Abstract
Eugenol is widely used in dentistry to relieve pain. We have recently demonstrated voltage-gated Na+ and Ca2+ channels as molecular targets for its analgesic effects, and hypothesized that eugenol acts on P2X3, another pain receptor expressed in trigeminal ganglion (TG), and tested the effects of eugenol by whole-cell patch clamp and Ca2+ imaging techniques. In the present study, we investigated whether eugenol would modulate 5'-triphosphate (ATP)-induced currents in rat TG neurons and P2X3-expressing human embryonic kidney (HEK) 293 cells. ATP-induced currents in TG neurons exhibited electrophysiological properties similar to those in HEK293 cells, and both ATP- and α ,β-meATP-induced currents in TG neurons were effectively blocked by TNP-ATP, suggesting that P2X3 mediates the majority of ATP-induced currents in TG neurons. Eugenol inhibited ATP-induced currents in both capsaicin-sensitive and capsaicin-insensitive TG neurons with similar extent, and most ATP-responsive neurons were IB4-positive. Eugenol inhibited not only Ca2+ transients evoked by α ,β-meATP, the selective P2X3 agonist, in capsaicin-insensitive TG neurons, but also ATP-induced currents in P2X3-expressing HEK293 cells without co-expression of transient receptor potential vanilloid 1 (TRPV1). We suggest, therefore, that eugenol inhibits P2X3 currents in a TRPV1-independent manner, which contributes to its analgesic effect.
Keywords: ATP, Eugenol, P2X receptor, Trigeminal ganglion neurons
INTRODUCTION
Eugenol has extensively been used in dentistry because of its ability to allay tooth pain, however, the molecular mechanisms of its action remain largely unexplained. We have recently demonstrated that inhibition of voltage-gated Na+ and Ca2+ channel currents by eugenol might contribute to its analgesic effects (Lee et al, 2005; Park et al, 2006; Chung et al, 2008). Furthermore, we showed that the activation of TRPV1 and inhibition of voltage-gated K+ channel currents might be involved in its stimulatory effects (Yang et al, 2003; Li et al, 2007), and that the inhibitory effects of eugenol on voltage-gated ion channels result from TRPV1 independent mechanisms although eugenol per se activates TRPV1. Given the fact that wide ranges of ion channels are modulated by eugenol, there could be another molecular target for eugenol.
P2X receptor is Ca2+-permeable cation channel which is activated by extracellular ATP which is released from damaged cells in the injured tissues. P2X receptors are involved in a variety of physiological responses in many different tissues and cell types, including trigeminal ganglion (TG) and dorsal root ganglion (DRG) neurons (Ralevic & Burnstock, 1998; Burnstock, 2007). Among seven different isoforms (P2X1~P2X7), P2X3 receptor is known to be predominantly expressed in subpopulations of nociceptive sensory neurons (Chen et al, 1995; Lewis et al, 1995; Xiang et al, 1998; Jiang & Gu, 2002). Immunohistochemical studies showed that the P2X3 receptors are found largely in the population of nociceptive neurons which are labeled with isolectin B4 (IB4) (Eriksson et al, 1998). Indeed, P2X receptors, particularly the P2X3 subtype, have attracted a great deal of interest as a potential target for the development of novel analgesics (Burnstock, 2006).
P2X3 is expressed in tooth pulp, thereby mediating the initiation of pain (Burnstock, 1996). P2X3 and P2X2/3 receptors on sensory afferents in tooth pulp appear to mediate nociception not only in rats but also in human (Cook & McCleskey, 1997; Cook et al, 1997; Alavi et al, 2001; Renton et al, 2003). P2X3 was also found to be co-expressed with TRPV1 receptor in small to medium-sized rat TG neurons and tooth pulp (Ichikawa & Sugimoto, 2004).
In this study, we hypothesized that P2X3 receptor would be inhibited by eugenol. We, therefore, examined its mode of action in rat nociceptive TG neurons and P2X3-expressing human embryonic kidney (HEK) 293 cells by using whole-cell patch clamp and Ca2+ imaging techniques.
METHODS
All experimental procedures for animal use were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), School of Dentistry, Seoul National University.
