Abstract
Sometimes, spinal cord injury (SCI) results in various chronic neuropathic pain syndromes that occur diffusely below the level of the injury. It has been reported that behavioral signs of neuropathic pain are expressed in the animal models of contusive SCI. However, the observation period is relatively short considering the natural course of pain in human SCI patients. Therefore, this study was undertaken to examine the time course of mechanical and cold allodynia in the hindpaw after a spinal cord contusion in rats for a long period of time (30 weeks). The hindpaw withdrawal threshold to mechanical stimulation was applied to the plantar surface of the hindpaw, and the withdrawal frequency to the application of acetone was measured before and after a spinal contusion. The spinal cord contusion was produced by dropping a 10 g weight from a 6.25 and 12.5 mm height using a NYU impactor. After the injury, rats showed a decreased withdrawal threshold to von Frey stimulation, indicating the development of mechanical allodynia which persisted for 30 weeks. The withdrawal threshold between the two experimental groups was similar. The response frequencies to acetone increased after the SCI, but they were developed slowly. Cold allodynia persisted for 30 weeks in 12.5 mm group. The sham animals did not show any significant behavioral changes. These results provide behavioral evidence to indicate that the below-level pain was well developed and maintained in the contusion model for a long time, suggesting a model suitable for pain research, especially in the late stage of SCI or for long term effects of analgesic intervention.
Keywords: Central neuropathic pain, Spinal cord injury, Mechanical allodynia, Spinal cord contusion, Cold allodynia
INTRODUCTION
Spinal cord injury (SCI) results in chronic neuropathic pain that interferes with the patient's rehabilitation as well as the loss of motor function. The prevalence of chronic central pain due to SCI was reported to vary with an average of 60~70% of cases. Various types of pain such as spontaneous burning pain, allodynia and hyperalgesia have been reported after a SCI (Beric et al, 1988; Boivie, 1989; Davidoff et al, 1991; Tasker & Loeser, 2001), however, the underlying mechanism is not fully understood, and there are a few treatments that provide reliable and effective relief (Yezierski, 1996; Tasker & Loeser, 2001). Therefore, SCI-induced central pain is one of the main problems in pain management (Boivie, 1989; Finnerup & Jensen, 2004).
One of the major stumbling blocks in understanding the mechanisms of neuropathic pain following a SCI is the difficulty in studying humans. SCI itself is not a very common disorder, thus making it difficult to study the state of central pain in humans due to the variety of etiology, the severity of injury, the time after SCI, and the responses to the pain treatments etc. Furthermore, the relative scarcity of animal studies makes it difficult to understand it, particularly compared with pain studies in other disorders.
Even though several animal models for SCI have been developed (Vierck et al, 2000), the contusion lesions by the Multicenter Animal Spinal Cord Injury Study (MASCIS) contusion device respond to early pharmacological interventions that are similar to human cord injuries (Behrmann et al, 1994; Constantini & Young, 1994; Rosenzweig & McDonald, 2004), and are close to a large number of human SCI (Bunge et al, 1997), suggesting that this model can be used to mimic a clinical situation.
Recently, experiments to elucidate the underlying mechanisms of pain and potential therapeutic intervention have been done using this model (Hook et al, 2007; Lee et al, 2007; Voda et al, 2007; Zhao et al, 2007). Even though SCI-induced pain may last for a long period of time and remission of pain has been reported to be rare in human patients (Stormer et al, 1997), experimental studies on animals were done mainly between 3 to 6 weeks after SCI. Earlier studies (Lindsey et al, 2000; Yoon et al, 2004) showed that neuropathic pain was maintained for 10 weeks after contusion injury, which is relatively short considering natural time course in human patients. In another SCI model such as hemisection injury, however, behavioral signs indicating neuropathic pain were expressed for 22~26 weeks (Kim et al, 2003).
Therefore, the aim of this study was to provide experimental data on the consequences for nociception of a spinal contusion injury in a weight-drop injury model using a MASCIS (NYU) impactor. More specifically, this study aimed to determine if those graded injuries (6.25 and 12.5 mm) influence the difference in the sensory threshold changes and the natural time course of the mechanical and cold allodynia over a longer observation period.
Preliminary data have been presented in an abstract form (Lee et al, 2004).
METHODS
Experimental animals
The experiments were conducted on male Sprague Dawley rats (Charles River, Biogenomics, Seoul, Korea) weighing between 200 and 240 gm. The animals were kept in a 12-h light /12-h dark cycle with the light on at 8:00 A.M. They were kept for least 7 days before surgery with access to water and food ad libitum. The procedures and protocols were in accordance with guidelines set by the Korea University College of Medicine Animals Research Policies Committee.
Spinal cord injury (SCI) and experimental groups
A total of 72 rats were contused by the New York University (NYU) impactor, released from a 6.25 mm (n=24) or a 12.5 mm (n=24), and sham group (n=24). Thus, 12 rats were randomly assigned to one of the three groups every week; 4 rats for the 6.25 mm group, 4 rats for the 12.5 mm group and 4 rats for the sham group. This was repeated for 6 weeks.
