Abstract
To determine the peripheral mechanisms involved in thermal sweating during the hot summers in July before acclimatization and after acclimatization in September, we evaluated the sweating response of healthy subjects (n=10) to acetylcholine (ACh), a primary neurotransmitter involved in peripheral sudomotor sensitivity. The quantitative sudomotor axon reflex test (QSART) measures sympathetic C fiber function after iontophoresed ACh evokes a measurable reliable sweat response. The QSART, at 2 mA for 5 min with 10% ACh, was applied to determine the directly activated (DIR) and axon reflex-mediated (AXR) sweating responses during ACh iontophoresis. The AXR sweat onset-time by the axon reflex was 1.50±0.32 min and 1.84±0.46 min before acclimatization in July and after acclimatization in September, respectively (p<0.01). The sweat volume of the AXR(1) [during 5 min 10% iontophoresis] by the axon reflex was 1.45±0.53 mg/cm2 and 0.98±0.24 mg/cm2 before acclimatization in July and after acclimatization in September, respectively (p<0.001). The sweat volume of the AXR(2) [during 5 min post-iontophoresis] by the axon reflex was 2.06±0.24 mg/cm2 and 1.39±0.32 mg/cm2 before and after acclimatization in July and September, respectively (p<0.001). The sweat volume of the DIR was 5.88±1.33 mg/cm2 and 4.98±0.94 mg/cm2 before and after acclimatization in July and September, respectively (p<0.01). These findings suggest that lower peripheral sudomotor responses of the ACh receptors are indicative of a blunted sympathetic nerve response to ACh during exposure to hot summer weather conditions.
Keywords: Heat acclimatization, Sweating, QSART, ACh
INTRODUCTION
In unacclimatized individuals, exposure to hot ambient conditions causes a high core temperature and an increased heart rate (Robinson et al, 1953; Wilkerson et al, 1986). However, prior heat exposure for lasting several consecutive days, improves heat resistance (Sato & Sato, 1983; Sato et al, 1990; Nielsen, 1998) by increasing sweat output, lowering the heart rate, and slowing the rise in the core temperature (Wyndham, 1967; Nadel et al, 1974; Ogawa & Sugenoya, 1993; Nielsen, 1998). Contrary to the phenomenon observed during short-term heat acclimation, when long-term heat acclimation is compared to short-term heat acclimation after exposure to equal amounts of heat, the subjects who were long-term heat acclimatized sweat less (Matsumoto et al, 1993; Ogawa & Sugenoya, 1993; Lee et al, 1997). When short-term heat acclimated Japanese subjects were compared to tropical Africans, an upward shift in the threshold core temperature for sweating and a decreased acetylcholine (ACh) sensitivity were observed in Africans (Lee et al, 1997). This led us to speculate that thermotolerance in the short-term heat-adapted temperate natives occurs through enhanced sweating, while thermotolerance in the tropical natives is achieved with a minimal amount of body fluid loss. Despite our extensive knowledge on the physiology of sweating, however, we do not fully understand why tropical natives maintain suppressed sweating activity in response to heat or ACh stimulation.
In the tropics, Africans appear to sweat less than Europeans during prolonged exercise in humid heat (Wyndham et al, 1964; Lee et al, 1997). The suppressed sweating in tropical natives (Matsumoto et al, 1993; Ogawa & Sugenoya, 1993; Lee et al, 1997, 2002; Bae et al, 2006) provides the advantage of preserving body fluid and osmoregulation so that individuals can better sustain thermoregulation (Sawka & Coyle, 1999). The mechanisms responsible for the reduction of dripped sweat with tropical acclimatization, however, are unknown.
The sweating mechanisms involve both central and peripheral activity, and heat acclimation is a process that is mediated by both the central nervous system and peripheral effectors (Horowitz, 1989). Although it is widely known that tropical natives maintain suppressed sweating during heat stress (Matsumoto et al, 1993; Ogawa et al, 1993; Lee et al, 1997, 2002, 2004, 2008; Bae et al, 2006), the precise site and nature of the sweat suppression has not been fully established. Experimental evidence has previously shown that peripheral mechanisms play a predominant role, compared to the central nervous system, in the suppression or augmentation of sweating (Collins & Weiner, 1962; Fox et al, 1962; Ogawa et al, 1982).
