Abstract
Ampicillin, a β-lactam antibiotic, has been reported to induce astrocytic glutamate transporter-1 which plays a crucial role in protecting neurons against glutamate excitotoxicity. We investigated the effect of ampicillin on neuronal damage in the mouse hippocampus and neostriatum following transient global forebrain ischemia. Male C57BL/6 mice were anesthetized with halothane and subjected to bilateral occlusion of the common carotid artery for 40 min. Ampicillin was administered post-ischemically (for 3 days) and/or pre-ischemically (for 3~5 days until one day before the onset of ischemia). Pre- and post-ischemic treatment with ampicillin (50 mg/kg/day or 200 mg/kg/day) prevented ischemic neuronal death in the medial CA1 area of the hippocampus as well as the neostriatum in a dose-dependent manner. In addition, ischemic neuronal damage was reduced by pre-ischemic treatment with ampicillin (200 mg/kg/day). In summary, our results suggest that ampicillin plays a functional role as a chemical preconditioning agent that protects hippocampal neurons from ischemic insult.
Keywords: Ampicillin, Neuroprotection, Hippocampus, Neostriatum, Global forebrain ischemia, Mice
INTRODUCTION
In animal models of cardiac arrest, transient global forebrain ischemia induces selective cell death in the brain (Kirino, 1982). The neuronal death following ischemic insult is mediated by several mechanisms, including glutamate excitotoxicity, oxidative stress, inflammation, and apoptosis (Lipton, 1999; Lo et al, 2005). Glutamate excitotoxicity is triggered earlier than the other mechanisms and influences the extent of ischemic injury (Benveniste, 1991).
Glutamate transporter-1 (GLT-1) is expressed predominantly in astrocytes and is responsible for the greatest proportion of glutamate uptake from the synaptic cleft in the adult forebrain (Zhou & Sutherland, 2004). Recently, it has been suggested that in rats subjected to transient forebrain ischemia, astrocytes in the hippocampal CA1 area lose their glutamate transport activity and immunoreactivity for GLT-1 within a few hours of reperfusion, resulting in ischemically induced neuronal death (Ouyang et al, 2007). Consistent with this dominant role of GLT-1, a GLT-1 knockout mouse showed increased susceptibility to acute forebrain injury (Watase et al, 1998). It has also been demonstrated that β-lactam antibiotics are potent stimulators of GLT-1 expression (Rothstein et al, 2005), and previous studies have reported that ceftriaxone, one of the β-lactam antibiotics, exerted a neuroprotective effect on ischemic injury in in vivo and in vitro experiments (Rothstein et al, 2005; Chu et al, 2007; Ouyang et al, 2007). However, whether ampicillin, another β-lactam antibiotic, protects brain tissue against ischemic damage remains to be determined.
Therefore, we investigated whether ampicillin protects neurons in the mouse hippocampus and neostriatum after transient global forebrain ischemia.
METHODS
Animals and transient global forebrain ischemia
All animal procedures were approved by the Ethics Committee of the Catholic University of Korea and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Male C57BL/6 mice (Koatec, Kyungki-do, Korea), weighing 20~25 g, were kept in cages under light-controlled conditions (lights on from 08:00 to 20:00 h) with access to food and water ad libitum.
Procedures to induce global forebrain ischemia were performed as described previously (Cho et al, 2006). Briefly, the bilateral common carotid arteries were occluded for 40 min. Then animals satisfying the selection criteria of both regional cerebral blood flow less than 15% of the baseline during bilateral common carotid artery occlusion and posterior communicating artery less than one third of the diameter of the basilar artery, applied to reduce interindividual variation, were killed three days after reperfusion. Rectal temperature was maintained at 37.5±0.5℃ on a heating pad during surgery. In the sham-treated groups, the bilateral common carotid arteries were not occluded, but were only isolated from the adjacent vagus nerve.