The contusion was performed at the T12 level using the NYU impactor under enflurane anesthesia (by mixture of 4% enflurane and 95% O2). Gas anesthesia was chosen over pentobarbital in order to reduce the risk of mortality, because the effective duration of pentobarbital is 2 to 3 hours even though the total operation time was approximately 20 min.
A laminectomy was performed at the spinal T10 segment and the spinal column was stabilized by clamping the spinous processes of T9 and T11 with tissue forceps. A 10 g weight was then released onto the spinal cord from a 6.25 (n=24) or 12.5 (n=24) mm height. After the spinal cord contusion, the musculature was sutured, the skin was autoclipped and the animals were allowed to recover from the anesthesia. A sham (n=24) operation was performed using only a laminectomy of the T10 vertebra without a spinal contusion. Four rats were housed in a cage. The bladders were manually expressed twice a day until spontaneous urination returned. In order to prevent a urinary tract infection, Unasyn® (Ampicillin/sulbactam, 100 mg/kg, Pfizer, Seoul, Korea) was injected intraperitoneally once a day for the first 3~5 days. Highly absorbent bedding was used to prevent sores and infections in the paralyzed animals.
Behavioral tests for sensory thresholds
All the behavioral tests were performed 1 day prior to surgery, 1, 2, 3, 4 and 7 days postoperatively (PO), and after that time, every week until 20 weeks PO and every other week until 30 weeks PO.
1. Mechanical allodynia
All the rats were tested to determine the withdrawal threshold of the hindpaw in response to the von Frey filaments from 0.41 gm to 15.1 gm (range: 0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50, and 15.10 g, Stoelting, Wood Dale, IL, U.S.A.). The rats were placed in a transparent plexiglass cubicle testing apparatus (size: 9.5×9.5×31.5 cm) on a metal mesh floor. After an acclimation period of 20 min, a series of calibrated von Frey filaments were applied perpendicularly to the plantar surface of both hindpaws with sufficient force to bend the filament for 3~5 sec. Brisk hindlimb withdrawal was considered to be a positive response. In the absence of a response, the filament of the next greater force was applied. After a positive response, the filament of the next lower force was applied. The tactile stimulus producing a 50% likelihood of a withdrawal response was calculated using the "up-down" method (Chaplan et al, 1994).
2. Cold allodynia
A response frequency to the application of 100% acetone to the plantar surface of the bilateral hindpaws was measured. The test procedures for cold allodynia of the paw are described elsewhere (Choi et al, 1994). Briefly, the rats were placed in a plexiglass box on a metal mesh floor, and acetone was applied to the plantar surface of the hindpaw. To accomplish this, an acetone bubble was formed at the end of a piece of small polyethylene tubing connected to syringe. The bubble was then allowed to slightly touch the heel. Acetone was quickly spread over the plantar surface of the hindpaw. The acetone was applied 5 times (once every 5 min) to each paw. The paw withdrawal and hindpaw licking response to the application of acetone were interpreted as a sign of cold allodynia. The frequency of paw withdrawal is expressed as a percentage; (# of frequencies of paw withdrawal / # of total trials)×100.
Behavioral test for locomotor function
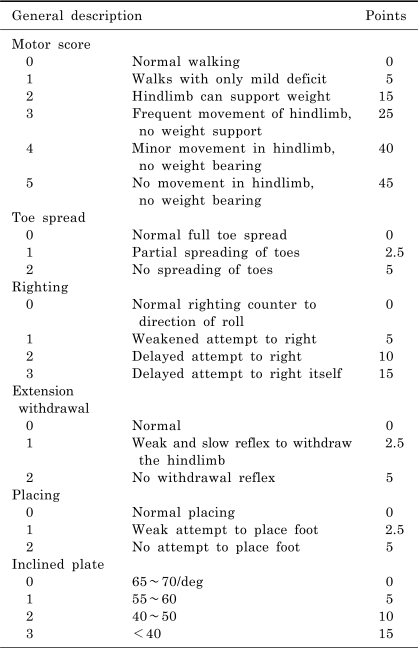
The locomotor function was assessed using the Basso, Beattie and Bresnahan (BBB) Locomotor Rating Scale (Basso et al, 1995), which is based on the locomotor ability following a SCI in rodent model. Briefly, the BBB scale is a 22-point scale that ranges from 0, indicating no observable hindlimb movement, to 21, which is a consistent coordinated gait with a parallel paw placement of the hindlimbs and a consistent trunk stability. The degree of motor impairment was also estimated by the combined behavioral score (CBS) (Gale et al, 1985) (Table 1). The motor function, toe spread, righting reflex, extension withdrawal reflex, placing reflex, and incline plate test were evaluated using the CBS scale, which ranges from 0 for a normal rat to 90 for a completely paralyzed rat.
Table 1.
Combined behavioral score (CBS) reported by Gale et al. (1985)
Statistical analysis
The withdrawal threshold and response frequency data from the right and left hindpaws were combined. All the values are expressed as a mean ± standard error of the mean. All statistical tests were evaluated at an alpha level of significance of 0.05. The Friedman repeated measures of analysis of the variance followed by multiple comparison tests were used to compare the behavioral test results before and after the SCI. The Kruskal and Wallis tests were used to compare the behavioral results obtained among the 3 groups.
