Abstract
To directly test if elevated glucocorticoids are required for fasting-induced regulation of growth hormone (GH)-releasing hormone (GHRH), GHRH receptors (GHRH-R) and ghrelin receptors (GHS-R) expression, male rats were bilaterally adrenalectomized or sham operated. After 7 days, animals were fed ad libitum or fasted for 48 h. Bilateral adrenalectomy increased hypothalamic GHRH to 146% and decreased neuropeptide Y (NPY) mRNA to 54% of SHAM controls. Pituitary GHRH-R and GHS-R mRNA levels were decreased by adrenalectomy to 30% and 80% of sham-operated controls. In shamoperated rats, fasting suppressed hypothalamic GHRH (49%) and stimulated NPY (166%) mRNA levels, while fasting increased pituitary GHRH-R (391%) and GHS-R (218%) mRNA levels. However, in adrenalectomized rats, fasting failed to alter pituitary GHRH-R mRNA levels, while the fasting-induced suppression of GHRH and elevation of NPY and GHS-R mRNA levels remained intact. In fasted adrenalectomized rats, corticosterone replacement increased GHRH-R mRNA levels and intensified the fasting-induced decrease in GHRH, but did not alter NPY or GHS-R response. These data suggest that elevated glucocorticoids mediate the effects of fasting on hypothalamic GHRH and pituitary GHRH-R expression, while glucocorticoids are likely not the major determinant in fasting-induced increases in hypothalamic NPY and pituitary GHS-R expression.
Keywords: Glucocorticoids, Fasting, Growth hormone-releasing hormone (GHRH), GHRH receptor, Growth hormone secretagogue (GHS) receptor, Adrenalectomy
INTRODUCTION
Food deprivation affects growth hormone (GH) secretion in all mammalian species studied to date (Tannenbaum et al, 1979; Ho et al, 1988; Fairhall et al, 1990; Thomas et al, 1990; Henricks et al, 1994; Stoving et al, 1999). However, the GH-secretory response to fasting is species dependent. In humans, nutrient deprivation results in a rise in circulating GH (Ho et al, 1988; Riedel et al, 1995; Stoving et al, 1999) accompanied by a paradoxical decrease in insulin-like growth factor-1 (IGF-1) due to GH resistance of peripheral target tissues (Ho et al, 1992; Gianotti et al, 2000). The increase in GH release is associated with an increase in pituitary sensitivity to GH-releasing hormone (GHRH) and a decrease in pituitary sensitivity to somatostatin (SRIH) (Gianotti et al, 1999). In contrast, in the fasted male rat, pulsatile GH release is dramatically reduced (Tannenbaum et al, 1979; Bruno et al, 1990; Janowski et al, 1993). The fasting-induced fall in GH is thought to be due to a decrease in hypothalamic GHRH expression (Bruno et al, 1990; Ghigo et al, 1997; Vuagnat et al, 1998; Carro et al, 1999) brought about by the inhibitory actions of neuropeptide Y (NPY) (Minami et al, 1998; Korbonits et al, 1999). Food deprivation in the rat leads to peripheral GH resistance and reduced IGF-1 (Bornfeldt et al, 1989), and increased pituitary sensitivity to GHRH (Sugihara et al, 1996). The fasting-induced increases in GHRH sensitivity is associated with an increase in pituitary GHRH receptor (GHRH-R) mRNA (Park et al, 2004) and an increase in pituitary GHRH binding (Sugihara et al, 1996), with a concomitant decline in pituitary SRIH receptor expression and ligand binding (Bruno et al, 1994; Park et al, 2004). Fasting also results in an increase in stomach ghrelin mRNA and pituitary ghrelin receptor (GHS-R) mRNA (Toshinai et al, 2001a; Kim et al, 2003; Park et al, 2004). Fasting-mediated increases in GH-stimulatory receptors (GHRH-R and GHS-R) and decreases in GH-inhibitory receptors (SRIH receptors) might be used as a mechanism to drive enhances GH release.
The fall in hypothalamic GHRH mRNA and rise in pituitary GHRH-R and GHS-R mRNA may be due to elevated glucocorticoids since experimental elevation of circulating glucocorticoids decreases hypothalamic GHRH mRNA (Senaris et al, 1996; Fife et al, 1996; Lam and Srivastava, 1997), while treatment of primary pituitary cell cultures with glucocorticoids increases GHRH-R (Miller and Mayo, 1997) and GHS-R (Tamura et al, 2000) mRNA. In order to directly test if the fasting-induced rise in glucocorticoids is responsible for fasting-induced changes in hypothalamic GHRH, pituitary GHRH-R and GHS-R mRNA, we compared the hypothalamic/pituitary response to fasting in sham-operated and adrenalectomized rats.
METHODS
Animals
Male Sprague-Dawley rats (7~8 weeks; 220~250 g) were used in the present study. They were housed under controlled environmental conditions (12-h light and 12-h dark). Food and tap water were available ad libitum. Experiments were conducted according to the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals. The in vivo study was approved by the Kyunghee University School of Medicine Animal Care Committee (Seoul, Korea).