Drug administration
Drugs were dissolved in normal saline. Schedules for ampicillin (Yungjin Pharmaceutical Co. Ltd, Hwa Sung, Korea) or saline injections were divided into two classes: the pre- and post-ischemic treatment group and the pre-ischemic treatment group. In animals allocated to the pre-ischemic and post-ischemic treatment group, ampicillin (50 mg/kg/day or 200 mg/kg/day) was administered both pre-ischemically for three days (from three days to one day before the onset of ischemia) and post-ischemically for three days. In the pre-ischemic treatment group, ampicillin (200 mg/kg/day) was injected for five days (from five days to one day before the onset of ischemia). For each control group, normal saline was administered in the same manner.
Fluoro-Jade and cresyl violet staining
Three days after transient forebrain ischemia, the animals were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) for 30 min, followed by 1.5 mL of latex solution to evaluate the plasticity of the posterior communicating artery. After the mice had been cooled on ice for one day, the brains were isolated and photographed with a digital camera (EOS 300D, Canon, Tokyo, Japan). They were then postfixed in 4% paraformaldehyde for 24 h and cut using a cryotome (20 µm sections) after they had been dehydrated with 30% sucrose in 0.1 M PB.
For cresyl violet staining, the sections were mounted on gelatin-coated slides and allowed to air-dry overnight. The mounted sections were rehydrated in distilled water, and submerged in 0.1% cresyl violet solution for 10 min. The sections were rinsed in 70% ethanol and dehydrated in graded series of ethanol, immersed in xylene, mounted in Canada balsam (Kanto chemical Co., Tokyo, Japan) and cover slipped.
In order to evaluate degenerating neurons, Fluoro-Jade staining was performed. Coronal sections of 20 µm in thickness were wet mounted then air dried on microscope slides. Slides were placed in distilled water for 1 min, then were oxidized by soaking in a solution of 0.06% KMnO4 for 7 min. Sections were stained in 0.001% Fluoro-Jade (Chemicon, Jefferson, USA) in 0.1% acetic acid for 30 min with shaking, then rinsed 3 times for 1 min in distilled water and dried for 1 h. Slides were cleared in xylene and coverslipped with para-xylene dimeric (Sigma Aldrich, Buchs, Schweiz) mounting medium.
Analysis of ischemic neuronal damage
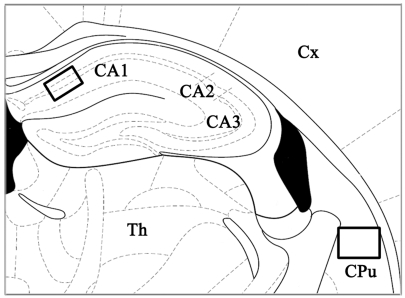
The severity of ischemic neuronal damage in the medial CA1 area of the hippocampus and the caudate-putamen (Fig. 1) was evaluated by an examiner blinded to the study groups and semiquantitatively assessed according to the method described previously (Murakami et al, 1998; Cho et al, 2006). Ischemic neuronal damage was measured on the following scale: no ischemically damaged neurons, grade 0; less than 30% ischemic neuronal damage, grade 1; 31~64% ischemic neuronal damage, grade 2; or 65~100% ischemic neuronal damage, grade 3.
Fig. 1.
Schematic presentation of the mouse hippocampus and caudate-putamen. Each rectangle indicates the position of two brain structures that were examined in the present study. Section is from -1.70 mm from bregma in the anteroposterior plane (adapted from Paxinos & Franklin, 2001). CA1, field CA1 of the hippocampus; CA2, field CA2 of the hippocampus; CA3, field CA3 of the hippocampus; CPu, caudate-putamen; Th, thalamus; Cx, cerebral cortex.
Statistical analysis
The semiquantitative measurements of ischemic neuronal damage were assessed using the χ2 test. Differences were considered to be significant when p<0.05.
