Abstract
The present study was aimed to determine whether there is an altered regulation of tubular transporters in gentamicin-induced nephropathy. Sprague-Dawley male rats (200~250 g) were subcutaneously injected with gentamicin (100 mg/kg per day) for 7 days, and the expression of tubular transporters was determined by immunoblotting and immunohistochemistry. The mRNA and protein expression of OAT was also determined. Gentamicin-treated rats exhibited significantly decreased creatinine clearance along with increased plasma creatinine levels. Accordingly, the fractional excretion of sodium increased. Urine volume was increased, while urine osmolality and free water reabsorption were decreased. Immunoblotting and immunohistochemistry revealed decreased expression of Na+/K+-ATPase, NHE3, NBC1, and AQP1 in the kidney of gentamicin-treated rats. The expression of OAT1 and OAT3 was also decreased. Gentamicin-induced nephropathy may at least in part be causally related with a decreased expression of Na+/K+-ATPase, NHE3, NBC1, AQP1 and OAT.
Keywords: Gentamicin, Sodium transporters, Aquaporin-1, Organic anion transporters
INTRODUCTION
Aminoglycoside antibiotics, including gentamicin, have widely been used in the treatment of Gram-negative bacterial infection. However, gentamicin may cause a dose-dependent nephrotoxicity (Bennett, 1997). Previous studies have shown that gentamicin is mainly concentrated in the proximal tubular cells, and the resultant renal dysfunction may be recognized as Fanconi-like syndrome of a proximal tubular damage (Pastoriza-Munoz et al, 1979; Gainza et al, 1997).
The tubular sodium reabsorption occurs through a two-step mechanism: Primary active transport out of the cell exerted by Na+/K+-ATPase at the basolateral membrane and passive entry at the luminal membrane via various sodium transporters (Besarab et al, 1976; Katz et al, 1979). In the proximal tubule, NHE3 provides a major route of luminal sodium transport across the membrane, whereas NBC1 serves as the exit across the basolateral membrane (Mahnensmith & Aronson, 1985; Preisig et al, 1987; Soleimani et al, 1987). On the other hand, OAT plays a crucial role in the excretion of a variety of potentially toxic compounds in the proximal tubule (van Montfoort et al, 2003).
However, despite the abnormalities in tubular function in gentamicin-induced nephropathy, the underlying molecular mechanisms remain to be elucidated. The unexpected adverse effects associated with gentamicin-induced nephropathy may occur as a consequence of altered regulation of these transporters. The present study was aimed at determining whether there are alterations of tubular transporters in the kidney following the use of gentamicin.
METHODS
Animals
Sprague-Dawley male rats, weighing 200~250 g, were used. The experimental protocol was approved by the Institutional Animal Care and Use Committee of Chonnam National University Medical School. The experimental group was treated with gentamicin (100 mg/kg per day, subcutaneously) for 7 days. Control rats were injected with vehicle only. The rats were maintained individually in the metabolic cage to allow urine collection for the measurement of sodium, creatinine and osmolality. They were killed for semiquantitative immunoblotting and immunohistochemical studies. Under anesthesia with ketamine, blood samples were collected from the inferior vena cava and analyzed for sodium, creatinine and osmolality. The right kidney was rapidly removed, and dissected cortex/OSOM was processed for immunoblotting. The left kidney was fixed by retrograde perfusion.
Another set of experiment was done for the assay of reverse transcriptase-PCR. The rats were decapitated under a conscious state, and their kidneys were rapidly taken and kept at -70℃ until assayed.
