Abstract
Human bone marrow mesenchymal stem cells (hBM-MSCs) represent a potentially valuable cell type for clinical therapeutic applications. The present study was designed to evaluate the effect of long-term culturing (up to 10th passages) of hBM-MSCs from eight individual amyotrophic lateral sclerosis (ALS) patients, focusing on functional ion channels. All hBM-MSCs contain several MSCs markers with no significant differences, whereas the distribution of functional ion channels was shown to be different between cells. Four types of K+ currents, including noise-like Ca+2-activated K+ current (IKCa), a transient outward K+ current (Ito), a delayed rectifier K+ current (IKDR), and an inward-rectifier K+ current (Kir) were heterogeneously present in these cells, and a TTX-sensitive Na+ current (INa,TTX) was also recorded. In the RT-PCR analysis, Kv1.1, heag1, Kv4.2, Kir2.1, MaxiK, and hNE-Na were detected. In particular, INa,TTX showed a significant passage-dependent increase. This is the first report showing that functional ion channel profiling depend on the cellular passage of hBM-MSCs
Keywords: Bone marrow, Stem cells, Functional ion channels, Tetrodotoxin-sensitive Na+ current, Passage-dependency
INTRODUCTION
Clinical trials evaluating hBM-MSCs have demonstrated the ability of these cells to repair the site of tissue damage in cartilage fractures (Hannouche et al, 2007), metabolic diseases (Karnieli et al, 2007), amyotrophic lateral sclerosis (Mazzini et al, 2006), and myocardial infarction (Tomita et al, 1999; Orlic et al, 2001).
The original report on MSCs by Friedenstein et al. (1974) demonstrated that the MSCs can be amplified to approximately 30 doublings in culture while maintaining the ability of differentiate into osteoblasts, chondrocytes, and adipocytes. However, DiGirolamo CM et al and other researchers have shown that hBM-MSCs cultured in vitro display a tendency to lose their proliferative potential, homing capability, and multipotentiality (Banfi et al, 2000; Rombouts & Ploemacher, 2003; Stenderup et al, 2003). Indeed, some reports have demonstrated that telomere length changes after each cell cycle division in BM-MSCs (Baxter et al, 2004; Bonab et al, 2006).
Ion channels are widely expressed in proliferative cells which can modulate the proliferation of cells by affecting the cell cycle (MacFarlane & Sontheimer, 2000; Ouadid-Ahidouch et al, 2004; Biagiotti et al, 2006). Recent studies from our group and others have demonstrated that multifunctional ion channels are heterogeneously expressed in various species of MSCs (Li et al, 2005; Deng et al, 2006; Li et al, 2006; Park et al, 2007; Tao et al, 2007). The present study was designed to investigate changes in the expression patterns of passage-dependent ion channels in undifferentiated hBM-MSCs from ALS patients using a whole cell patch clamp technique and real time RT-PCR.
METHODS
Study population and hBM-MSC cultures
The donors were eight ALS patients: six males aged 35~63 years and two females aged 61 years. All patients signed informed written consent forms. This study was approved by the Institutional Review Board at Hanyang University (Seoul, Korea).
To isolate hBM-MSCs, bone marrow was obtained from the iliac crests of ALS patients. Mononuclear cells were isolated using a density gradient (Sigma-Aldrich, St. Louis, MO, density 1.077 g/mL). After 3 days, non-adherent cells were removed and the culture medium was changed twice per week. During in vitro passage (up to 10th), the cells were expanded for successive passages until they were confluent.
Flow cytometry analysis and differentiation of hBM-MSCs
Cells were analyzed by flow cytometry (FACSCalibur A, BD Biosciences, San Jose, CA), as previously described (Park et al, 2007). Cell-surface epitopes were evaluated using anti-human antibodies: CD29-fluorescein isothiocyanate (FITC), CD105-FITC, HLA-DR-FITC, CD34-phycoerythrin (PE), CD45-PE (Serotec, Oxford, UK), CD44-FITC (DakoCytomation, Glostrup, Denmark), and CD73-PE (BD Bioscience, Franklin Lakes, NJ). Mouse isotype antibodies (FITC, PE) served as controls (Serotec). Adipogenic and osteogenic differentiations were performed according to the manufacturer's instructions using a Human Mesenchymal Stem Cell Functional Identification Kit (R&D systems, Minneapolis, MN).