RESULTS
Dose-dependent effect of pre- and post-ischemic treatment with ampicillin on ischemic neuronal death
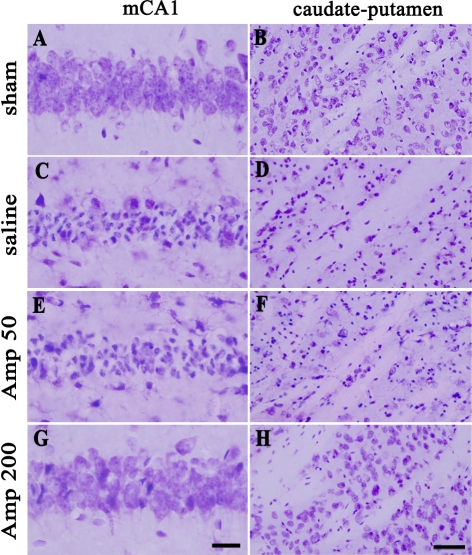
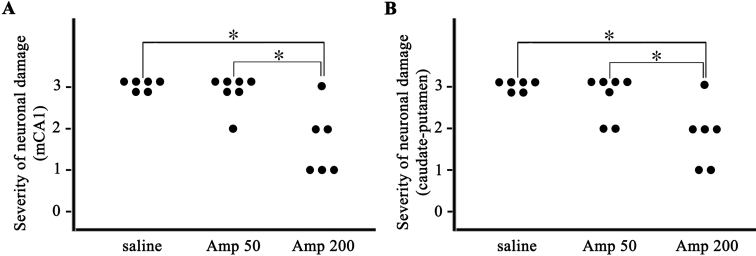
As can be seen in Fig. 2, pre- and post-ischemic treatment with ampicillin (50 mg/kg/day or 200 mg/kg/day) for six days protected neurons in the medical CA1 area of the hippocampus and the neostriatum. In the saline-treated group, neuronal damage was significant in the medial CA1 area of the hippocampus and the caudate-putamen three days after transient forebrain ischemia, compared with that in the sham-operated group (A, B, C and D of Fig. 2). Low-dose ampicillin (50 mg/kg/day) slightly, but not significantly, reduced ischemic neuronal damage compared with that in the saline control group (C, D, E and F of Fig. 2). High-dose ampicillin (200 mg/kg/day) significantly reduced ischemic neuronal damage relative to that in the saline control group (C, D, G and H of Fig. 2 and Fig. 3A, B, p<0.05).
Fig. 2.
Representative photomicrographs of cresyl-violet-stained ischemic neuronal damage in the hippocampus (A, C, E, G) and the caudate-putamen (B, D, F, H) three days after transient global forebrain ischemia. sham, sham-operated group; saline, saline-treated group; Amp 50, ampicillin (50 mg/kg/day)-treated group; Amp 200, ampicillin (200 mg/kg/day)-treated group. Scale bar=20 µm (A, C, E, G), 50 µm (B, D, F, H).
Fig. 3.
Pre- and post-ischemic treatment with ampicillin reduced the severity of ischemic neuronal damage in the medial CA1 area (mCA1) of the hippocampus and the caudate-putamen after transient global forebrain ischemia in a dose-dependent manner. Ampicillin (200 mg/kg/day)-treated group significantly reduced the severity of neuronal damage than saline-treated group or ampicillin (50 mg/kg/day)-treated group. saline, saline-treated group (n=6); Amp 50, ampicillin (50 mg/kg/day)-treated group (n=6); Amp 200, ampicillin (200 mg/kg/day)-treated group (n=6). *p<0.05 by χ2 test.
Neuroprotective effect of pre-ischemic treatment with ampicillin on ischemic neuronal damage
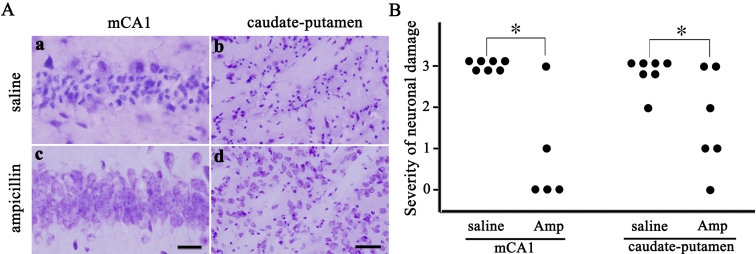
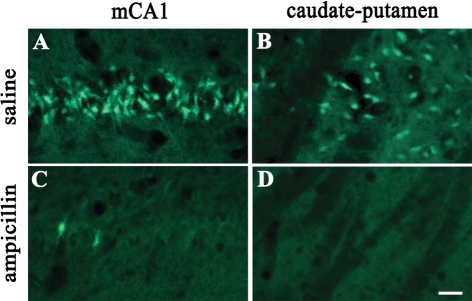
We further investigated whether ampicillin has neuroprotective effects by pre-ischemic treatment. In the pre-ischemic ampicillin (200 mg/kg/day, for 5 days) group, neuronal damage in the hippocampus and the caudate-putamen was significantly reduced three days after transient global forebrain ischemia, compared with that of the control group (Fig. 4A and B, p<0.05). Moreover, Fluoro-Jade labeled cells were abundantly observed in the CA1 subfield of the hippocampus and the caudate-putamen of the pre-ischemic saline group, while those were hardly seen in the pre-ischemic ampicillin group (Fig. 5).
