Abstract
Enantiomers of N-substituted benzofuro[2,3-c]pyridin-6-ols have been synthesized and the subnanomolar affinity and potent agonist activity of the known racemic N-phenethyl substituted benzofuro[2,3-c]pyridin-6-ol can now be ascribed to the 4aS,9aR enantiomer. The energy minimized structures suggest that the active enantiomer bears a greater three-dimensional resemblance to morphine than to an ostensibly structurally similar oxide-bridged phenylmorphan. Structural features of the conformers of N-substituted benzofuro[2,3-c]pyridin-6-ols were compared to provide the rationale for their binding affinity.
Keywords: Oxide-bridged 5-phenylmorphans, N-phenethyl-substituted para-d-isomer, conformers, nitrogen inversion, opioid receptor binding, functional assay
Introduction
In 1989, Hutchison, et al.,3 published the synthesis of racemic N-substituted benzofuro[2,3-c]pyridin-6-ols and noted that one of them, the N-phenethyl derivative of the cis-benzofuropyridin-6-ol had high μ-opioid affinity (Ki = 0.9 nM) and potent antinociceptive activity. These compounds appeared to have marked structural resemblance to the oxide-bridged phenylmorphans. We published our initial work on the synthesis of the 12 racemic topologically rigid N-methyl substituted a- through f-oxide-bridged phenylmorphans (Figure 1) in 1984,4, 5 and have recently reported the preparation of the final pair of oxide-bridged phenylmorphans.4-13 Some of the N-phenethyl analogues have been synthesized, as well, and their pharmacological activity determined.12, 13 A few of the enantiomers11 of the racemates have also been examined. The topology of the ortho-b, -d,a and –f isomers has been reported13 and comparisons have previously been made to a highly potent azabicyclo[3.3.1]nonane μ-opioid agonist.14 We believed that we could gain information about the three-dimensional shape of a molecule required for it to interact with a specific opioid receptor if any of the 12 oxide-bridged racemic phenylmorphans was found to have agonist or antagonist activity. There was no way then, or now, to determine the agonist or antagonist conformation of a ligand acting at an opioid receptor.
Figure 1.
Twelve racemic N-methyl substituted oxide-bridged phenylmorphan isomers
In order to gain additional insight into the ligand-receptor interaction we thought it would prove useful to find new rigid ligands with relatively few conformational shapes that could interact with opioid receptors as agonists and/or antagonists. We decided to explore the benzofuro[2,3-c]pyridin-6-ols3 because they appeared to be fairly rigid, partial structures of the oxide-bridged phenylmorphans. We synthesized the most potent racemic compound (rac-cis-2-phenethyl-4a-ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol, 22, Scheme 3) on a larger scale than previously reported, obtained its enantiomers (16 and 23) and determined which of those enantiomers was responsible for the high opioid receptor affinity. New N-substituted compounds with chiral side chains (17, 18, 24, and 25) were also prepared and evaluated to see if chiral N-hydroxyphenethyl moieties affect binding to opioid receptors. We then examined whether the active enantiomer topologically resembled any of our rigid oxide-bridged phenylmorphans utilizing quantum chemistry and a superposition study.
Scheme 3.a.
a Reagents and conditions: (a) 10% Pd/C, H2, 37% formic acid, MeOH, room temperature, 4 h; (b) (2-bromoethyl)benzene, K2CO3, DMF, 50 °C, 20 h; (c) 48% HBr, reflux, 1 h, then NH4OH, CH2Cl2; (d) 48% HBr, reflux, 30 min, then NH4OH, CH2Cl2; (e) 48% HBr, HOAc, reflux, 7.5 h; (f) (S) (−)-styrene oxide, toluene, reflux, 72 h; (g) (R) (+)-styrene oxide, toluene, reflux, 72 h.
Chemistry
Synthesis and Resolution
The starting material, 2-hydroxy-5-methoxypropiophenone, (1, Scheme 1) was isolated as a by-product by Shulgin and Dyer,15 and their procedure for the preparation of 1 was used by Hutchison et al.3 We modified the procedure15 to obtain 1 in high yield on a large scale from the reaction between propionyl chloride and 1,4-dimethoxybenzene, by using aluminum chloride with nitromethane as the solvent. Selective O-demethylation of the intermediate product, 2,5-dimethoxypropriophenone, was required in this reaction to obtain the desired monomethoxy compound 1, and preliminary experiments with BBr3 gave unsatisfactory results. The use of anhydrous aluminum chloride in dry nitromethane at 60 °C gave, in a rapid reaction, the desired product 1 in 84% yield, after work-up. At a lower temperature (10 °C) the reaction resulted in the formation of only the 2,5-dimethoxypropriophenone interme diate, and little of the desired O-demethylated product 1.
Scheme 1a.
a Reagents and conditions: (a) Propionyl chloride, AlCl3, CH3NO2, 60 °C; (b) BrCH2CO2Et, K2CO3, acetone, reflux, 30 h; (c) (EtO)2POCH2CN, NaH, THF, 0 °C, 2 h; (d) NaH, EtOH, reflux, 15 min; (e) H2, 5% Pt/C, acetic acid, 50 psi, room temperature; (f) 60% NaH in mineral oil, EtOH, reflux, 8.5 h; (g) NaBH4; (CH3O)2SO2, THF, reflux 48 h, purified through oxalate salt (oxalic acid, acetone), followed by KOH (aqueous) and distillation of the free base at 183 °C. in vacuo (68%).
* The yield of (±)-7 after the combination steps of e and f was about 60%.
The reaction sequence to the known racemic secondary amine 8 (Scheme 1) was similar to that used by Hutchison et al.,3 but modified for the larger scale synthesis. The yield in the conversion of 1 to the propiophenone (2) was improved from 65%3 to 90% by running the reaction in acetone, monitoring its completion by TLC and NMR, and by crystallization using hexane and isopropanol. Borane reduction16 of the amide 7 gave the racemic cis-6-methoxy amine 8. Compound 8 was treated with (S)-(+)-mandelic acid to give the salt with the (+)-amine base 9 (Scheme 2). From the mother liquors of the recrystallization of the salt, material enriched in the (−)-enantiomer 10 was obtained, converted to the base, and treatment with (R)-(−)-mandelic acid gave the desired salt with the (−)-amine base 10 (Scheme 2). The enantiomers, (+)-9 and (−)-10 were obtained from their mandelate salts on basification. The melting points of these enantiomers were similar, and their optical rotations about equal and opposite.
Scheme 2.a.
a Reagents and conditions: (a) (S)-(+)mandelic acid, EtOAc/Et2O (recrystallized from acetonitrile:Et2O (1:1) (1x), EtOAc:Et2O (1:1) (1x), and acetonitrile:Et2O (1:1) (1x)), free-based with NaOH (2N), extracted by CHCl3, remove solvent, high vacuum distillation at 130 °C; (b) (R)-(−)mandelic acid, EtOAc/Et2O (recrystallized from EtOAc:Et2O (1:1) (1x), acetonitrile:Et2O (1:1) (2x)), 68% recovery. Free-based with NaOH (2N), extracted with CHCl3, remove solvent, high vacuum distillation at 125 °C.
Derivatization of (+)-9 or (−)-10 with R-(+)-1-phenethylisocyanate to form the diastereomeric ureas17 enabled the determination of the optical purity of these amines. The optical purity of 9 was established by the disappearance of the chemical shift at δ 1.453, and that of 10 by the disappearance of the chemical shift at δ 1.408. Their optical purity was estimated to be ca. 99% e.e.17
The chirality of (−)-10 was determined to be 4aS,9aR by X-ray crystallographic analysis of the salt (−)-10•R-(−)-mandelate (Figure 2). N-Alkylation of the secondary amine 4aR,9aS-9 or 4aS,9aR-10 (Scheme 3) gave the dextrorotary N-methyl (4aR,9aS-11) and N-phenylethyl analogue (4aR,9aS-12) and their levorotatory relatives (4aS,9aR-18 and 4aS,9aR-19). Cleavage of the aromatic methoxy group with 48% HBr gave the phenols, 4aR,9aS-14 and 4aR,9aS-15 and 4aS,9aR-21 and 4aS,9aR-22 (Scheme 3). The (+)-S- and R-2′-hydroxyphenethyl compounds 4aR,9aS-16 and 4aR,9aS-17 and the (−)-S- and R-2′-hydroxyphenethyl compounds 4aS,9aR-23 and 4aS,9aR-24 (Scheme 3) were obtained from the 4aR,9aS-amino phenol 13 and the 4aS,9aR-amino phenol 20, respectively. These phenols (4aR,9aS-13 and 4aS,9aR-20) were prepared by 48% HBr cleavage of the aromatic methoxy secondary amine compounds 4aR,9aS-9 and 4aS,9aR-10. The various chiral compounds were generally prepared in moderate to high yields.
Figure 2.
X-ray crystallographic structure of the R-(−)-mandelic acid salt of 4aS,9aR-(−)-cis-4a-ethyl-6-methoxy-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((−)-10). Displacement ellipsoids are shown at the 50% level.