Preparation of TG neurons
TG neurons from neonatal rats were prepared as previously described (Park et al, 2006). Briefly, trigeminal ganglion neurons were isolated from neonatal (4- to 7-day-old) Sprague-Dawley rats (Orient BIO, INC., Gapyeong-City, Korea). Dissected TG were incubated in 3 ml of HBSS (Invitrogen, Carlsbad, CA, USA) containing 0.25% trypsin (Invitrogen) at 37℃ for 30 min. Neurons were then dissociated by trituration with a series of sterile Pasteur pipettes, re-suspended in TG media containing apyrase (2 units/ml; type VII; Sigma), and then plated onto glass coverslips previously coated with a solution of 0.1 mg/ml poly-L-ornithine (Sigma, St. Louis, MO, USA). Neurons were maintained in a humidified atmosphere of 95% air/5% CO2 at 37℃ and were used for patch clamp recording within 6 and 8 hrs after being plated.
Transient transfection of HEK293 cells
cDNAs encoding the P2X3 subunits were expressed alone or cotransfected with TRPV1 in HEK 293 cells. HEK293 cells were cultured in Dulbecco's modified Eagle's medium (WelGene, Inc., Daegu-city, Korea), supplemented with 10% heat-inactivated fetal bovine serum containing penicillin and streptomycin (Invitrogen). Cells reaching 60~80% confluency were used for transient cDNAs of P2X3 transfections with the cationic liposome method with 1 µg of supercoiled plasmid cDNA per 1×105 cells mixed with 2.5 µl enhancer and 3 µl WelFect-EX™ (WelGene) in 0.5 ml of serum-free medium. Green fluorescence protein (GFP) (Clontech Laboratories, Inc., CA, USA) was co-transfected with P2X receptors as an expression marker. After 5 hrs at 37℃, the medium was replaced with normal HEK293 medium, and cells were incubated for another 28~36 hrs before electrophysiological experiments.
Electrophysiological recordings
Whole-cell voltage-clamp recordings were performed for the measurement of ATP-induced currents with an Axopatch-1C amplifier (Axon Instruments, Union City, CA, USA). The patch electrodes and puffer pipettes were pulled from borosilicate capillaries (Chase Scientific Glass, Inc., Rockwood, TN, USA). When the pipettes were filled with the solution, their resistance was 2~4 MΩ. The kinetics of ATP-induced inward currents was determined with the decay time which was calculated as the time taken for current to fall from 90% to 10% of the peak (decay time90~10%). The extracellular solution contained (mM): NaCl 147, KCl 3, MgCl2 1, CaCl2 2, glucose 13, HEPES 10, and pH adjusted to 7.4 with NaOH. The pipette solution contained (mM): NaCl 145, glucose 10, HEPES 10, and EGTA 10, pH adjusted to 7.3 with NaOH. The resting potential was held at -60 mV. All the experiments were performed at room temperature. At the end of each recording in TG neurons, 10 µg/ml IB4-FITC was applied to the bath chamber for 10 min, and then rinsed for 10 min in extracellular solution. IB4-FITC staining was visualized with standard FITC filters.
Intracellular calcium imaging
Intracellular calcium imaging was performed as described previously (Yang et al, 2003). Briefly, neurons were loaded with fura-2 AM (2 µM; Molecular Probes, Eugene, OR) for 45 min at 37℃ in serum free medium. The cells were plated onto poly-L-ornithine-coated coverslips, mounted onto the chamber, placed on an inverted microscope (Olympus IX70, Japan) and perfused continuously with extracellular solution at 3 ml/min. Cells were illuminated with a 175-watt xenon arc lamp, and excitation wavelengths (340/380 nm) were selected by a Lambda DG-4 monochromator wavelength changer (Shutter Instrument, Novato, CA). Intracellular free calcium concentration ([Ca2+]i) was measured by digital video microfluorometry with an intensified CCD camera (CasCade, Roper Scientific, Trenton, NJ) coupled to a microscope and software (Metafluor, Universal Imaging Corp., Downingtown, PA) on a Pentium 4 computer.