Bilateral adrenalectomy and food deprivation
To study the effect of adrenalectomy, animals were bilaterally adrenalectomized or sham-operated by the dorsal approach under ketamine (100 mg/kg)/xylazine (15 mg/kg) anesthesia between 10:00 and 12:00 h. After the surgery they were individually housed. Adrenalectomized rats were maintained on 0.9% NaCl in their drinking water to compensate for the loss of mineralocorticoids. Animals were killed after 7 days. To study the effect of adrenalectomy on fasting-induced changes in hypothalamic and pituitary components of GH-axis, sham-operated and adrenalectomized animals were supplied food ad libitum or fasted for 24, 48 or 72h. All groups were allowed free access to water. To study the effect of corticosterone replacement, adrenalectomized rats were provided with vehicle (0.5% ethanol in saline) or corticosterone (40µg/ml in saline with 0.5% ethanol) immediately after the operation. The corticosterone was first dissolved in ethanol before being diluted to the concentrations in saline. After 7 days, food was removed from half of the vehicle-treated and half of the corticosterone-treated animals for 48 h. All animals were sacrificed by decapitation and anterior pituitaries, hypothalami, and sera were collected. The hypothalamic block was cut in half horizontally to produce a basal portion (medialbasal hypothalamus, MBH) which contained the arcuate nucleus (ARC), and a dorsal portion (dorsalmedial hypothalamus, DMH) which contained the paraventricular nucleus (PVN) as previously described (Hanson et al, 1997).
Measurement of glucose, GH, IGF-1 and corticosterone concentrations
Glucose levels were measured by GlucoDr Blood Glucose Meter (Allmedicus, Korea; maximal reading 600 mg/dl). Serum GH concentrations were measured by rat GH RIA kit (Amersham Biosciences Co., NJ, USA). Total serum IGF-1 levels were assayed using a rat IGF-1 ELISA kit (Amersham) after acid/ethanol extraction according to the manufacturer's instructions. Serum corticosterone concentrations were assessed using the ELISA kit (Neogene CO., MI, USA). ADX animals with serum corticosterone levels above 10 ng/ml were removed from the study.
RNA isolation
Total hypothalamic and pituitary RNA was recovered using standard procedure previously reported (Kamegai et al, 1998a; Kamegai et al, 1998b). RNA was then precipitated with isopropanol, and the pellet was washed with 70% ethanol, air dried, and dissolved in sterile DEPC water. The concentration and purity of RNA were determined by NanoDrop spectrophotometer (NanoDrop Technologies, Inc., DE, USA) at OD 260/280 nm.
Real-time reverse transcription (RT)-PCR of hypothalamic GHRH, SRIH and NPY, pituitary GHRH-R, GHS-R and GH mRNA
One microgram of total hypothalamic pituitary RNA was used as a template to generate cDNA by RT with random hexamer priming. The resultant cDNA was amplified using the LightCycler (Roche Diagnosties Ltd. Lewes, UK). Realtime PCR analysis was carried out with SYBR Green I and primers (for SRIH, NPY, GH, GHRH-R and β-actin) or hybridization probes and primers (for GHRH and GHS-R). The sequences of primers and probes for GHRH (GenBank Accession No. M73486) were as follows: GHRH, sense; 5'-CAA TTA TAT GCC CGC AAA CT-3', antisense; 5'-TGG GTC TTT ATT GTA TTC ACA G-3', probe1; 5'-GCA GGG ATT CCC AAG GAT GAA G (Fluo)-3', probe2; 5'-(Red640) TTC AGC GGA GGC TTG AGC (Phosphate)-3'. The sequences of primers for SRIH (M25890) and NPY (M15880) were as follows: SRIH, sense; 5'-AGC TGAGCAGGA CGA GATGAG-3', antisense; 5'-GGG CTG GGA GTT AAG GAA GAG-3', and NPY, sense; 5'-GCA GAG GAC ATG GCC AGA TAC-3', antisense; 5'-GGA CAG GCA GAC TGG TTT CAC-3'. The PCR primer and hybridization probe sequences used for determination of the GH, GHRH-R and GHS-R mRNA levels have been previously described (Park et al, 2004; Kim et al, 2006). The level of expression of each mRNA and their estimated crossing points (CP) in each sample were determined relative to the standard preparation using the LightCycler computer software (v. 3.5). PCR amplification was performed with a series of standards prepared by successive dilutions and a linear standard curve was automatically generated. A standard curve was constructed for each PCR run.
Statistical analysis
All data were expressed as mean±S.E.M. All experiments were repeated at least two times. Comparisons between groups were made by student's t-test or one-way ANOVA and Holm-Sidak method for multiple comparisons using SigmaStat for Windows Version 3.10 (Systat Software, Inc., Point Richmond, CA), and p<0.05 was considered significant. All comparisons were made between samples on the same real-time PCR run.