We have hypothesized that suppressed sweating in tropical natives results from the blunted sensitivity of the sweat glands to ACh, because of continuous exposure to the tropical environment. The primary purpose of this study was to examine the differences in the responsiveness of the active sweat gland output after ACh stimulation under thermoneutral conditions during the hot summer after acclimatization in September compared to exposure to the hot summer weather before acclimatization in July. Quantification of the sudomotor axon reflex test (QSART) is a useful method for evaluating postganglionic sympathetic fibers. The QSART measures sympathetic C fiber function after iontophoresed ACh evokes a measurable reliable sweat response, and also provides accurate quantification of the sudomotor axon reflex-mediated (AXR) response in healthy people. In our previous study (Bae et al, 2006; Lee et al, 2008), the sweating response was measured directly from the activated (DIR: muscarinic receptor-mediated sweating activity) and indirectly activated (AXR: nicotinic receptor-mediated sweating activity) sweat responses resulting from the QSART. A possible mechanism for this new QSART which in stimulated by dynamometry is through the central hypothalamic preoptic sweat-mediated pathway (Vetrugno et al, 2003). Therefore, muscarinic receptors (mAChRs) are those membrane-bound ACh that are more sensitive to muscarine than nicotine (Ishii and Kurachi, 2006). We found that peripheral cholinergic signals might be the primary sites responsible for conferring short-term (exposure to hot summer seasons) heat acclimatization during July and September.
METHODS
Subjects
Ten healthy male subjects, who lived all of their lives in the city of Cheonan, the Republic of Korea, volunteered for this study. All research subjects were students at the Soonchunhyang University between 10~11 July and 11~12 September 2007 (first and second experimental term within approximately 70 days). As shown in Fig. 1, for 60 days before the experiment and during the experimental period, they lived in Cheonan (Chungnam). The physical characteristics of the subjects were as follows: mean height, 173.8±4.8 cm; mean weight, 67.17±6.58 kg; mean age, 21.2±3.6 years; and mean body surface area (BSA), 1.81±0.12 m2. Each subject provided written informed consent after the purpose and experimental procedures as well as any potential risks were thoroughly discussed. The protocol complied with the Helsinki Declaration of 1975. Cheonan of the Republic of Korea is located in the deep south (126° 52'N, 33.38'E) and the deep north (130° 4'N, 43.0'E). The mean annual ambient temperature is 11.60℃ with 73.6% relative humidity (data from the Republic of Korea Meteorological Administration, 2007).
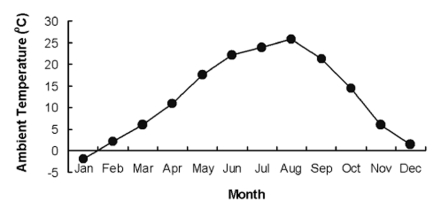
Fig. 1.
Monthly mean ambient temperatures in Cheonan (126 52'N, 33.38'E; Republic of Korea). Cheonan is located in a temperate zone, with cold winters and hot summers. The mean ambient temperature of the experimental period was -1.9℃ in the winter (January) and 25.8℃ in the summer (August) of 2007.
Measurements and procedures
All experiments were carried out in an automated climate chamber (24.0±0.5℃, relative humidity 40±3%, and <1 m/sec air velocity) between 2 and 5 p.m. Upon arrival to the climate chamber, the subjects were dressed in light clothing and rested quietly for 60 min before the experiment commenced. The QSART (Low et al, 1983; Chemali et al, 2001; Bae et al, 2006; Lee et al, 2004, 2008) was performed to quantitatively evaluate glandular ACh-sensitivity. The QSART capsule consists of three concentric compartments. Ach, iontophoretically applied, stimulates directly the underlying sweat glands in the outer compartment, while the glands of the skin in the central compartment of the capsule are activated indirectly via axon reflexes. The sweating response was measured from the directly activated and axon reflex-mediated sweat responses, resulting from the iontophoresis. Two sets of QSART capsules were attached to the volar aspect of the forearm with rubber bands, one at the mid-portion between the wrist and elbow joints, and the other 10 cm proximal to the first capsule. The outer compartment of the first capsule was filled with 10% ACh (Ovisot; Daiichi Pharmaceutical Co., Ltd., Japan) solution. Two mA of direct current were applied for 5 min between an electrode on the ACh cell (anode) and a flexible plate-electrode (cathode, HV-BIGPAD; Omron, Kyoto, Japan) attached to the forearm skin just proximal to the wrist joint. The central compartment of the ACh capsule served as the site of the sudomotor axon reflex, AXR (1) [sweating during 5 min 10% iontophoresis] and measurement during the 5 min of iontophoresis (Lee et al, 2004, 2008). Immediately after the cessation of current loading, the sweat capsules were detached and the skin covered with the ACh capsule was wiped; and then the position of the capsules was exchanged. This procedure took <30 sec. The data were acquired for another 5 min to permit simultaneous observation of DIR and AXR (2) [sweating during 5 min post-iontophoresis] sweating. The data, including sweat onset-time, latent period for sweating after current loading, sweat volume for more then 5 min, area under the sweating curve, 0~5 min for AXR (1) and 6~11 min for AXR (2), and DIR, were used for the analysis (Lee et al, 2002, 2004, 2008).