Protein preparation and Western blot analyses
The kidneys were homogenized in ice-cold isolation solution containing 0.3 M sucrose, 25 mM imidazole, 1 mM EDTA, 8.5 µM leupeptin, and 1 mM phenylmethylsulfonyl fluoride, with pH 7.4. The homogenates were centrifuged at 1,000 g for 15 min at 4℃ to remove whole cells, nuclei and mitochondria. The total protein concentration was measured (Pierce BCA protein assay reagent kit, Pierce, Rockford, IL). All samples were adjusted with isolation solution to have same final protein concentrations, solubilized at 65℃ for 15 min in SDS-containing sample buffer, and then stored at -20℃. To confirm equal loading of protein, an initial gel was stained with Coomassie blue. SDS-PAGE was performed on 8% or 12.5% polyacrylamide gels. The proteins were transferred by gel electrophoresis (Bio-Rad Mini Protean II, Bio-Rad; Hercules, CA, USA) onto nitrocellulose membranes (Amersham Pharmacia Biotech, Hybond ECL RPN3032D; Little Chalfont, UK). The blots were subsequently blocked with 5% milk in PBST (80 mM Na2HPO4, 20 mM Na2HPO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 hr and incubated overnight at 4℃ with anti-rabbit polyclonal antibodies against anti-mouse monoclonal antibody against Na+/K+-ATPase α1 subunit (Upstate Biotechnology; Lake Placid, NY, USA), NHE3, NBC1 (Alpha Diagnostic; San Antonio, TX, USA), AQP1 (Alomone Laboratories; Jerusalem, Israel), OAT1 (Alpha Diagnostic; San Antonio, TX, USA) or anti-β-actin antibody (Santa Cruz Biotechnology Inc; Santa Cruz, CA, USA). The membranes were then incubated for 2 hr with anti-rabbit or anti-mouse horseradish peroxidase-conjugated antibodies. The labeling was visualized by an enhanced chemiluminescence system.
Reverse transcriptase-PCR
The kidneys were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was extracted with chloroform, precipitated with isopropanol, washed with 70% ethanol, and then redissolved in distilled water. The RNA concentration was determined by the absorbance read at 260 nm. cDNA was generated at 50℃ for 30 min and then the samples were denatured at 94℃ for 2 min. PCR amplification was performed in 40 cycles of 94℃ for 15 s, then 55℃ for 30 s and 72℃ for 60 s. The final elongation step was 72℃ for 10 min. For OAT1, the primers were 5'-agagtcacagagccctgcat-3' (sense) and 5'-gcccaggctgtagacatagc-3' (antisense), resulting in 402 bp RT-PCR-product. For OAT3, the primers were 5'-tcctggtgggtaccagagtc-3' (sense) and 5'-ctgcat ttctgaaggcacaa-3' (antisense), resulting in a 468 bp RT-PCR-product.
Immunohistochemistry
The kidneys were fixed by in vivo perfusion of the abdominal aorta with cold 3% paraformaldehyde (pH 7.4) for 3 min. The tissue was dehydrated in graded ethanol and left overnight in xylene. After embedding in paraffin, tissue sections were made at 2 µm and mounted on gelatin-coated glass slides.
The sections were de-waxed with xylene and rehydrated with graded ethanol. Sections had endogenous peroxidase activity blocked with 0.5% H2O2 in absolute methanol for 10 min. In a microwave oven the sections were boiled in target retrieval solution (1 mM Tris, pH 9.0, with 0.5 mM EGTA) for 10 min. After cooling, nonspecific binding was blocked with 50 mM NH4Cl in PBS for 30 min followed by 3×10 min with PBS blocking-buffer containing 1% BSA, 0.05% saponin and 0.2% gelatin. The sections were incubated overnight with primary antibody (diluted in PBS with 0.1% BSA and 0.3% Triton-X-100) at 4℃. The sections were washed 3×10 min with PBS wash-buffer containing 0.1% BSA, 0.05% saponin and 0.2% gelatin and incubated with horseradish peroxidase conjugated secondary antibody for 1 hr at room temperature. After 3×10 min rinses with PBS wash-buffer the sites with antibody-antigen reaction were visualized with a brown chromogen produced within 10 min by incubation with 0.05% 3, 3'-diaminobenzidine tetrachloride dissolved in distilled water with 0.1% H2O2. Mayers hematoxylin was used for counterstaining and coverslips were mounted with hydrophobic medium after dehydration. The microscopy was carried out using an Olympus light microscope (Olympus, Tokyo, Japan).
Reagents and statistical analysis
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless stated otherwise. Data are expressed as means±SEM. The statistical significance of differences between groups was determined by unpaired t-test.