RESULTS
There were a total of 72 rats that were either contused by the MASCIS/NYU impactor released from a 6.25 mm (n=24) or 12.5 (n=24) or underwent sham operation (n=24). Every week, 12 rats (4 rats assigned to 6. 25 mm group, 4 rats to 12.5 mm group and 4 rats to sham group) were examined. The same protocol was repeated for 6 weeks. One rat in the 6.25 mm group died in the 22 week post-operatively (PO), and five rats in 12.5 mm group died in the 11, 13, 22, and 28 week PO. In the sham operated group, one rat died in the 24 week PO. Finally there were 23 rats in the 6.25 mm group, 19 rats in the 12.5 mm group and 23 rats in the sham operated group for entire 30 weeks after SCI. The manual expression of the urinary bladder was needed for approximately four days in the 6.25 mm group and for seven days in the 12.5 mm group.
General behavioral status and locomotor function
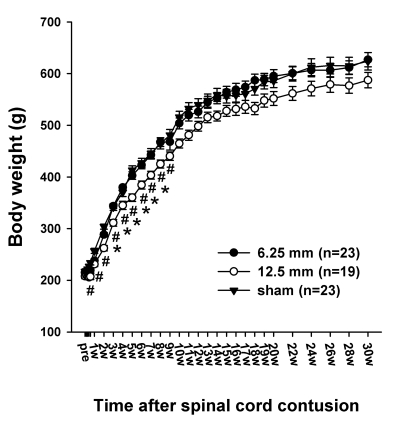
The weight of the rats was measured using digital scales for 30 weeks after the spinal cord contusion. The body weights of all the animals including the sham group were unchanged for the four days after the contusion or sham operation. Since then, the rats steadily gained weight without any noticeable trouble. There was no difference in body weight between the sham group and the 6.25 mm group. From the 3 days PO to 9 weeks PO, the body weight of the 12.5 mm group was significantly lower than that of the sham group (p<0.05) from the 3 days PO to 9 weeks PO and lower than that of the 6.25 mm group from the 3 weeks PO to 8 weeks PO (Fig. 1).
Fig. 1.
Time course of the body weight in the sham control (sham), 6.25 mm contusion, and 12.5 mm contusion groups. The average weight in all groups was approximately 220 g before the contusion. The rats in both the 6.25 and 12.5 mm groups showed an immediate reduction in body weight after the spinal contusion. Two weeks after the SCI, the weight of the rats in the 12.5 mm group (n=19) was significantly lower than that of the rats in the 6.25 mm group (n=23) or sham-operated group (n=23). There was no significant difference in weight between the sham group and the 6.25 mm group for the entire 30 weeks. The sharps (#) indicate the values significantly different between the sham and the 12.5 mm groups, and the asterisks (*) indicate the values significantly different between the 6.25 mm group and 12.5 mm group. The time after the spinal cord contusion indicates weeks (W) following spinal contusion, except for "pre", which represents the preoperative test period.
Immediately after the spinal cord contusion, the rats in the 6.25 and 12.5 mm groups showed hindlimb paralysis (Fig. 2 and 3). In the 6.25 mm group, most of the rats could not move three joints (hip, knee, and ankle) in the hindlimbs with ranges of motion less than 50% on 1 and 2 days PO. Most rats in this group showed behavioral improvement between 3 days PO and 3 weeks PO. Most of the rats produced extensive hindlimb joint movements 3 days PO and showed plantar stepping on 7 days PO. By 3 weeks PO, the rats were usually stepping consistently and were well coordinated, and the BBB locomotor score was 17±0.6. Since then, there was no significant change in locomotor recovery. The CBS score in this group was 26.3 ± 1.7 on the 1 week PO, however, as the coordination of walking improved, the CBS score decreased markedly to 13.8 ± 1.8 on 2 weeks PO and the score was maintained at approximately 5 from 4 weeks PO. The rats in this group could spread toes almost perfectly when the observer lifted up the rats within the first week, and had a complete withdrawal reflex and a placing reflex in the second week after the SCI. The rats could not hold their body in an inclined plate with 40 degrees 1 day PO but could stand up on the incline 4 days PO and most rats could keep their posture at an inclination of 55° from one week PO. The rats recovered almost all the hindlimb muscle power that could stand up to an inclination of 65 degrees 5 weeks after the contusion, which was similar to the normal rats.
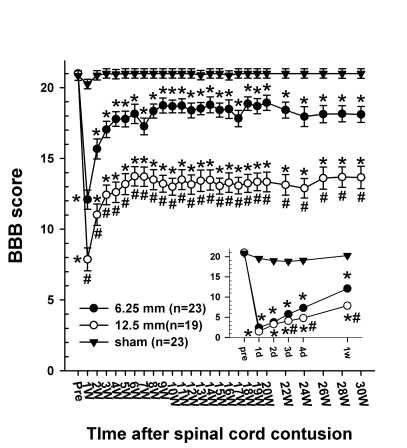
Fig. 2.
