppressor (NS) cells, Natural killer cells, Ehrlich carcinoma (EC)
INTRODUCTION
Alpha-fetoprotein (AFP) is a well-known tumor marker, and its production has been shown to correlate with the development of hepatocarcinoma, embryonic carcinoma, tumors of the yolk sac, teratocarcinoma, some stromal tumors of the testis and ovary, hepatoblastoma, and flat-cellular liver cancer (Abelev, 1993). One of the most important functions of AFP is its immunosuppressive activity (Cardoso et al, 1991; Chakraborty and Mandal, 1993; Semeniuk et al, 1999), and such activity may be important in the inhibition of cancer immunity during tumor development. However, the cellular and molecular mechanisms for the immunosuppressive activity of AFP are not known.
We hypothesized that AFP participates in natural killer (NK) cell inhibition during tumor development by stimulating of the so-called natural suppressor (NS) cells. NS cells belong to the class of immature hematopoietic progenitors that non-specifically inhibit various immune responses (Sykes and Strober, 1999). The likelihood of bone marrow (BM) and spleen NS cells to suppress immune function is significantly augmented during the development of cancer and experimental tumors (Subiza et al, 1989; Young et al, 1992; Yamaguchi et al, 1994).
In this study, we examined the direct influence of AFP on three subpopulations of NS cells obtained from the BM of mice, and found that AFP induced anti-proliferative and NK inhibitory activity. A single injection of AFP led to an increase of spontaneous NS activity of intact mice and inhibition of NK cell activity, thereby significantly accelerating the growth of Ehrlich carcinomas (EC). Our data suggest that AFP in AFP-producing tumors, can activate NS cells, thereby inhibiting cancer immunity and enhancing tumor progression.
METHODS
Animals
CBA male mice (8~10 week old) were used in the experiments.
Reagents
Percoll, RPMI 1640 medium, histamine dihydrochloride, sodium bicarbonate, L-glutamine, gentamycin, DMSO, and MTT were purchased from Sigma (St. Louis, MO, USA). Human rIL-2 was purchased from Biotech Co (Saint-Petersburg, Russia). WEHI-3BD cell conditioned medium which was tested on IL-3 activity was prepared in our laboratory. Fetal calf serum (FCS) was purchased from Sigma (St. Louis, MO, USA). [3H] Uridine was purchased from Isotop Co (Saint-Petersburg, Russia).
Purification of AFP
AFP was obtained from human cord-placental blood serum as described earlier (Sinenko et al, 1999) by two-step affinity chromatography: 1) on rabbit anti-AFP antibodies, and 2) on rabbit antibodies against adult human serum proteins.
Cell lines
PAI, WEHI-3BD, K-562, and FDC-P1 Muv127 cell lines were maintained in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine and 30 µg/ml gentamicin under standard cell culture conditions.
Preparation of IL-3 activity
Exponentially growing WEHI-3BD cells were cultivated for 48 h in the absence of FCS at 37℃ and 5% CO2. Cell free culture supernatant was tested for IL-3 activity by an IL-3 dependent FDC-P1 Muv127 cell line and stored at -70℃ before use.
BM separation on percoll density gradients
BM cells were obtained from the femurs and tibias by flushing as described elsewhere (Subiza et al, 1989). BM cells were separated by isopycnic centrifugation for 20 min at 400 g on discontinuous percoll gradients consisting of five steps with the following buoyant densities: 1.033, 1.060, 1.076, 1.090, and 1.100 g/ml (Belyaev et al, 1993). For preparation of the percoll densities desired, we followed the methods described in the Pharmacia Fine Chemicals AB brochure (Uppsala, Sweden). After centrifugation, the cells were subdivided into six fractions, with the last fraction remaining at the bottom of the tube. The cells from the interface between 1.076 and 1.090 g/ml were collected, washed and additionally separated on percoll gradients consisting of two steps: 1.90 and 1.080 g/ml. Three cell fractions with buoyant densities of 1.080, 1.090 and >1.090 (tube bottom) g/ml were collected, washed, calculated, and used as NS1, NS2 and NS3 fractions.