Drugs
Apyrase, ATP, α,β-meATP, TNP-ATP, capsaicin, eugenol, and IB4-FITC were purchased from Sigma. ATP and α,β-meATP were applied via a glass 'puffer' pipette (1 µm tip diameter, 5 psi) using a Picopump (Medical Systems Corporation, PLI-100, Greenvale, NY, USA). The tip of the puffer pipette was positioned down-stream from the cell with respect to the direction of flow of the superfusing solution, and temporarily repositioned to a point about 15 µm from the cell for the period of application only. ATP was applied for 1s three times with the interval of 90s. Eugenol and capsaicin were dissolved in dimethylsulfoxide (DMSO) to make a stock solution, diluted to their final concentration in the extracellular solution, and then applied by gravity through a bath perfusion system. The final concentration of DMSO was less than 0.1% (v/v), which did not affect membrane currents and [Ca2+]i (Yang et al, 2003). Eugenol was applied after 10s of the first ATP application until the third ATP application, and the ATP-induced currents were compared with those observed in other cells without eugenol pretreatment. The sensitivity of eugenol-responsive cells to capsaicin was determined by the induction of inward currents by 1 µM capsaicin after the exposure of the cells to eugenol. The perfusion rate of bath solution was unchanged (4 ml/min) during the experiment.
Statistical analyses
Data are expressed as mean±SEM. ANOVA and Student's t test were used to determine the differences using the software Origin 6.0 (Northampton, MA, USA). Differences were considered to be significant when p value was less than 0.05.
RESULTS
P2X3 mediates most of the ATP-induced currents in rat TG neurons
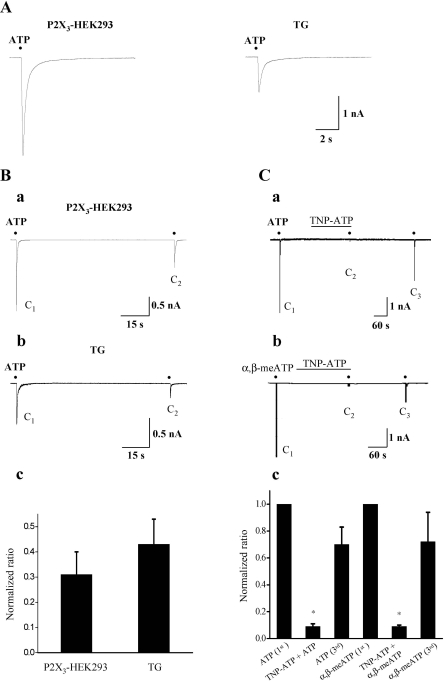
Using rat small-sized (<25 µm) TG neurons, we first characterized ATP-induced currents by comparison of those measured in P2X3-expressing HEK293 cells. In 72% (60/83) of neurons tested, application of 10 µM ATP induced inward currents at the holding potential of -60 mV, in which 93% of (n=56/60) neurons showed fast activation and inactivation with decay time90-10% of 212 ms, comparable to the kinetic properties of ATP-induced currents in P2X3-expressing HEK293 cells (decay time90-10%=225 ms) (Fig. 1A). The rest of neurons tested (n=4/60) showed fast activation and slow inactivation with decay time90-10%>500 ms (data not shown). In both TG neurons and P2X3-expressing HEK293 cells, recovery from desensitization was also extremely slow, so that the second application of ATP (C2) after 90s interval produced significantly smaller currents compared to the first application (C1) (Fig. 1Ba and Bb). The magnitude of the first 10 µM ATP-induced currents in P2X3-expressing HEK293 cells (3.25±0.50 nA, n=60) was much bigger than that of TG neurons (1.01±0.21 nA, n=10) (P<0.05), however, the normalized amplitude ratio (C2/C1) was not significantly different from each other (28±9% versus 39±10%, p>0.05) (Fig. 1Bc). When small-sized TG neurons were exposed to 1 µM TNP-ATP, a selective antagonist of P2X3, ATP-induced currents were blocked by 91±2% (n=5, p<0.05) (Fig. 1Ca and Fig. 1Cc). Moreover, α,β-meATP (100 µM), the selective agonist of P2X3 receptors, induced inward currents with similar current profile and amplitude, which were abolished by 1 µM TNP-ATP (91±1%, n=5, p<0.05) (Fig. 1Cb and Fig. 1Cc).
Fig. 1.
Comparison of current profiles of 10 µM ATP-induced currents in P2X3-expressing HEK293 cells and rat TG neurons. (A) Representative current traces activated by 10 µM ATP in P2X3-expressing HEK293 cells (left, n=60) and rat TG neurons (right, n=56). (B) Records of 10 µM ATP-induced currents activated by twice applications of 10 µM ATP with the interval of 90s in a P2X3-expressing HEK293 cell (a) and rat TG neuron (b). The normalized amplitude ratio (C2/C1) in P2X3-expressing HEK293 cells and that in TG neurons (c). (C) Inhibition of 10 µM ATP (a)- and 100 µM α,β-meATP (b)-induced currents by 1 µM TNP-ATP in small TG neurons. The summary of the inhibition of TNP-ATP on ATP (n=5)- and α,β-meATP (n=5)-induced currents (mean±SEM, p<0.05) (c). Black points indicate the time point of ATP or α,β-meATP application.