Time course of the BBB score following the spinal cord injury in three groups of rats. The inset shows the BBB score for the initial 7 days after spinal contusion for clarification. Before the SCI, the BBB score of all groups was 21. Every rat showed normal gaiting. On the first day after the SCI, the BBB scores decreased markedly to 2.4 ± 0.4 in the 6.25 mm groups and 1.5 ± 0.4 in the 12.5 mm group. By 3 weeks after the SCI, BBB scores gradually increased in both groups and were maintained for the remaining 27 weeks. Since 2 weeks after the SCI, the difference in the BBB score between the 6.25 mm group and the 12.5 mm group had obviously been sustained until 30 weeks after the SCI (p<0.05). The final BBB score of the 6.25 mm group was 18.1 ± 0.6 and that of the 12.5 mm group was 13.7 ± 0.8. The asterisks (*) of each group indicate the values that are significantly different from the values in the sham group and sharps (#) indicate the values significantly different between the 6.25 mm group and the 12.5 mm group. The time after the spinal cord contusion indicates days (D) and weeks (W) following the spinal contusion, except for "pre", which represents the preoperative test period.
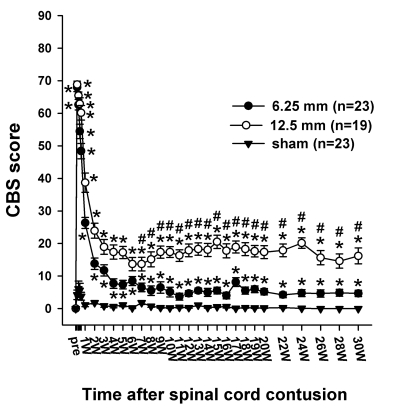
Fig. 3.
Time course of the CBS score (max.=90) following the spinal cord injury in the three groups. The CBS score of all the rats in pre-operation was 0 in the three groups. One day after the contusion injury, the CBS scores increased to 67.9 ± 1.3 in the 6. 25 mm group and 68.8 ± 1.1 in the 12.5 mm group. The CBS scores include the motor score, toe spread, righting reflex, extension withdrawal reflex, placing reflex and incline test. The asterisks (*) of each group indicate the values significantly different from the sham group, and the sharps (#) I ndicate the values significantly different between the 6.25 mm group and 12.5 mm group. The time after the spinal cord contusion indicates weeks (W) following spinal contusion, except for "pre", which represents the preoperative test period.
The rats in the 12.5 mm group showed no hindlimb joint movement 1 day PO and produced gradual motor recovery. All the rats could step on the plantar surface of the hindpaws (BBB scale>10) 2 weeks PO. Most of the rats showed weight-supported plantar stepping with occasional or frequent forelimb and hindlimb coordination. In this group, the CBS score was 38.7 ± 3.0 on 1 week PO and 23.9 ± 2.3 on 2 week PO. The CBS scores did not drop to less than 15 for the entire 30 weeks after the SCI, because rats did not recover to the level of coordinated walking. In this case, the motor score, which is one component consisting of the CBS score, was >15. The toe spread of the hindpaws recovered quite rapidly, however, the motor function, the extension withdrawal reflex, the placing reflex, and the inclined plane test recovered incompletely over the 30 week period after the SCI. The righting reflex of all the animals was normal pre- and post T12 injury.
Behavioral signs of allodynia
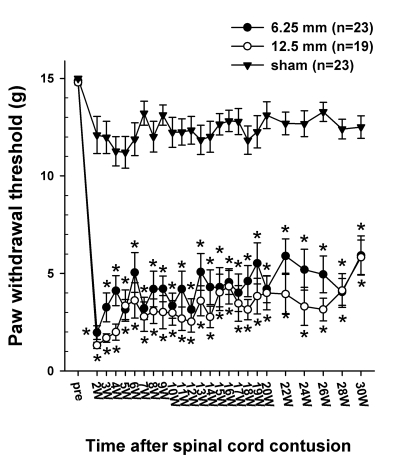
The behavioral tests for mechanical and cold allodynia were applied when the hindlimbs of the injured rats could support their weight. The BBB score at this time was 15.7 ± 0.7 and 12.4 ± 0.7 in the 6.25 mm and 12.5 mm groups, respectively. Fig. 4 shows the time course of the withdrawal thresholds to the mechanical stimulation applied to the plantar surface. Before the SCI, the paw withdrawal threshold in the 6.25 mm, 12.5 mm group and sham groups were 14.9 ± 0.1 g, 14.8 ± 0.1 g and 14.9 ± 0.1 g, respectively. However, after the spinal contusion from the 6.25 mm and 12.5 mm height, both hindpaws became responsive by 2 weeks PO, while the threshold dropped to approximately 2 g. The withdrawal thresholds remained low for up to 30 weeks PO and were significantly different from the pre-injury values. The decrease in the withdrawal threshold was interpreted as being a behavioral sign of mechanical allodynia. The sham control rats showed some reduction in the threshold, however, this was not significantly different from the value obtained before the SCI. The withdrawal threshold tended to increase as the time increased, nervertheless, this was still low. For example, on the 20 week PO, 17 out of 23 rats in the 6.25 mm group had thresholds lower than 4.0 g, whereas 14 out of 19 rats in the 12.5 mm group had threshold less than 4.0 g, which is normally regarded as an innocuous stimulus.
