Abstract
Purpose
The aim of this study is to determine the expression and localization of integrin α5β1 in human retinal pigment epithelium (RPE) and its ability to modulate RPE cell attachment, proliferation, migration, and F-actin cytoskeleton distribution.
Methods
Expression/localization of α5β1 were analyzed on human RPE by immunoblot/immunofluorescence. SearchLight technology was used to detect fibronectin secretion. RPE attachments to different substrates were determined using CytoMatrix™ screening kits. BrdU incorporation and wound-healing assays were used to test hfRPE proliferation and migration. F-actin cytoskeleton was visualized with phalloidin.
Results
α5β1 was detected in native adult and fetal human RPE. The α5-subunit is predominantly localized at the apical membrane of hfRPE, while the β1-subunit is uniformly detected at the apical/basolateral membranes. We also found that hfRPE cultures secrete significant amounts of fibronectin to the apical bath. JSM6427, a specific integrin α5β1 antagonist significantly inhibited hfRPE cell attachment to fibronectin, but not laminin, or collagen I or IV. JSM6427 also showed a strong inhibitory effect on bFGF, PDGF-BB or serum induced cell migration and proliferation. Furthermore, JSM6427 induced significant disruption of the F-actin cytoskeleton of dividing RPE cells but had no effect on quiescent cells.
Conclusions
The apical localization of α5β1 and the secretion of fibronectin to the apical bath suggest the presence of an autocrine loop that can guide the migration of RPE. The strong inhibitory effects of JSM6427 on human RPE cell attachment, proliferation, and migration is probably mediated by F-actin cytoskeletal disruption in proliferating cells and suggests a potential clinical use of this compound in proliferative retinopathies.
Keywords: Integrin α5β1, fibronectin, attachment, migration, proliferation, cytoskeleton
Introduction
Integrins are a family of heterodimeric plasma membrane proteins important for cell-cell and cell-extracellular matrix interaction.1, 2 They consist of variable α and β subunits, whose combination determine receptor specificity. Thus far, 19 different integrin α and eight β subunits have been identified that form at least 25 distinct α/β heterodimers. Integrins provide a link between their extracellular ligand and the cytoskeleton and help modulate various signaling pathways including, cell adhesion, migration, differentiation, angiogenesis, and wound healing.2
The retinal pigment epithelium (RPE) is a monolayer of hexagonal cells located between the distal retina and the choroidal blood supply and forms the outer blood-retina barrier. By participation in the visual cycle, photoreceptor outer segment phagocytosis, and transport of nutrients, ions, and fluid between the distal retina and the choriocapillaris, the RPE helps maintain the health and integrity of the distal retina.3-5 Studies of RPE cells have shown a wide spectrum of integrin receptor expression, 6, 7 however the precise localization and possible polarized distribution of these receptors are controversial or unknown. 8 9 Proliferative vitreoretinopathy (PVR) is the most common cause of surgical failure in the treatment of reghmatogenous retinal detachment characterized by the formation of epi- and sub-retinal membranes on the neuroretinal surface.10-12 There is accumulating evidence that the formation of a PVR membrane is a chronic wound-healing process characterized by extracellular matrix (ECM) accumulation, cell adhesion, migration and proliferation. Proliferative vitreoretinopathy affected membranes are composed of mixed population of cells. The RPE cell has been identified as critical part of PVR membranes in light/electron microscopy and immohistochemistry studies, and is thought to play an important role in the development and onset of PVR.13-16 Detachment of RPE cells from their subretinal monolayer after retinal injury, for example, retinal detachment or trauma, appears to be the crucial event in early stage of PVR. These detached RPE cells proliferate, migrate, and attach to ECM highly enriched in *-fibronectin.13, 17
Integrin α5β1 is a specific receptor of fibronectin through its arginine-glycine-aspartic acid (RGD) binding site.2 Together they play an important role in the development of the vascular system during embryogenesis.18 Knock out of the gene encoding the α5 subunit leads to lethal vasculature and cardiac defects.19 Similarly, knockout mice for fibronectin died early during development from a variety of defects including an improperly formed vasculature.20 Interestingly, fibronectin and integrin α5β1 were upregulated in growth factor or tumor-induced neovascularization,21 whereas expression of integrin α5β1 was low in quiescent vascular cells.22, 23 Robbin et al.24, 25 showed the presence of the α5 subunit on pigmented cells (probably of RPE origin) from PVR membrane and concluded that its presence was abnormal when compared to the lack of α5 staining on normal retina consistent with recent finding (Zahn et al., 2009 submitted). JSM6427 is a specific inhibitor of integrin α5β126 and recent studies indicated that systemic administration of JSM6427 suppressed laser-induced choroidal neovascularization in which integrin α5β1 was upregulated.27 Similar inhibitory effect was also observed in hypoxia-induced neovascularization.28 Most recently, Farber et al.29 reported the attenuation effect of this compound on glioma cell growth.
The present experiments show that α5β1 is mainly localized to the apical membrane of primary human fetal RPE culture and that fibronectin is constitutively secreted to the apical bath suggesting an autocrine signaling pathway that can mediate proliferative disease. We also show that the inhibitory effects of JSM6427 on dividing RPE cells are most likely mediated through F-actin which supports a therapeutic role for this antagonist.