The sweat rates were measured by the capacitance hygrometer-ventilated capsule method (Lee et al, 2004; Bae et al, 2006). In brief, nitrogen gas flowed into each compartment with a constant flow rate of 0.3 l/min. The change in the relative humidity of the effluent gas was detected by a hygrometer (H211; Technol Seven, Yokohama, Japan). The oral (sublingual) and skin temperatures just beside and 10 cm away from the ACh capsule were monitored using thermistors (PXK-67; Technol Seven) connected to the data logger (K-720; Technol Seven). The sweating rates and temperatures were recorded with a PC (PC9801; NEC, Japan) every 5 sec (Lee et al, 2004, 2008). Transepidermal water loss (TEWL) was measured (µg/cm2/min) with a Tewameter (model No. TM 210; Courage and Khazaka, Germany) according to the manufacturer's recommendations as well as described in detail by Pinnagoda et al, (1990). Briefly, the measurement involved placing the probe device containing on the volar aspect of the forearm skin.
Statistical analysis
The values are presented as means±standard deviation (SD). Statistical significance was assessed by the paired Student's t-test to compare the data obtained before acclimatization in July and after acclimatization in September and by one-way ANOVA for repeated measures. Statistical significance was accepted at the 0.05 level.
RESULTS
A data recording of sweating activity and temperature changes due to ACh iontophoresis
The QSART (10% ACh at 2 mA for 5 min) was applied iontophoretically. As shown in Fig. 2, the nicotinic indirect axon reflex [AXR (1)] sweating was initiated after a latent period (sweat onset time of axon reflex) and it reached a plateau phase within a few minutes. After the end of iontophoresis, the muscarinic activity of direct sweating of DIR was sustained, while whereas AXR (2) sweating declined to the baseline. The forearm skin surface temperature near capsule A (ACh iontophoresis) tended to rise following the onset of sweating, however, no changes were observed in the skin temperature near capsule B (ACh non-iontophoresis from 15 cm) throughout the experiment. In the resting condition (pre-iontophoresis), the forearm skin surface temperature was 0.31℃ higher before acclimatization in July (33.22±0.47℃) than after acclimatization in September (32.91±0.59℃, p<0.01). The forearm local skin surface temperature in the proximity of capsule A (ACh iontophoresis) increased during ACh iontophoresis (from pre-test to post-test) both before acclimatization in July and after acclimatization in September, and the differences were statistically significant (p<0.01). However, the forearm local skin temperature (0.35℃ higher) near Capsule 1 (ACh iontophoresis) was different after iontophoresis (from pre-test to post-test) before acclimatization in July (0.96℃ rise; p<0.001) when it was increased more, compared to after acclimatization in September (0.61℃ rise; p<0.001).
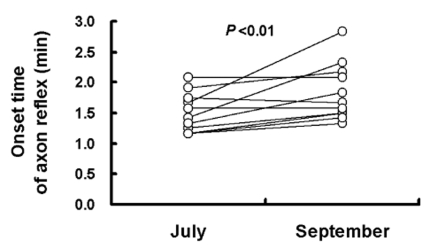
Fig. 2.
Sweat onset-time of axon reflex was 1.50±0.32 min and 1.84±0.46 min in human subjects before acclimatization in July and after acclimatization in September, respectively. The experimental period was during the hottest months of the summer (between July and August; Fig. 1). Values are presented as means (n=10)±SD. Statistical significance was set at p<0.001.
The oral temperature was slightly elevated throughout the recording period. However, there was no observed influence of the ACh iontophoresis on the oral temperature before acclimatization in July or after acclimatization in September.
Onset time, nicotinic indirect axon reflex, and muscarinic direct sweating with the QSART
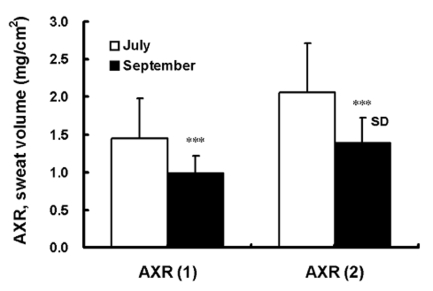
As shown in Fig. 2, the sweat onset time of the axon reflex (nicotinic receptor-mediated) was 0.34 min later after acclimatization in September (1.84±0.46 min) compared to before acclimatization in July (1.50±0.32 min, p<0.01). The nicotinic receptor-mediated sweating activity [indirect axon reflex AXR (1) and AXR (2)] and the muscarinic receptor-mediated sweating activity (direct response of DIR), in terms of the sweat volume, were as follows: AXR (1), 149%; AXR (2), 148% (p<0.001, Fig. 3) and 118% (p<0.01, Fig. 4). There was a significant decrease before acclimatization in July compared to after acclimatization in September. These results indicate that there was suppressed thermal sweating after acclimatization in September compared to before acclimatization in July.