Fig. 4.
Pre-ischemic treatment with ampicillin diminishes neuronal damage in the medial CA1 area of the hippocampus and caudate-putamen three days after transient forebrain ischemia in the mouse. A: Representative photomicrographs of cresyl-violet-stained ischemic neuronal damage in the medial CA1 (mCA1) of the hippocampus and caudate-putamen. Scale bar=20 µm (a, c), 50 µm (b, d). B: Pre-ischemic treatment with ampicillin reduced the severity of ischemic neuronal damage in two brain regions. saline, saline-treated group (n=6); Amp, ampicillin (200 mg/kg/day)-treated group (n=6). *p<0.05 by χ2 test.
Fig. 5.
Fluoro-Jade (FJ) staining of a representative coronal brain section showing the medial CA1 area (mCA1) of the hippocampus and the caudate-putamen three days after transient forebrain ischemia. Pre-ischemic treatment with ampicillin (200 mg/kg/day) prevented ischemic neurodegeneration. Scale bar=20 µm (A, B, C, D).
DISCUSSION
This study showed that pre-ischemic treatment with ampicillin for five days until one day before the onset of ischemia provides neuroprotection against transient forebrain ischemia. On the contrary, post-ischemic treatment with ampicillin for three days, starting 10 min before the onset of ischemia, had no neuroprotective effect (data not shown). Because the plasma concentration of ampicillin declines exponentially, with a half life of approximately 70 min in mice (English et al, 1984), we can infer that the ampicillin-induced neuroprotective effect is not the result of a direct mechanism, but is instead mediated by the upregulation of an endogenous defense mechanisms against ischemic insult. Two lines of evidence support this possibility. Huber et al. (1999) reported that in vitro pre-treatment with ampicillin (1 h before hypoxia) induces an increase in cellular hypoxic tolerance. It has also been reported that pre-treatment with ceftriaxone, one of the β-lactam antibiotics, exerts a neuroprotective effect in in vivo and in vitro models of brain ischemia and stroke (Chu et al, 2007; Lipski et al, 2007). Taken together, these data suggest that pre-ischemic treatment with ampicillin acts as a chemical pre-conditioning agent, resulting in the prevention of the ischemic neuronal damage induced by transient global forebrain ischemia.
Recently, it has been demonstrated that many β-lactam antibiotics are potent stimulators of GLT-1 expression (Rothstein et al, 2005). The major forebrain astroglial glutamate transporter, GLT-1, plays an essential role in removing the glutamate that rapidly increases in the synaptic cleft within several minutes of ischemia/reperfusion (Anderson & Swanson, 2000; Rao et al, 2001; Swanson et al, 2004). It has been suggested that the neuroprotective effect of β-lactam antibiotics in vivo and in vitro is mediated by the induction of GLT-1 (Ji et al, 2005; Rothstein et al, 2005; Chu et al, 2007; Lipski et al, 2007; Lee et al, 2008). For instance, ceftriaxone, one of β-lactam antibiotics, induced upregulation of GLT-1 transduction and transcription along with the reduction of the mRNA levels of matrix metalloproteinase-9, tumor necrosis factor α and Fas ligand, thereby prevented neuronal cell death in an experimental stroke model (Chu et al, 2007). Moreover, Rothstein et al. (2005) reported that GLT-1 expression was increased up to 2 fold by ampicillin, compared to the control. Therefore, we could raise the possibility that the upregulation of GLT-1 induced by pre-ischemic treatment with ampicillin plays an important role in the acquisition of brain ischemic tolerance. Further studies are needed to confirm this hypothesis.