Materials and Methods
Cell culture and reagents
The research followed the tenets of the Declaration of Helsinki and the NIH Institutional Review Board. Fetal eyes (gestation, 16-18 weeks) were obtained from Advanced Bioscience resources (Alameda, CA) and adult eyes were obtained from Analytical Biological Services Inc. (Wilmington, DE). Human fetal retinal pigment epithelial (hfRPE) cells were isolated and cultured as described previously.30 The culture medium was changed every 3 days, and passages 1-2 were used for all studies. For immunofluorescence localization and fibronectin secretion experiments, cells were seeded in transwells (Costar 0.4 μm pores, polyester membrane, Corning Incorporated, Corning, NY) and maintained for 6-8 weeks before experiment.
3-(2-[1-alkyl-5-[(pyridine-2-ylamino)-methyl]-pyrrolidin-3-yloxy]-acetylamino)-2-(alkyl-amino)-propionic acid (JSM6427), a selective integrin α5β1 antagonist26 and 3-(4-(3-arylureido)phenoxy)butanamido)-3-arylic-propanoic acid (JSM8009), an inactive control compound were supplied by Jerini AG Company.
Immunoblot analysis
Confluent monolayers of primary cultures of hfRPE (6-8 weeks) and native human adult RPE cells were lysed using RIPA buffer (Sigma-Aldrich, St. Louis, MO) supplemented with proteinase inhibitor cocktail (Roche, Indianapolis, IN). Cell lysate was centrifuged at 14,000 g for 10 minutes and the supernatant was collected. Protein concentration was determined using BCA™ protein assay (Pierce Biotechnology, Rockford, IL). Protein (8 -30 μg) was electrophoresed on a 4 - 12% Bis-Tris NuPAGE gel under non-reduced condition and electroblotted onto the nitrocellular membranes using XCell II™ Blot Module (Invitrogen, Carlsbad, CA). Nonspecific binding sites were blocked with StartingBlock™ T20 (TBS) (Pierce Biotechnology) and membranes were probed with rabbit anti-integrin α5 polyclonal antibody (Cat: AB1928), mouse anti-human integrin β1 monoclonal antibody (Clone: LM534) and mouse anti-human integrin α5β1 monoclonal antibody (Clone: JBS5) (Chemicon International Inc., Temecula, CA). Membranes were then incubated with horseradish peroxidase (HRP) conjugated secondary antibody (Pierce Biotechnology) and developed with Supersignal® West Dura Extended Duration Substrate (Pierce Biotechnology) and imaged using an Autochemie™ system (UVP, Upland, CA).
Immunofluorescence localization
For localization experiments, primary antibodies against integrin α5, β1 subunits, α5β1, or ZO-1 (Invitrogen) were labeled with Zenon technology following manufacturer's instructions (Invitrogen). Primary cultures (6-8 weeks) of hfRPE monolayers on transwells were fixed with 4% formaldehyde (Ted Pella Inc., Redding, CA), permeabilized for 10 minutes with 0.2% Triton X-100 (Sigma-Aldrich), and blocked with a signal enhancer (Image-iT FX; Invitrogen). RPE monolayers were incubated with antibodies pre-labeled with fluorophores and normal mouse serum or rabbit serum was used as the negative control. Samples were mounted on glass slides with antifade reagent containing DAPI (Prolong Gold; Invitrogen) and imaged with a Zeiss Axioplan 2 microscope with apotome using Axiovision 3.4 software (Carl Zeiss AG, Germany).
Attachment Assay
Cell attachment assays were performed using CytoMatrix™ screening kit (Chemicon International Inc.) following manufacturer's instructions. Briefly, 96 well plates were coated with fibronectin, vitronectin, laminin, collagen I, collagen IV or BSA (negative assay control for each plate). Subconfluent hfRPE cells were pretreated with integrin α5β1 antagonist JSM6427 (0.1, 0.4, 1.6, 6.3, 25, 100 μM) or inactive control compound JSM8009 for 16 hours. Cell were collected using trypsin and resuspended in Serum free medium (SFM; MEM-α modified medium (Sigma-Aldrich) containing non-essential amino acids (Sigma-Aldrich) and Glutamine-Penicillin-Streptomycin) (Invitrogen) (2 × 105 cells/ml) containing JSM6427 or JSM8009. Cells were seeded to pre-coated 96 well plate (100 μl/well) and put back to CO2 incubator (37 °C) for 60 minutes. Plates were washed with PBS containing Ca2+/Mg2+ and then 0.2% crystal violet in 10% ethanol (100 μl/well) was added and incubated for 5 minutes at room temperature. After washing with PBS, solubilization buffer (A 50/50 mixture of 0.1M NaH2PO4, pH 4.5 and 50 % ethanol) was added (100 μl/well). Plates were placed on a shaker for 10 minutes until the cell-bound stain was completely solubilized. Absorbance of the cells was measured at 540-570 nm with a Safire 3 spectrophotometric microplate reader (Tecan Trading AG, Switzerland).
Bromodeoxyuridine (BrdU) incorporation assay
Prior to the experiments, Primaria™ 96 well tissue culture plates were coated with 10 μg/ml fibronectin (Sigma-Aldrich). RPE cells were seeded in tissue culture plates (2.5 × 103/well) for 24 hours, and then serum starved in SFM for another 16 hours. Cells were then treated with different concentrations of JSM6427 or inactive control compound JSM8009. SFM was used as the negative control and SFM supplemented with 5% serum were used as positive control. After 48 hours treatment, RPE cells were incubated with BrdU for another 24 hours. The proliferation rate was evaluated using Cell proliferation ELISA BrdU Kit (Roche). Similar experiments were also performed using non-coated tissue culture plates and proceeded as above. Quadruplicates were used for each condition and the experiments were repeated using cell cultures from two different donors.