Results and Discussion
Five compounds (20 - 24) were examined for their efficacy as μ-agonists in the [35S]GTP-γ-S functional assay (Table 1). In that assay, the secondary amine 20 was found to be a weak partial agonist (ED50 = 150 nM) and had weak antagonist activity (Ke = 95 nM), the N-methyl substituted pyridin-6-ol 21 had good affinity for the μ-receptor (Ki = 16 nM) but had little efficacy in the functional assay, 23 was noted to be a weak partial agonist, compound 24 a weak agonist, and the 4aS,9aR-N-phenethyl substituted pyridin-6-ol (22) was, as expected, a potent agonist with very high affinity for the μ-receptor (Ki = 0.7 nM). As shown in Table 1, the 4aS,9aR compounds (all levorotatory) were found to have much higher affinity than the comparable 4aR,9aS compounds (all dextrorotatory). Clearly, the activity of the racemic mixtures examined by Hutchison et al.,3 reflected the activity of the compounds with the 4aS,9aR stereochemistry. The 4aS,9aR-N-phenethyl compound 22 had subnanomolar affinity for the μ-opioid receptor, and slightly more than 100 fold less affinity at the δ- and κ-receptors. Interestingly, introduction of a hydroxy substituent in the N-phenethyl side-chain decreased affinity for the μ-receptor 10 fold with the hydroxy in the S-configuration (4aS,9aR,2′S-23), and over 100 fold when the hydroxy was in the R-configuration (4aS,9aR,2′R-24).
Table 1.
[3H] Opioid Receptor Binding Dataa for cis-Benzofuro[2,3-c]pyridin-6-ols and Functional Data ([35S]GTP-γ-S)b for Selected Compounds
| Ki (nM) | |||||
|---|---|---|---|---|---|
| 4aS,9aR | μc | δd | κe | μ-Agonism Emax %f | ED50 (nM) |
| 20g | 48 ± 2.9 | - | - | 97 ± 4 | 150 ± 25 |
| 21 | 15.9 ± 1 | 1350 ± 90 | 450 ± 34 | 45 ± 2 | 241 ± 47 |
| 22 | 0.70 ± 0.06 | 75 ± 8.8 | 88 ± 8 | 84 ± 4 | 24 ± 6 |
| 23 (2′S) | 9.3 ± 0. 7 | 443 ± 32 | 413 ± 44 | 100 ± 3 | 560 ± 60 |
| 24 (2′R) | 80 ± 5 | 807 ± 45 | 1090 ± 60 | 36 ± 2 | 480 ± 90 |
| 4aR,9aS | μc | δd | κe | ||
| 13 | 3500 ± 417 | - | - | - | - |
| 14 | 1330 ± 50 | 4270 ± 240 | > 10,000 | - | - |
| 15 | 200 ± 9 | 2690 ± 210 | 2325 ± 160 | - | - |
| 16 (2′S) | 600 ± 34 | 2490 ± 230 | 5870 ± 470 | - | - |
| 17 (2′R) | 1330 ± 80 | 3100 ± 90 | 9500 ± 780 | - | - |
Assays22 were conducted using CHO cells, which were stably transfected and express the μ-, δ- or κ-opiate receptors respectively. All results n=3. For comparison, the Ki of morphine at μ = 2.55 ± 0.01 nM;
[35S]GTP-γ-S binding was performed using CHO hMOR cells which express the human μ,-opiate receptor. All values n=3;
[3H]DAMGO binding,
[3H]DADLE binding,
[3H]U69,593 binding;
The Emax is the extrapolated maximal stimulation where 100% is defined as the stimulation produced by 10 μM DAMGO (DAMGO ED50 at μ = 42 ± 4 nM);
Secondary amine 20 is a μ-antagonist, Ke = 95 ± 16 nM ([35S]GTP-γ-S assay with CHO hMOR cells, n = 5).
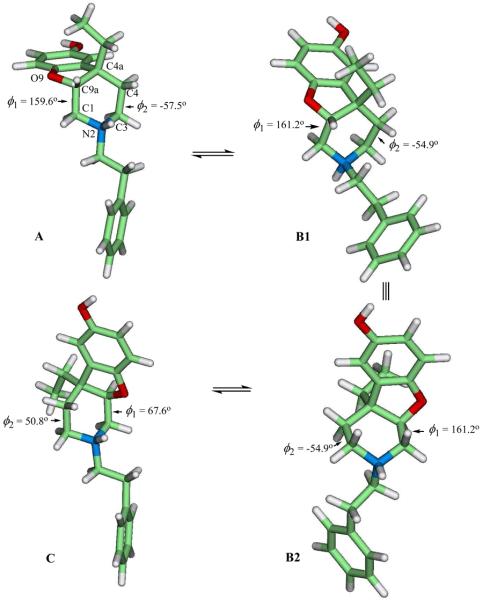
Figure 3 illustrates the geometry optimized conformers of the high affinity N-phenethyl compound (22) in the gaseous phase in its protonated form; the active form of 22 is likely to be protonated at physiological pH. For the comparison of the energetics, the Gibbs free energy of each conformer was calculated at 298.15 K from its respective fully optimized geometry in CHCl3. The topology of A, except for the equatorial N-phenylethyl substituent, is essentially identical to the X-ray structure of 10 as evidenced by the less than 0.03 Å root mean square deviation (rmsd) between the heavy atoms of both the dihydrofuran and the piperidine rings. Conformer B1 is epimeric to A, and was obtained by nitrogen inversion; it is 2.6 kcal/mol less stable relative to A, a consequence of the change in the orientation of the N-phenylethyl from equatorial to axial. This nitrogen inversion in solution is likely to proceed through deprotonation followed by reprotonation. B2 was obtained by ∼180° rotation of B1 to point both the hydrogen on the nitrogen atom and the dihydrofuran ring of B1 out of the plane. A conformational change of the piperidine ring in B2 or B1 of Figure 3 via the variation of the dihedral angle of O9-C9a-C1-N2 (ϕ1) and C4a-C4-C3-N2 (ϕ2) leads to conformer C with the equatorially-oriented N-phenylethyl moiety; conformer C is slightly (0.26 kcal/mole) more stable than A, and this stability may arise from the charge interaction between the dihydrofuran oxygen and the hydrogen on the nitrogen atom as evidenced by their distance of 2.4 Å. Note that A can undergo the piperidine conformation change first, followed by the nitrogen inversion to conformer C. Both the reaction pathway and the energy barrier between the two conformers A and C are not known, and are currently being investigated with the ab-initio Replica Path method.18 Nonetheless, considering that both the nitrogen inversion energy barrier and the conformation energy barrier of the neutral N-methyl piperidine are known to be less than 11 kcal/mol,19, 20 22 may well undergo a rapid interconversion between A and C, and thus is likely to exist as a conformer mixture at room temperature.
Figure 3.
Geometry optimized conformers of the protonated 4aS,9aR-(−)-cis-4a-ethyl-2-(2-phenylethyl)-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol (22) in the gaseous phase. Topology of A is essentially identical with the X-ray structure of 10 in Figure 2 (without the N- phenylethyl moiety). Conformer B1 is epimeric to A with respect to the nitrogen, B2 is another representation of B1 via a 180° rotation, and C is formed by a conformational change of the piperidine ring of B2 or B1. ϕ1 is the dihedral angle of O9-C9a-C1-N2 and ϕ2 is the dihedral angle of C4a-C4-C3-N2.
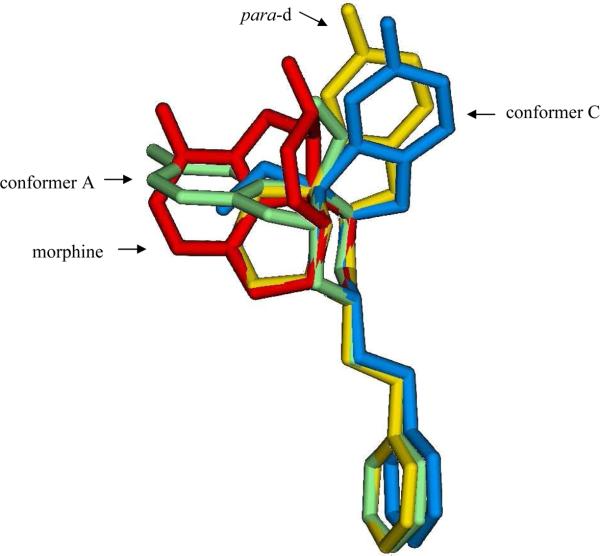
Energetics suggest that 22 exists in CHCl3 predominantly in the form of A and C (> 95%) at room temperature. To identify the conformer most likely to be recognized at the μ-opioid receptor, the heavy atoms of the piperidine rings of A, C and the oxide-bridged para-d phenylmorphan isomera (that was found to be structurally more similar to 22 than any of the other oxide-bridged phenylmorphans) were fitted to morphine with the equatorial N-methyl as shown in Figure 4. Their respective rmsd value of the fitting is 0.13 Å, 0.12 Å, and 0.07 Å. The dihydrofuran ring of conformer C overlaps well with that of the para-d isomer that is known to bind poorly to the μ-opioid receptor (Ki = 1220 nM).13 This indicates that conformer C, although thermochemically more stable than A, is not recognized by the receptor, and thus the subnanomolar affinity of 22 (Ki at μ = 0.7 nM) is likely to arise from conformer A. The benzofuran ring in conformer A overlaps better with the benzofuran ring of morphine than that of the para-d isomer (Figure 4). The affinity of 22 for the μ-receptor is much closer to that of morphine (Ki = 2.55 ± 0.1 nM) than to the para-d isomer.