Fig. 4.
Time course of 50% withdrawal threshold to the graded von Frey filaments stimulation applied to the plantar surface of the hindpaw. The average paw withdrawal threshold before the contusion was approximately 15 g. However, following the contusion injury, the withdrawal thresholds to von Frey stimulation decreased markedly to 1.96 ± 0.35 g in the 6.25 mm group and 1.31 ± 0.16 g in the 12.5 mm group at 2 weeks PO and was slightly increased with the lapse of time. Asterisks (*) indicate the values significantly different from the pre-operative value. The time after the spinal cord contusion indicates weeks (W) following spinal contusion, except for "pre", which represents the preoperative test period.
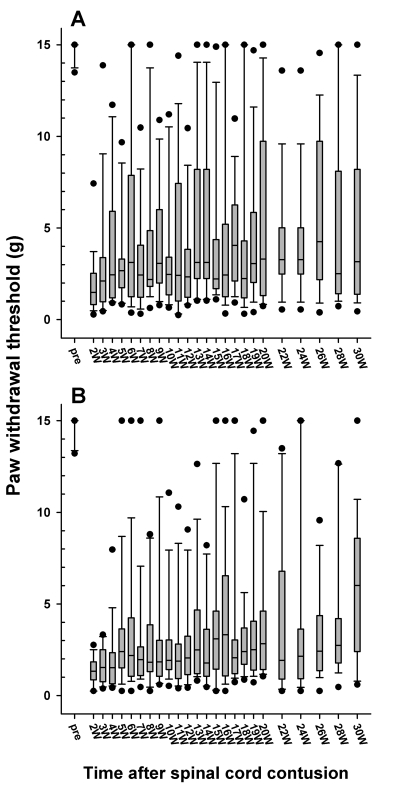
Fig. 5 shows the distribution of the withdrawal threshold to the mechanical stimulation after 6.25 and 12.5 mm injury, respectively. There was no significant difference in the magnitude of the withdrawal threshold reduction and the incidence of allodynia between the 6.25 mm and 12.5 mm groups. Also there was no significant difference in threshold between the right and left paws.
Fig. 5.
Follow-up of the paw withdrawal thresholds to mechanical stimulation in the 6.25 (A) and 12.5 mm (B) injury groups, which are expressed as a box plot. The data are expressed as a box plots with median (horizontal bar) and percentile points (25~75%; box) and 10~90% (error bar). The time after the spinal cord contusion indicates weeks (W) following the spinal contusion, except for "pre", which represents the preoperative test period.
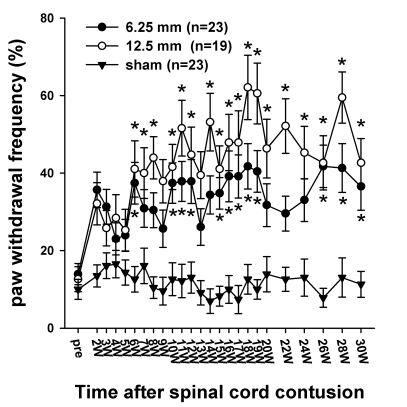
The rats were also tested for the presence of cold allodynia (Fig. 6). Cold allodynia was determined by measuring the paw withdrawal frequency after the application of acetone. Brisk paw withdrawal and licking, and lifting and shaking the hindpaw were regarded as positive responses. These responses were usually accompanied with turning head to the direction of the stimulus, guarding and hyperextending hindlimbs, which were not included in the positive response. Prior to injury, the withdrawal frequencies in the spinal contusion groups were 13.9 ± 2.7% in the 6.25 mm group and 12.6 ± 3.2% in the 12.5 mm group. Two weeks after the SCI, the withdrawal frequencies in both hindpaws to the application of acetone increased to 35.7 ± 4.6% and 32.1 ± 5.4% respectively, but the increase in the withdrawal frequency was not significantly different from the pre-injury value (p>0.05). The response frequencies tended to increase steadily, showing significantly increased responses compared to pre-injury value, especially in the 12.5 mm group during 6~30 weeks PO and in the 6.25 mm group during 10~20 weeks PO. Various aversive responses to the application of acetone were observed more frequently and more severely in the 12.5 mm group than in the 6.25 mm group. Even though the responses to the application of acetone increased after the SCI, they were slowly developed and not consistently elevated as opposed to that observed with mechanical allodynia. The sham group did not produce any significant changes.
Fig. 6.
Time course of cold sensitivity. The frequencies of the foot withdrawals to cold stimuli caused by the application of acetone to the hindpaw are depicted. Before surgery, the withdrawal frequencies were 13.9 ± 2.7% in the 6.25 mm group and the 12.6 ± 3.2% in 12.5 mm group. Two weeks after the SCI, the withdrawal frequencies to the application of acetone in the 6.25- and 12.5 mm groups increased to 35.7 ± 4.6% and 32.1 ± 5.4%, respectively. The withdrawal responses in the 12.5 mm group were generally higher than those in the 6.25 mm, however, there was no difference between the two experimental groups. The asterisks (*) indicate the values significantly different from the pre-operative value. The time after the spinal cord contusion indicates weeks (W) following the spinal contusion, except for "pre", which represents the preoperative test period.