Wound healing assay
The wound healing assay was used to study the effects of integrin α5β1 antagonist on hfRPE cell migration, as described previously.31 hfRPE cells (100 × 103 cells/well) were seeded into fibronectin coated or uncoated Primaria™ 24 well tissue culture plates and grown for 4 weeks to confluence. Cell proliferation was suppressed by incubation with 10 μg/ml mitomycin C (Sigma-Aldrich) for 2 hour before all the experiments. A circular denuded area (7 mm in diameter) was made in each well using a custom designed cell scraper. Cells were treated with JSM6427 or inactive control compound JSM8009 (100, 400 μM) for 48 hours; cells were fixed in cold methanol and stained with ethidium homodimer-1 (EthD-1) (Invitrogen). To test the effects of JSM6427 on growth factor-induced cell migration, cells were treated with EGF, PDGF-BB, bFGF or combination of growth factors and JSM6427 (50 μM) in SFM condition. Cell migration was quantitated by counting the average number of cells that migrated into the denuded area in 16 microscope fields surrounding the circumference of the denuded area. Each condition was tested in triplicates and repeated using cells from different donors. Cell viability was evaluated using a Live/Dead Viability/Cytotoxicity Kit (Invitrogen).
F-actin cytoskeleton staining
Confluent monolayers of cells grown in transwell for 6-8 weeks were scratched with 1ml pipette tips, washed, and then grown for 24 hours. Cells were then treated with integrin α5β1 antagonist JSM6427 or inactive compound JSM8009 (100, 400 μM) for another 24 hours. Cells were fixed with 4% formaldehyde, permeabilized for 10 minutes with 0.2% Triton X-100, and blocked with a signal enhancer (Image-iT FX; Invitrogen). Cells were then stained with Alexa Fluor® 488 conjugated phalloidin (Invitrogen) and Alexa Fluor® 555 conjugated mouse anti-human ZO-1 antibody. Samples were mounted using Prolong Gold antifade reagent containing DAPI and imaged with a Zeiss Axioplan 2 microscope with apotome using Axiovision 3.4 software.
Fibronectin secretion
Confluent monolayers of hfRPE cultured in transwell for 6-8 weeks were washed with SFM, incubated overnight and then replaced with fresh SFM. Cells were cultured for another 24 hours and supernatants from both apical and basal compartments were collected. Fibronectin secretion level was assayed using a commercial technology (SearchLight; Pierce Biotechnology) as described previously.32 Quadruplicate were used and the final concentrations were adjusted to normalize for the volume difference in apical and basal compartments of the transwell.
Statistical Analysis
Data are expressed as mean ± sem.; statistical significance (Student's test, unpaired, two-tailed) was accepted as P < 0.05.
Results
Localization of integrin α5β1 and fibronectin secretion in hfRPE
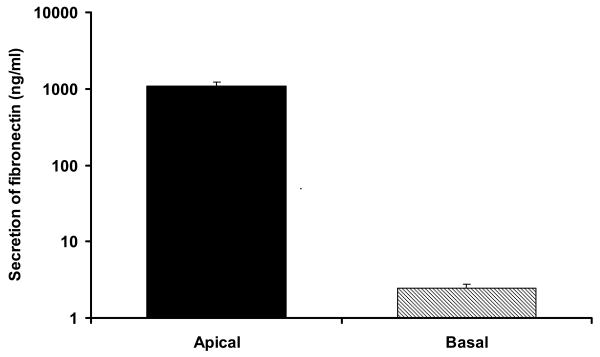
Integrin α5, β1 subunits were detected in native human adult RPE and native and cultured hfRPE by microarray analysis (Wang F, et al. IOVS 2006; 47: ARVO E-Abstract 2855). In Figure 1, the protein expression of α5, β1 subunits was further confirmed by immunoblots, which show antibody specific bands of 140 kDa (α5) and 110 kDa (β1). Figure 2 shows the immunofluorescence staining of α5, β1 subunits on hfRPE. Nuclei are stained with DAPI (blue), the tight junction marker ZO-1 is stained in red (panel A) or green (panel B, C), while the α5 subunits are stained in green (panel A), β1 subunits in red (panel B) and α5β1 in red (panel C). The middle part of each panel is an en face view of the monolayer shown as a maximum intensity projection through the Z-axis. It also shows a uniform hexagonal pattern of ZO-1, typical of epithelial cells. Integrin α5, β1, subunits appears as punctuate staining visible throughout the cells. The top and right side of each panel shows a cross section through the Z-plane. In these cross-sections, ZO-1 serves as a tight junction marker separating the apical and basolateral sides of the epithelial cells. Nuclei (blue) are located close to the basal side and serve as a marker to help define basal localization. High gain images of the cross section through the Z-plane, are shown at the top of each panel. Integrin α5 subunit (panel A) was mainly detected on the apical side, although some expression can be detected at the basolateral membrane. In contrast, integrin β1 subunit was uniformly detected at both the apical and basolateral membranes. In separate experiments using another antibody that targets α5β1, localization was detected mainly at the apical membrane consistent with the localization of α5 (panel C). Fibronectin is a specific ligand for this receptor and as shown in Figure 3, intact monolayers of hfRPE constitutively secrete significant amounts of fibronectin to the apical bath (1.1 μg/ml). This combination suggests a possible autocrine signaling pathway at the apical membrane.
Figure 1.