Estimation of NS cell suppressive activity
BM cells (2.0×106/ml), separated on percoll gradients, were incubated at 37℃ and 5% CO2 in the presence of one of the inducers (IL-2, IL-3, histamine, or AFP) for 3 h at the following concentrations: rIL-2 at 50 IU/ml, IL-3 activity at two thirds of total culture medium volume and 10-5 M histamine, and 100 µg/ml AFP. The cells were then centrifuged at 150 g for 10 min. The culture medium was replaced with fresh medium and the cells were cultivated for another 48 h. The cell free culture supernatants were added to the mouse myeloma cells PAI (5.0×104/ml) at a ratio of 1:1 (v/v) and cultivated for 24 h. Four hours before the end of incubation, MTT solution was added to a final concentration of 0.5 mg/ml (Carmichael et al, 1987). The culture medium was then removed and crystals of formazan were solubilized in DMSO. The absorbancy of the converted dye was measured at 570 nm. The untreated myeloma cells were used as a control. The suppressive activity (SA) of the culture supernatants was calculated as follows:
SA=[(Control Test)/Control]×100 (%)
Determination of NK activity
NK activity of the mononuclear cells was estimated by a direct cytotoxic assay, using K562 cells as a target. Thus, K562 cells were labeled with 3H-Uridine (3 µCi/ml) for 12 h under standard cell culture conditions. Mononuclear cells as a source of NK cells were separated from the spleen cell suspension by centrifugation on a percoll gradient (ρ=1.065 g/ml) for 20 min at 1,400 g and 4℃. The cells were then washed and incubated on plastic Petri dishes for 2 h at 37℃ to remove adherent cells. The remaining non-adherent cells were adjusted to 107 cells/ml and incubated in the presence of equal volumes of NS cell supernatants or culture medium for 16 h. After incubation, the cells were centrifuged, washed and adjusted to 5.0×106 cells/ml. Next, 100 µl of both effector and target cells (labeled K562) were mixed at a ratio of 50:1 and then incubated for 4 h in round-bottomed microtiter plates under standard cell culture conditions. The cells from each well were carefully removed and transferred onto GF filters (Schleicher and Schuell, Dassel, Germany). The filters were washed consecutively in PBS (5 mM NaH2PO4, and 150 mM NaCl, pH 7.4), 5% TCA and 80% ethanol, and then dried. The radioactivity retained by the non-lysed cells was determined using a liquid scintillation β-counter (Beckman, USA). K562 cells supplied with cell culture medium, instead of the effector cells, were used as a control. The NK cytolytic activity (CLA) was calculated as follows:
CLA=[(Control Test)/Control]×100 (%)
The relevance of human cell line (K562) for estimation of NK activity in mice was confirmed by a Cellular DNA Fragmentation ELISA kit (Boehringer Mannheim GmbH, Germany). The correlation of the CLA estimated by the two methods (radioactive and non-radioactive) was r=0.96 (not shown).
In vivo studies of AFP influence
The in vivo experiments were performed using four groups of mice, with six mice per group: 1) 3 days after subcutaneous AFP injection (100 µg), 2) bearing 14-day EC, 3) bearing 45-day EC, and 4) bearing 14-day EC after subcutaneous AFP injection. The mice bearing EC were prepared by a single subcutaneous inoculation of 105 EC cells (Subiza et al, 1989). The BM cells obtained from each animal within a group were mixed before the cell separation procedure.
Statistics
The experiments were performed at least three times and representative data (average value±SD) are presented. The Student's t test was used to determine the significance of the differences between experimental values.
RESULTS
Suppressive activities of different NS fractions
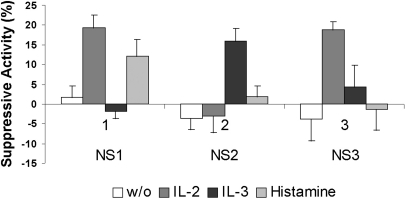
None of the NS1, NS2 and NS3 fractions showed any spontaneous production of NS factors to inhibit myeloma cell culture growth (Fig. 1). The fractions however, the fractions differed in their response to the inducers. The NS1 cells produced suppressive factors only under the influence of IL-2 or histamine, the NS2 cells responded only to IL-3, and the NS3 cells were sensitive only to IL-2, but not to histamine. These data suggest that different NS cell fractions have different suppressive activities.