Figure 4.
Overlay of the piperidine ring of conformers A and C of compound 4aS,9aR-(−)-22, and the para-d oxide-bridged phenylmorphan, onto that of morphine, illustrating the topographical similarity of conformer A with morphine and conformer C with the para-d oxide-bridged phenylmorphan that has little affinity for the μ-opioid receptor. Hydrogen atoms are not shown.
This work has enabled us to determine the enantiomer responsible for the μ-affinity and activity of the racemic N-substituted benzofuro[2,3-c]pyridin-6-ols.3 In addition, we have compared the structures of the energy minimized conformers of the most active enantiomer 22 and the various oxide-bridged phenylmorphans, determining that the activity and affinity of 22 was not likely to be due to conformer C that is structurally similar to the para-d oxide-bridged phenylmorphan. Compound 22, however, was found to have a less stable conformer A, topologically similar to morphine, that could be responsible for its activity. Also, we synthesized new N-derivatives with a hydroxyl substituent on the phenylethyl side-chain (23 and 24), and determined that an hydroxyl moiety on that side-chain hindered binding and reduced efficacy.
Experimental Section
All reactions were performed in oven-dried glassware under an argon atmosphere. Some of the large scale reactions were magnetically stirred. All melting points were determined on a Thomas-Hoover capillary melting point apparatus and are uncorrected. The optical rotation data were obtained on a Perkin Elmer polarimeter model 341 or on a Jasco DIP-370 digital polarimeter (589nm). 1H NMR (in CDCl3 with TMS at δ 0.0 ppm) spectra were recorded on a Varian XL-300 spectrometer at 300-MHz, and on a Varian AS-400 spectrometer at 400-MHz. Mass spectra (EI) and high-resolution mass spectra (HRMS) were obtained using a JEOL SX102 instrument. Thin layer chromatography (TLC) was performed on precoated plates of silica gel GF (0.25 mm, F254, Alltech) using various gradients of CHCl3/MeOH containing 1% NH4OH or gradients of ethyl acetate/n-hexanes. Visualization was accomplished under UV or by staining in an iodine chamber. Flash chromatography was conducted using silica gel (230-400 mesh, Merck). Elemental analyses were performed by Atlantic Microlabs Inc., Norcross, GA, to determine the purity of the compounds and were within ±0.4% of theoretical values, confirming ≥95% purity.
2-Hydroxy-5-methoxypropiophenone (1)
In a 5 L 3-necked flask fitted with a mechanical stirrer, thermometer, nitrogen purge and pressure equalizing funnel was placed 500 mL of dry nitromethane. The stirred solvent was cooled to −20 °C and 277.82 g (2.08 mol, 1.15 eq) of anhydrous AlCl3 was added slowly (exothermic) keeping the temperature < 0 °C with a dry ice-acetone bath. A 50 mL portion of nitromethane was used to wash in the AlCl3. Propionyl chloride (201.05 g, 2.17 mol, 1.20 equiv) was then slowly added keeping the temperature < 0 °C. A mixture of 150 mL of nitromethane and 1,4-dimethoxybenzene (250.0 g, 1.81 mol) was heated to solution. This solution was added slowly to the reaction mixture beginning at − 20 °C and the temperature was allowed to rise to 10 °C. A 50 mL portion of nitromethane was used as wash in. This is a very rapid reaction. TLC (petroleum ether :Et2O, 3:1) of an aliquot quenched with H2O gave essentially a single spot indicating the intermediate product 2,5-dimethoxypropiophenone, no starting material, and only a trace of 1. The stirred mixture was slowly and cautiously heated to reflux with the rapid evolution of HCl and methyl chloride. At 60 °C TLC showed ∼ 2 : 1 mixture of intermediate and the desired 1. After 1.5 h of reflux, TLC showed the absence of the intermediate product. The reaction mixture was cooled and slowly quenched with 500 mL of H2O and the nitromethane evaporated under water aspirator vacuum. The mixture was extracted with CHCl3 (600 mL, 2 × 200 mL) and the combined CHCl3 washed with 250 mL of H2O and evaporated in vacuo. The dark residue was vacuum distilled at 120-125/1-2 mm to give 288.84 g of yellow oil. This was dissolved in CH3OH (722 mL) and treated dropwise with H2O (577 mL) (CH3OH:H2O, 2.5:2) to give crystalline material (cooling to a final temperature of 15 °C). This was filtered and washed with mixture of CH3OH (360 mL) and H2O (289 mL) (CH3OH:H2O, 1.25:1) at 15 °C and dried on the filter to constant weight to give 273.9 g (84%) of 1, mp 45-46 °C (lit15 47-48 °C); NMR (CDCl3, 300 MHz): δ 1.25 (3 H, t, J = 7.2 Hz), 3.02 (2 H, q, J = 7.2 Hz), 3.80 (3 H, s), 6.93 (1 H, d, J = 9.0 Hz), 7.10 (1 H, dd, J = 3.0, 9.0 Hz), 6.72 (1 H, d, J = 3.0 Hz).
2-(Carbethoxymethoxy)-5-methoxypropiophenone (2)
A mixture of 2-hydroxy-5-methoxypropiophenone (114.5 g), ethyl bromoacetate (74 mL), and powdered anhydrous K2CO3 (131.5 g) in anhydrous acetone (900 mL) was refluxed and vigorously stirred under argon for 30 h. The completion of the reaction was initially monitored by TLC (hexane:EtOAc, 60:40) and after about 20 h, by NMR. The reaction mixture was filtered and concentrated to afford 2 (173.9 g) as a yellow solid. Compound 2 was dissolved in hexane (690 mL) with heating, and the solution was cooled to room temperature. Isopropanol (170 mL) was added to solubilize the oily material that came out of solution. A crystalline solid precipitated, and the solution was cooled to 0 °C to complete the crystallization. The solid was filtered and washed three times with the solvent mixture (hexane, 690 mL and isopropanol, 170 mL, 0 °C). The air dried product (2, 147.8 g) was obtained as an ivory-colored crystalline solid in 90% yield, mp 61-62.5 °C (lit3 mp 58-60 °C). NMR (CDCl3, 300 MHz): δ 1.18 (3H, t, J = 7.4 Hz), 1.30 (3H, t, J = 7.2 Hz), 3.12 (2H, quartet, J = 7.2 Hz), 4.27 (2H, q, J = 7.2 Hz), 4.66 (2H, s), 6.79 (1H, d, J = 9.0 Hz), 6.97 (1H, dd, J = 3.3, 9.0 Hz), 7.26 (1H, d, J = 3.3 Hz); MS m/z 267 [M + H]+.
(E,Z)-2-(Carbethoxymethoxy)-5-methoxy-β-ethylcinnamonitrile (3)
The procedure of Hutchison et al.3 was modified for use on a larger scale. Compound 2 (147.77 g) in THF (350 mL) was added to a pre-reacted mixture of sodium hydride (60% in mineral oil, 26.64 g), diethyl(cyanomethyl)phosphonate (96 mL), and anhydrous THF (500 mL). The reaction was complete after 2 h at 0 °C (monitored by TLC (hexane:EtOAc, 60:40)), to give 3 (141.35 g, 88.3%) as a yellow oil that was used without further purification. MS m/z 290 [M + H]+.
rac-2-Carbethoxy-3-(cyanomethyl)-3-ethyl-5-methoxy-2,3-dihydrobenzofuran (4)
A mixture of compound 3 (130.7 g), EtOH (anhydrous, 18.5 mL), and sodium hydride (60% in mineral oil, 1.807 g) was refluxed for 15 min. The completion of the reaction was monitored by NMR. The yield of the 4 was improved by modifying the work-up procedure of Hutchison et al.3 After cooling to room temperature, EtOH was evaporated from the reaction mixture, which was then mixed with ice water (100 mL) and extracted with CH2Cl2 (100 mL × 3). The crude product (130.69 g, 100%) was used to synthesize 5 (cis-(2R*,3S*)-ethyl 3-(2-aminoethyl)-3-ethyl-5-methoxy-2,3-dihydrobenzofuran-2-carboxylate) and 6 (trans-(2R*,3R*)-ethyl 3-(2-aminoethyl)-3-ethyl-5-methoxy-2,3-dihydrobenzofuran-2-carboxylate).