Western blot analysis identifying constitutive expression of integrin α5β1 in human RPE. 10 μg (native adult RPE) or 30 μg (hfRPE) of protein were loaded and electrophoresed. In each sample, prominent antibody specific bands (black arrow) for α5, β1 subunits are shown at approximately 140 and 110 kDa, respectively.
Figure 2.
Immunofluorescence localization of integrin α5β1 in hfRPE. Central part of each panel is an en face view of a cell culture monolayer shown as a maximum intensity projection through the Z-axis. Top and right side of each panel is a cross section through the Z-plane. Nuclei were stained with DAPI (blue) and ZO-1 tight junction marker (red in panel A, green in panel B, C). High gain images of the cross section through the Z-plane are shown at the top of each panel. Integrin α5 (green; panel A) was mainly detected on the apical side and integrin β1 (red; panel B) was detected at both the apical and basolateral membranes. Anti-α5β1 antibody staining (red, panel C) shows that integrin α5β1 was mainly detected on the apical membrane.
Figure 3.
Fibronectin secretion in hfRPE. Searchlight Technology was used to detect secretion of fibronectin to apical and basal baths. Final concentrations were adjusted to normalize for the volume difference in apical and basal baths of the Transwell assembly. Secretion to apical bath ≈ 450-fold greater than to basal bath (n = 4).
JSM6427, a specific α5β1 antagonist, inhibits RPE attachment to fibronectin
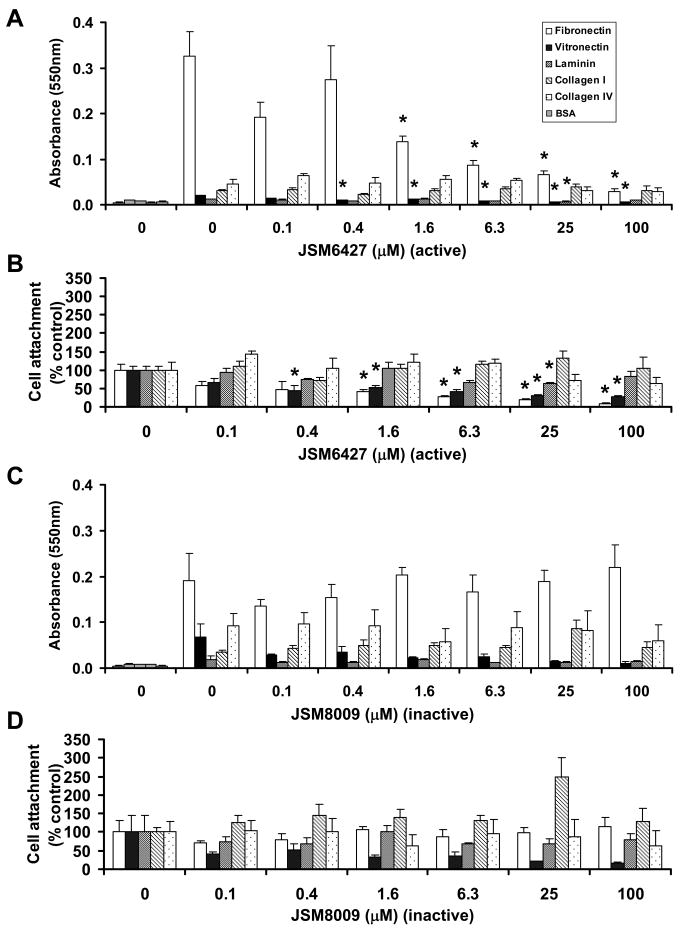
The data summarized in Figure 4B shows that this antagonist significantly inhibits RPE cell attachment to fibronectin in the range from ≈ 2 to 100 μM (P < 0.05); this inhibitory effect is monotonic with concentration. In contrast, an inactive form of this inhibitor (JSM8009) showed no effect over the entire range tested from 0.1 to 100 μM (Fig 4C, D). Although the attachment to vitronectin was also inhibited by JSM6427 in the range from 0.4 to 100 μM (P < 0.02), the level of RPE attachment to fibronectin, in absolute terms, as measured colormetrically (METHODS), was ≈ 16-fold greater than the attachment to vitronectin (Fig 4A). The observations that JSM6427 has no significant effects on RPE attachment to collagen I, IV, laminin and vitronectin confirm the specificity of JSM6427 for α5β1.
Figure 4.