Fig. 3.
The AXR (1) sweat volume of the axon reflex was 1.46±0.53 mg/cm2 and 0.98±0.24 mg/cm2 in human subjects before acclimatization in July and after acclimatization in September, respectively. Comparison of the AXR (2) sweat volume of the axon reflex was 2.06±0.24 mg/cm2 and 1.39±0.32 mg/cm2 in human subjects before acclimatization in July and after acclimatization in September, respectively. AXR=axon reflex-mediated (indirectly activated) sweating during (nicotinic receptor-mediated sweating activity). AXR (1)=0~5 min and AXR (2)=6~11 min. Values are presented as means (n=10)±SD. Statistical significance was set at ***p<0.001.
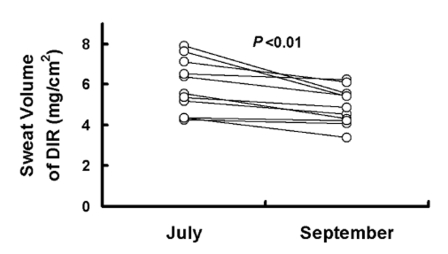
Fig. 4.
The DIR sweat volume (directly activated 6~11 min) was 5.88±1.33 mg/cm2 and 4.98±0.94 mg/cm2 in human subjects before acclimatization in July and after acclimatization in September, respectively. DIR=directly activated sweating during (muscarinic receptor-mediated sweating activity). Values are presented as means (n=10)±SD. Statistical significance was set at p<0.01.
Volume of transepidermal evaporative water loss
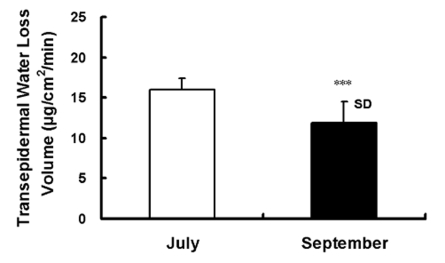
Fig. 5 illustrates the evaporation on the forearm skin surface humidity near capsule A (ACh iontophoresis). The volume of skin evaporative loss was 15.96±1.33 µg/cm2/min and 11.90±2.50 µg/cm2/min before acclimatization in July and after in September, respectively (p<0.001). The volume of transepidermal evaporative water loss by evaporative activity decreased by 25% of the suppressed thermal sweating after acclimatization in September compared to before acclimatization in July.
Fig. 5.
Sweating activity (forearm local skin evaporative loss volume) was 15.96±1.33 µg/cm2/min and 11.90±2.50 µg/cm2/min in human subjects before acclimatization in July and after acclimatization in September, respectively. Values are presented as the means (n=10)±SD. Statistical significance was set at ***p<0.001.
DISCUSSION
The inhibition or suppression after acclimatization in September was expected because of the adjustment to heat compared to the results obtained in July before acclimatization. As shown in Fig. 1, the mean July-to-August ambient temperatures above 24.5° influences the short-term adjustment to (exposure of hot summer seasons) heat. In order to clarify the mechanisms of short-term (exposure to hot summer seasons) adjustments before acclimatization in July and after acclimatization in September, we compared the QSART results (AXR and DIR) before acclimatization in July to after acclimatization in September.
The results of this study demonstrate suppressed peripheral sweat gland activity after acclimatization in September in response to iontophoretically-administered ACh compared to before acclimatization in July. When the data after acclimatization in September were compared to the data obtained from short-term heat adjustments before acclimatization in July, there was a lower volume of evaporative loss (25%), a lower forearm local skin temperature (0.31℃), a lower sweat output of AXR (1) 148%-AXR (2) 148% and DIR 118%, and a longer sweat onset time (0.34 min later) after acclimatization in September.
ACh is the primary neurotransmitter that elicits sweating after nerve stimulation or agonist administration. The sympathetic innervation of sweat glands is unusual in that the postganglionic fibers release ACh rather than norepinephrine, the transmitter found at other sympathetic synapses. ACh is also necessary for the development and maintenance of secretory responsiveness (Leblanc & Landis, 1986; Weihe et al, 1996). Sweat gland innervation also contains choline acetyltransferase, the synthetic enzyme for ACh, and the vesicular ACh transporter, but not catecholamines (Leblanc & Landis, 1986; Weihe et al, 1996). Furthermore, muscarinic agonists mimic the ability of nerve stimulation to elicit sweating, and nerve-evoked sweating can be blocked by muscarinic antagonists (Vilches et al, 1995).