DISCUSSION
This study showed that the hindpaw withdrawal thresholds to von Frey stimulation decreased after the spinal contusion, indicating that mechanical allodynia developed below the level of the injury and was maintained for a long time. Chronic pain perseveres in human patients for many years after initial SCI, and only 4~6% of patients experience spontaneous remission (Siddall et al, 2003; Cruz-Almeida et al, 2005). Therefore, SCI-induced pain is substantially life-long pain. Animal models mimicking human SCI are needed to find out their characteristics including temporal aspects of pain. Rats with SCI induced by hemisection showed behavioral signs of mechanical allodynia for 22~26 weeks (Christensen et al, 1996; Kim et al, 2003) and earlier studies (Lindsey et al, 2000; Yoon et al, 2004) showed that behavioral signs of neuropathic pain were expressed for 10 weeks. In this study, rats with contusive injury showed decreased hindpaw withdrawal thresholds to mechanical stimulation for 30 weeks PO. The duration of neuropathic pain in peripheral nerve injury models is comparable to that in SCI models (Choi et al, 1994; Decosterd & Woolf, 2000).
The incidence and withdrawal threshold in the rats with 6.25 mm and 12.5 mm injuries were not different. Recently, it was reported that rats with mild injuries produced more robust mechanical allodynia following a SCI by a NYU impactor (Yoon et al, 2004). They observed mechanical and cold allodynia for 10 weeks after the SCI, and that rats with 6.25 mm injury showed more consistent and severe allodynia. In contrast to those reports, Lindsey et al, (Lindsey et al, 2000) reported that rats with 25 mm-injuries showed the most consistent decrease in the hindpaw withdrawal threshold to von Frey hair stimulation among 6.25, 12.5 and 25 mm injury groups. They reported that the withdrawal threshold decreased to approximately 15 g following a 12.5 mm injury, which is much higher than the values obtained in this study. The sources of this discrepancy are unclear at present, nevertheless, differences in rat strain, gender, and handling may have to be considered. In this study, an attempt was made to test the mechanical allodynia in rats with 25 mm injury. However, the behavioral tests for the below-level pain could not be reliably performed due to the poor motor response required for the assessment of the sensory test. At two weeks PO, which was the initial test point in this study, the rats with the 25 mm injury usually could not stand on their plantar surfaces, which was a prerequisite for the test in our setting. Recently, rats with 25 mm injury have occasionally been used for pain research, especially 4 weeks after contusive injury (Berrocal et al, 2007; Zhao et al, 2007). At this time, the degree of motor recovery reaches the level that plantar placement of the hindpaw is possible (BBB score is around 10). Therefore, once rats can stand with their plantar surface, conventional plantar approach with von Frey filaments for sensory tests can be applicable.
The rats with 12.5 mm injury showed patterns and degrees of mechanical allodynia similar to the rats with 6.25 mm injury even though the lesions in the spinal cord were apparently more extensive (Basso et al, 1996a). We did not use 25 mm injury in this study, but a recent report (Zhao et al, 2007) using rats with 25 mm drop-injury by NYU impactor showed that hindpaw withdrawal threshold was 2~4 g, which is similar to the values obtained in this study. It is not known whether severer injury increases the chance of developing pain or produces more severe type of pain; severe spinal lesions with total destruction of the ascending sensory systems have been shown to be not followed by pain syndromes (Beric, 1993) and residual intact spinothalamic pathways may contribute to neuropathic pain (Wasner et al, 2008), indicating significance of remaining ascending pathways in neuropathic pain. Even the 6.25 mm drop injury by NYU impactor causes the least injury and produces approximately half the loss of the corresponding spinal cord (Basso et al, 1996a). Therefore, it is not clear whether the 6.25 mm drop is truly a mild injury. Although a drop higher than 25 mm produces more extensive damage to the corresponding spinal cord, rats with those injuries cannot reliably be tested, particularly for the below-level pain, because of their poor motor performance especially during the first few weeks. Even if the rats with 50 mm injury or an even more severe injury have indeed a robust below-level pain, it would be difficult to detect this in the paralyzed animals because behavioral tests for pain are almost entirely dependent on their behaviors. This is an obvious obstacle in expressing pain in animal models that are made to mimic the human disease state. Accordingly, it is speculated that 1) the incidence and/or severity of pain after a contusive SCI would be similar to the spinal cord within some range of damage as in the present results; 2) the incidence and/or severity of pain may be dependent on the site of the lesion which is involved in the critical structures producing neuropathic pain. Yezierski et al. (1998) reported excessive grooming behavior to suggest at-level pain and mechanical hypersensitivity in the hindlimbs to indicate below-level pain following quisqualate injection into the spinal cord, and suggested that sparing laminae I and II was essential for the rat to express an excessive grooming behavior. However, they could not draw any conclusion as to what was the critical lesion to produce mechanical hypersensitivity in the hindlimb, and could not correlate the severity of cord damage and the magnitude of mechanical hypersensitivity. Therefore, more studies are needed to determine the effects of the lesion size and the regions on the characteristics of pain after a SCI.