Effects of integrin α5β1 antagonist on hfRPE attachment to fibronectin, vitronectin, laminin, collagen I and IV. A, B. Cells treated with various concentrations of JSM6427 (0.1-100 μM) or C, D inactive control compound JSM8009. BSA coated wells serve as a negative assay control for each plate (gray shaded bars). For comparison we provide both the original raw data before normalization (A, C) and the data normalized to untreated control (B, D). JSM6427 significantly inhibited RPE cell attachment to Fibronectin. (P < 0.05; n = 4)
JSM6427 inhibits RPE cell proliferation and migration
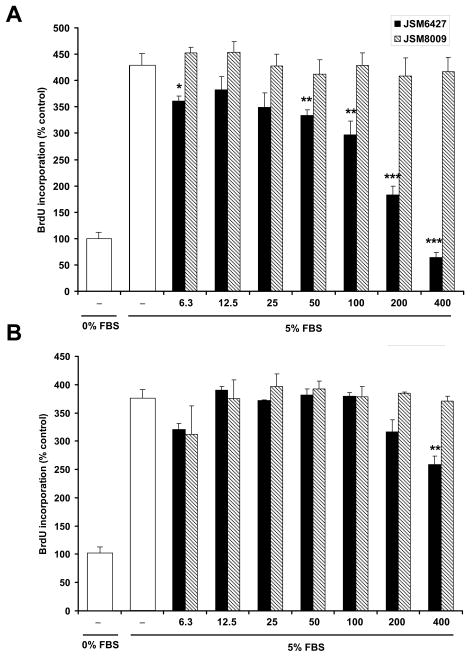
The data summarized in Figure 5A shows that JSM6427 (solid bars), but not JSM8009 (hatched bars) significantly inhibits the serum-induced proliferation of hfRPE cells cultured on fibronectin pre-coated 96 well plates (72 hours). This inhibition extends in a dose-dependent manner from ≈ 6 to 400 μM with some variability at the lower concentrations. At all concentrations of the inactive analogue, there was no significant decrease of FBS-induced cell proliferation. In contrast, the active compound (JSM6427) caused monotonic and significant inhibition starting at 50 μM. The effect of the inactive compound (JSM8009) was constant over all concentrations which serves as a control for the effect of the active compound and validates its specificity. In a separate set of experiments (Fig 5B), in the absence of coating, we found that the effect of the inactive analogue is also constant over all tested concentrations. In this case, the active compound was much less effective and exhibited significant inhibition of proliferation only at the highest concentration. This inhibitory effect can be assumed to be due to constitutive fibronectin secretion by RPE (Fig 3). These observations reflect a specific blockade of RPE proliferation by integrin α5β1 antagonist and highlights the importance of the fibronectin substrate.
Figure 5.
Dose response of integrin α5β1 antagonist on hfRPE proliferation (6.3 - 400 μM). A. Experiments performed on fibronectin coated plate. B. Experiments performed on uncoated plate. JSM6427 dose-dependently inhibited the proliferation of hfRPE cultured on plate coated with fibronectin. Open bars in A, B are controls for 0 and 5% FBS. * P<0.05, ** P<0.01, *** P<0.001 compared to control (5% FBS).
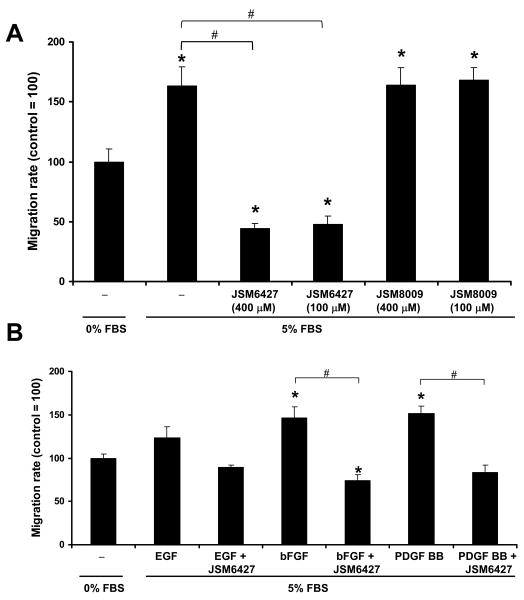
Figure 6A shows that JSM6427 completely abolished the stimulatory effect of 5% serum on hfRPE migration. In contrast, the inactive control compound, JSM8009, showed no effect on cell migration. In addition, 50 μM JSM6427 significantly inhibited bFGF and PDGF-BB induced hfRPE cell migration (Figure 6B). In all of these experiments, cell viability was evaluated and confirmed using an appropriate assay. Figure 7 shows that the percentage of viable cells was more than 96% in every examined group and this percentage was not significantly decreased after incubation with JSM6427 or the control compound (JSM8009).
Figure 6.
Effects of integrin α5β1 antagonist on hfRPE migration. A. Integrin α5β1 antagonist JSM6427 (100 μM, 400 μM) completely abolished the stimulatory effect of 5% serum induced hfRPE migration (uncoated plate). B. JSM6427 (50 μM) significantly inhibited bFGF and PDGF-BB induced hfRPE migration on fibronectin coated plates. * P<0.05, compared to SFM control. # P<0.05
Figure 7.
Cell cytotoxicity assay. JSM6427 had no appreciable effect on cell viability, up to 400 μM.
JSM6427 disrupts hfRPE F-actin cytoskeleton
Images (A-C) in Figure 8 show immunofluorescence staining of post-confluent monolayers of hfRPE. In all images, F-actin filaments were stained with phalloidin (green), junctional complexes were stained with ZO-1 (red), and nuclei were stained with DAPI (blue). Neither integrin antagonist, JSM6427 (B) nor control compound JSM8009 (C) had any appreciable effect on F-actin cytoskeleton of quiescent post-confluent RPE cells compared to untreated control. The middle set of images (D-F) are from cells that are in the process of reaching confluence and like the post-confluent cells, do not show any effect of treatment with either JSM6427 or its control, JSM8009. In contrast, the lower sets of images (G-L) illustrate cells that are non-confluent along a wound edge. For these cells, addition of JSM6427 caused the aggregation of F-actin filaments around the circumference of the cell (H, K white arrow). Panel II shows an enlarged view of images J, K and L showing the effect of JSM6427 on cytoskeleton organization and ZO-1 distribution. To better visualize the ZO-1 staining, images M, N, and O only show the red and blue channels. In addition to the circumferential accumulation of F-actin, JSM6427 also causes the loss of the “interdigitated” structure of ZO-1 observed in the regions of cell - cell contact (Fig 8 panel II, compare inserts in M and N). Taken together, panels I and II indicate that this antagonist can only affect actively dividing cells.
Figure 8.