Fig. 1.
Production of suppression factors (suppressive activity) by three NS cell fractions with and without (w/o) influence of inducers. Data are shown as mean±SD.
AFP induces NS activity
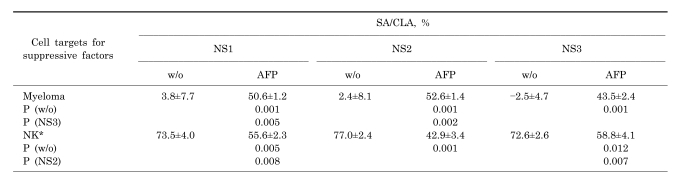
As shown in Table 1, AFP induced significant suppressive activity in all the NS fractions. NS3 activity was lower than bothNS1 and NS2. NK inhibitory activity was not observed in the untreated NS fractions, whereas it occurred only under the influence of AFP. In the presence of AFP, NS2 cells produced significantly more NK inhibitory activity than NS1 or NS3 cells did.
Table 1.
Antiproliferative and NK inhibitory activity of NS cell fractions. Suppressive activity of different NS cell fractions either under influence or without (w/o) influence of AFP, which inhibits both myeloma cell proliferation (SA) and NK cytolytic activity (CLA)
*CLA of NK cells without any influence was 73.8±1.7%. Cytolytic activity (CLA) was calculated as follows: CLA=[(Control Test)/Control]×100 (%). Data are shown as mean±SD.
NK activity 3 days after a single AFP injection
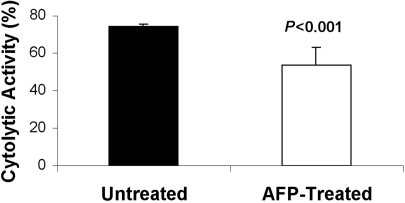
The effect of AFP on the cytolytic activity of NK cells was studied 3 days after a single AFP injection. Fig. 2 shows that there was a significant reduction of cytolytic NK activity (p<0.001) 3 days after a single injection of AFP (100 µg) into mice (n=6).
Fig. 2.
Cytolytic activity of spleen NK cells obtained from untreated and AFP-treated mice. Data are shown as mean±SD.
Effect of AFP injection and tumor growth on the suppressive activity of NS cell fractions
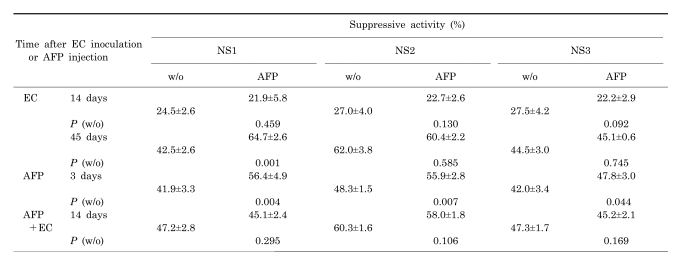
As shown in Table 2, subcutaneous inoculation of EC induced the ability of NS cells to spontaneously produce suppressive factors. Suppressive activity was increased as the tumor grew, whereas NS cells failed to respond to AFP. Similar activity of NS cells was also observed in intact the mice 3 days after a single injection of AFP. However, all three NS cells could increase their suppressive activity in response to AFP stimulation. Inoculation of EC cells into mice which had been pretreated with AFP showed increase of spontaneous activity of all NS fractions, and this increase was much more than that observed in the absence of AFP. In this case, the loss of sensitivity of all NS fractions to AFP was observed.
Table 2.
Spontaneous or AFP-induced NS activity after EC cell inoculation or AFP injection. Suppressive activity (SA) to inhibit myeloma cell proliferation by different NS cell fractions obtained from EC bearing - or intact mice after a single AFP injection
Data are shown as mean±SD.