rac-cis-4a-Ethyl-6-methoxy-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]-pyridin-1-one (7)
A mixture of 4 (130.69 g, 1 equiv) and Pt/C (5% Pt/C powder, 63.05% H2O, 71.55 g) in acetic acid (727 mL) was hydrogenated at 50 psi at room temperature for 40 h (reaction completion monitored by TLC (EtOAc)). The mixture was filtered, concentrated, and then poured into ice water. Ammonium hydroxide (28%, 50 mL) was used to adjust the pH ∼9. The solution was then extracted with CH2Cl2 (200 mL × 4), dried over MgSO4 and concentrated in vacuo to give 7. The residual material containing 5 (cis-(2R*,3S*)-ethyl 3-(2-aminoethyl)-3-ethyl-5-methoxy-2,3-dihydrobenzofuran-2-carboxylate) and 6 (trans-(2R*,3R*)-ethyl 3-(2-aminoethyl)-3-ethyl-5-methoxy-2,3-dihydrobenzofuran-2-carboxylate), but enriched with 5, (104.0 g) was mixed with sodium hydride (60% in mineral oil, 1.66 g) in EtOH (anhydrous, 78 mL) in order to epimerize 6 to 5. The mixture of 5 + 6 was refluxed under argon for 8.5 h (reaction completion monitored by TLC (EtOAc)), concentrated, and redissolved in EtOAc (350 mL) and H2O (200 mL). After the separation of the organic phase, the aqueous phase was extracted with EtOAc (150 mL × 3), followed by CH2Cl2 (150 mL). The combined organic phase was dried with brine (150 mL × 2) and concentrated in vacuo to give additional 7 (109.1 g) as a solid. The crude 7 (105 g) was dissolved in Et2O (300 mL) at room temperature by mechanical stirring for 2 h. The solution was then cooled to 0 °C, filtered, washed with cold Et 2O (300 mL), solvent removed in vacuo, and the product was air dried. Pure 7 (60.2 g, 57%) was obtained as a beige colored solid, mp 117.5 °C (lit3 mp 122-123 °C). TLC (a 1 : 1 mixture of CHCl3 and the mixture CHCl3:MeOH: 28% NH4OH, 90:9:1) indicated that the impurities remained in the ether filtrate. NMR (CDCl3, 300MHz): δ 0.87 (3H, t, J = 7.6 Hz), 1.74 (2H, m), 2.04 (2H, m), 3.21 (2H, m), 3.77 (3H, s), 4.66 (1H, s), 6.60 (1H, d, J = 2.7 Hz), 6.61 (1H, brs), 6.70 (1H, dd, J = 2.6, 8.6 Hz), 6.79 (1H, d, J = 8.7 Hz); MS m/z 248 [M + H]+.
rac-cis-4a-Ethyl-6-methoxy-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine (8)
In a dry, 2 L three-necked round-bottomed flask equipped with stirrer bar, thermometer, dropping funnel and reflux condenser, was placed THF (3Å molecular sieve treated, 490 mL) and lactam 7 (49.2 g, 0.199 mol), under argon. Sodium borohydride (60.2 g, 1.592 mol) was added to the stirred mixture, followed by dimethyl sulfate (150.6 mL, 1.592 mol) dissolved in THF (3Å molecular sieve treated, 160 mL), over a period of 1 h. Stirring was continued for an additional 30 min with the temperature maintained at 35∼45 °C. The mixture was then refluxed for two days (reaction completion monitored by TLC (CHCl3:CH3OH:28% NH4OH, 90:9:1)). Methanol (250 mL) was added slowly to quench the reaction. The reaction mixture was then concentrated to a foam and redissolved in CH3OH (700 mL). The methanol solution was saturated with hydrogen chloride gas, then refluxed overnight. The mixture was then concentrated and partitioned in H2O (500 mL) and Et2O (400 mL). The aqueous phase was then washed with another portion of Et2O (300 mL). The combined etherate wash was extracted again with H2O (200 mL) to reduce the loss of the product. At the end, the combined aqueous phase was basified with NH4OH (120 mL) to pH 9∼9.5, extracted with CHCl3 (500 mL × 4), and concentrated to afford the crude racemic amine (8, 56.21g). Oxalic Acid (21.69 g, 0.24 mol) and crude amine 8 (56.21 g) were dissolved in acetone (400 mL). The clear yellow solution was stirred until the solid oxalate salt precipitated from the solution. After cooling to 0 °C, the oxalate was filtered, washed with cold acetone (300 mL) and dried in vacuo to afford 8•oxalate (50.48 g). The oxalate was placed in a separatory funnel, dissolved in H2O (200 mL), and a KOH aqueous solution (40.2 g in 160 mL H2O) added. The mixture was shaken to give 8 as the free base, and CHCl3 (200 mL × 4) was added to extract the amine. The first two CHCl3 extracts were combined, washed with H2O (200 mL), then filtered through celite. The third extract was washed with that H2O (200 mL), filtered through celite and the celite filter-cake was washed with CHCl3. This was repeated again with the fourth CHCl3 extract. The organic solvent was removed in vacuo to give 8 (42.16 g, 81%). Compound 8 was distilled under water aspirator vacuum at 183 °C to give the pure racemic amine 8 (35.2 g, 68%) as a colorless viscous oil. MS m/z 234 [M + H]+.
4aR,9aS-(+)-cis-4a-Ethyl-6-methoxy-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((+)-9)
Racemic amine 8 (33.58 g, 0.144 mol) and S-(+) mandelic acid (22.34 g, 0.147 mol) were heated to solution in EtOAc (91 mL) in a 250 mL beaker. Ether (105 mL) was then slowly added to the hot solution. Scratching or a seed crystal was used to induce crystallization. Another portion of Et2O (27 mL) was added to the boiling mixture. Scratching was continued until crystallization ended. The solution was cooled in a chilled water bath and another portion of Et2O (4.5 mL) was added. An oil appeared with this addition and a small amount of EtOAc was added to resolubilize the oil. The solid was filtered and washed with a portion of a mixture of EtOAc (91 mL) and Et2O (136.5 mL), and was briefly vacuum dried (to avoid drying any oil on the crystals). Three additional washes used the remaining amount of the rinsing solvent mixture. Ether (25 mL) was used as a final rinse to dry the filter cake. The white solid (30.55 g) was recrystallized 3x from EtOAc or acetonitrile to give pure (+)-9•S-(+)-mandelate (17.96 g, 64%), mp 144.5-145.5 °C. The mandalate salt was dissolved in CHCl3 (40 mL) and NaOH (2N, 45 mL) was used to convert it to the free base. The aqueous phase was extracted with CHCl3 (3x). The combined organic phase was washed with H2O (30 mL), and the solvent removed in vacuo to give (+)-9 as the free base (11.23 g). High vacuum distillation at 120-125 °C gave a quantitative yield of (+)-9 (10.88 g) as a colorless viscous oil. The oil slowly crystallized to a white solid, mp 50-51 °C. [α]20D +79.5° (c 1.014, CDCl3); NMR (CDCl3, 300 MHz): δ 0.86 (3H, t, J = 7.4 Hz), 1.58-1.84 (4H, m), 2.65 (1H, ddd, J = 4.2, 8.1, 12.6 Hz), 2.79 (1H, ddd, J = 4.5, 6.6, 11.1 Hz), 2.94 (1H, dd, J = 5.2, 13.6 Hz), 3.09 (1H, dd, J = 4.4, 13.6 Hz), 3.77 (3H, s), 4.30 (1H, t, J = 5.0 Hz), 6.68-6.63 (2H, m), 6.72 (1H, m). Anal. Calcd for (C14H19NO2): C, H, N.
A portion of the amine (+)-9 was derivatized with R-(+)-1-phenethylisocyanate17 to give the diastereomeric urea, and the chemical shifts at δ 1.453 and 1.408 were examined. The optical purity of (+)-9 (ca. 99% e.e.)17 was determined by the disappearance of the chemical shift at δ 1.453, and that of (−)-10 (ca. 99% e.e.)17 by the disappearance of the chemical shift at δ 1.408. The chirality of (+)-9 as 4aR,9aS was established by the X-ray crystallographic examination of the R-(−)-mandelic acid salt of enantiomer (−)-10.
4aS,9aR-(-)-cis-4a-Ethyl-6-methoxy-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((-)-10)
The mother liquors from the preparation of the 4aR,9aS-(+) isomer, enriched with the 4aS,9aR-(−)-enantiomer, were combined and free-based with 28% NH4OH (13.5 mL) until the pH was around 9∼9.5. Chloroform (80 mL × 4) was used to extract the amine and the solvent removed in vacuo to give an amine mixture (23.0 g). NMR of the derivatized amine mixture indicated that it was about a 3:2 mixture of the 4aS,9aR and the 4aR,9aS isomers, respectively. The amine mixture (23.0 g, 0.098 mol) and R-(−)-mandelic acid (14.98 g, 0.098 mol) were heated to solution in EtOAc (62 mL) in a 250 mL beaker. The solution was cooled, and Et2O (86 mL) was added slowly until an oil began to separate. Scratching was used to induce crystallization. The solution was cooled with chilled H2O until the crystallization ended. The crystals were washed as described for enantiomer (+)-9 (EtOAc, 60 mL and Et2O, 86 mL) to give the (−)-10•R-(−)-mandelate (25.58 g) as a white solid. The NMR of the derivatized free-based amine showed ∼88% of the desired enantiomer. The solid was recrystallized 3x from EtOAc or acetonitrile to give the pure enantiomer (68% yield, mp 145-146 °C), as determined by the NMR spectra of the derivatized free-based amine with R-(+)-1-phenethylisocyanate. The chirality of (−)-10 was determined by X-ray diffraction analysis of the (−)-10•R-(−)-mandelate to be 4aS,9aR. The R-(−)-mandelate salt (13.34 g, 34.6 mmol) was dissolved in CHCl3 (40 mL) and free-based using NaOH (2N, 35 mL, 69.2 mmol). This was worked up as with the mandelate salt of (+)-9 to give the amine (−)-10 (8.21 g) that was distilled under high vacuum at 130 °C to afford pure (−)-10 (8.01 g) in quantitative yield as a colorless viscous oil that crystallized very slowly on long standing, mp 50-51 °C. [α]20D-79.4° (c 1.166, CDCl3); NMR (CDCl3, 300 MHz): δ 0.86 (3H, t, J = 7.4 Hz), 1.58-1.84 (4H, m), 2.65 (1H, ddd, J = 4.2, 8.1, 12.6 Hz), 2.79 (1H, ddd, J = 4.5, 6.6, 11.1 Hz), 2.94 (1H, dd, J = 5.2, 13.6 Hz), 3.09 (1H, dd, J = 4.4, 13.6 Hz), 3.77 (3H, s), 4.30 (1H, t, J = 5.0 Hz), 6.68-6.63 (2H, m), 6.72 (1H, m). Anal. Calcd for (C14H19NO2): C, H, N.