Electrophysiological recordings and chemicals
The hBM-MSCs from eight samples were used for the ionic current studies using a whole cell patch clamp technique, as previously described (Park et al, 2007). The experiments were conducted at room temperature. Four-aminopyridine (4-AP), verapamil, and tetraethylammonium (TEA) were ordered from Sigma-Aldrich and tetrodotoxin (TTX) and iberiotoxin was purchased from Tocris (Ellisville, MO).
RT-PCR and Quantitative RT - PCR
The hBM-MSCs from three samples were used for RT-PCR analysis performed according to a previously described procedure (Park et al, 2007). The forward and reverse PCR oligonucleotide primers chosen to amplify the cDNA are listed in Table 1. The cDNA at 2 µl aliquots was amplified by a DNA thermal cycler (Bio-Rad Laboratories, Hercules, CA) in a 25 µl reaction mixture; the mixture was annealed at 50~61℃ (1 minute), extended at 72℃ (2 minutes), and denatured at 95℃ (45 seconds) for 30~35 cycles. Quantitative RT-PCR was performed with the 7,500 fast quantitative PCR System (Applied Biosystems, Foster City, CA) using SYBR Green master mix (Takara Bio Inc., Shiga, Japan). During each cycle, the accumulation of PCR products was detected by monitoring the increase in reporter dye fluorescence from dsDNA-bound SYBR Green. GAPDH was used as a control.
Table 1.
List of ion channel primers used for RT-PCR
Abbreviations: Acc. No., accession number.
Data analysis and statistics
Nonlinear-fitting programs (Origin, Northampton, MA) were used and statistical results are presented as mean±standard error of the mean (SEM). Paired and unpaired Student's t-tests were used as appropriate to evaluate the statistical significance of differences between two groups of means. p-Values were considered to indicate statistical significance.
RESULTS
Expression of stem cell markers and differentiation potential in hBM-MSCs
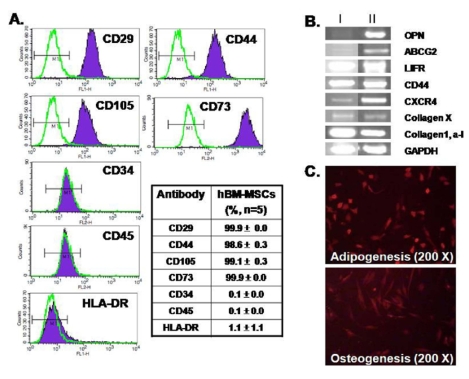
MSCs-specific surface markers (Weiss et al, 2006) present on hBM-MSCs at the 4th and 7th passages were analyzed by flow cytometry (Fig. 1A). The cells were positive for integrin markers (CD29), adhesion molecules (CD44), and MSCs markers (CD105, CD73) and were negative for hematopoietic (CD34, CD45) and major histocompatibility antigen (HLA-DR). We performed RT-PCR analysis to evaluate the expression of stem cell-specific genes. As shown in Fig. 1B, hBM-MSCs expressed markers of the osteogenic state (OPN), undifferentiated state (LIFR, ABCG2), mesoderm state (CXCR4, CD44, collagen X), and extracellular matrix molecules (collagen1, alpha1). The expression pattern of surface proteins and genes on our hBM-MSCs preparations was consistent those obtained by the Cambrex Corporation (Park et al, 2007) and the results indicate that these cells are primitive MSC populations. There were no significant differences among samples from the eight individual donors or among cells from different passages. To evaluate the differentiation capacity of hBM-MSCs, the cells were cultured in adipogenic and osteogenic medium for 3 weeks. Fig. 1C shows the capacity of hBM-MSCs to differentiate into adipocytes (upper) and osteoblasts (lower).
Fig. 1.
Characterization of hBM-MSCs. (A) Flow cytometry analysis showing that hBM-MSCswere positive for CD29, CD44, CD105, and CD73 and were negative for CD34, CD45, and HLA-DR. The table shows mean values (%). (B) hBM-MSCs expressed markers for OPN, LIFR, ABCG2, CXCR4, CD44, collagen X, collagen1 and alpha1. The hBM-MSCs RNAs were obtained from different donors (I and II, n=4) (C) Differentiation capacity of hBM-MSCs to adipocytes (upper) and osteoblasts (lower). 200× magnification.