Eugenol inhibits ATP-induced P2X currents in both capsaicin-sensitive and capsaicin-insensitive TG neurons
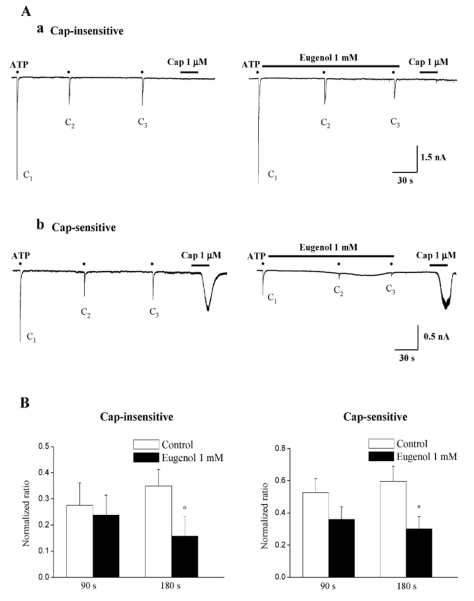
We then tested whether eugenol inhibited ATP-induced currents in rat TG neurons. Because ATP-induced currents exhibited profound desensitization (See Fig. 1) and had a tendency to show wide variation of responses by repetitive application of ATP even after the long-term washout (>10 min between applications), we employed the analytical method of Gerevich et al (2007) and compared the normalized amplitude ratio (C2/C1 and C3/C1) of ATP-induced currents with the values of the control and eugenol pretreatment. When 1 mM eugenol was applied for 90s, eugenol did not inhibit 10 µM ATP-induced P2X currents in both capsaicin-sensitive and capsaicin-insensitive TG neurons (versus the first application p>0.05) (Fig. 2A and 2B). However, 3 min application of 1 mM eugenol inhibited ATP-induced P2X currents in both capsaicin-sensitive (50±7% versus the first application, n=10) and capsaicin-insensitive (55±8% versus the first application, n=15) neurons (Fig. 2B, p<0.05). The magnitude of the inhibition of ATP-induced P2X currents by 1 mM eugenol was similar between capsaicin-insensitive and capsaicin-sensitive TG neurons (Fig. 2B, p>0.05). Eighty-four% (n=42/50) of ATP-responsive neurons, including both capsaicin-sensitive and capsaicin-insensitive neurons, were IB4-positive (data not shown).
Fig. 2.
Eugenol inhibited ATP-induced P2X currents in both capsaicin-insensitive and capsaicin-sensitive rat TG neurons. (A) Representative current traces of 10 µM ATP-induced P2X currents under control (Aa and Ab, left), and eugenol (1 mM) (Aa and Ab, right) in both capsaicin-insensitive (Aa) and capsaicin-sensitive (Ab) rat TG neurons. (B) The summary of the inhibition of ATP-induced P2X currents in both capsaicin-insensitive (Ba) and capsaicin-sensitive (Bb) rat TG neurons. The amplitude of second (C2) and third currents (C3) was normalized compared to the first one (C1). Eugenol-induced inhibition in capsaicin-insensitive neurons (n=15) was similar to that obtained in capsaicin-sensitive neurons (n=10) (mean±SEM, p>0.05). Black points indicate the time point of ATP application.
Eugenol inhibits α ,β-meATP-induced Ca2+ transients in rat TG neurons
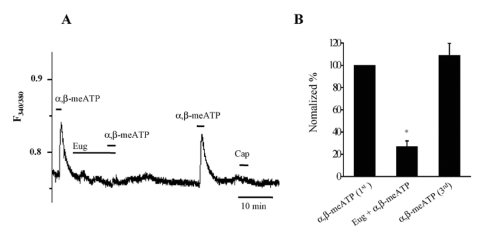
To determine specific inhibitory effects on P2X3 receptors and involvement of TPRV1 in the actions of eugenol, we examined the effects of eugenol on α,β-meATP-induced Ca2+ transients in capsaicin-insensitive TG neurons. One hundred µM α,β-meATP induced Ca2+ transients only in small-sized TG neurons, which was abolished by eugenol (Fig. 3A and 3B, 27±5% versus the first Ca2+ transient, n=9, p<0.05) in a reversible manner.