4aR,9aS-(+)-cis-4a-Ethyl-6-methoxy-2-methyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((+)-11)
Pd/C (10 wt %, 21.3 mg) was added in an argon atmosphere to (+)-9 (233 mg) that was dissolved in MeOH (10 mL). The argon was evacuated and re-added 3x. Formaldehyde solution (37% aqueous, 89 μL) was added and the argon replaced by hydrogen via a hydrogen balloon connected to the flask through a needle (16 gauge, 1½). The reaction was complete after 4 h at room temperature (monitored by TLC (CHCl3:MeOH:28% NH4OH, 90:9:1)). The mixture was then filtered through celite, the filtered catalyst was rinsed with ethanol, and the solvent removed in vacuo. Column chromatography (CHCl3:MeOH:28% NH4OH, 90 :9:1) afforded (+)-11 (247 mg, 100%) as a colorless viscous oil that crystallized on standing, mp 50-51 °C. [α]20D +89.3 (c 0.750, CDCl3); NMR (CDCl3, 300 MHz): δ 0.84 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.3 Hz), 1.69 (1H, sextet, J = 7.2 Hz), 1.78 (1H, ddd, J = 4.2, 9.3, 13.8 Hz), 2.02 (1H, ddd, J = 3.4, 5.8, 13.8 Hz), 2.12 (1H, ddd, J = 3.4, 9.4, 14.6 Hz), 2.25 (3H, s), 2.30 (1H, dd, J = 6.9, 12.3 Hz), 2.42 (1H, m), 2.75 (1 H, ddd, J = 1.4, 5.4, 12.3 Hz), 3.77 (3H, s), 4.45 (1H, dd, J = 5.3, 6.8 Hz), 6.65 (2H, m), 6.72 (1H, m). MS m/z 248 [M + H]+. Anal. Calcd for (C15H21NO2): C, H, N.
4aR,9aS-(+)-cis-4a-Ethyl-6- methoxy-2-(2-phenylethyl)-1 ,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine (+)-12)
Compound (+)-9 (234 mg) was mixed with 2-bromoethylbenzene (611 mg), K2CO3 (166 mg) in anhydrous DMF (5 mL). The mixture was heated at 50 °C for 20 h. After the completion of the reaction (monitored by TLC, CHCl3:EtOAc, 10:3), the mixture was quenched with H2O (50 mL), extracted with EtOAc (50 mL × 3). The combined organic phase was washed with H2O (50 mL), followed by brine (50 mL), dried with Na2SO4, and filtered through celite. The solvent was removed in vacuo to give crude (+)-12 which was purified by column chromatography (CHCl3:EtOAc, 5:1). The product (+)-12 (205 mg, 61%) was obtained as a white solid, mp 84-86.5 °C. [α]20D +113.7° (c 0.750, CDCl3); NMR (CDCl3, 300 MHz): δ 0.83 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.2 Hz), 1.70 (1H, sextet, J = 7.2 Hz), 1.80 (1H, ddd, J = 4.2, 9.9, 14.1 Hz), 2.07 (1H, ddd, J = 3.3, 5.4, 14.1 Hz), 2.21 (1H, dt, J = 3.3, 11.4 Hz), 2.36 (1H, dd, J = 7.2, 12.0 Hz), 2.59 (3H, m), 2.79 (2H, m), 2.94 (1H, ddd, J = 1.5, 6.0, 12.0 Hz), 3.78 (3H, s), 4.48 (1H, dd, J = 5.7, 7.2 Hz), 6.66 (2H, m), 6.73 (1H, m), 7.15-7.29 (5H, m); MS m/z 338 [M + H]+. Anal. Calcd for (C22H27NO2•0.1 H2O): C, H, N.
4aR,9aS-(+)-cis-4a-Ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((+)-13)
Compound 9 (250 mg) was dissolved in hydrobromic acid (2.5 mL, 48%). The mixture was refluxed for about 1 h and the completion of the reaction was monitored by TLC (CHCl3:MeOH :28% NH4OH, 90:9:1). The acidic solution was then basified with 28% NH4OH to pH∼10, extracted with CH2Cl2 (15 mL × 4), and concentrated in vacuo to afford crude (+)-13. Column chromatography (CHCl3:MeOH:28% NH4OH, 90:9:1) purification afforded 4aR,9aS-(+)-13 (220 mg, 95%) as a white solid, mp 165-166 °C. [α]24D +103.0° (c 0.930, CDCl3); NMR (CDCl3, 300 MHz): δ 0.86 (3H, t, J = 7.4 Hz), 1.64 (1H, sextet, J = 7.3 Hz), 1.66-1.82 (3H, m), 2.66 (1H, ddd, J = 4.8, 8.4, 12.6 Hz), 2.80 (1H, ddd, J = 4.5, 6.6, 12.6 Hz), 2.96 (1H, dd, J = 5.1, 13.8 Hz), 3.08 (1H, dd, J = 4.2, 13.8 Hz), 4.30 (1H, t, J = 4.8 Hz), 6.58 (2H, m), 6.66 (1H, m); MS m/z 220 [M + H]+. Anal. Calcd for (C13H17NO2•0.1 H2O): C, H, N.
4aR,9aS-(+)-cis-4a-Ethyl-2-methyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((+)-14)
Compound (+)-11 (212 mg) was dissolved in hydrobromic acid (48%, 2.5 mL). The mixture was refluxed for about 30 min. The completion of the reaction was monitored by TLC (CHCl3:MeOH:28% NH4OH, 90:9:1). The mixture was concentrated in vacuo to give a viscous brownish glassy residue. It was dissolved in mixture of H2O (5 mL) and 28% NH4OH (1 mL), extracted with CH2Cl2 (10 mL × 3) and the solvent removed in vacuo. Column chromatography (CHCl3:MeOH:28% NH4OH, 90:9:1) afforded (+)-14 (169 mg, 85%) as a white solid, mp 159-162 °C. [α]28D +92.7 (c 0.780, CDCl3); NMR (CDCl3, 300 MHz): δ 0.84 (3H, t, J = 7.5 Hz), 1.53 (1H, sextet, J = 7.3 Hz), 1.61 (1H, sextet, J = 7.3 Hz), 1.78 (1H, ddd, J = 3.9, 9.3, 13.6 Hz), 2.01 (1H, ddd, J = 3.4, 5.8, 13.8 Hz), 2.13 (1H, ddd, J = 3.3, 9.6, 12.2 Hz), 2.25 (3H, s), 2.32 (1H, dd, J = 7.2, 12.0 Hz), 2.44 (1H, m), 2.76 (1H, ddd, J = 1.4, 5.6, 12.3 Hz), 4.45 (1H, dd, J = 5.6, 6.8 Hz), 6.58 (2H, m), 6.66 (1H, m); MS m/z 234 [M + H]+. Anal. Calcd for (C14H19NO2•0.1 H2O): C, H, N.
4aR,9aS-(+)-cis-4a-Ethyl-2-phenylethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((+)-15)
Compound (+)-12 (362.0 mg) was dissolved in a mixture of hydrobromic acid (3.7 mL, 48%) and acetic acid (16.5 mL). The mixture was refluxed for about 7.5 h with the completion of the reaction monitored by TLC (CHCl3:EOAc, 10: 3). The acidic solution was then concentrated under aspirator vacuum, redissolved in H2O (15 mL), basified with 28% NH4OH to pH∼10, extracted with CH2Cl2 (20 mL × 3), and the solvent removed in vacuo to get crude (+)-15. Column chromatography (CHCl3:EtOAc, 10:3) afforded pure 4aR,9aS-(+)-15 (304 mg, 88%) as a white solid, mp 171.5-173 °C. [α]24D +115.8° (c 0.810, CDCl3); NMR (CDCl3, 300 MHz): δ 0.83 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.2 Hz), 1.69 (1H, sextet, J = 7.2 Hz), 1.80 (1H, ddd, J = 4.2, 9.9, 14.1 Hz), 2.05 (1H, ddd, J = 3.4, 5.6, 9.0 Hz), 2.22 (1H, dt, J = 3.3, 10.5 Hz), 2.37 (1H, dd, J = 7.4, 11.8 Hz), 2.59 (3H, m), 2.79 (2H, m), 2.94 (1H, ddd, J = 1.5, 6.0, 12.0 Hz), 4.48 (1H, dd, J = 6.0, 7.2 Hz), 6.58 (2H, m), 6.66 (1H, m), 7.15-7.29 (5H, m); MS m/z 324 [M + H]+. Anal. Calcd for (C21H25NO2): C, H, N.