For forearm sweating, the active sweat gland density and the glandular sweat output primarily determine the total sweat output (Kondo et al, 2001). Our data suggest that the sensitivity to ACh may be blunted after acclimatization in September. This blunting may have developed because of repeated stimulation of the sweat glands by heat-mediated release of endogenous ACh in a hot and humid environment after acclimatization to short-term summer-season climates. The related finding of a depressed sensitivity of the sweat glands, following repeated ACh administration, supports this claim. Chen and Elizondo (1974) observed the depression of thermal sweating after repeated ACh iontophoresis, in support of our earlier data that showed that the suppression of the sweat gland response in a significant contributor to the suppression of sudomotor function in tropical natives (Matsumoto et al, 1997; Lee et al, 1997, 2002; Bae et al, 2006). In contrast to these observations, short-term heat acclimation increases the activity of the sweat glands. In short-term heat acclimation trials, Taylor (1986) and Sato et al. (1990) observed increased sweat output, an increased size of the eccrine sweat glands, and increased sensitivity of the sweat glands to methacholine stimulation. These findings indicate that short- and long-term acclimatization are mediated by different physiological mechanisms. Furthermore, the liberal sweating that occurs after weeks of heat exposure in unacclimated subjects has been attributed to an increase in the sensitivity of the sweat glands to thermal and hormonal stimuli, and an increase in the size and/or number of active sweat glands (Nielsen 1998).
Another important adaptive feature after acclimatization in September is the maintenance of low resting oral and skin temperatures. In our previous study, to clarify the mechanisms of heat acclimatization to tropical climates (Lee et al, 2008), changes in the oral temperature after a 30 min heat load on the legs were compared between subjects in Chiang Mai (a tropical region) and Nagasaki (a temperate region), and our recent study (Lee et al, 2007) also had similar aims and measurements. The results in both studies showed that the subjects in the tropical region were found to have significantly lower mean skin and body temperatures than the subjects in the temperate region. The tropical subjects are able to maintain lower core and skin temperatures compared to temperate subjects due, in part, to a lower metabolic rate resulting from lower thyroxine secretion (Onaka et al, 1978). Both the core and skin temperatures have a significant influence on the sweating response, not only as input to the central regulatory mechanism, but also locally with effects on the activity of the sweat glands. For example, local skin heating facilitates transmitter release at the neuroglandular junction and augments glandular responsiveness to the transmitter (Ogawa & Asayama, 1986). Lee et al. (1997) and Low et al. (1983) demonstrated an increase in the sweating response when the skin temperature was increased. Similarly, skin vasodilatation induced by iontophoretically applied ACh causes sweat expulsion (Sugenoya et al, 1995; Morris & Shore, 1996). In addition, non-evaporated heat dissipation from the body to the air occurs when the skin temperature is higher than the air temperature, since the amount of heat dissipation is proportional to the temperature gradient between the skin surface and the air (Yoshimura, 1960).
In this study, we observed 0.31℃ higher skin temperature before acclimatization in July (33.22℃), than after acclimatization in September (32.91℃). However, the forearm local skin temperature (0.35℃ higher) near capsule 1 (ACh iontophoresis) was different after iontophoresis (from pre-test to post-test) before acclimatization in July (0.96℃ rise), when it was increased more than after acclimatization in September (0.61℃ rise). We asked question of whether the higher skin temperature at the site of ACh iontophoresis before acclimatization in July (0.31℃ higher) played any role in the higher sweating activity. However, in a temperature-controlled study in which the skin on one forearm was heated to 40.5±5℃, while the contralateral arm was maintained at 33~34℃, (a difference of 6.5℃), the sweating increased by only 7% (Chen and Elizondo, 1974). Considering the fact that the skin temperature difference in our study was only 0.31℃, and the difference in the sweat volume was DIR 18%-AXR (1) 49%-AXR (2) 48% higher, we concluded that the higher skin temperature before acclimatization in July compared to after acclimatization in September was insufficient to account for the large difference in sweat output. These results, therefore, indicate that sensitivity to ACh after acclimatization in September was reduced, consistent with recent findings of Bae et al. (2006) and Lee et al. (2008).
Even though a high skin temperature promotes non-evaporative heat dissipation, forearm local skin temperature before acclimatization in July was higher, and the recorded site of ACh iontophoresis was higher after acclimatization in September. The response of tropical human skin at the site of ACh iontophoresis raises the possibility that, when the skin was heat-challenged, the tropical natives could maintain a higher skin temperature, thereby resulting in a higher non-evaporative heat loss, as reported previously (Tsujita & Hori, 1978). The results for September (tropical region) are consistent with our previous results (Matsumoto et al, 1993; Lee et al, 1997, 2007, 2008).