Phalloidin staining of F-actin cytoskeleton. Post-confluent monolayer of hfRPE cells were scratched and either untreated or treated with 100 μM of JSM6427 or JSM8009 for 48 hours. Panel I: Image A-C, post-confluent area, D-F, sub-confluent area, G-L, non-confluent (edge area). Nuclei were stained with DAPI (blue), ZO-1 tight junction marker (red) and phalloidin staining of F-actin (green). White arrows indicate areas of JSM6427 –induced cytoskeletal changes. Panel II: Enlarged view of images J, K and L showing the effect of JSM6427 on cytoskeleton organization and ZO-1 distribution. Images including the red and blue channels are also shown in the bottom of panel II for better visualization of ZO-1(arrow head directs attention to the insert which shows detail of the ZO-1 interdigitation).
Discussion
Human fetal RPE cultures constitutively secrete fibronectin to the apical bath. In Figure 3, the much lower protein secretion to the basal, compared to the apical bath probably occurred because the ECM coated porous plastic support, per se, can limit secretion of large proteins, such as fibronectin (≈ 440 kDa). Fibronectin is a specific ligand for integrin α5β1 that is present on both native and cultured human RPE cells. The α5 subunit is mainly located at the RPE apical membrane while the β1 subunit is localized to both the apical and basolateral membranes. The integrin α5β1 antagonist JSM6427 significantly and specifically inhibited hfRPE cell attachment to fibronectin; it also inhibited bFGF and PDGF-BB induced cell migration and serum induced cell proliferation. In dividing RPE cells this antagonist significantly altered the F-actin cytoskeleton, but had no effect on F-actin filaments of quiescent cells.
Reports on the presence and localization of α5 in RPE cells have been inconsistent, even within species. For example, using immunohistochemistry techniques, Brem et al. found no α5 in adult native human RPE cells.25 while another study used immunofluorescence to detect integrin α5 at the basal surface of the RPE.33 Anderson et al, used non-permeabilized adult human RPE cell cultures and found the fibronectin receptor to be localized to the plasma membrane of apical microvilli in vitro and a similar result was obtained using native monkey RPE.8 A later report using permeabilized adult monkey RPE cells with access to the basolateral surface showed fibronectin receptor (α5β1) localization at the basolateral surface.7 The present experiments employed confluent, high resistance primary cultures of well characterized human fetal RPE 30, 32, 34-36 to reexamine the question of localization. A panel of antibodies (α5 subunit, β1 subunit and α5β1) and immunoblots show that the integrin α5 and β1 subunits are expressed on both native human RPE and primary cultures of hfRPE; immunofluorescence experiments show that α5 is localized mainly on the apical membrane, while β1 is localized on both the apical and basolateral membranes of hfRPE. In addition, microarray experiments show that mRNA expression of integrin α5 and β1 subunits are much higher in primary culture of hfRPE cells compared to the native human RPE cells (Wang F, et al. IOVS 2006; 47: ARVO E-Abstract 2855). Also the mRNA expression of β1 is much higher than α5 indicating other β1 integrins in human RPE.
The difference in the behavior of primary culture of hfRPE and native human adult RPE may be related to the matrix coating, which contains various ECM components, including fibronectin. The high level of hydrocortisone in the culture media could increase integrin expression level.37 Early reports suggest that the expression level of integrin α5β1 can be significantly affected by RPE cell state - actively dividing cells express higher integrin α5β1 compared to the quiescent cells.33 The protein levels of integrin α5 and β1 subunits in native RPE may depend on donor age and non-reported systemic diseases; this conclusion is based on our limited sample of adult human eyes (n = 2). In another study, Zarbin et al. assessed the differences in expression of integrin subunits in fetal and human adult RPE. Integrin subunits α1, α2, α3, α4 and α5 (mRNA) was significantly lower in uncultured aged native RPE, compared to primary cultured cells. In fetal RPE, α2, α3, α5, β4, and β5 subunit mRNAs were significantly lower in uncultured compared to passaged cultured cells.38 Taken together, these data indicate that α5β1 integrin expression correlates closely with proliferative capacity of RPE cells.
The interface between photoreceptors and the RPE is the site of a weak, but functionally important adhesive interaction. Disruption of this interface can lead to a number of proliferative and degenerative changes, concluding in photoreceptor cell death.39, 40 The apical distribution of integrin α5 subunit suggests a possible role for integrin α5β1 in the attachment of RPE to the neuroretina. The apical microvilli of native RPE cells ensheath the photoreceptor outer segments and phagocytose large numbers of shed ROS tips each day.41 Mechanisms involving αvβ5 integrins and other receptors have been implicated in the phagocytosis by RPE.42-44 Zhao et al.45 have presented data indicating that α5β1 integrin plays a role in the phagocytosis of fibronectin by sub-confluent RPE. Whether or not this conclusion holds for confluent/native RPE remains to be determined.
Cell attachment of RPE cells onto fibronectin, but not collagen I or IV, laminin or vitronectin was significantly inhibited by a specific α5β1 antagonist, JSM6427 (1700-fold greater for α5β1 than other integrins; Zahn et al. 2009 submitted). In diseases such as PVR46, 47, PDR48 or AMD49, normally quiescent RPE cells can reenter the cell cycle and initiate migration and proliferation. In vitro, integrin α5β1 mediated cell adhesion to fibronectin is particularly efficient in supporting mitogen-dependent proliferation of fibroblastic, epithelial, and endothelial cells in vitro.50 In our studies, JSM6427 significantly inhibited bFGF and PDGF-BB induced RPE cell migration as well as cell proliferation suggesting this small molecule as a possible therapeutic agent.