4aR,9aS,2′S-(+)-cis-2-(2′-Hydroxy-2′-phenylethyl)-4a-ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((+)-16)
Compound (+)-13 (306 mg), (S)-(−) styrene oxide (352.1 mg), and toluene (anhydrous, 9 mL) were reacted under an argon atmosphere. The mixture was refluxed for 72 h. The completion of the reaction was monitored by TLC (hexane:EtOAc, 1:1). After completion, the mixture was concentrated in vacuo. Column chromatography (hexane:EtOAc, 3:2) afforded (+)-16 (265 mg, 56%) as a white solid, mp 163.0-166.0 °C. [α]27D +173.0° (c 0.770, CDCl3); NMR (CDCl3, 300 MHz): δ 0.84 (3H, t, J = 7.4 Hz), 1.58 (1H, sextet, J = 7.2 Hz), 1.71 (1H, sextet, J = 7.2 Hz), 1.85 (1H, ddd, J = 6.2, 8.7, 14.4 Hz), 2.04 (1H, dt, J = 4.4, 14.1 Hz), 2.37 (1H, dd, J = 6.8, 12.2 Hz), 2.49 (4H, m), 3.13 (1H, dd, J = 5.4, 12.0 Hz), 4.46 (1H, dd, J = 5.2, 6.8 Hz), 4.68 (1H, dd, J = 5.6, 8.2 Hz), 6.59 (2H, m), 6.67 (1H, m), 7.21-7.34 (5H, m); MS m/z 340 [M + H]+. Anal. Calcd for (C21H25NO3): C, H, N.
4aR,9aS,2′R-(+)-cis-2-(2′-Hydroxy-2′-phenylethyl)-4a-ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((+)-17)
Compound (+)-13 (179 mg), (R)-(+) styrene oxide (206 mg), and toluene (anhydrous, 5 mL) were reacted under an argon atmosphere as in (+)-16. The product (+)-17 (182 mg, 66%) was obtained as a white solid, mp 144.0-146.0 °C. [α]26D +85.4° (c 0.785, CDCl3); NMR (CDCl3, 300 MHz): δ 0.84 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.2 Hz), 1.68 (1H, sextet, J = 7.2 Hz), 1.80 (1H, ddd, J = 4.4, 10.2, 14.6 Hz), 2.07 (1H, dt, J = 4.1, 12.3 Hz), 2.21 (1H, td, J = 3.5, 14.2 Hz), 2.41 (1H, dd, J = 10.5, 12.6 Hz), 2.50 (1H, dd, J = 3.9, 12.6 Hz), 2.58 (1H, dd, J = 6.9, 12.0 Hz), 2.85 (2H, m), 4.49 (1H, dd, J = 5.6, 6.8 Hz), 4.72 (1H, dd, J = 3.4, 10.0 Hz), 6.59 (2H, m), 6.65 (1H, m), 7.22-7.34 (5H, m); MS m/z 340 [M + H]+. Anal. Calcd for (C21H25NO3•0.2H2O): C, H, N.
4aS,9aR-(−)-cis-4a-Ethyl-6-methoxy-2-methyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((−)-18)
Compound (−)-10 (450 mg) was dissolved in MeOH (19 mL) and the flask was flushed 3× with argon. Pd/C (5 wt %, 82.0 mg) was added under the argon atmosphere, and the flask was again flushed with argon 3×. Formaldehyde solution (37% aqueous, 172 μL) was added and the reaction was carried out as with (+)-11, except that the reaction was completed in 4.5 h to give (−)-18 (451 mg, 95%) as a colorless viscous oil that crystallizes on standing, mp 50-51 °C. [ α]27D −89.5° (c 0.810, CDCl3); NMR (CDCl3, 400 MHz): δ 0.84 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.3 Hz), 1.71 (1H, sextet, J = 7.2 Hz), 1.78 (1H, ddd, J = 4.3, 9.2, 13.6 Hz), 2.02 (1H, ddd, J = 3.6, 6.4, 14.0 Hz), 2.12 (1H, ddd, J = 3.4, 9.2, 12.0 Hz), 2.25 (3H, s), 2.32 (1H, dd, J = 7.0, 12.2 Hz), 2.42 (1H, m), 2.75 (1H, ddd, J = 1.4, 5.2, 12.0 Hz), 3.77 (3H, s), 4.45 (1H, dd, J = 5.4, 7.0 Hz), 6.65 (2H, m), 6.72 (1H, m); MS m/z 248 [M + H]+. Anal. Calcd for (C15H21NO2): C, H, N.
4aS,9aR-(−)-cis-4a-Ethyl-6-methoxy-2-(2-phenylethyl)-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((−)-19)
Compound (−)-10 (234 mg) was reacted as shown in the preparation of (+)-12, except that the mixture was quenched with H2O (50 mL), and 28% NH4OH was used to adjust the pH∼10. Ethyl acetate (50 mL × 3) was used to extract the product. The combined organic phase was washed with H2O (50 mL), followed by brine (50 mL), dried with MgSO4, and filtered through celite. After rotary evaporation, the crude product was purified by column chromatography (CHCl3:EtOAc, 5:1). The product (242 mg, 72%) was obtained as a white solid, mp 92-93.5 °C. [α]27D −113.5° (c 0.755, CDCl3); NMR (CDCl3, 400 MHz): δ 0.83 (H, t, J = 7.5 Hz), 1.59 (1H, sextet, J = 7.2 Hz), 1.72 (1H, sextet, J = 7.2 Hz), 1.80 (1H, ddd, J = 4.2, 10.0, 14.2 Hz), 2.07 (1H, ddd, J = 3.2, 5.6, 14.0 Hz), 2.21 (1H, dt, J = 3.4, 10.6 Hz), 2.36 (1H, dd, J = 7.2, 12.2 Hz), 2.59 (3H, m), 2.79 (2H, m), 2.94 (1H, ddd, J = 1.5, 6.0, 12.0 Hz), 3.78 (3H, s), 4.48 (1H, dd, J = 5.6, 7.2 Hz), 6.66 (2H, m), 6.73 (1H, m), 7.15-7.29 (5H, m); MS m/z 338 [M + H]+. Anal. Calcd for (C22H27NO2): C, H, N.
4aS,9aR-(−)-cis-4a-Ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((−)-20)
Compound (−)-10 (350 mg) was dissolved in hydrobromic acid (3.5 mL, 48%), and the reaction run as shown for (+)-13 to give (−)-20 (325mg, 100%) as a beige colored solid, mp 169-171 °C. [α]27D −100.6° (c 0.815, CDCl3); NMR (CDCl3, 400 MHz): δ 0.86 (3H, t, J = 7.5 Hz), 1.64 (1H, sextet, J = 7.2 Hz), 1.70 (1H, ddd, J = 5.3, 9.2, 14.4 Hz), 1.74 (1H, sextet, J = 7.2 Hz), 1.78 (1H, dt, J = 4.3, 14.0 Hz), 2.66 (1H, ddd, J = 4.3, 7.7, 12.4 Hz), 2.80 (1H, ddd, J = 4.3, 6.1, 10.8 Hz), 2.96 (1H, dd, J = 4.8, 13.6 Hz), 3.09 (1H, dd, J = 4.0, 14.0 Hz), 4.30 (1H, t, J = 4.8 Hz), 6.57 (2 H, m), 6.66 (1H, m). MS m/z 220 [M + H]+. Anal. Calcd for (C14H19NO2): C, H, N.
4aS,9aR-(−)-cis-4a-Ethyl-2-methyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((−)-21)
Compound (−)-18 (226.4 mg) was dissolved in hydrobromic acid (48%, 2.7 mL). The mixture was refluxed for about 30 min. The completion of the reaction was monitored by TLC as in (+)-14. The mixture was then neutralized with 28% NH4OH to pH∼10 and worked up as in (+)-14 to give (−)-21 (198 mg, 93%) as a white solid, mp 161-163 °C. [α]27D −98.9° (c 0.825, CDCl3); NMR (CDCl3, 400 MHz): δ 0.84 (3H, t, J = 7.5 Hz), 1.56 (1H, sextet, J = 7.3 Hz), 1.70 (sextet, J = 7.3 Hz), 1.78 (1H, ddd, J = 4.0, 9.2, 13.6 Hz), 2.01 (1H, ddd, J = 3.4, 6.0, 14.4 Hz), 2.13 (1H, ddd, J = 3.2, 9.6, 12.0 Hz), 2.26 (3H, s), 2.32 (1H, dd, J = 7.2, 12.0 Hz), 2.44 (1H, m), 2.76 (1H, ddd, J = 1.2, 5.2, 12.4 Hz), 4.45 (1H, dd, J = 5.6, 7.0 Hz), 6.58 (2H, m), 6.66 (1H, m). MS m/z 234 [M + H]+. Anal. Calcd for (C14H19NO2): C, H, N.
4aS,9aR-(−)-cis-4a-Ethyl-2-(2-phenylethyl)-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((−)-22)
Compound (−)-19 (201.0 mg) was dissolved in a hydrobromic acid (2.0 mL, 48%) and acetic acid (10 mL) mixture. The reaction was run and purified as in (+)-15 to give (−)-22 (150 mg, 78%) as a white solid, mp 172.0-173.5 °C. [α]27D −120.4° (c 0.785, CDCl3); NMR (CDCl3, 400 MHz): δ 0.83 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.2 Hz), 1.71 (1H, sextet, J = 7.2 Hz), 1.80 (1H, ddd, J = 4.2, 9.9, 14.0 Hz), 2.05 (1H, ddd, J = 3.2, 5.2, 14.0 Hz), 2.22 (1H, dt, J = 3.3, 10.7 Hz), 2.37 (1H, dd, J = 7.2, 12.0 Hz), 2.59 (3H, m), 2.79 (2H, m), 2.94 (1H, ddd, J = 1.5, 6.0, 12.0 Hz), 4.48 (1H, dd, J = 5.6, 7.2 Hz), 6.58 (2H, m), 6.66 (1H, m), 7.15-7.29 (5H, m); MS m/z 324 [M + H]+. Anal. Calcd for (C21H25NO2): C, H, N.