Another important feature of long-term thermal acclimation may be the density of the sweat glands. It has been reported that the total number of active sweat glands remains unchanged throughout life, after the age of about 2.5 years (Kuno, 1956). A difference in sweat gland density has been reported in people who live in different climate zones. For example, the total number of active sweat glands in Filipinos (tropical residents) is higher than in Japanese (temperate residents; Kuno, 1956). Therefore, the total number of active sweat glands is thought to be dependent on the climate where an individual lived during early life. However, the size or number of active sweat glands can change after exposure to heat (Nielsen, 1998). According to Low et al. (1983), the QSART with 10% ACh and 2 mA of direct current maximally stimulate sweat gland, nevertheless, they could not confirm this finding in short-term (exposure to hot summer seasons) heat acclimatization. It is possible that 10% ACh is insufficient to activate all sweat glands after acclimatization in September. On the other hand, the use of higher concentrations of ACh is not justified, since physiological ACh concentrations at the nerve terminals are often much lower. Furthermore, the anatomy of the sweat glands after acclimatization in September, compared to before acclimatization in July, requires additional investigation. Sato and Sato (1983) reported a significant correlation between methacholine sensitivity and the size of the sweat gland, sweat rate per gland, sweat rate per unit length of the secretory tubule, and sweat rate per glandular unit. The sweat glands from individuals who are defined as poor sweaters are smaller, have a lower secretory activity both in vivo and in vitro, and have decreased methacholine sensitivity. Whether these parameters played a role in the observations reported in this study requires further investigation.
Based on the data provided in this study, the differences in the cooling mechanisms seem to be the key determinant of the differences in heat susceptibility before acclimatization in July compared to after acclimatization in September. The cooling system after acclimatization in September is more economical in terms of water conservation, and it provides more efficient plasma preservation during heat stress. This may enable tropical natives to endure heat stress better in terms of body fluid maintenance and osmoregulation. We have previously suggested a concept of economical sweating as a means of thermal adaptation (Matsumoto et al, 1993; Ogawa & Sugenoya, 1993; Bae et al, 2006). Earlies studies by our as well as other groups indicated that the preservation of body fluids plays an important role in thermoregulatory responses (Lee et al, 1997; Cheuvront & Haymes, 2001). Hypohydration increases heat storage by reducing blood flow to the skin, as well as sweating rate, and subsequent hypertonicity further contributes to reduced heat loss and increased heat storage. In addition, hypovolemia and displacement of blood to the skin makes it difficult to maintain the central venous pressure, therefore, the cardiac output for simultaneous support of metabolism and thermoregulation as well (Sawka et al, 2001). This results in the maintenance of maximal sustainable heat loss with greater ease after acclimatization in September. However, the preservation of body fluids through reduced sweating has its disadvantages. Reduced sweating after acclimatization in September may hamper heat loss since the evaporation of sweat from a wetted skin facilitates heat loss; there are limits of opportunities to lose dry heat when ambient humidity is high.
It is not obvious whether the data obtained by measurements of forearm sweating activity is the representative of whole body. The density and capacity of the sweat glands may differ from one body region to another (Sato and Sato, 1983; Shibasaki et al, 1997; Kondo et al, 1998). However, the forearm sweat glands have widely been used to approximate sweating activity of whole body (Wyndham et al, 1964; Wyndham, 1967; Chen & Elizondo, 1974; Ogawa et al, 1982; Kondo et al, 2001).
In conclusion, the results of this study suggest that lower peripheral sudomotor responses to ACh receptors are indicative of blunted sympathetic nerve responsiveness (effects of short-term heat acclimatization) to ACh sensitivity with exposure to hot summer weather conditions.
ACKNOWLEDGEMENTS
The authors wish to thank the subjects whose participation made this study possible.