Physiological relevance
We demonstrated the inhibitory effect of an integrin α5β1 antagonist on RPE cell attachment, migration and proliferation. This inhibition is accompanied by a concomitant reorganization of RPE cytoskeleton with distinctive features including the aggregation of F-actin filaments around the circumference of the cell, resembling the quiescent state. Activated, migrating cells at the wound edges often adopt a more fibroblast-like phenotype that forms actin stress fibers. These stress fibers terminate in focal adhesions which contain integrins that anchor the cells to the extracellular matrix. Intracellularly focal adhesions contain numerous structural and signaling molecules. These are needed to transmit integrin-mediated signals into the cell during proliferation, apoptosis or migration. Disrupting these structures leads to the inactivation of these integrin-mediated signaling pathways. Actin is a major component of the cytoskeleton-forming microfilaments and, together with microtubules and intermediate filaments, mediate cell movement.51, 52 Depending on their structure, integrin receptors can interact with ECM molecules, cytoskeleton, and related proteins such as talin.53 For example, β4 has a unique and very large cytoplasmic domain that can link to keratin filaments while β1 possesses a short cytoplasmic domain that connects with actin-based filaments.54 This unique difference creates a transmembrane link between the extracellular matrix and the cell cytoskeleton, and adds a regulatory function in a variety of adhesion-related cellular events. It is worth noting that JSM6427 showed significant disruption of F-actin cytoskeleton of dividing RPE cells, but had no effect on F-actin filaments of quiescent cells. We also found that JSM6427 had no effect on mitochondrial membrane potential or cell apoptosis of quiescent cells (not shown). Therefore, JSM6427 may provide therapeutic benefit under certain pathophysiological conditions, such as PVR, PDR or AMD.
We show that primary cultures of human fetal RPE constitutively secrete fibronectin to the apical bath to create an autocrine signaling loop with low levels of α5β1 localized to the apical membrane. The present experiments lead us to speculate that in vivo, fibronectin secretion may populate the subretinal space. Following a disease stimulus this secretion would be increased along with a α5β1 receptor expression increase and this combination of events could provide the basis for RPE cell proliferation and migration in proliferative retinopathies.
Acknowledgments
It is our pleasure to thank Roland Stragies (Jerini AG) for the synthesis of test compounds. This work was supported by the Intramural Research Program of the National Institutes of Health, National Eye Institute.
References
- 1.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. [Google Scholar]
- 4.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 5.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 6.Elner SG, Elner VM. The integrin superfamily and the eye. Invest Ophthalmol Vis Sci. 1996;37:696–701. [PubMed] [Google Scholar]
- 7.Anderson DH, Johnson LV, Hageman GS. Vitronectin receptor expression and distribution at the photoreceptor-retinal pigment epithelial interface. J Comp Neurol. 1995;360:1–16. doi: 10.1002/cne.903600102. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DH, Guerin CJ, Matsumoto B, Pfeffer BA. Identification and localization of a beta-1 receptor from the integrin family in mammalian retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1990;31:81–93. [PubMed] [Google Scholar]
- 9.Chen W, Joos TO, Defoe DM. Evidence for beta 1-integrins on both apical and basal surfaces of Xenopus retinal pigment epithelium. Exp Eye Res. 1997;64:73–84. doi: 10.1006/exer.1996.0183. [DOI] [PubMed] [Google Scholar]
- 10.Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch Ophthalmol. 1981;99:1445–1454. doi: 10.1001/archopht.1981.03930020319025. [DOI] [PubMed] [Google Scholar]
- 11.Hiscott PS, Grierson I, McLeod D. Retinal pigment epithelial cells in epiretinal membranes: an immunohistochemical study. Br J Ophthalmol. 1984;68:708–715. doi: 10.1136/bjo.68.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiscott P, Sheridan C, Magee RM, Grierson I. Matrix and the retinal pigment epithelium in proliferative retinal disease. Prog Retin Eye Res. 1999;18:167–190. doi: 10.1016/s1350-9462(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 13.Charteris DG. Proliferative vitreoretinopathy: pathobiology, surgical management, and adjunctive treatment. Br J Ophthalmol. 1995;79:953–960. doi: 10.1136/bjo.79.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinores SA, Campochiaro PA, Conway BP. Ultrastructural and electron-immunocytochemical characterization of cells in epiretinal membranes. Invest Ophthalmol Vis Sci. 1990;31:14–28. [PubMed] [Google Scholar]
- 15.Morino I, Hiscott P, McKechnie N, Grierson I. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br J Ophthalmol. 1990;74:393–399. doi: 10.1136/bjo.74.7.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz D, de la Cruz ZC, Green WR, Michels RG. Proliferative vitreoretinopathy. Ultrastructural study of 20 retroretinal membranes removed by vitreous surgery. Retina. 1988;8:275–281. [PubMed] [Google Scholar]
- 17.Casaroli Marano RP, Vilaro S. The role of fibronectin, laminin, vitronectin and their receptors on cellular adhesion in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994;35:2791–2803. [PubMed] [Google Scholar]