RESULTS
Urinary parameters
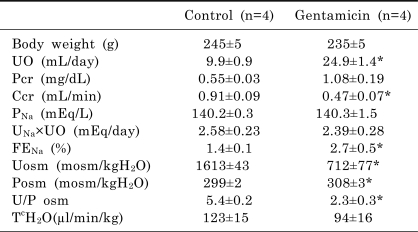
Table 1 summarizes the urinary parameters. Gentamicin-treated rats exhibited significantly decreased creatinine clearance along with increased plasma creatinine levels. The fractional urinary excretion of sodium was increased. Accordingly, the urine volume was increased. Urine osmolality, urine to plasma osmolality ratio and free water reabsorption were decreased.
Table 1.
Urinary parameters in control and gentamicin-treated rats
Values are expressed as mean±SEM. The values were measured onthe last day of experiment. UO, urine output; Pcr, plasma creatinine; Ccr, creatinine clearance; PNa, plasma sodium; UNa×UO, urinary sodium excretion; FENa, fractional excretion of sodium; Uosm, urine osmolality; Psom, plasma osmolality; U/P osm, urine to plasma osmolality ratio; TcH2O, solute-free water absorption. *p<0.05, compared with control.
Expression of Na+/K+-ATPase, NHE3, NBC1, and AQP1
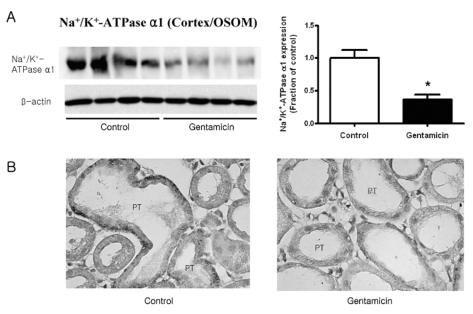
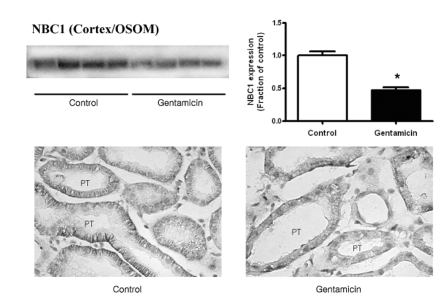
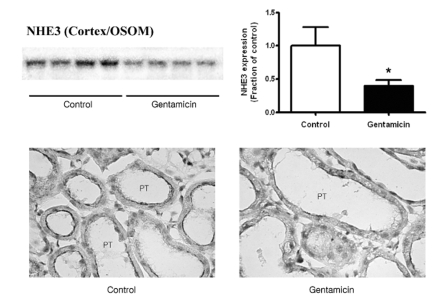
Fig. 1~3 show the expression of Na+/K+-ATPase, NHE3, and NBC1 proteins, respectively. The expression of Na+/K+-ATPase, NHE3, and NBC1 was markedly decreased in parallel in gentamicin-treated rats. The immunolabeling of Na+/K+-ATPase and NBC1 was mainly observed in the basolateral plasma membrane of proximal tubules (Fig. 1 & 3), while that of NHE3 appeared in the apical membrane (Fig. 2). Being consistent with the immunoblotting data, there were decreases of their immunolabeling in the proximal tubule (Fig. 1~3).
Fig. 1.
Expression of Na+/K+-ATPase in cortex/OSOM. (A) The expression of Na+/K+-ATPase was significantly decreased in gentamicin-treated rats, while that of β-actin remained unaltered. Each column represents mean±SEM of 4 rats (*p<0.05, compared with control). (B) Immunostaining of Na+/K+-ATPase showed decreased expression in gentamicintreated rats. Magnification ×400.
Fig. 3.
Expression of NBC1 in cortex/OSOM. (A) The protein expression of NBC1 was significantly decreased in gentamicin-treated rats. Each column represents mean±SEM of 4 rats (*p<0.05, compared with control). (B) Immunostaining of NBC1 showed decreased expression in gentamicin-treated rats. Magnification ×400.
Fig. 2.
Expression of NHE3 in cortex/OSOM. (A) The protein expression of NHE3 was significantly decreased in gentamicin-treated rats. Each column represents mean±SEM of 4 rats (*p<0.05, compared with control). (B) Immunostaining of NHE3 showed decreased expression in gentamicin-treated rats. Magnification ×400.