RESULTS
Effect of adrenalectomy
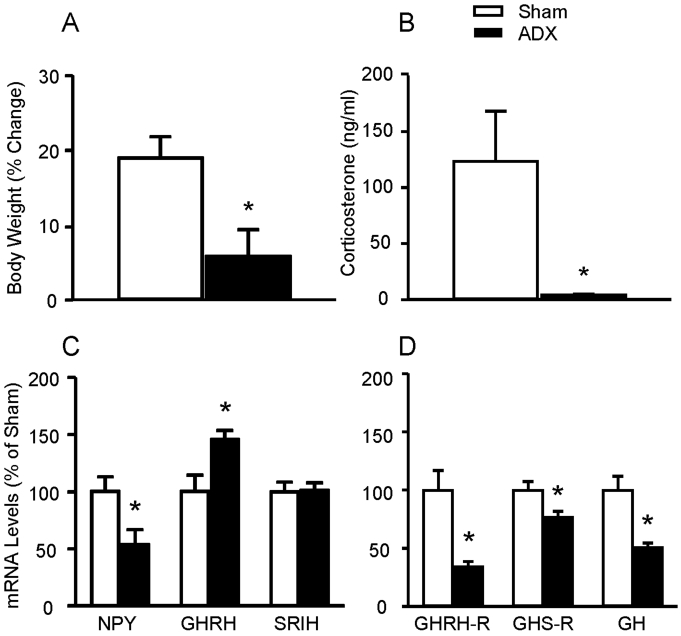
The percent change of body weight gain (from presurgery to the time the animals were sacrificed) was 19 (3% in sham-operated vs. 6 (4% in adrenalectomized animals (p<0.05, n=5/group) (Fig. 1A). Plasma corticosterone levels were significantly reduced in adrenalectomized rats (4±2 ng/ml, n=5) compared with sham-operated animals (123±101 ng/ml, n=5, p<0.05) (Fig. 1B). Hypothalamic NPY mRNA levels in adrenalectomized rats were 54±13% (p<0.05) of sham-operated controls, whereas GHRH mRNA levels increased to 146±8% (p<0.05) by adrenalectomy. Hypothalamic SRIH mRNA levels were not changed by adrenalectomy (Fig. 1C). Pituitary GHRH-R, GHS-R, and GH mRNA levels were decreased to 30±3% (p<0.01), 80±8% (p<0.05), and 50 (4% of sham-operated animals (Fig. 1D).
Fig. 1.
Comparison of the body weight (A) and plasma corticosterone levels (B) of sham-operated (Sham) and adrenalectomized (ADX) animals and effect of adrenalectomy on hypothalamic (C) and pituitary (D) gene expression. Body weight was displayed % of change between immediately before operation and immediately before sacrifice. Serum corticosterone was measured by ELISA. Hypothalamic and pituitary gene expressions were measured by real-time RT-PCR. Data are mean±S.E.M., n=5 animals/group. *p<0.05.
Effect of fasting in adrenalectomized or sham-operated rats
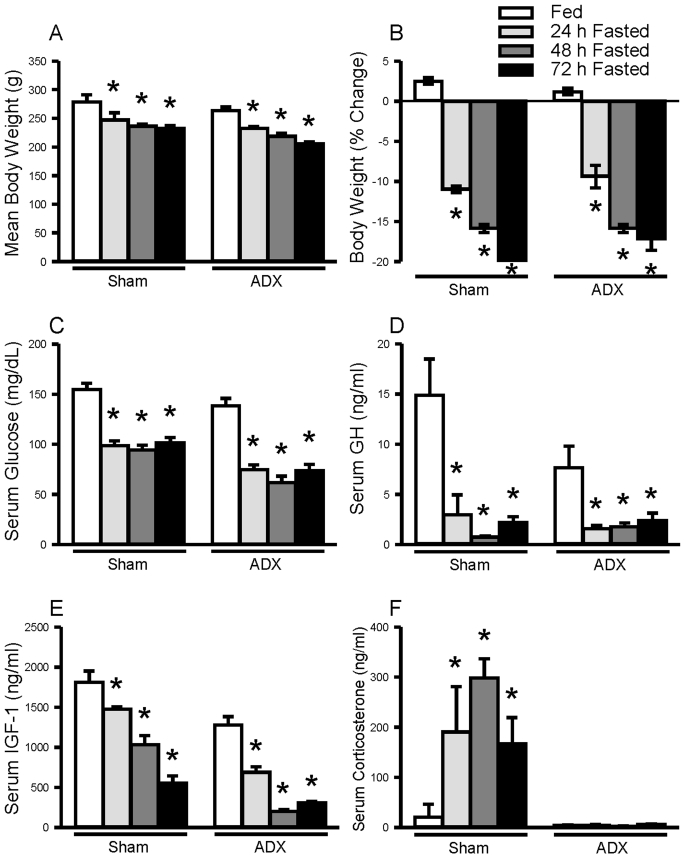
As shown in Fig. 2, fasting resulted in a progressive and comparable weight loss in sham-operated (n=5 animals/group) and adrenalectomized rats (n=5 animals/group). This reduction of body weight is associated with significant elevation of serum corticosterone levels in sham-operated animals, while corticosterone levels were not changed in response to fasting in adrenalectomized rats. Regardless of adrenal status, blood glucose, circulating GH and IGF-1 levels were significantly suppressed.
Fig. 2.