4aS,9aR,2′S-(−)-cis-2-(2′-Hydroxy-2-phenylethyl)-4a-ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((−)-23)
Compound (−)-20 (300 mg), (S)-(−)-styrene oxide (345.2 mg), and toluene (anhydrous, 8 mL) were reacted and purified as in (+)-16 to give (−)-23 (289 mg, 62.3%) as a beige color solid, mp 141.0-143.0 °C. [α]25D −88.5° (c 0.850, CDCl3); NMR (CDCl3, 400 MHz): δ 0.84 (3H, t, J = 7.4 Hz), 1.59 (1H, sextet, J = 7.3 Hz), 1.70 (1H, sextet, J = 7.3 Hz), 1.81 (1H, ddd, J = 4.2, 10.4, 14.6 Hz), 2.08 (1H, dt, J = 4.2, 14.8 Hz), 2.20 (1H, td, J = 3.0, 10.6 Hz), 2.41 (1H, t, J = 10.8 Hz), 2.50 (1H, dd, J = 3.4, 12.6 Hz), 2.57 (1H, dd, J = 7.2, 12.4 Hz), 2.86 (2H, m), 4.49 (1H, dd, J = 5.2, 6.8 Hz), 4.72 (1H, dd, J = 3.4, 10.6 Hz), 6.60 (2H, m), 6.66 (1H, m), 7.21-7.38 (5H, m); MS m/z 340 [M + H]+. Anal. Calcd for (C21H25NO3): C, H, N.
4aS,9aR,2′R-(−)-cis-2-(2′-Hydroxy-2-phenylethyl)-4a-ethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol ((−)-24)
Compound (−)-20 (283.5 mg), (R)-(−)-styrene oxide (326.2 mg), and toluene (anhydrous, 8 mL) were reacted and purified as shown for (+)-16 to give (−)-24 (255 mg, 58%) as a white solid, mp 165.0-168.0 °C. [α]27D -177.0° (c 0.775, CDCl3); NMR (CDCl3, 400 MHz): δ 0.84 (3H, t, J = 7.5 Hz), 1.58 (1H, sextet, J = 7.2 Hz), 1.71 (1H, sextet, J = 7.2 Hz), 1.85 (1H, ddd, J = 5.3, 9.2, 14.4 Hz), 2.04 (1H, dt, J = 4.3, 14.0 Hz), 2.37 (1H, dd, J = 6.8, 12.4 Hz), 2.48 (4H, m), 3.14 (1H, dd, J = 5.2, 12.4 Hz), 4.46 (1H, dd, J = 5.3, 6.9 Hz), 4.68 (1H, dd, J = 5.2, 8.8 Hz), 6.59 (2H, m), 6.67 (1H, m), 7.21-7.35 (5H, m); MS m/z 340 [M + H]+. Anal. Calcd for (C21H25NO3•0.3H2O): C, H, N.
X-ray Crystal Structure of 4aS,9aR-(−)-cis-4a-ethyl-6-methoxy-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridine ((−)-10)•R-(−)-mandelic acid
Single-crystal X-ray diffraction data on the (R)-(−) mandelic acid salt of the enantiomer 10 were collected using MoK± radiation and a Bruker APEX 2 CCD area detector. The structure was solved by direct methods and refined by full-matrix least squares on F2 values using the programs found in the SHELXTL suite (Bruker, SHELXTL v6.10, 2000, Bruker AXS Inc., Madison, WI). Parameters refined included atomic coordinates and anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms on carbons were included using a riding model [coordinate shifts of C applied to H atoms] with C-H distance set at 0.96 Å.
A 0.504 × 0.112 × 0.037 mm crystal of (−)-10)•R-(−)-mandelic acid was prepared for data collection coating with high viscosity microscope oil (Paratone-N, Hampton Research). The oil-coated crystal was mounted on a MicroMesh mount (MiTeGen, Ithaca, NY) and transferred to the cold stream (113 °K) on the diffractometer. The crystal was monoclinic in space group P21 with unit cell dimensions a = 11.1970(7) Å, b = 6.2958(4) Å, c = 13.8939(9) Å, and β= 96.147(1)°. Corrections were applied for Lorentz, polarization, and absorption effects. Data were 98.9% complete to 29.28° θ (approximately 0.73 Å) with an average redundancy of 3.76. The asymmetric unit contained a single molecule of (−)-10 and a single molecule of (−)-mandelic acid.
Quantum Chemisty and Superposition Study
The geometry optimization for the conformers of compound 22, the para-d oxide-bridged phenylmorphan, and morphine, in their protonated forms, was done in the gaseous phase with the density functional theory at the level of B3LYP/6-31G*.21 The conformers of 22 were also fully optimized in CHCl3 with the polarized continuum model with the UAKS parameters set to compare their energetics, which include the zero-point correction as well as the enthalpy and the entropy contribution at 298.15 K. For the superposition study, the optimized structures in the gaseous phase were overlaid onto morphine with the rigid fit of Quanta 2008 (Accelrys) using the heavy atoms of the piperidine ring as a common docking site.
Binding and Efficacy assays. Cell culture and membrane preparation
As noted previously,22 the recombinant CHO cells (hMOR-CHO, hDOR-CHO and hKOR-CHO) were produced by stable transfection with the respective human opioid receptor cDNA, and were provided by Dr. Larry Toll (SRI International, CA). The cells were grown on plastic flasks in DMEM (100%) (hDOR-CHO and hKOR-CHO) or DMEM/ F-12 (50%/50%) medium (hMOR-CHO) containing 10% FBS, and G-418 (0.10-0.2 mg/mL) under 95% air/5% CO2 at 37 °C. Cell monolayers were harvested and frozen at −80 °C.
[35S]GTP-γ-S binding assays
On the day of the assay, cells were thawed on ice for 15 min and homogenized using a polytron in 50 mM Tris-HCl, pH 7.4, containing 4 μ/mL leupeptin, 2 μ/mL chymostatin, 10 μ/mL bestatin and 100 μ/mL bacitracin. The homogenate was centrifuged at 30,000 × g for 10 min at 4 °C, and the supernatant discarded. The membrane pellets were resuspended in binding buffer and used for [35S]GTP-γ-S binding assays. [35S]GTP-γ-S binding was determined as described previously.23 Briefly, test tubes received the following additions: 50 μL buffer A (50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA), 50 μL GDP in buffer A/0.1% BSA (final concentration = 10 μM), 50 μL drug in buffer A/0.1% BSA, 50 μL [35S]GTP-γ-S in buffer A/0.1% BSA (final concentration = 50 pM), and 300 μL of cell membranes (50 μg of protein) in buffer B. The final concentrations of reagents in the [35S]GTP-γ-S binding assays were: 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM DTT, 10 μM GDP and 0.1% BSA. Incubations proceeded for 3 h at 25 °C. Nonspecific binding was determined using GTP-γ-S (40 μM). Bound and free [35S]-GTP-γ-S were separated by vacuum filtration through GF/B filters. The filters were punched into 24-well plates to which was added 0.6 mL LSC-cocktail (Cytoscint). Samples were counted, after an overnight extraction, in a Trilux liquid scintillation counter at 27% efficiency.
Supplementary Material
Acknowledgement
The research of the Drug Design and Synthesis Section, CBRB, NIDA & NIAAA, was supported by the NIH Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism, and NIDA supported the research of the Clinical Psychopharmacology Section. We thank Dr. John Lloyd (NIDDK) for the mass spectral data, Dr. Amy Newman (NIDA) for the use of her laboratory for some of this work, and NIDA for support of the Xray crystallographic studies (NIDA contract Y1-DA6002). The quantum chemical study utilized PC/LINUX clusters at the Center for Molecular Modeling of the NIH (http://cit.nih.gov), and this research was supported by the NIH Intramural Research Program through the Center for Information Technology.
Footnotes
Abbreviations: para-d-oxide-bridged phenylmorphan, (3R*,6aS*11aR*)-1,3,4,5,6,11a-hexahydro-2H-3,6a-methanobenzofuro[2,3-c]azocin-8-ol; EI, electron impact mass spectra; HRMS, high-resolution mass spectra; TLC, thin layer chromatography
Dedicated to the 100th anniversary of the Division of Medicinal Chemistry.
Supporting Information Available: Elemental analysis and crystallographic data. This material is available free of charge via the Internet at http://pubs.acs.org. Atomic coordinates for compounds (−)-10)•R-(−)-mandelic acid have been deposited with the Cambridge Crystallographic Data Centre (deposition number 723854). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK [fax: +44(0)-1223-336033 or deposit@ccdc.cam.ac.uk].