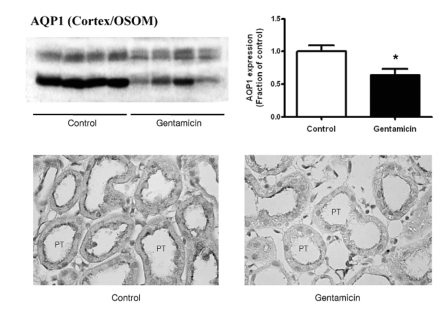
Fig. 4 shows the expression of AQP1. It was markedly decreased in gentamicin-treated rats. Being consistent with the immunoblotting data, its immunolabeling was decreased in the proximal tubule.
Fig. 4.
Expression of AQP1 in cortex/OSOM. (A) The protein expression of AQP1 was significantly decreased in gentamicin-treated rats. Each column represents mean±SEM of 4 rats (*p<0.05, compared with control). (B) Immunostaining of AQP1 showed decreased expression in gentamicin-treated rats. Magnification ×400.
Expression of OAT
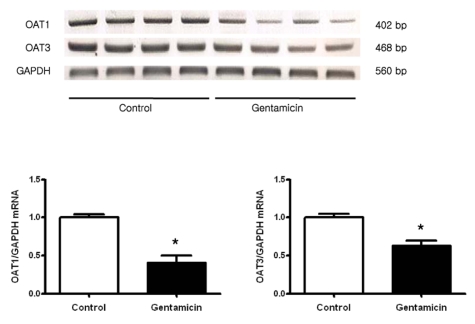
Fig. 5 shows the expression of OAT1 and OAT3 mRNA. Both OAT1 and OAT3 were markedly decreased in gentamicin-treated rats. The protein expression of OAT1 was also decreased (Fig. 6A). Immunohistochemistry of OAT1 revealed its labeling in the basolateral membrane of proximal tubule cells, the degree of which was markedly decreased in gentamicin-treated rats (Fig. 6B).
Fig. 5.
Expression of OAT1 and OAT3 mRNA in whole kidney. Each column represents mean±SEM of 4 rats (*p<0.05, compared with control).
Fig. 6.
Expression of OAT1 in cortex/OSOM. (A) The protein expression of OAT1 was significantly decreased in gentamicin-treated rats. Each column represents mean±SEM of 4 rats (*p<0.05, compared with control). (B) Immunostaining of OAT1 showed decreased expression in gentamicin-treated rats. Magnification ×400.
DISCUSSION
Following the treatment with gentamicin, the expression of sodium transporters (Na+/K+-ATPase, NHE3, NBC1) was significantly decreased, thereby increasing the fractional excretion of sodium and decreasing glomerular filtration rate. Therefore, the impaired tubular sodium reabsorption may be attributed to the downregulation of sodium transporters. Furthermore, it is likely that a decreased activity of sodium transporters in the proximal nephron would result in an increased distal delivery of sodium to activate the tubuloglomerular feedback, thereby decreasing glomerular filtration rate.
Accordingly, the urine volume was increased, and the free water reabsorption decreased. The AQP water channels have been known to play an important role in the urinary concentration, in which AQP1 provides the major route for water reabsorption in the proximal nephron. In the present study, the expression of AQP1 was indeed decreased, being consistent with our earlier findings (Lee et al, 2001). The urinary concentration defect may in part be attributed to the decreased abundance of AQP1 in gentamicin-induced nephropathy.
The present study also showed that the expression of OAT1 and OAT3 in the cortex was decreased in gentamicin-treated rats. Their basolateral localization constitutes the terminal step in a tertiary active transport system dependent on an inward-directed Na+-gradient to drive the uptake of α-ketoglutarate, which is then exchanged for organic anions (Sweet et al, 2003). The inward movement of Na+ drives the uptake of α-ketoglutarate by Na+/dicarboxylate cotransporter 3 (Sweet, 2005). Taken together, the downregulation of OAT1 and OAT3 may be attributed to a downregulation of Na+/K+-ATPase which results in an attenuation of inward Na+ gradient and hence the uptake of α-ketoglutarate.
It is unclear whether the altered regulation of these transporters is directly due to gentamicin-induced tubular effects or secondarily as a consequence of other foregoing effects. It has been suggested that an altered renal hemodynamics, such as renal vasoconstriction, may contribute to the gentamicin-mediated nephropathy (Baylis et al, 1977). We have recently shown that the treatment with gentamicin increases the expression of endothelin-1 in the kidney (Bae et al, 2007), and that an ischemic injury may cause a downregulation of sodium transporters in the kidney (Bae et al, 2008). Taken together, the decreased abundance of sodium transporters may in part be causally related with the renal hypoperfusion, which remains to be further elucidated.