Effect of fasting on body weight (A, B), serum glucose (C), circulating growth hormone (GH) (D), insulin-like growth factor (IGF)-1 (E) and corticosterone (F) in sham-operated (Sham) and adrenalectomized (ADX) rats. Serum glucose levels were measured by GlucoDr Blood Glucose Meter. Serum GH concentrations were measured by GH RIA. Serum IGF-1 and corticosterone levels were measured by ELISA. Values are expressed as mean±SEM., n=5 animals/group. *, indicates values differ from respective fed controls; *, p<0.05.
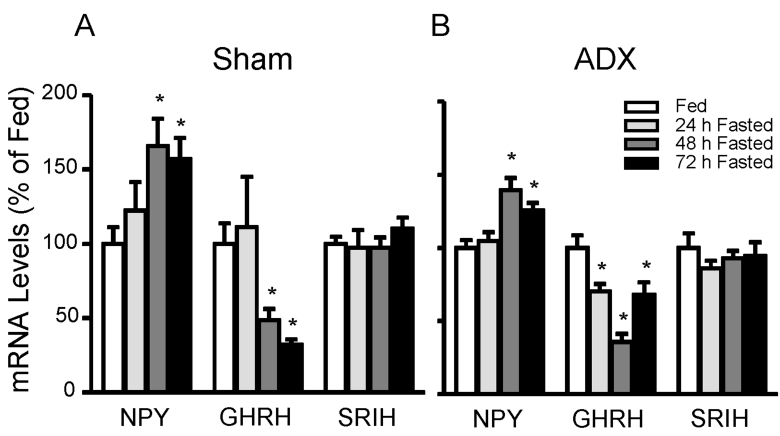
In sham-operated animals (n=5 animals/group), fasting suppressed hypothalamic GHRH mRNA levels to 49(8% (48 h; p<0.05) and 32±4% (72 h; p<0.01) of fed controls, whereas NPY mRNA levels increased in response to 48h and 72h fasting to 166±18% (p<0.05) and 157±13% (p<0.05), respectively (Fig. 3A). In the absence of endogenous glucocorticoids (n=5 animals/group), fasting suppressed GHRH and increased NPY (Fig. 3B) as observed in shamoperated rats suggesting fasting-induced changes in GHRH and NPY are glucocorticoids-independent. SRIH mRNA levels were not changed by fasting in both groups.
Fig. 3.
Effect of fasting on hypothalamic neuropeptide Y (NPY), growth hormone-releasing hormone (GHRH), and somatostatin (SRIH) mRNA levels in sham-operated (A) or adrenalectomized (B) animals. Animals were fasted for 24 h, 48 h, 72 h or supplied food ad libitum (fed). Both groups were allowed free access to water or 0.9% NaCl solution (adrenalectomized group). Hyperthalamic mRNA levels were measured by real-time RT-PCR and adjusted with β-actin. The graph is expressed as a percentage of fed controls. Data are mean±S.E.M., n=5 animals/group. *, indicates values differ from respective fed controls; *, p<0.05.
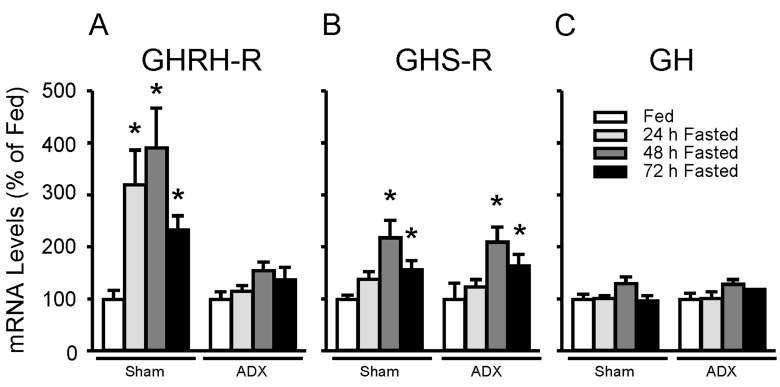
Fig. 4 represents the effect of fasting on pituitary GHRH-R, GHS-R, and GH mRNA levels in sham-operated (n=5 animals/group) and adrenalectomized rats (n=5 animals/group). GHRH-R mRNA levels were significantly increased in 48h fasted sham-operated animals to 391±76% (p<0.01) of fed controls, whereas fasting failed to increase GHRH-R in fasted adrenalectomized rats (Fig. 4A). In contrast, 48h fasting significantly increased pituitary GHS-R mRNA levels in sham-operated (218±33%, p<0.01) as well as adrenalectomized (210±29%, p<0.01) animals (Fig. 4B). Despite the decline in circulating GH, pituitary GH mRNA levels did not differ between fed and fasted sham-operated and adrenalectomized animals (Fig. 4C) consistent with our previous report (Park et al, 2004).
Fig. 4.
Effect of fasting on pituitary growth hormone (GH)-releasing hormone receptor (GHRH-R) (A), GH secretagogue receptor (GHS-R) (B), and GH (C) mRNA levels in adrenalectomized or shamoperated animals. Animals were fasted for 24 h, 48 h, 72 h or supplied food ad libitum (fed). Both groups were allowed free access to water or 0.9% NaCl solution (adrenalectomized group). GHRH-R, GHS-R, and GH mRNA levels were measured by real-time RT-PCR and adjusted with β-actin. The graph is expressed as a percentage of fed controls. Data are mean±S.E.M., n=5 animals/group. *, indicates values differ from respective fed controls; *, p<0.05.