Functional ion channel recordings in hBM-MSCs by whole cell patch clamp
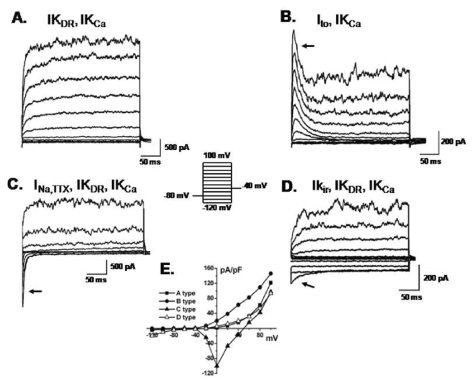
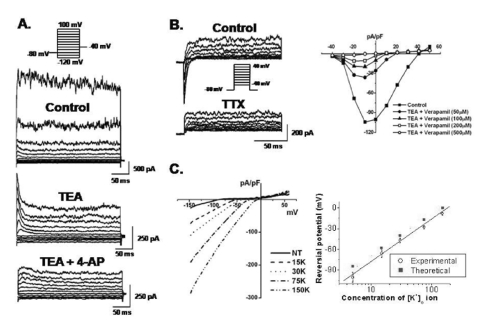
Whole cell patch clamp recordings were made from the 2nd~10th passages obtained from eight samples. Membrane currents were elicited by 300 ms voltage steps between -120 and +100 mV from a holding potential of -80 mV in hBM-MSCs, as illustrated in Fig. 2. The hBM-MSCs displayed four types of K+ currents: IKDR, IKCa, Ito, and Kir. The IKCa usually coexisted with IKDR in most hBM-MSCs (462 out of 476 cells, 97.1%, Fig. 2A) and was effectively inhibited by treatment with 5 mM TEA (Fig. 3A). Ito was detected in only 3.7% of the cells (18 out of 476 cells, Fig. 2B). Ito was substantially inhibited by 300 µM 4-AP, but remained unaffected by TEA (Fig. 3A, middle). Another type of K+ current, inward-rectifier K+ current, were evoked using a ramp voltage from -150 to +60 mV for 400 ms at a holding potential of 0 mV (4.4%, 21 out of 476 cells, Fig. 2D, arrow). The I-V relationships were plotted by ramp voltage-induced inward currents against membrane potentials in bath solutions containing 5, 15, 30, 75, and 150 mM K+, as indicated (Fig 3C). The reversal potential was positively shifted as external K+ concentration increased, suggesting that K+ induces this current. The plot of the reversal potential as a function of [K+]o showed that the slope was 66.04±5.55 mV/10-fold change in [K+]o (Fig. 3C right panel, n=6). The current was blocked in a dose-dependent manner by Ba2+, a Kir-specific blocker (IC50 at -100 mV; 162.2±22.2 µM).
Fig. 2.
Different patterns of membrane currents recorded in hBM-MSCs. Current traces elicited by the voltage step (inset) in hBM-MSCs. (A) a slowly activating current similar to IKDR at potentials from +20 to +100 mV that coexisted with IKCa. (B) Ito (arrow) with IKCa. (C) INa,TTX (arrow) with IKDR and IKCa. (D) IKir (arrow) with IKDR and IKCa. (E) Current-voltage relationships were plotted from A, B, C and D.
Fig. 3.
Pharmacological effects of functional ion channels in hBM-MSCs. (A) Membrane currents were recorded in the presence of 10 mM TEA or co-application of TEA and 300 µM 4-AP. (B) INa,TTX was continuously recorded under control conditions and in the presence of TTX. INa,TTX was blocked by TTX (left panel) and also blocked by verapamil (right panel). (C) The I-V relationships of Kir were obtained by ramp voltage induced currents against membrane potentials in bath solutions containing 5, 15, 30, 75, and 150 mM K+ as indicated (left panel). Reversal potentials from six patches were observed and plotted as a function of external [K+] concentrations (right panel). The dotted line represents the slope from the Nernst equation (slope, 58 mV/decade). Experimental values (O) were fitted by linear regression (slope, 66 mV/decade).
A Na+ current was found in a small population of cells using our experimental voltage protocols for outward currents (11.5%, 55 out of 476 cells, Fig. 2C, arrow). This inward current was blocked by TTX (INa,TTX, Fig. 3B). Interestingly, INa,TTX was also blocked by verapamil (Fig 3B right panel), a Ca2+ channel antagonist but nifedipine, a L-type Ca2+ channel blocker, demonstrated no blocking effect (data not shown). These findings suggest that the current is a TTX-sensitive Na+ current and is sensitive to verapamil.