References
- 1.Iyer MR, Deschamps JR, Jacobson AE, Rice KC. Probes For Narcotic Receptor Mediated Phenomena. 38. An Expeditious Synthesis of rac-cis-4a-Ethyl-2-methyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol and rac-cis-2-Methyl-4a-phenethyl-1,2,3,4,4a,9a-hexahydrobenzofuro[2,3-c]pyridin-6-ol. Heterocycles. 2009;79:1061–1072. doi: 10.3987/COM-09-S(D)84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Rothman RB, Dersch C, Jacobson AE, Rice KC. The Synthesis and Opioid Binding Affinity of Optically Pure Benzofuro[2,3-c]pyridin-6-ols. Abstracts of Papers, 236th American Chemical Society National Meeting; American Chemical Society: Washington, DC; Philadelphia, PA. August 17-21, 2008; 2008. 2008; Abstract MEDI 189. [Google Scholar]
- 3.Hutchison AJ, de Jesus R, Williams M, Simke JP, Neale RF, Jackson RH, Ambrose F, Barbaz BJ, Sills MA. Benzofuro[2,3-c]pyridin-6-ols: synthesis, affinity for opioid-receptor subtypes and antinociceptive activity. J. Med. Chem. 1989;32:2221–2226. doi: 10.1021/jm00129a031. [DOI] [PubMed] [Google Scholar]
- 4.Burke TR, Jr., Jacobson AE, Rice KC, Silverton JV. Probes for narcotic receptor mediated phenomena. 4. Synthesis of (±)-2,3,4,5,6,6a-hexahydro-3-methyl-8-hydroxy-1H-4,11b-methanobenzofuro[2,3-d]azocine, an oxide-bridged 5-(m-hydroxyphenyl)morphan. J. Org. Chem. 1984;49:1051–1056. [Google Scholar]
- 5.Burke TR, Jr., Jacobson AE, Rice KC, Silverton JV. Probes for Narcotic Receptor Mediated Phenomena. 6. Synthesis of (±)-(1α,4aα,9aβ)-1,3,4,9a-Tetrahydro-2-methyl-2H-1,4-propanobenzofuro[2,3-c]pyridin-8-ol, an Oxide-bridged 5-(3-Hydroxyphenyl)morphan. J. Org. Chem. 1984;49:2508–2510. [Google Scholar]
- 6.Yamada K, Flippen-Anderson JL, Jacobson AE, Rice KC. Probes for Narcotic Receptor Mediated Phenomena; 29: Synthesis of rac-(4R,6aR,11bR)-3-Methyl 2,3,4,5,6,6a hexahydro-1H 4,11b-methanobenzofuro[3,2-d]azocin-10-ol, the para-a Oxide-bridged Phenylmorphan Isomer, and a New Route to rac-(4R,6aR,11bR)-3-Methyl-2,3,4,5,6,6a-hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocin-8-ol, the ortho-a oxide-bridged phenylmorphan isomer. Synthesis-Stuttgart. 2002:2359–2364. [Google Scholar]
- 7.Linders JTM, Mirsadeghi S, Flippen-Anderson JL, George C, Jacobson AE, Rice KC. Probes for Narcotic Receptor Mediated Phenomena. Part 30. Synthesis of rac-(3R,6aS,11aR)-2-Methyl-1,3,4,5,6,11a-hexahydro-2H-3,6a-methanobenzofuro[2,3-c]azocin-8-ol, an Epoxy Isomer of 5-Phenylmorphan. Helv. Chim. Acta. 2003;86:484–493. [Google Scholar]
- 8.Tadic D, Linders JTM, Flippen-Anderson JL, Jacobson AE, Rice KC. Probes for narcotic receptor mediated phenomena. Part 31: Synthesis of rac-(3R,6aS,11aS)-2-methyl-1,3,4,5,6,11a-hexahydro-2H-3,6a-methanobenzofuro[2,3-c]azocine-10-ol, and azocine-8-ol, the ortho-c and the para-c oxide-bridged phenylmorphan isomers. Tetrahedron. 2003;59:4603–4614. [Google Scholar]
- 9.Kodato S, Linders JTM, Gu X-H, Yamada K, Flippen-Anderson JL, Deschamps JR, Jacobson AE, Rice KC. Synthesis of rac-(1R,4aR,9aR)-2-methyl-1,3,4,9a-tetrahydro-2H-1,4a-propanobenzofuro[2.3-c]pyridin-6-ol. An Unusual Double Rearrangement Leading to the ortho- and para–f Oxide-Bridged Phenylmorphan Isomers. Org. & Biomolec. Chem. 2004;2:330–336. doi: 10.1039/b312633c. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto A, Przybyl AK, Linders JTM, Kodato S, Tian XR, Deschamps JR, George C, Flippen-Anderson JL, Jacobson AE, Rice KC. Probes for narcotic receptor-mediated phenomena. 33. Construction of a strained trans-5,6-ring system by displacement of a nitro-activated aromatic fluorine. Synthesis of the penultimate oxide-bridged phenylmorphans. J. Org. Chem. 2004;69:5322–5327. doi: 10.1021/jo040159k. [DOI] [PubMed] [Google Scholar]
- 11.Zezula J, Jacobson AE, Rice KC. A novel divergent synthesis of ortho-hydroxy-e and -f oxide-bridged 5-phenylmorphans. Heterocycles. 2007;71:881–889. [Google Scholar]
- 12.Zezula J, Singer LB, Przybyl AK, Hashimoto A, Dersch CM, Rothman RB, Deschamps J, Lee YS, Jacobson AE, Rice KC. Synthesis and pharmacological effects of the enantiomers of the N-phenethyl analogues of the ortho and para e- and f-oxide-bridged phenylmorphans. Org. & Biomol. Chem. 2008;6:2868–2883. doi: 10.1039/b803433h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurimura M, Liu H, Sulima A, Przybyl AK, Ohshima E, Kodato S, Deschamps JR, Dersch C, Rothman RB, Lee YS, Jacobson AE, Rice KC. Probes for Narcotic Receptor Mediated Phenomena. 37. Synthesis and Opioid Binding Affinity of the Final Pair of Oxide-Bridged Phenylmorphans, the ortho- and para-b Isomers and Their N-Phenethyl Analogues, and the Synthesis of the N-Phenethyl Analogues of the ortho- and para-d Isomers. J. Med. Chem. 2008;51:7866–7881. doi: 10.1021/jm800913d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiebel AC, Lee YS, Bilsky EJ, Giuvelis D, Deschamps JR, Parrish DA, Aceto MD, May EL, Harris EM, Coop A, Dersch CM, Partilla JS, Rothman RB, Jacobson AE, Rice KC. Probes for Narcotic Receptor Mediated Phenomena. 34. Synthesis and Structure-Activity Relationships of a Potent μ-Agonist δ-Antagonist and an Exceedingly Potent Antinociceptive in the Enantiomeric C9-Substituted 5-(3-Hydroxyphenyl)-N-phenylethylmorphan Series. J. Med. Chem. 2007;50:3765–3776. doi: 10.1021/jm061325e. [DOI] [PubMed] [Google Scholar]
- 15.Shulgin AT, Dyer DC. Psychotomimetic phenylisopropylamines. 5. 4-Alkyl-2,5-dimethoxyphenylisopropylamines. Journal of Medicinal Chemistry. 1975;18:1201–1204. doi: 10.1021/jm00246a006. [DOI] [PubMed] [Google Scholar]
- 16.Bell HM, Vanderslice CW, Spehar A. Reduction of organic halogen compounds by sodium borohydride. The Journal of Organic Chemistry. 1969;34:3923–3926. [Google Scholar]
- 17.Greiner E, Folk JE, Jacobson AE, Rice KC. A novel and facile preparation of bremazocine enantiomers through optically pure N-norbremazocines. Bioorganic & Medicinal Chemistry. 2004;12:233–238. doi: 10.1016/j.bmc.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y-S, Pike VW, Hodoscek M. Identification of the Transition States in the Inversion of 1,4-Benzodiazepines with the Ab Initio Replica Path Method. The Journal of Physical Chemistry A. 2008;112:1604–1611. doi: 10.1021/jp077738o. [DOI] [PubMed] [Google Scholar]
- 19.Belostotskii AM, Goren Z, Gottlieb HE. N-Inversion-Associated Conformational Dynamics Is Unusually Rapid in Morphine Alkaloids. J. Nat. Prod. 2004;67:1842–1849. doi: 10.1021/np049895+. [DOI] [PubMed] [Google Scholar]
- 20.Eliel EL, Kandasamy D, Yen C-Y, Hargrave KD. Conformational analysis. 39. Carbon-13 NMR spectra of saturated heterocycles. 9. Piperidine and N-methylpiperidine. J. Am. Chem. Soc. 1980;102:3698–3707. [Google Scholar]
- 21.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision D.01. Pittsburgh PA: 2004. [Google Scholar]
- 22.Fontana G, Savona G, Rodriguez B, Dersch CM, Rothman RB, Prisinzano TE. Synthetic studies of neoclerodane diterpenoids from Salvia splendens and evaluation of opioid receptor affinity. Tetrahedron. 2008;64:10041–10048. doi: 10.1016/j.tet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu H, Hashimoto A, Rice KC, Jacobson AE, Thomas JB, Carroll FI, Lai J, Rothman RB. Opioid Peptide Receptor Studies. 14. Stereochemistry Determines Agonist Efficacy and Intrinsic Efficacy in the [35S]GTP-γ-S Functional Binding Assay. Synapse. 2001;39:64–69. doi: 10.1002/1098-2396(20010101)39:1<64::AID-SYN9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.