Effect of corticosterone replacement in ad libitum fed and fasted adrenalectomized rats
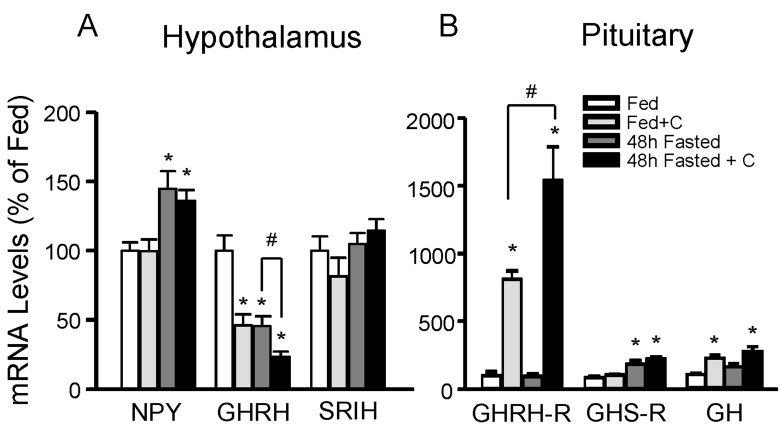
In order to examine the direct effect of glucocorticoids in adrenalectomized rats, vehicle or corticosterone was given to adrenalectomized animals for 7 days immediately after the operation. Vehicle or corticosterone-replaced adrenalectomized rats (n=5 animals/group) were 48h fasted or fed ad libitum. Fig. 5 represents the effect of corticosterone replacement in fed and 48h fasted adrenalectomized rats. Hypothalamic NPY mRNA levels were not changed by corticosterone replacement in fed and fasted adrenalec tomized rats. In contrast, GHRH mRNA levels were significantly decreased by corticosterone replacement in fed adrenalectomized rats. Furthermore, corticosterone replacement in fasted adrenalectomized animals augmented fasting-induced suppressive effect of fasting (Fig. 5A). At the pituitary levels, GHRH-R and GH mRNA levels were significantly increased by corticosterone replacement in both fed and fasted adrenalectomized animals whereas corticosterone had no significant effect on fed and fasted mRNA levels of GHS-R in adrenalectomized rats (Fig. 5B).
Fig. 5.
Effect of corticosterone (C) on pituitary (A) and hypothalamic (B) gene expression in adrenalectomized animals. Animals were fasted for 48 h or supplied food ad libitum (fed). Subsets of fed or 48 h fasted rats were provided with 40µg/ml of corticosterone containing water from immediately after operation to immediately before sacrifice. Hypothalamic and pituitary mRNAs were measured by real-time RT-PCR. Data are mean±S.E.M., n=5 animals/group. *, indicates values differ from respective fed controls; *, p<0.05, #, p<0.05.
DISCUSSION
In the present study we examined the effects of adrenalectomy and fasting on hypothalamic neuropeptide expression and found that fasting suppressed GHRH expression regardless of adrenal status, however the effect of fasting on GHRH was blunted in adrenalectomized rats, suggesting a minor role of glucocorticoids in fasting-induced decrease in GHRH. This observation appears to be contrast to the previous reports (Senaris et al, 1996; Lam and Srivastava, 1997) that experimental elevation of glucocorticoids decreases hypothalamic GHRH mRNA levels. Therefore, we decided to examine the direct effect of corticosterone on fasting-induced changes in hypothalamic neuropeptide expression in the absence of endogenous glucocorticoids. In the present study, adrenalectomized rats were provided with 40µg/ml of corticosterone in their drinking water. Providing adrenalectomized rats this corticosterone solution has been reported to result in plasma corticosterone levels comparable to those of sham-operated controls (Wilkinson et al, 1981). Indeed, corticosterone replacement did suppress GHRH expression in the ad libitum fed adrenalectomized rats, supporting the tonic inhibitory role of glucocorticoids on GHRH. Interestingly, corticosterone intensified the fasting-induced decline in GHRH expression. Taken together, our results suggest that elevated glucocorticoids mediate the effects of fasting on hypothalamic GHRH expression, and other factors, which are increased by food deprivation, are involved in fasting-induced suppression of GHRH.
In contrast to the changes in GHRH, NPY expression was not changed by corticosterone replacement in both fed and fasted adrenalectomized animals. Given the observation that NPY expression was increased in the absence of endogenous glucocorticoids, we suggest that glucocorticoids are likely not the major determinant in the rise of NPY by fasting. The question remains; what is responsible for fasting-induced upregulation of hypothalamic NPY expression? A plausible candidate is ghrelin. Circulating ghrelin levels are increased during fasting and refeeding decreases circulating ghrelin levels (Ariyasu et al, 2001; Toshinai et al, 2001b; Kim et al, 2003). NPY neurons express the GHS-R (Willesen et al, 1999) and central administration of ghrelin increases NPY (Shintani et al, 2001; Kamegai et al, 2001). We might speculate that ghrelin is, at least in part, responsible for the upregulation of NPY expression during fasting. Given the fact that NPY suppresses GHRH expression (Bruno et al, 1990; Ghigo et al, 1997; Vuagnat et al, 1998; Carro et al, 1999), fasting-induced increase in ghrelin might play a role in the downregulation of GHRH expression in adrenalectomized rats during food deprivation. The role of free fatty acids, which are also increased with fasting, in the changes of neuropeptide expression during food deprivation remains to be determined. However, it is unlikely responsible for the upregulation of NPY during fasting in that experimental elevation of circulating free fatty acids suppress GH release (Casaneuva et al, 1987; Alvarez et al, 1991; Maccario et al, 1994) and central administration of oleic acid inhibits food intake via the suppression of hypothalamic NPY expression (Obici et al, 2002).