- 18.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Francis SE, Goh KL, Hodivala-Dilke K, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 20.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons-Wingerter P, Kasman IM, Norberg S, et al. Uniform overexpression and rapid accessibility of alpha5beta1 integrin on blood vessels in tumors. Am J Pathol. 2005;167:193–211. doi: 10.1016/s0002-9440(10)62965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnussen A, Kasman IM, Norberg S, Baluk P, Murray R, McDonald DM. Rapid access of antibodies to alpha5beta1 integrin overexpressed on the luminal surface of tumor blood vessels. Cancer Res. 2005;65:2712–2721. doi: 10.1158/0008-5472.CAN-04-2691. [DOI] [PubMed] [Google Scholar]
- 24.Robbins SG, Brem RB, Wilson DJ, et al. Immunolocalization of integrins in proliferative retinal membranes. Invest Ophthalmol Vis Sci. 1994;35:3475–3485. [PubMed] [Google Scholar]
- 25.Brem RB, Robbins SG, Wilson DJ, et al. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci. 1994;35:3466–3474. [PubMed] [Google Scholar]
- 26.Stragies R, Osterkamp F, Zischinsky G, et al. Design and synthesis of a new class of selective integrin alpha5beta1 antagonists. J Med Chem. 2007;50:3786–3794. doi: 10.1021/jm070002v. [DOI] [PubMed] [Google Scholar]
- 27.Umeda N, Kachi S, Akiyama H, et al. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69:1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- 28.Maier AK, Kociok N, Zahn G, et al. Modulation of hypoxia-induced neovascularization by JSM6427, an integrin alpha5beta1 inhibiting molecule. Curr Eye Res. 2007;32:801–812. doi: 10.1080/02713680701553052. [DOI] [PubMed] [Google Scholar]
- 29.Farber K, Synowitz M, Zahn G, et al. An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci. 2008;39:579–585. doi: 10.1016/j.mcn.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy TL, Sakamoto T, Hinton DR, et al. Migration of retinal pigment epithelium cells in vitro is regulated by protein kinase C. Exp Eye Res. 1995;60:683–695. doi: 10.1016/s0014-4835(05)80010-7. [DOI] [PubMed] [Google Scholar]
- 32.Shi G, Maminishkis A, Banzon T, et al. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49:4620–4630. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proulx S, Guerin SL, Salesse C. Effect of quiescence on integrin alpha5beta1 expression in human retinal pigment epithelium. Mol Vis. 2003;9:473–481. [PubMed] [Google Scholar]
- 34.Economopoulou M, Hammer J, Wang FE, Fariss R, Maminishkis A, Miller SS. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1454–1463. doi: 10.1167/iovs.08-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voloboueva LA, Killilea DW, Atamna H, Ames BN. N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration. FASEB J. 2007;21:4077–4086. doi: 10.1096/fj.07-8396com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, Maminishkis A, Wang FE, Miller SS. PDGF-C and -D induced proliferation/migration of human RPE is abolished by inflammatory cytokines. Invest Ophthalmol Vis Sci. 2007;48:5722–5732. doi: 10.1167/iovs.07-0327. [DOI] [PubMed] [Google Scholar]
- 37.Haslam SZ, Woodward TL. Reciprocal regulation of extracellular matrix proteins and ovarian steroid activity in the mammary gland. Breast Cancer Res. 2001;3:365–372. doi: 10.1186/bcr324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarbin MA. Analysis of retinal pigment epithelium integrin expression and adhesion to aged submacular human Bruch's membrane. Trans Am Ophthalmol Soc. 2003;101:499–520. [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA. The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci. 1981;21:10–16. [PubMed] [Google Scholar]
- 40.Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983;24:927–942. [PubMed] [Google Scholar]
- 41.Sarangarajan R, Apte SP. Melanization and phagocytosis: implications for age related macular degeneration. Mol Vis. 2005;11:482–490. [PubMed] [Google Scholar]
- 42.Finnemann SC. Role of alphavbeta5 integrin in regulating phagocytosis by the retinal pigment epithelium. Adv Exp Med Biol. 2003;533:337–342. doi: 10.1007/978-1-4615-0067-4_42. [DOI] [PubMed] [Google Scholar]
- 43.Boyle D, Tien LF, Cooper NG, Shepherd V, McLaughlin BJ. A mannose receptor is involved in retinal phagocytosis. Invest Ophthalmol Vis Sci. 1991;32:1464–1470. [PubMed] [Google Scholar]
- 44.Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci. 1996;109(Pt 2):387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- 45.Zhao MW, Jin ML, He S, Spee C, Ryan SJ, Hinton DR. A distinct integrin-mediated phagocytic pathway for extracellular matrix remodeling by RPE cells. Invest Ophthalmol Vis Sci. 1999;40:2713–2723. [PubMed] [Google Scholar]
- 46.Ryan SJ. The pathophysiology of proliferative vitreoretinopathy in its management. Am J Ophthalmol. 1985;100:188–193. doi: 10.1016/s0002-9394(14)75004-4. [DOI] [PubMed] [Google Scholar]
- 47.Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–241. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- 48.Esser P, Heimann K, Bartz-schmidt KU, et al. Apoptosis in proliferative vitreoretinal disorders: possible involvement of TGF-beta-induced RPE cell apoptosis. Exp Eye Res. 1997;65:365–378. doi: 10.1006/exer.1997.0341. [DOI] [PubMed] [Google Scholar]
- 49.Miller H, Miller B, Ryan SJ. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest Ophthalmol Vis Sci. 1986;27:1644–1652. [PubMed] [Google Scholar]
- 50.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 51.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 54.Lussier C, Basora N, Bouatrouss Y, Beaulieu JF. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc Res Tech. 2000;51:169–178. doi: 10.1002/1097-0029(20001015)51:2<169::AID-JEMT8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]