Acceleration of tumor growth in mice pretreated with AFP
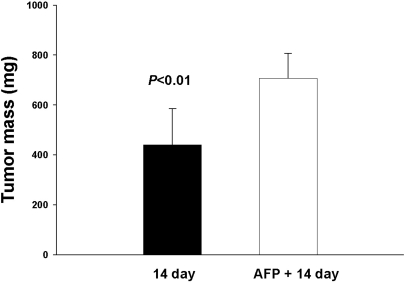
We also examined the effect of AFP injection into mice on the growth of subcutaneous EC. As shown in Fig. 3, pretreatment of mice with AFP accelerated EC growth by approximately 60% at the 14th day.
Fig. 3.
Influence of a single AFP injection on Ehrlich carcinoma (EC) growth. Tumor mass was observed with and without AFP injection after EC inoculation at the 14th day. Data are shown as mean±SD.
DISCUSSION
Convincing evidence for a negative role of NS cells in tumor progression has been described (Subiza et al, 1989; Young et al, 1992; Yamaguchi et al, 1994). However, many studies in this field are limited by the absence of satisfactory methods to obtaining, identify and estimate NS cell activity. The phenotype heterogeneity of NS cells also causes additional difficulties. According to earlier studies, at least two NS cell populations belong to myelomonocytic precursors. One of them is activated with IFN- and IL-2 (Holda et al, 1986; Angulo et al, 1995), whereas the other is activated by exposure to IL-3 and GM-CSF (Young et al, 1990; Moore et al, 1992). In the present work, we successfully obtained three NS cell fractions, and each fraction showed different sensitivity to the inducers tested (IL-2, IL-3 and histamine). We also showed that the separation method of NS cells herein, described was suitable not only for BM cells from different mice and rat strains, but also for rodent spleen cells and human peripheral blood cells.
Several previous studies have shown the stimulating effect of AFP on NS cell activity. For example, AFP induced both mice BM and NS cell proliferation and had an immunosuppressive effect on maternal anti-fetal T cell autoreactivity (Hoskin and Murgita, 1989). Chicken AFP led to reduction of NK activity and accelerated tumor growth in quails, presumably through NS cell stimulation (Yamada and Hayami, 1983).
In this study, AFP induced the production of both anti-proliferative and NK inhibitory factors in all NS fractions. Considering strong correlation existed between these activities, we conclude that both effects were mediated by the same factor. Similar effects were obtained in vivo, where a single injection of AFP led to 20% reduction of NK cell activity and the occurrence of increased spontaneous NS activity in all NS fractions. All NS subpopulations retained their ability to respond to AFP. Our results indicate a different response in cases when tumor was inoculated. EC growth was accompanied by an increase of spontaneous NS activity and loss of NS cell reactivity to AFP. The cause of this phenomenon is not clear. However, acceleration of tumor growth, decrease of NK activity and simultaneous increase of NS activity after AFP injection suggest that AFP suppresses tumor immunity and facilitates tumor growth. The present results suggest that AFP in AFP-producing tumors, can activate NS cells, thereby inhibiting cancer immunity and enhancing tumor progression.
ACKNOWLEDGEMENT
This work was supported by a grant from "Fund of Kazakhstan Science" (FKS 3-3-3.10-5(92).