In the present study, we observed that pituitary GHRH-R expression was decreased by adrenalectomy and fasting clearly increased GHRH-R mRNA levels in the shamoperated animals consistent with previous reports (Lam et al, 1996; Sugihara et al, 1996; Miller and Mayo, 1997; Ohyama et al, 1998; Park et al, 2004). However, in this report, the fasting-induced rise in GHRH-R expression was completely blocked in the adrenalectomized rats and corticosterone replacement dramatically increased GHRH-R in both fed and fasted groups. Other investigators also have reported that glucocorticoid treatment increases GHRH-R expression in sham-operated and adrenalectomized animals (Lam et al, 1996; Miller and Mayo, 1997). Taken together, our data clearly demonstrate that the rise in glucocorticoid levels in the fasted state is absolutely required for the up-regulation of pituitary GHRH-R expression in response to fasting.
In agreement with previous reports (Tamura et al, 2000; Thomas et al, 2000), adrenalectomy decreased pituitary GHS-R mRNA levels but failed to alter the fasting-induced rise in GHS-R expression. Furthermore, corticosterone replacement in fed and fasted adrenalectomized rats had no significant effect on GHS-R mRNA levels indicating that glucocorticoids are likely not the major determinant in fasting-induced increase in GHS-R expression. Our results appear to be in opposition to the hypothesis that the fasting-induced rise in glucocorticoids is responsible for the increase in GHS-R. In fact, it has been reported that dexamethasone treatment increases GHS-R mRNA levels in vivo and in vitro (Tamura et al, 2000; Thomas et al, 2000). However, it should be noted that the methods used to replace glucocorticoids differed. In this study, corticosterone was added in drinking water.
In conclusion, our data suggest that elevated glucocorticoid levels in the fasted state are absolutely required for the increase in pituitary GHRH-R expression and may play a modulatory role in regulating hypothalamic GHRH. The results of the current study also suggest that glucocorticoids are not the major determinant in fasting-induced rises in hypothalamic and pituitary GHS-R expression.
ACKNOWLEDGEMENT
This work was supported by Kyunghee University (Grant No. 20031114).
ABBREVIATIONS
- GH
growth hormone
- GHRH
growth hormone-releasing hormone
- GHRH-R
growth hormone-releasing hormone receptor
- GHS-R
growth hormone secretagogue receptor
- NPY
neuropeptide Y
- SRIH
somatostatin
References
- 1.Alvarez CV, Mallo F, Burguera B, Cacicedo L, Dieguez C, Casanueva FF. Evidence for a direct pituitary inhibition by free fatty acids of in vivo growth hormone responses to growth hormone-releasing hormone in the rat. Neuroendocrinology. 1991;53:185–189. doi: 10.1159/000125716. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 3.Bornfeldt KE, Arnqvist HJ, Enberg B, Matthews LS, Norstedt G. Regulation of insulin-like growth factor-1 and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol. 1989;122:651–656. doi: 10.1677/joe.0.1220651. [DOI] [PubMed] [Google Scholar]
- 4.Bruno JF, Olchovsky D, White JD, Leidy JW, Song J, Berelowitz M. Influence of food deprivation in the rat on hypothalamic expression of growth hormone-releasing factor and somatostatin. Endocrinology. 1990;127:2111–2116. doi: 10.1210/endo-127-5-2111. [DOI] [PubMed] [Google Scholar]
- 5.Bruno JF, Xu Y, Song J, Berelowitz M. Pituitary and hypothalamic somatostatin receptor subtype messenger ribonucleic acid expression in the food-deprived and diabetic rat. Endocrinology. 1994;135:1787–1792. doi: 10.1210/endo.135.5.7956902. [DOI] [PubMed] [Google Scholar]
- 6.Carro E, Senaris RM, Seoane LM, Frohman LA, Arimura A, Casaneuva FF, Dieguez C. Role of GHRH and somatostatin on leptin-induced GH secretion. Neuroendocrinology. 1999;69:3–10. doi: 10.1159/000054397. [DOI] [PubMed] [Google Scholar]
- 7.Casaneuva FF, Villanueva L, Dieguez C, Diaz Y, Cabranes JA, Szoke B, Scanlon MF, Schally AV, Fernandez-Cruz A. Free fatty acids block growth hormone (GH) releasing hormone-stimulated GH secretion in man directly at the pituitary. J Clin Endocrinol Metab. 1987;65:634–642. doi: 10.1210/jcem-65-4-634. [DOI] [PubMed] [Google Scholar]
- 8.Fairhall KM, Gabrielsson BG, Robinson ICAF. Effect of food withdrawal and insulin on growth hormone secretion in the guinea pig. Endocrinology. 1990;127:716–723. doi: 10.1210/endo-127-2-716. [DOI] [PubMed] [Google Scholar]