Brain penetration is a prerequisite for the neuroprotective efficacy of ampicillin. A previous study reported that ampicillin is a relatively lipophilic antibiotic and can cross the blood-brain barrier into the brain (Lutsar et al, 1998). Ampicillin has proved clinically effective against a variety of brain infections, including bacterial meningitis and brain abscesses (Kanra, 2002). High doses of ampicillin (12 g/day for adults) are effective in the treatment of bacterial meningitis (Quagliarello & Scheld, 1997). An ampicillin dose of 200 mg/kg in a 20 g mouse (as given pre-ischemically in the present study), which provided neuroprotection against ischemic insult, can be regarded as equivalent to a dose of 1.2 g in a 70 kg human, according to the interspecies scaling relationship: Dhuman=Danimal (Whuman/Wanimal)0.7, where D denotes the dose of ampicillin in milligrams and W denotes weight in kilograms (Mordenti & Chappell, 1989). Therefore, these data suggest that pre-ischemic treatment with ampicillin is sufficiently potent to induce ischemic tolerance at or below the clinically relevant dose.
In conclusion, our results show that pre- and post-ischemic treatment with ampicillin has neuropretective effects against ischemic neuronal death in a dose-dependent manner. In addition, pre-ischemic treatment with ampicillin protects neurons in the CA1 area of the hippocampus and the caudate-putamen against transient global forebrain ischemia in the mouse.
ACKNOWLEDGEMENT
This research was supported by the Korea Science and Engineering Foundation grant R13-2002-005-04002-0.
ABBREVIATIONS
- GLT-1
glutamate transporter-1
- PB
phosphate buffer
References
- 1.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 2.Benveniste H. The excitotoxin hypothesis in relation to cerebral ischemia. Cerebrovasc Brain Metab Rev. 1991;3:213–245. [PubMed] [Google Scholar]
- 3.Cho KO, Kim SK, Cho YJ, Sung KW, Kim SY. A simple method for predicting hippocampal neurodegeneration in a mouse model of transient global forebrain ischemia. Korean J Physiol Pharmacol. 2006;10:167–172. [Google Scholar]
- 4.Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 5.English AR, Girard D, Haskell SL. Pharmacokinetics of sultamicillin in mice, rats, and dogs. Antimicrob Agents Chemother. 1984;25:599–602. doi: 10.1128/aac.25.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber R, Kasischke K, Ludolph AC, Riepe MW. Increase of cellular hypoxic tolerance by erythromycin and other antibiotics. Neuroreport. 1999;10:1543–1546. doi: 10.1097/00001756-199905140-00027. [DOI] [PubMed] [Google Scholar]
- 7.Ji HF, Shen L, Zhang HY. Beta-lactam antibiotics are multipotent agents to combat neurological diseases. Biochem Biophys Res Commun. 2005;333:661–663. doi: 10.1016/j.bbrc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Kanra G. Experience with ampicillin/sulbactam in severe infections. J Int Med Res. 2002;30(1) Suppl:20A–30A. doi: 10.1177/14732300020300S104. [DOI] [PubMed] [Google Scholar]
- 9.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 13.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 14.Lutsar I, McCracken GH, Friedland IR. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 1998;27:1117–1128. doi: 10.1086/515003. [DOI] [PubMed] [Google Scholar]
- 15.Mordenti J, Chappell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokinetics and new drug development. New York: Pergamon Press; 1989. pp. 42–96. [Google Scholar]
- 16.Murakami K, Kondo T, Kawase M, Chan PH. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998;780:304–310. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. London: Academic Press; 2001. [Google Scholar]
- 19.Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708–716. doi: 10.1056/NEJM199703063361007. [DOI] [PubMed] [Google Scholar]
- 20.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 22.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 23.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 2004;24:6301–6306. doi: 10.1523/JNEUROSCI.1404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]