Cold allodynia following a SCI was reported in spinal ischemic (Hao et al, 1996), hemisection (Kim et al, 2003) and excitotoxic (Brewer & Yezierski, 1998) injury models by applying ethyl chloride or acetone to the skin or by the observation of behavioral changes on a cold plate. In this study, acetone was applied to the plantar surface of the foot, and the frequency of foot withdrawals was recorded. This method has previously been used to quantify cold allodynia in rats (Choi et al, 1994). The present findings that normal rats were rarely responsive to the application of acetone, and that the rats showed increased reactivity to acetone with an associated behavior such as licking and shaking after SCI indicate that cold allodynia develops in the hindlimb. Frequencies of foot withdrawals to acetone were increased on 2 week PO, which was not high enough to get statistical significance. The response frequencies tended to steadily increase, showing significantly increased responses compared to pre-injury value, especially after 6 or 10 weeks PO in 12.5 mm group and 6.25 mm group, respectively. Compared to mechanical allodynia, development of cold allodynia was delayed. Recently, Voda et al. (2007) reported that rats with 12.5 mm injury by NYU impactor exhibited increased responses to acetone application from 1 weeks PO to 5 week PO, the end of their observation. The sources of discrepancies, especially for the early phase after SCI, are not known. Nevertheless, cold allodynia may develop soon after SCI and maintain for a long time. Lindsey et al. (2000) also reported that contusion rats showed an increased hindpaw withdrawal frequency and decreased withdrawal latency to an ice probe (0℃). However, these behaviors are regarded as behavioral signs of cold hyperalgesia because normal rats lifted their foot 40~60% in response to the ice probe stimulation.
Although several animal models of a SCI have been developed to explore its pathophysiology (Ramer et al, 2000; Vierck et al, 2000; Rosenzweig & McDonald, 2004), a pain study on animal models has only been carried out relatively recently (Hao et al, 1992; Christensen et al, 1996; Yezierski et al, 1998), compared with other fields such as degeneration or regeneration. The contusion model is regarded as a model comparable to human SCI (Metz et al, 2000; Ramer et al, 2000; Rosenzweig & McDonald, 2004). The NYU impactor, which allows a 10 g rod drop from the predetermined heights, is one of devices that produces contusion injury. Its advantages include standardization of the injury and correlation between levels of injury and various indexes of actual spinal cord damages such as lesion volume (Gruner, 1992; Constantini and Young, 1994; Basso et al, 1996b; Metz et al, 2000). Among several SCI animal models, this model is considered to be a proper model for assessment of therapeutic efficacy (Ramer et al, 2000). Therefore, long term observation of sensory changes, especially neuropathic pain, would be a ground for further study.
In summary, contusive SCI produces behavioral changes to indicate below-level neuropathic pain such as mechanical and cold allodynia in the hindpaw. The severity and incidence of pain were not significantly different between two different degrees of contusion. This study provides behavioral evidence to show that below-level pain is well developed and maintained for a long time in the contusion model, which parallels human traumatic SCI. It may also provide basic data to explore pathophysiology of pain mechanisms or drug responses over time.
ACKNOWLEDGEMENT
This work was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ8-PG1-01CN01-0034).
ABBREVIATIONS
- BBB
basso, beattie and bresnahan
- CBS
combined behavioral score
- MASCIS
multicenter animal spinal cord injury study
- NYU
new york university
- SCI
spinal cord injury
References
- 1.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 2.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996a;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 3.Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996b;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 4.Behrmann DL, Bresnahan JC, Beattie MS. Modeling of acute spinal cord injury in the rat: neuroprotection and enhanced recovery with methylprednisolone, U-74006F and YM-14673. Exp Neurol. 1994;126:61–75. doi: 10.1006/exnr.1994.1042. [DOI] [PubMed] [Google Scholar]
- 5.Beric A. Central pain: "new" syndromes and their evaluation. Muscle Nerve. 1993;16:1017–1024. doi: 10.1002/mus.880161004. [DOI] [PubMed] [Google Scholar]
- 6.Beric A, Dimitrijevic MR, Lindblom U. Central dysesthesia syndrome in spinal cord injury patients. Pain. 1988;34:109–116. doi: 10.1016/0304-3959(88)90155-8. [DOI] [PubMed] [Google Scholar]
- 7.Berrocal Y, Pearse DD, Singh A, Andrade CM, McBroom JS, Puentes R, Eaton MJ. Social and environmental enrichment improves sensory and motor recovery after severe contusive spinal cord injury in the rat. J Neurotrauma. 2007;24:1761–1772. doi: 10.1089/neu.2007.0327. [DOI] [PubMed] [Google Scholar]
- 8.Boivie J. On central pain and central pain mechanisms. Pain. 1989;38:121–122. doi: 10.1016/0304-3959(89)90229-7. [DOI] [PubMed] [Google Scholar]