- 9.Fife SK, Brogan RS, Giustina A, Wehrenberg WB. Immunocytochemical and molecular analysis of the effects of glucocorticoid treatment on the hypothalamic-somatotropic axis in the rat. Neuroendocrinology. 1996;64:131–138. doi: 10.1159/000127109. [DOI] [PubMed] [Google Scholar]
- 10.Ghigo MC, Torsello A, Grilli R, Luoni M, Locatelli V, Muller EE. Effects of GH and IGF-I administration on GHRH and somatostatin mRNA levels: I. A study on ad libitum fed and starved adult male rats. J Endocrinol Invest. 1997;20:144–150. doi: 10.1007/BF03346893. [DOI] [PubMed] [Google Scholar]
- 11.Gianotti L, Pincelli AI, Scacchi M, Rolla M, Bellitti D, Arvat E, Lanfranco F, Torsello A, Ghigo E, Cavagnini R, Muller EE. Effects of recombinant human insulin-like growh factor I administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH secretion in anorexia nervosa. J Clin Endocrinol Metab. 2000;85:2805–2809. doi: 10.1210/jcem.85.8.6743. [DOI] [PubMed] [Google Scholar]
- 12.Gianotti L, Rolla M, Arvat E, Belliti D, Valleto MR, Ferdeghini M, Ghigo E, Mueller EE. Effect of somatostatin infusion on the somatotrope responsiveness to growth hormone-releasing hormone in patients with anorexia nervosa. Biol Psychiat. 1999;45:334–339. doi: 10.1016/s0006-3223(98)00039-0. [DOI] [PubMed] [Google Scholar]
- 13.Hanson ES, Levin N, Dallman MF. Elevated corticosterone is not required for the rapid induction of neuropeptide Y expression by an overnight fast. Endocrinology. 1997;138:1041–1047. doi: 10.1210/endo.138.3.4995. [DOI] [PubMed] [Google Scholar]
- 14.Henricks DM, Jenkins TC, Ward JR, Krishnan CS, Grimes L. Endocrine responses and body composition changes during feed restriction and realimentation in young bulls. J Anim Sci. 1994;72:2289–2297. doi: 10.2527/1994.7292289x. [DOI] [PubMed] [Google Scholar]
- 15.Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KGMM, Thorner MO. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988;81:968–975. doi: 10.1172/JCI113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho PJ, Friberg RD, Barkan AL. Regulation of pulsatile growth hormone secretion by fasting in normal subjects and patients with acromegaly. J Clin Endocrinol Metab. 1992;75:812–819. doi: 10.1210/jcem.75.3.1517371. [DOI] [PubMed] [Google Scholar]
- 17.Janowski BA, Ling NC, Giustina A, Wehrenberg WB. Hypothalamic regulation of growth hormone secretion during food deprivation in the rat. Life Sci. 1993;52:981–987. doi: 10.1016/0024-3205(93)90534-a. [DOI] [PubMed] [Google Scholar]
- 18.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- 19.Kamegai J, Unterman TG, Frohman LA, Kineman RD. Hypothalamic/pituitary-axis of the spontaneous dwarf rat: autofeedback regulation of growth hormone (GH) includes suppression of GH releasing-hormone receptor messenger ribonucleic acid. Endocrinology. 1998a;139:3554–3560. doi: 10.1210/endo.139.8.6136. [DOI] [PubMed] [Google Scholar]
- 20.Kamegai J, Wakabayashi I, Miyamoto K, Unterman TG, Kineman RD, Frohman LA. Growth hormone (GH)-dependent regulation of pituitary GH secretagogue receptor (GHS-R) mRNA levels in the spontaneous dwarf rat. Neuroendocrinology. 1998b;68:312–318. doi: 10.1159/000054379. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Sohn S, Lee M, Jung J, Kineman RD, Park S. Differential responses of the growth hormone axis in two rat models of streptozotocin-induced insulinopenic diabetes. J Endocrinol. 2006;188:263–270. doi: 10.1677/joe.1.06501. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, Cho BY, Lee HK. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14:1317–1320. doi: 10.1097/01.wnr.0000078703.79393.d2. [DOI] [PubMed] [Google Scholar]
- 23.Korbonits M, Little JA, Forsling L, Tringali G, Costa A, Navarra P, Trainer PJ, Grossman AB. The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol. 1999;11:521–528. doi: 10.1046/j.1365-2826.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 24.Lam KS, Lee MF, Tam SP, Srivastava G. Gene expression of the receptor for growth hormone-releasing hormone is physiologically regulated by glucocorticoids and estrogen. Neuroendocrinology. 1996;63:475–480. doi: 10.1159/000127075. [DOI] [PubMed] [Google Scholar]
- 25.Lam KS, Srivastava G. Gene expression of hypothalamic somatostatin and growth hormone-releasing hormone in dexamethasone-treated rats. Neuroendocrinology. 1997;66:2–8. doi: 10.1159/000127212. [DOI] [PubMed] [Google Scholar]