Fig. 3.
Eugenol inhibited α,β-meATP-induced Ca2+ transients in rat TG neurons. (A) α,β-meATP (100 µM) induced Ca2+ transients in small-sized TG neurons. Eugenol (1 mM) abolished α,β-meATP-induced Ca2+ transients in capsaicin (0.5 µM)-insensitive TG neurons. (B) The summary of the inhibition of α,β-meATP-induced Ca2+ transients by eugenol in capsaicin-insensitive TG neurons (mean±SEM, n=9, p<0.05). The amplitude changes of Ca2+ transients induced by second (combined application with eugenol) and third (3rd) α,β-meATP applications were normalized compared to the first one (1st).
Inhibition of ATP-induced currents by eugenol is TRPV1 independent
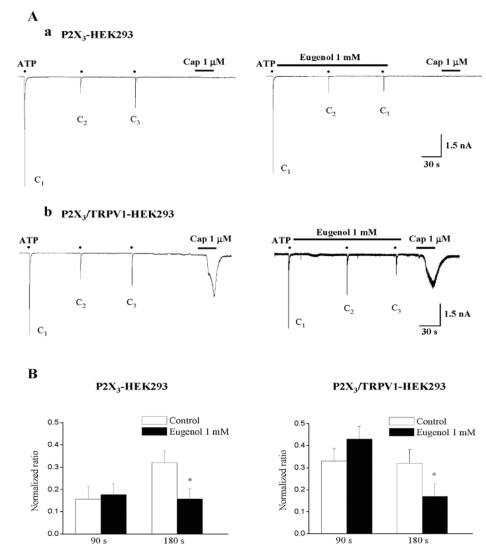
We tested the effects of 1 mM eugenol on ATP-induced currents in P2X3-expressing HEK293 cells (Fig. 4Aa). Consistent with the above TG results, the 90s application of 1 mM eugenol did not inhibit 10 µM ATP-induced currents, while 180s application of 1 mM eugenol inhibited 10 µM ATP-induced currents (51±7% versus the first application, n=13) (Fig. 4B left, p<0.05). Likewise, exposure to eugenol for 180s, but not for 90s, blocked ATP-induced currents in P2X3/TRPV1-coexpressing cells (47±6%, n=12) (Fig. 4B right), and the extent of the inhibition was comparable to that obtained in only P2X3-expressing cells (p>0.05). Capsaicin induced inward currents only in P2X3/TRPV1-coexpressing HEK293 cells tested (Fig. 4Ab), but did not in P2X3-expressing HEK293 cells.
Fig. 4.
Eugenol inhibited ATP-induced P2X3 currents in both P2X3-expressing and P2X3/TRPV1-coexpressing HEK293 cells. (A) Representative current traces of 10 µM ATP-induced P2X3 currents under control (Aa and Ab, left), and eugenol (1 mM) (Aa and Ab, right) in both P2X3-expressing (Aa) and P2X3/TRPV1-coexpressing (Ab) HEK293 cells. (B) The summary of the inhibition of ATP-induced P2X3 currents in both P2X3-expressing (Aa) and P2X3/TRPV1-coexpressing (Ab) HEK293 cells. The amplitude of second (C2) and third currents (C3) was normalized compared to the first one (C1). Eugenol-induced inhibition in P2X3-expressing cells (n=13) was similar to that obtained in P2X3/TRPV1-coexpressing cells (n=12) (mean±SEM, p>0.05). Black points indicate the time point of ATP application.
DISCUSSION
In the present study, we found that eugenol inhibited ATP-induced currents in nociceptive TG neurons, suggesting that P2X receptor could be an another molecular target for eugenol in addition to voltage-gated Ca2+ and Na+ channels which have previously been demonstrated (Lee et al, 2005; Park et al, 2006; Chung et al, 2008). In agreement with the actions of eugenol on voltage-sensitive Ca2+ and Na+ channels, modulation of P2X3 receptor by eugenol was TRPV1-independent as well.