ABBREVIATIONS
- QSART
quantitative sudomotor axon reflex test
- ACh
acetylcholine
- AXR
axon reflex-mediated
- DIR
directly activated
- BSA
body surface area
References
- 1.Bae JS, Lee JB, Matsumoto T, Othman T, Min YK, Yang HM. Prolonged residence of temperate natives in the tropics producesa suppression of sweating. Pflugers Arch-Eur J Physiol. 2006;453:67–72. doi: 10.1007/s00424-006-0098-x. [DOI] [PubMed] [Google Scholar]
- 2.Chemali KR, Gorodeski R, Chelimsky TC. Alpha-adrenergic supersensitivity of the sudomotor nerve in complex regional pain syndrome. Ann Neurol. 2001;49:453–459. [PubMed] [Google Scholar]
- 3.Chen YW, Elizondo RS. Peripheral modification of thermoregulatory function during heat acclimation. J Appl Physiol. 1974;37:367–373. doi: 10.1152/jappl.1974.37.3.367. [DOI] [PubMed] [Google Scholar]
- 4.Cheuvront SN, Haymes EM. Ad libitum fluid intakes and thermoregulatory responses of female distance runners in three environments. J Sports Sci. 2001;19:845–854. doi: 10.1080/026404101753113796. [DOI] [PubMed] [Google Scholar]
- 5.Collins KJ, Weiner JS. Observations on arm-bag suppression of sweating and its relationship to thermal sweating "fatigue". J Physiol London. 1962;161:538–556. doi: 10.1113/jphysiol.1962.sp006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox RH, Jack JW, Kidd DJ, Rosenbaum S. Observations in climatic chambers. London, Ret: Army Personal Research Committee, Med Res Coun; 1962. No. APRC 6, 25. [Google Scholar]
- 7.Horowitz M. Heat acclimation: A continuum of process. In: Mercer J, editor. Thermal Physiology. Amsterdam: Elsevier Science Publishers B.V; 1989. pp. 445–450. [Google Scholar]
- 8.Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Curr Pharm Des. 2006;12:3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 9.Kondo N, Shibasaki M, Aoki K, Koga S, Inoue Y, Crandall CG. Function of human eccrine sweat glands during dynamic exercise and passive heat stress. J Appl Physiol. 2001;90:1877–1881. doi: 10.1152/jappl.2001.90.5.1877. [DOI] [PubMed] [Google Scholar]
- 10.Kondo N, Takano S, Aoki K, Shibasaki M, Tominaga H, Inoue Y. Regional differences in the effect of exercise intensity on thermoregulatory sweating and cutaneous vasodilation. Acta Physiol Scand. 1998;164:71–78. doi: 10.1046/j.1365-201X.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuno Y. Human Perspiration. Springfield, Illinois: Charles C Thomas Publisher; 1956. [Google Scholar]
- 12.Leblanc G, Landis SC. Development of choline acetyltransferase activity in the cholinergic sympathetic innervation of sweat glands. J Neurosci. 1986;6:260–265. doi: 10.1523/JNEUROSCI.06-01-00260.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JB, Matsumoto T, Othman T, Kosaka M. Suppression of the sweat gland sensitivity to acetylcholine applied iontophoretically in tropical Africans compared to temperate Japanese. Trop Med. 1997;39:111–121. [Google Scholar]
- 14.Lee JB, Othman T, Lee JS, Quan FS, Choi JH, Yang HM, Min YK, Matsumoto T, Kosaka M. Sudomotor modifications by acclimatization of stay in temperate Japan of Malaysian native tropical subjects. Jan J Tropical Medicine Hygiene. 2002;30:295–299. [Google Scholar]
- 15.Lee JB, Bae JS, Lee MY, Yang HM, Min YK, Song HY, Ko KK, Kwon JT, Matsumoto T. The change in peripheral sweating mechanisms of the tropical Malaysian who stays in Japan. J Thermal Biol. 2004;29:743–747. [Google Scholar]
- 16.Lee JB, Bae JS, Shin YO, Kang JC, Matsumoto T, Aliopv AT, Alipov GK, Kim WJ, Min YK, Yang HM. Long-term tropical residency diminishes central sudomotor sensitivities in male subjects. Korean J Physiol Pharmacol. 2007;11:233–237. [Google Scholar]
- 17.Lee JB, Bae JS, Yang HM, Min YK. Tropical Malaysians and temperate Koreans exhibit significant differences in sweating sensitivity to iontophoretically administered acetylcholine. Int J Biometeorol. 2008;52 doi: 10.1007/s00484-008-0197-9. Online 02 December. [DOI] [PubMed] [Google Scholar]
- 18.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Kosaka M, Yamauchi M, Tsuchiya K, Ohwatari N, Motomura M, Otomasu K, Yang GJ, Lee JM, Boonayathap U, Praputpittaya C, Yongsiri A. Study on mechanisms of heat acclimatization due to thermal sweating -Comparison of heat-tolerance between Japanese and Thai subjects. Trop Med. 1993;35:23–34. [Google Scholar]
- 20.Matsumoto T, Taimura A, Yamauchi M, Lee JB, Kosaka M, Pongchaidecha A, Praputpittaya C, Gomonchareonsiri S, Boonayathap U, Sugenoya J. Long-term heat acclimatization in tropical inhabitants. In: Nielsen BJ, Nielsen R, editors. Thermal Physiology. Copenhagen: August Krough Institute; 1997. p. 69. Abstract. [Google Scholar]