- 9.Brewer KL, Yezierski RP. Effects of adrenal medullary transplants on pain-related behaviors following excitotoxic spinal cord injury. Brain Res. 1998;798:83–92. doi: 10.1016/s0006-8993(98)00398-9. [DOI] [PubMed] [Google Scholar]
- 10.Bunge RP, Puckett WR, Hiester ED. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv Neurol. 1997;72:305–315. [PubMed] [Google Scholar]
- 11.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 13.Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- 14.Constantini S, Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg. 1994;80:97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- 15.Cruz-Almeida Y, Martinez-Arizala A, Widerstrom-Noga EG. Chronicity of pain associated with spinal cord injury: A longitudinal analysis. J Rehabil Res Dev. 2005;42:585–594. doi: 10.1682/jrrd.2005.02.0045. [DOI] [PubMed] [Google Scholar]
- 16.Davidoff G, Roth EJ, Casey KL. Clinical characteristics of central (dysesthetic) pain in spinal cord injury patients. In: Casey KL, editor. Pain and central nervous system disease: The central pain syndromes. New York: Raven; 1991. pp. 77–83. [Google Scholar]
- 17.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 18.Finnerup NB, Jensen TS. Spinal cord injury pain-mechanisms and treatment. Eur J Neurol. 2004;11:73–82. doi: 10.1046/j.1351-5101.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 19.Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- 20.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- 21.Hao JX, Xu XJ, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Photochemically induced transient spinal ischemia induces behavioral hypersensitivity to mechanical and cold stimuli, but not to noxious-heat stimuli, in the rat. Exp Neurol. 1992;118:187–194. doi: 10.1016/0014-4886(92)90035-o. [DOI] [PubMed] [Google Scholar]
- 22.Hao JX, Yu W, Xu XJ, Wiesenfeld-Hallin Z. Capsaicin-sensitive afferents mediate chronic cold, but not mechanical, allodynia-like behavior in spinally injured rats. Brain Res. 1996;722:177–180. doi: 10.1016/0006-8993(96)00216-8. [DOI] [PubMed] [Google Scholar]
- 23.Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW. The impact of morphine after a spinal cord injury. Behav Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Yoon YW, Hong SK, Na HS. Cold and mechanical allodynia in both hindpaws and tail following thoracic spinal cord hemisection in rats: time courses and their correlates. Neurosci Lett. 2003;343:200–204. doi: 10.1016/s0304-3940(03)00377-x. [DOI] [PubMed] [Google Scholar]
- 25.Lee KH, Suh-Kim H, Choi JS, Jeun SS, Kim EJ, Kim SS, Yoon DH, Lee BH. Human mesenchymal stem cell transplantation promotes functional recovery following acute spinal cord injury in rats. Acta Neurobiol Exp (Wars) 2007;67:13–22. doi: 10.55782/ane-2007-1628. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Jung J, Kim J, Kim K, Hong SK, Yoon YW. Behavioral signs of pain in a rat spinal contusion model. Program No. 981.4. Washington, DC: Society for Neuroscience; 2004. Abstract Viewer/Itinerary Planner. [Google Scholar]
- 27.Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- 28.Metz GA, Curt A, van de MH, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17. doi: 10.1089/neu.2000.17.1. [DOI] [PubMed] [Google Scholar]
- 29.Ramer MS, Harper GP, Bradbury EJ. Progress in spinal cord research - a refined strategy for the International Spinal Research Trust. Spinal Cord. 2000;38:449–472. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- 30.Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17:121–131. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 32.Stormer S, Gerner HJ, Gruninger W, Metzmacher K, Follinger S, Wienke C, Aldinger W, Walker N, Zimmermann M, Paeslack V. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997;35:446–455. doi: 10.1038/sj.sc.3100411. [DOI] [PubMed] [Google Scholar]
- 33.Tasker RR, Loeser JD. Central Pain States. In: Loeser JD, editor. Bonica's Management of Pain. Philadelphia: Lippincott Williams Wilkins; 2001. pp. 433–457. [Google Scholar]
- 34.Vierck CJ, Jr, Siddall P, Yezierski RP. Pain following spinal cord injury: animal models and mechanistic studies. Pain. 2000;89:1–5. doi: 10.1016/S0304-3959(00)00463-2. [DOI] [PubMed] [Google Scholar]
- 35.Voda J, Hama A, Sagen J. FK506 reduces the severity of cutaneous hypersensitivity in rats with a spinal cord contusion. Neurosci Res. 2007;58:95–99. doi: 10.1016/j.neures.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–2400. doi: 10.1093/brain/awn169. [DOI] [PubMed] [Google Scholar]
- 37.Yezierski RP. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 1996;68:185–194. doi: 10.1016/s0304-3959(96)03178-8. [DOI] [PubMed] [Google Scholar]
- 38.Yezierski RP, Liu S, Ruenes GL, Kajander KJ, Brewer KL. Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain. 1998;75:141–155. doi: 10.1016/S0304-3959(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 39.Yoon YW, Dong H, Arends JJ, Jacquin MF. Mechanical and cold allodynia in a rat spinal cord contusion model. Somatosens Mot Res. 2004;21:25–31. doi: 10.1080/0899022042000201272. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]