All P2X subtypes, except P2X7, are present in TG neurons (Xiang et al, 1998). We also found mRNA expression of all P2X receptors in TG neurons (unpublished data). Among P2X receptors, however, P2X3 which is localized predominantly on small nociceptive sensory neurons in TG and DRG (Chen et al, 1995; Lewis et al, 1995; Xiang et al, 1998) has been shown to be the main P2X subtype that plays an important role in peripheral pain mechanisms in sensory neurons (Burnstock, 1996; Burnstock & Wood, 1996). Thus, we focused on P2X3 receptor, and our results provided strong evidences that P2X3 receptor is the main P2X subtype in TG neurons, not only activated by ATP but also modulated by eugenol.
First, ATP-induced currents were observed in small-sized TG neurons, and most of these ATP-sensitive TG neurons tested were IB4-positive, which was consistent with previous studies (Eriksson et al, 1998; Dunn et al, 2001). Second, ATP-induced currents exhibited typical elecrophysiological properties of P2X3 channel. The current profile of ATP-induced currents in rat TG neurons was similar to that in P2X3-expressing HEK293 cells, whereas very different from that in P2X1, P2X2, and P2X4-expressing HEK293 cells (North, 2002; Li et al, 2008), Besides, ATP-induced currents were rapidly desensitized upon repetitive ATP applications. These characteristics are in good agreement with previous studies (Luo et al, 2006). Third, both ATP- and α,β-meATP-induced currents were effectively blocked by TNP-ATP. Lastly, α,β-meATP induced Ca2+ transients in small-sized TG neurons. Taken together, these results suggest that P2X3 is the major P2X receptors expressed in nociceptive TG neurons, and that eugenol might exert its inhibitory effects on P2X3 recepors in rat TG neurons.
Eugenol inhibited ATP-induced P2X currents in nociceptive TG neurons in a TRPV1 independent manner, which is consistent with eugenol action on voltage-sensitive channels (Lee et al, 2005; Park et al, 2006; Li et al, 2007; Chung et al, 2008). Inhibition of ATP-induced P2X currents by eugenol was observed in both capsaicin-sensitive and capsaicin-insensitive TG neurons. In addition, eugenol inhibited α,β-meATP-induced Ca2+ transients in capsaicin-insensitive TG neurons. By using heterologous expression system, it was also found that TRPV1 involvement was not prerequisite for the effect of eugenol on P2X3 receptors. All these results indicate TRPV1-independent actions of eugenol on P2X3 receptor.
Because of abolishment of ATP-induced hyperalgesia in mice lacking TRPV1 receptors, the interactions between P2 and TRPV1 have previously been suggested (Caterina et al, 2000). Indeed, TG and DRG neurons expressing TRPV1 receptors also express P2X3 receptors (Guo et al, 1999; Ichikawa & Sugimoto, 2004). However, our results which were observed in both TG neurons and heterologous expression system argue against the involvement of TRPV1 in P2X3 modulation by eugenol, and detailed mechanisms remain to be elucidated. In contrast to the action of eugenol to voltage-sensitive channels, it was of an interest to note that action of eugenol on P2X was somehow delayed in both TG and heterologous expression system, suggesting a possibility of indirect interaction between eugenol and P2X which may involve other molecules.
P2X3 has been shown to be expressed in tooth pulp (Burnstock, 1996; Cook et al, 1997). ATP, which might be released from mechanically-stimulated odontoblasts or damaged pulpal cells in the mild inflamed pulp, could initiate tooth pain. Thus, our finding that eugenol inhibited P2X3 receptors provides a plausible explanation of why eugenol is therapeutically applicable in this clinical situation. Indeed, the range of eugenol concentration (~10-4 to 10-3 M) used in the present study is within the range of eugenol concentration in the cavity preparation below ZOE (Craig & Powers, 2002).
In summary, in addition to the inhibition of voltage-gated Na+ and Ca2+ channels, the inhibition of P2X3 channels by eugenol, which is TRPV1-independent, could be an additional underlying mechanism of its analgesic effects.
ACKNOWLEDGEMENT
This research was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-313-E00465). We thank Dr. North RA (Faculty of Life Sciences, The University of Manchester, UK) for the kind gift of plasmid cDNAs of P2X3.