- 21.Morris SJ, Shore A. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanism. J Physiol. 1996;496:531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadel ER, Pandolf KB, Roberts MF, Stojwijk JAJ. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515–520. doi: 10.1152/jappl.1974.37.4.515. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen B. Heat acclimation-mechanisms of adaptation to exercise in the heat. Int J Sports Med. 1998;19(Suppl 2):S154–S156. doi: 10.1055/s-2007-971984. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa T, Asayama M, Miyagawa T. Effects of sweat gland training by repeated local heating. Jpn J Physiol. 1982;32:971–981. doi: 10.2170/jjphysiol.32.971. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Asayama M. Quantitative analysis of the local effect of the skin temperature on sweating. Jpn J Physiol. 1986;36:417–422. doi: 10.2170/jjphysiol.36.417. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Sugenoya J. Pulsatile sweating and sympathetic sudomotor activity. Jpn J Physiol. 1993;43:275–289. doi: 10.2170/jjphysiol.43.275. [DOI] [PubMed] [Google Scholar]
- 27.Onaka S, Hori S, Saito N, Shiraki K, Migasena P, Yoshihara H. The role of food habits in physiological adaptations of inhabitants of Southeast Asia to the habitat of tropical countries. Progr Hum Nutr. 1978;2:219–235. [Google Scholar]
- 28.Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 29.Robinson SM, Turrel ES, Belding HS, Hovath SM. Rapid acclimatization to work in hot climates. Am J Physiol. 1953;140:168–176. [Google Scholar]
- 30.Sato F, Owen M, Matthes R, Sato K, Gisolfi CV. Functional and morphological changes in the eccrine sweat gland with heat acclimation. J Appl Physiol. 1990;69:232–236. doi: 10.1152/jappl.1990.69.1.232. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Dobson RL. Regional and individual variations in the function of the human eccrine sweat gland. J Invest Derm. 1970;54:443–449. doi: 10.1111/1523-1747.ep12259272. [DOI] [PubMed] [Google Scholar]
- 32.Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol. 1983;245:R203–R208. doi: 10.1152/ajpregu.1983.245.2.R203. [DOI] [PubMed] [Google Scholar]
- 33.Sawka MN, Coyle EF. Influence of body water and blood volume on thermoregulation and exercise performance in the heat. Exerc Sport Sci Rev. 1999;27:167–218. [PubMed] [Google Scholar]
- 34.Sawka MN, Montain SJ, Latzka WA. Hydration effects on thermoregulation and performance in the heat. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:679–690. doi: 10.1016/s1095-6433(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 35.Shibasaki M, Inoue Y, Kondo N, Iwata A. Thermoregulatory responses of prepubertal boys and young men during moderate exercise. Eur J Appl Physiol Occup Physiol. 1997;75:212–218. doi: 10.1007/s004210050150. [DOI] [PubMed] [Google Scholar]
- 36.Sugenoya J, Ogawa T, Jmai K, Ohnishi N, Natsume K. Cutaneous vasodilatation synchronize with sweat expulsions. Eur J Appl Physiol Occup Physiol. 1995;71:33–40. doi: 10.1007/BF00511230. [DOI] [PubMed] [Google Scholar]
- 37.Taylor NA. Eccrine sweat glands. Adaptations to physical training and heat acclimation. Sports Med. 1986;3:387–397. doi: 10.2165/00007256-198603060-00001. [DOI] [PubMed] [Google Scholar]
- 38.Tsujita J, Hori S. Comparative studies on sweating reflex, number of active sweat gland and body temperature of subtropical natives and temperate natives. Jpn J Trop Med Hyg. 1978;6:157–165. [Google Scholar]
- 39.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 40.Vilches J, Navarro X, Verdu E. Functional sudomotor responses to cholinergic agonists and antagonists in the mouse. J Auton Nerv Syst. 1995;55:105–111. doi: 10.1016/0165-1838(95)00033-t. [DOI] [PubMed] [Google Scholar]
- 41.Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc Natl Acad Sci USA. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkerson WJ, Young RJ, Melius JM. Investigation of a fatal heatstroke. Am Ind Hyg Assoc J. 1986;47:A493–A494. A496. [PubMed] [Google Scholar]
- 43.Wyndham CH, Strydom NB, Munro A, Macpherson RK, Metz B, Schaff G, Schieber J. Heat reactions of Caucasians in hot dry and hot humid climates. J Appl Physiol. 1964;19:607–612. doi: 10.1152/jappl.1964.19.4.607. [DOI] [PubMed] [Google Scholar]
- 44.Wyndham CH. Effect of acclimatization on the sweat rate/rectal temperature relationship. J Appl Physiol. 1967;22:27–30. doi: 10.1152/jappl.1967.22.1.27. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimura H. Essential Problems in Climatic Physiology. Kyoto: Nankodo; 1960. Acclimatization to heat and cold; pp. 61–106. [Google Scholar]