Passage-dependent mRNA expression of ion channels in hBM-MSCs
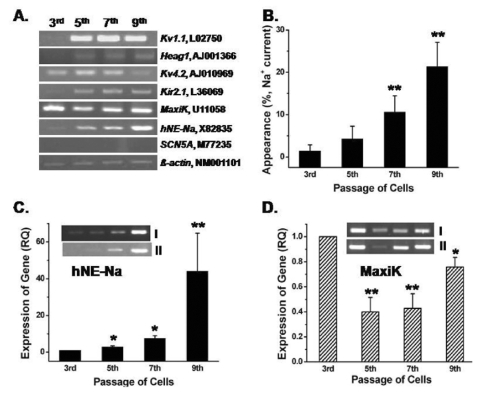
During the electrophysiological studies of hBM-MSCs (2nd~10th passages) from eight samples, we found that the recording rate of Na+ currents, in particular, varied according to the passage number. Therefore, we investigated the mRNA levels present during different passages in three hBM-MSCs samples by RT-PCR and quantitative RT-PCR analysis. MaxiK for IKCa, Kv4.2 for Ito, Kv1.1 and heag1 for IKDR, hNE-Na for INa,TTX, and Kir2.1 for Kir were detected in hBM-MSCs (Fig. 4A). β-actin was used as the control. The gene expression levels of all channels were low at an early passage (3rd), except for the MaxiK gene. Relative quantities of the Kv1.1, heag1, and Kir2.1 genes and MaxiK remain unchanged from the 5th through the 9th passage, but not hNE-Na (Fig. 4A). The "recording rate (%)" of INa,TTX increased almost three-fold more in late passages compared to early passages based on whole cell patch configurations (Fig. 4B). The "recording rate (%)" of INa,TTX was determined by the number of Na+ current recordings by dividing the totally achieved of whole cell patch configurations. MaxiK gene levels (RQ) also displayed a visible change between passages, but the variation was not as extreme as that observed for the hNE-Na gene levels (RQ) by quantitative RT-PCR (n=3, each from three samples, Fig. 4C and 4D). Representative passage-dependent hNE-Na and MaxiK gene expression patterns (RT-PCR) from two different samples are displayed in the inset of Fig. 4C and 4D.
Fig. 4.
Comparison of mRNA expression of ion channels between passages. (A) Kv1.1, heag1 (for IKDR), Kv4.2 (for Ito), Kir2.1 (for Kir), MaxiK (for IKCa) and hNE-Na (for INa.TTX) were detected in hBM-MSCs, but not SCN5A (for TTX-resistant INa). β-actin was used as the control. (B) The "recording rate (%)" of INa,TTX in various passage of hBM-MSCs. (C) Relative passage-dependent mRNA quantities of the hNE-Na gene by quantitative RT-PCR (n=3). The inset in Fig. 4C shows a representative result for the hNE-Na gene from two different samples. (D) Relative passage-dependent MaxiK gene expression levels in hBM-MSCs. Inset in Fig. 4D displays representative passage-dependent MaxiK channel gene expression patterns from two different samples. *p<0.05, **p<0.005 when compared with each 3rd passage group.
DISCUSSION
In our study, all cells isolated from the BM of ALS patients exhibited typical MSC characteristics (morphology, differentiation capacity, and expression of a typical set of surface proteins and genes). Each hBM-MSCs obtained from ALS patients showed a passage dependent gradual increase in Na+ channel expression, which may provide additional information for autologous cell based therapy.
Comparisons of the distribution and function of ion channels in BM-MSCs
Four types of K+ current (IKCa, IKDR, Ito and Kir) and one type of inward current (INa.TTX) were detected using the patch clamp technique in hBM-MSCs from ALS patients. This is the first study of channel expression in continuously passaged hBM-MSCs. The expression of multiple ion channels indicates possible functions of these different channels in the cellular physiological activity of hBM-MSCs. Deng et al reported that IKDR decreases during cell cycle progression in rat BM-MSCs, while IKCa increases during progression from the G1 to S phase (Deng et al, 2007). Other reports showed that inhibition of K+ currents by treatment with K+ channel blockers, TEA and clofilium, also inhibited the hMSCs proliferation (Balana et al, 2006). These reports suggest that ion channels may participate in the proliferation of stem cells.