ABBREVIATIONS
- ATP
Adenosine 5'-triphosphate
- DRG
dorsal root ganglion
- HEK293
human embryonic kidney 293
- IB4
isolectin B4
- TG
trigeminal ganglion
- TRPV1
transient receptor potential vanilloid 1
References
- 1.Alavi AM, Dubyak GR, Burnstock G. Immunohistochemical evidence for ATP receptors in human dental pulp. J Dent Res. 2001;80:476–483. doi: 10.1177/00220345010800021501. [DOI] [PubMed] [Google Scholar]
- 2.Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 9.Chung G, Rhee JN, Jung SJ, Kim JS, Oh SB. Modulation of CaV2.3 calcium channel currents by eugenol. J Dent Res. 2008;87:137–141. doi: 10.1177/154405910808700201. [DOI] [PubMed] [Google Scholar]
- 10.Cook SP, McCleskey EW. Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology. 1997;36:1303–1308. doi: 10.1016/s0028-3908(97)00132-9. [DOI] [PubMed] [Google Scholar]
- 11.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 12.Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 13.Craig RG, Powers JM. Restorative dental materials. St. Louis: Mosby; 2002. Biocompatibility of dental materials; pp. 154–155. [Google Scholar]
- 14.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson J, Bongenhielm U, Kidd E, Matthews B, Fried K. Distribution of P2X3 receptors in the rat trigeminal ganglion after inferior alveolar nerve injury. Neurosci Lett. 1998;254:37–40. doi: 10.1016/s0304-3940(98)00656-9. [DOI] [PubMed] [Google Scholar]
- 16.Gerevich Z, Zadori Z, Müller C, Wirkner K, Schröder W, Rubini P, Illes P. Metabotropic P2Y receptors inhibit P2X3 receptorchannels via G protein-dependent facilitation of their desensitization. Br J Pharmacol. 2007;151:226–236. doi: 10.1038/sj.bjp.0707217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa H, Sugimoto T. The co-expression of P2X3 receptor with VR1 and VRL-1 in the rat trigeminal ganglia. Brain Res. 2004;998:130–135. doi: 10.1016/j.brainres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Gu J. Expression of adenosine triphosphate P2X3 receptors in rat molar pulp and trigeminal ganglia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:622–626. doi: 10.1067/moe.2002.128973. [DOI] [PubMed] [Google Scholar]
- 20.Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MH, Yeon KY, Park CK, Li HY, Fang Z, Kim MS, Choi SY, Lee SJ, Lee S, Park K, Lee JH, Kim JS, Oh SB. Eugenol inhibits calcium currents in dental afferent neurons. J Dent Res. 2005;84:848–851. doi: 10.1177/154405910508400913. [DOI] [PubMed] [Google Scholar]
- 22.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can accountfor ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 23.Li HY, Oh SB, Kim JS. Pharmacological and electrophysiological characterization of rat P2X currents. IJOB. 2008;33:1–5. [Google Scholar]
- 24.Li HY, Park CK, Jung SJ, Choi SY, Lee SJ, Park K, Kim JS, Oh SB. Eugenol inhibits K+ currents in trigeminal ganglion neurons. J Dent Res. 2007;86:898–902. doi: 10.1177/154405910708600918. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Yin GF, Gu YZ, Liu Y, Dai JP, Li C, Li ZW. Characterization of three types of ATP-activated current in relation to P2X subunits in rat trigeminal ganglion neurons. Brain Res. 2006;1115:9–15. doi: 10.1016/j.brainres.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 26.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 27.North RA. The P2X3 subunit: a molecular target in pain therapeutics. Curr Opin Investig Drugs. 2003;4:833–840. [PubMed] [Google Scholar]
- 28.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park CK, Li HY, Yeon KY, Jung SJ, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Eugenol inhibits sodium currents in dental afferent neurons. J Dent Res. 2006;85:900–904. doi: 10.1177/154405910608501005. [DOI] [PubMed] [Google Scholar]
- 30.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 31.Renton T, Yiangou Y, Baecker PA, Ford AP, Anand P. Capsaicin receptor VR1 and ATP purinoceptor P2X3 in painful and nonpainful human tooth pulp. J Orofac Pain. 2003;17:245–250. [PubMed] [Google Scholar]
- 32.Sarrami N, Pemberton MN, Thornhill MH, Theaker ED. Adverse reactions associated with the use of eugenol in dentistry. Br Dent J. 2002;193:257–259. doi: 10.1038/sj.bdj.4801539. [DOI] [PubMed] [Google Scholar]
- 33.Tsuda M, Ueno S, Inoue K. In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br J Pharmacol. 1999;127:449–456. doi: 10.1038/sj.bjp.0702582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- 35.Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, Kim JS, Oh SB. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res. 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]