- 26.Maccario M, Procopio M, Loche S, Cappa M, Martina V, Camanni F, Ghigo E. Interaction of free fatty acids and arginine on growth hormone secretion in man. Metabolism. 1994;43:223–226. doi: 10.1016/0026-0495(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 27.Miller TL, Mayo KE. Glucocorticoids regulate pituitary growth hormone-releasing hormone receptor messenger ribonucleic acid expression. Endocrinology. 1997;138:2458–2465. doi: 10.1210/endo.138.6.5184. [DOI] [PubMed] [Google Scholar]
- 28.Minami S, Kamegai J, Sugihara H, Suzuki N, Wakabayashi I. Growth hormone inhibits its own secretion by acting on the hypothalamus through its receptors on neuropeptide Y neurons in the arcuate nucleus and somatostatin neurons in the periventricular nucleus. Endocr J. 1998;48(Suppl):19–26. doi: 10.1507/endocrj.45.suppl_s19. [DOI] [PubMed] [Google Scholar]
- 29.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 30.Ohyama T, Sato M, Ohye H, Murao K, Niimi M, Takahara J. Effects of adrenalectomy and glucocorticoid receptor antagonist, RU38486, on pituitary growth hromone-releasing hormone receptor gene expression in rats. Peptides. 1998;19:1063–1067. doi: 10.1016/s0196-9781(97)00472-5. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Sohn S, Kineman RD. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J Endocrinol. 2004;180:369–378. doi: 10.1677/joe.0.1800369. [DOI] [PubMed] [Google Scholar]
- 32.Riedel M, Hoeft B, Blum WF, von zur Muhlen A, Brabant G. Pulsatile growth hormone secretion in normal-weight and obese men: differential metabolic regulation during energy restriction. Metabolism. 1995;44:605–610. doi: 10.1016/0026-0495(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 33.Senaris RM, Lago F, Coya R, Pineda J, Dieguez C. Regulation of hypothalamic somatostatin, growth hormone-releasing hormone, and growth hormone receptor messenger ribonucleic acid by glucocorticoids. Endocrinology. 1996;137:5236–5241. doi: 10.1210/endo.137.12.8940340. [DOI] [PubMed] [Google Scholar]
- 34.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 35.Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, Kristiansen J, Hagen C. Jointly amplified basal and pulasatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-Insulin-like growth factor 1 axis. J Clin Endocrinol Metab. 1999;84:2056–2063. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- 36.Sugihara H, Emoto N, Shibasaki T, Minami S, Wakabayashi I. Increased pituitary growth hormone-releasing factor (GRF) receptor messenger ribonucleic acid expression in food-deprived rats. Brain Res. 1996;742:355–358. doi: 10.1016/s0006-8993(96)01100-6. [DOI] [PubMed] [Google Scholar]
- 37.Tamura H, Kamegai J, Sugihara H, Kineman RD, Frohman LA, Wakabayashi I. Glucocorticoids regulate pituitary growth hormone secretagogue receptor gene expression. J Neuroendocrinol. 2000;12:481–485. doi: 10.1046/j.1365-2826.2000.00446.x. [DOI] [PubMed] [Google Scholar]
- 38.Tannenbaum GS, Rorstad O, Brazeau P. Effects of prolonged food deprivation on the ultradian growth hormone rhythm and immunoreactive somatostatin tissue levels in the rat. Endocrinology. 1979;104:1733–1738. doi: 10.1210/endo-104-6-1733. [DOI] [PubMed] [Google Scholar]
- 39.Thomas GB, Bennett PA, Carmignac DF, Robinson ICAF. Glucocorticoid regulation of growth hormone (GH) secretagogue-induced growth responses and GH secretagogue receptor expression in the rat. Growth Horm IGF Res. 2000;10:45–52. doi: 10.1054/ghir.1999.0138. [DOI] [PubMed] [Google Scholar]
- 40.Thomas GB, Mercer JE, Karalis T, Rao A, Cummins JT, Clarke IJ. Effect of restricted feeding on the concentrations of growth hormone (GH), gonadotropins, and prolactin (PRL) in plasma, and on the amounts of messenger ribonucleic acid for GH, gonadotropin subunits, and PRL in the pituitary glands of adult ovariectomized ewes. Endocrinology. 1990;126:1361–1367. doi: 10.1210/endo-126-3-1361. [DOI] [PubMed] [Google Scholar]
- 41.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001a;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 42.Toshinai K, Mondal SS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001b;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 43.Vuagnat BA, Pierroz DD, Lalaoui M, Englaro P, Pralong FP, Blum WF, Aubert ML. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology. 1998;67:291–300. doi: 10.1159/000054326. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson CW, Engeland WC, Shinsako J, Dallman MF. Nonsteroidal adrenal feedback demarcates two types of pathways to CRF-ACTH release. Am J Physiol. 1981;240:E136–E145. doi: 10.1152/ajpendo.1981.240.2.E136. [DOI] [PubMed] [Google Scholar]
- 45.Willesen MG, Kirstensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]