Passage dependent increases in the expression of Na+ channel in hBM-MSCs
Voltage-gated Na+ channels have been well known to be responsible for action potential initiation and propagation in excitable cells, including nerve, muscle, and cardiac cells. Therefore, the Na+ channel may serve important functions during hBM-MSCs differentiation as it plays a distinct function in cardiac and neuronal cells. In the present study, we found that TTX-sensitive Na+ current (responsible for hNE-Na) increased approximately three-fold during later compared to earlier cell passages, whereas a TTX-resistant Na+ current (responsible for SCN5A) was not detected in our hBM-MSCs. This noticeable increase of the TTX-sensitive Na+ current could lead to the activation of other channels via depolarization, which results from an influx of Na+ ions into cells. Alternatively, the facilitated excitation of cells may serve to activate the intracellular signaling system. Taken together, the increment of Na+ channels must be considered before using them. Based on the above results, we suggest that the TTX-sensitive Na+ channel is significantly increased in during late passages of hBM-MSCs and that passage-dependent variation in functional ion channel expression might play a role in proliferation and/or differentiation.
Further investigation is needed to elucidate the role of Na+ channels in the differentiation and proliferation of hBM-MSCs and to study the dependence of ion channel profiles on passages in the other mesenchymal stem cell lines.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund, KRF 2007-532-E00001) and by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R11-2008-014-02001-0).
ABBREVIATIONS
- hBM-MSCs
Human bone marrow mesenchymal stem cells
- ALS
amyotrophic lateral sclerosis
- IKCa
Ca2+-activated K+ current
- Ito
transient outward K+ current
- IKDR
delayed rectifier K+ current
- Kir
inward-rectifier K+ current
- INa,TTX
TTX-sensitive Na+ current
References
- 1.Balana B, Nicoletti C, Zahanich I, Graf EM, Christ T, Boxberger S, Ravens U. 5-Azacytidine induces changes in electrophysiological properties of human mesenchymal stem cells. Cell Res. 2006;16:949–960. doi: 10.1038/sj.cr.7310116. [DOI] [PubMed] [Google Scholar]
- 2.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells:Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 3.Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 4.Biagiotti T, D'Amico M, Marzi I, Di Gennaro P, Arcangeli A, Wanke E, Olivotto M. Cell renewing in neuroblastoma: electrophysiological and immunocytochemical characterization of stem cells and derivatives. Stem Cells. 2006;24:443–453. doi: 10.1634/stemcells.2004-0264. [DOI] [PubMed] [Google Scholar]
- 5.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng XL, Lau CP, Lai K, Cheung KF, Lau GK, Li GR. Cell cycle-dependent expression of potassium channels and cell proliferation in rat mesenchymal stem cells from bone marrow. Cell Prolif. 2007;40:656–670. doi: 10.1111/j.1365-2184.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng XL, Sun HY, Lau CP, Li GR. Properties of ion channels in rabbit mesenchymal stem cells from bone marrow. Biochem Biophys Res Commun. 2006;348:301–309. doi: 10.1016/j.bbrc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 9.Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, Petite H, Sedel L, Vacanti JP. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13:87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- 10.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–2844. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 11.Li GR, Deng XL, Sun H, Chung SS, Tse HF, Lau CP. Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells. 2006;24:1519–1528. doi: 10.1634/stemcells.2005-0307. [DOI] [PubMed] [Google Scholar]
- 12.Li GR, Sun H, Deng X, Lau CP. Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells. 2005;23:371–382. doi: 10.1634/stemcells.2004-0213. [DOI] [PubMed] [Google Scholar]
- 13.MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, Boccaletti R, Testa L, Livigni S, Fagioli F. Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res. 2006;28:523–526. doi: 10.1179/016164106X116791. [DOI] [PubMed] [Google Scholar]
- 15.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 16.Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004;287:C125–C134. doi: 10.1152/ajpcell.00488.2003. [DOI] [PubMed] [Google Scholar]
- 17.Park KS, Jung KH, Kim SH, Kim KS, Choi MR, Kim Y, Chai YG. Functional expression of ion channels in mesenchymal stem cells derived from umbilical cord vein. Stem Cells. 2007;25:2044–2052. doi: 10.1634/stemcells.2006-0735. [DOI] [PubMed] [Google Scholar]
- 18.Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- 19.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Tao R, Lau CP, Tse HF, Li GR. Functional ion channels in mouse bone marrow mesenchymal stem cells. Am J Physiol Cell Physiol. 2007;293:C1561–C1567. doi: 10.1152/ajpcell.00240.2007. [DOI] [PubMed] [Google Scholar]
- 21.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(Suppl 19):II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 22.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]