ABBREVIATIONS
- AFP
alpha-fetoprotein
- BM
bone marrow
- NS
natural suppressor
- IL
interleukin
- NK
natural killer
- EC
Ehrlich carcinoma
- CLA
cytolytic activity
- SA
suppressive activity
References
- 1.Abelev GI. Alpha-fetoprotein biology. In: Skulachev VP, editor. Soviet Science Review (section D) Physicochemical Biology. New York: Harwood Acad Publ; 1993. pp. 85–109. [Google Scholar]
- 2.Angulo I, Rodriguez R, Garcia B, Medina M, Navarro J, Subiza JL. Involvement of nitric oxide in bone marrow-derived natural suppressor activity. J Immunol. 1995;155:15–26. [PubMed] [Google Scholar]
- 3.Belyaev NN, Zakiryanova GK, Khegai LA, Beklemishev AB. Analysis of antimitogenic and histamine binding functions of bone marrow cell isopycnic fractions. Immunology. 1993;3:15–17. (Rus.) [Google Scholar]
- 4.Cardoso E, Valdez G, Comini E, Matera L. Effect of human alpha-fetoprotein on native and in vitro-stimulated NK activity. J Clin Lab Immunol. 1991;34:183–188. [PubMed] [Google Scholar]
- 5.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semi-automated colorimetric assay: assessment of chemosensitivity testing. Cancer Research. 1987;47:936–942. [PubMed] [Google Scholar]
- 6.Chakraborty M, Mandal C. Immuno-supressive effect of human alpha-fetoprotein: a cross species study. Immunol Invest. 1993;22:329–339. doi: 10.3109/08820139309063412. [DOI] [PubMed] [Google Scholar]
- 7.Holda, JH, Maier T, Claman HN. Natural suppressor activity in graft-vs-host spleen and normal bone marrow is augmented by IL-2 and interferon-γ. J Immunol. 1986;137:3538–3543. [PubMed] [Google Scholar]
- 8.Hoskin DW, Murgita RA. Specific maternal anti-fetal lymphoproliferative responses and their regulation by natural immunosuppressive factors. Clin Exp Immunol. 1989;76:262–267. [PMC free article] [PubMed] [Google Scholar]
- 9.Moore SC, Theus SA, Barnett JB, Soderberg LS. Cytokine regulation of bone marrow natural suppressor cell activity in the suppression of lymphocyte function. Cell Immunol. 1992;141:398–408. doi: 10.1016/0008-8749(92)90158-l. [DOI] [PubMed] [Google Scholar]
- 10.Semeniuk DJ, Boismenu R, Tam J, Weissenhofer W, Murgita RA. Evidence that immunosuppression is an intrinsic property of the alpha-fetoprotein molecule. Adv Exp Med Biol. 1999;383:255–269. doi: 10.1007/978-1-4615-1891-4_27. [DOI] [PubMed] [Google Scholar]
- 11.Sinenko SA, Belyaev NN, Kuzovlev VD, Khakimzhanov AA. The immunochemical distinctions of human-α fetoprotein preparations as revealed with novel monoclonal antibodies. Reports MS-AS RK. 1999;1:72–80. [Google Scholar]
- 12.Subiza JL, Vinuela JE, Rodriguez R, Gil J, Figueredo MA, De la Concha EG. Development of splenic natural suppressor (NS) cells in Ehrlich tumor-bearing mice. Int J Cancer. 1989;44:307–314. doi: 10.1002/ijc.2910440220. [DOI] [PubMed] [Google Scholar]
- 13.Sykes M, Strober S. Hematopoietic cell transplantation. 2nd ed. Malden: Blackwell Science; 1999. Mechanisms of tolerance. [Google Scholar]
- 14.Yamada A, Hayami M. Suppression of natural killer cell activity by chicken alpha-fetoprotein in Japanease quails. J Natl Cancer Inst. 1983;70:735–738. [PubMed] [Google Scholar]
- 15.Yamaguchi V, Takashima I, Funakoshi M, Kawami H, Toge T. Defective natural killer activity in gastric cancer patients: possible involvement of suppressor factor receptor. In Vivo. 1994;8:279–283. [PubMed] [Google Scholar]
- 16.Young MR, Young ME, Wright MA. Stimulation of immunesuppressive bone marrow cells by colony-stimulated factors. Exp Hematol. 1990;18:806–811. [PubMed] [Google Scholar]
- 17.Young MR, Wright MA, Coogan M, Young ME, Bagash J. Tumor-derived cytokines induce bone marrow suppressor cells that mediate immunosuppression through transforming growth factor beta. Cancer Immunol Immunother. 1992;35:14–18. doi: 10.1007/BF01741049. [DOI] [PMC free article] [PubMed] [Google Scholar]