In conclusion, the downregulation of Na+/K+-ATPase, NHE3, NBC1, and AQP1 in the proximal tubule may contribute to the impairment of tubular sodium reabsorption and concentrating ability. The decreased OAT abundance would result in an impaired secretion of organic anions.
ACKNOWLEDGMENT
This work was supported by grant (R01-2006-000-10554-0) from the Basic Research Program of the Korea Science & Engineering Foundation, and Chonnam National University (2007).
ABBREVIATIONS
- NHE3
type 3Na+/K+ exchangers
- NBC1
type 1 Na+/HCO3- cotransporters
- AQP1
aquaporin-1
- OAT
organic anion transporters
- OSOM
outer stripe of outer medulla
References
- 1.Bae EH, Kim IJ, Oh YW, Bae WK, Park JW, Ma SK, Kim NH, Choi KC, Lee J, Kim SH, Kim SW. Increased expression of endothelin-1 and CYP11B2 in gentamicin-induced nephropathy in rat kidney. Korean J Nephrol. 2007;26:660–668. [Google Scholar]
- 2.Bae EH, Lee KS, Lee J, Ma SK, Kim NH, Choi KC, Frokiaer J, Nielsen S, Kim SY, Kim SZ, Kim SH, Kim SW. Effects of alpha-lipoic acid on ischemia-reperfusion-induced renal dysfunction in rats. Am J Physiol Renal Physiol. 2008;294:F272–F280. doi: 10.1152/ajprenal.00352.2007. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C, Rennke HR, Brenner BM. Mechanisms of the defect in glomerular ultrafiltration associated with gentamicin administration. Kidney Int. 1977;12:344–353. doi: 10.1038/ki.1977.121. [DOI] [PubMed] [Google Scholar]
- 4.Bennett WM. Drug nephrotoxicity: an overview. Ren Fail. 1997;19:221–224. doi: 10.3109/08860229709026280. [DOI] [PubMed] [Google Scholar]
- 5.Besarab A, Silva P, Epstein FH. Multiple pumps for sodium reabsorption by the perfused kidney. Kidney Int. 1976;10:147–153. doi: 10.1038/ki.1976.88. [DOI] [PubMed] [Google Scholar]
- 6.Gainza FJ, Minguela JI, Lampreabe I. Aminoglycoside-associated Fanconi's syndrome: an underrecognized entity. Nephron. 1997;77:205–211. doi: 10.1159/000190274. [DOI] [PubMed] [Google Scholar]
- 7.Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979;237:F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Yoo KS, Kang DG, Kim SW, Choi KC. Gentamicin decreases the abundance of aquaporin water channels in rat kidney. Jpn J Pharmacol. 2001;85:391–398. doi: 10.1254/jjp.85.391. [DOI] [PubMed] [Google Scholar]
- 9.Mahnensmith RL, Aronson PS. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985;56:773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- 10.Pastoriza-Munoz E, Bowman RL, Kaloyanides GJ. Renal tubular transport of gentamicin in the rat. Kidney Int. 1979;16:440–450. doi: 10.1038/ki.1979.149. [DOI] [PubMed] [Google Scholar]
- 11.Preisig PA, Ives HE, Cragoe EJ, Jr, Alpern RJ, Rector FC., Jr Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest. 1987;80:970–978. doi: 10.1172/JCI113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soleimani M, Grassi SM, Aronson PS. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987;79:1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweet DH. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol Appl Pharmacol. 2005;204:198–215. doi: 10.1016/j.taap.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol. 2003;284:F763–F769. doi: 10.1152/ajprenal.00405.2002. [DOI] [PubMed] [Google Scholar]
- 15.van Montfoort JE, Hagenbuch B, Groothuis GM, Koepsell H, Meier PJ, Meijer DK. Drug uptake systems in liver and kidney. Curr Drug Metab. 2003;4:185–211. doi: 10.2174/1389200033489460. [DOI] [PubMed] [Google Scholar]